Multivariate Imaging for Fast Evaluation of In Situ Dark Field Microscopy Hyperspectral Data
Abstract
:1. Introduction
2. Results and Discussion
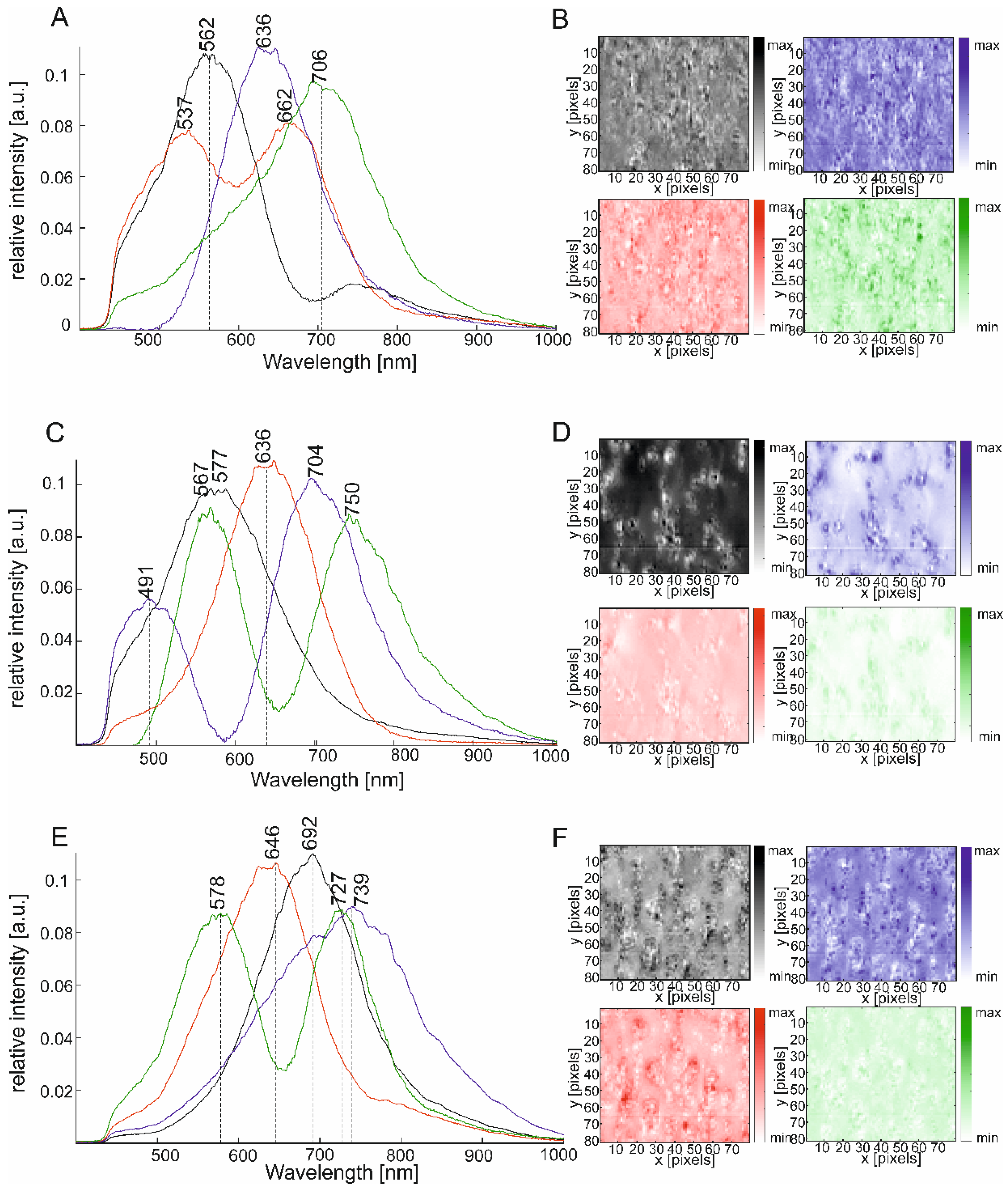
2.1. Exploring Large Dark Field Scattering Data Sets of Silver Nanoparticles
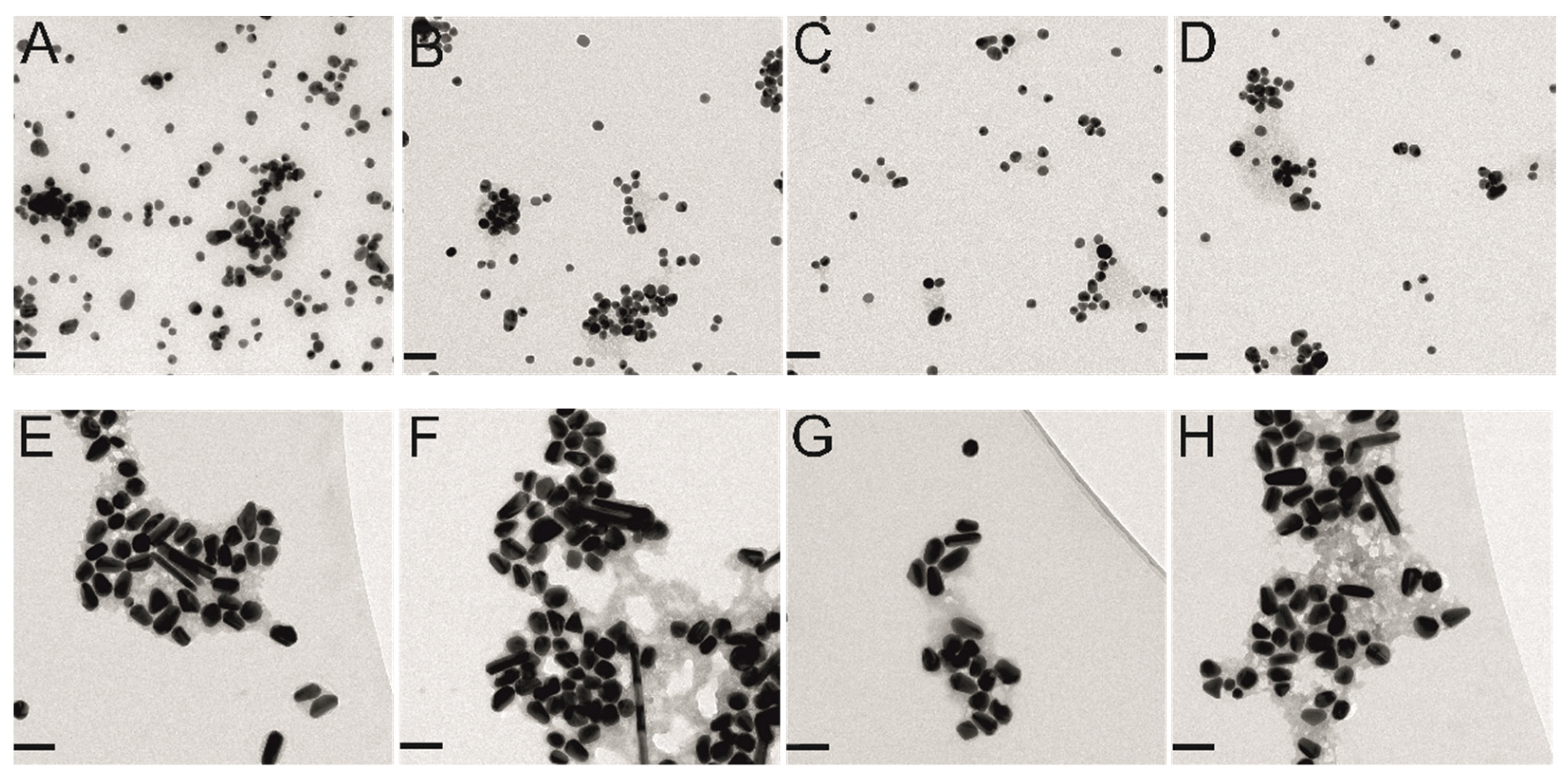
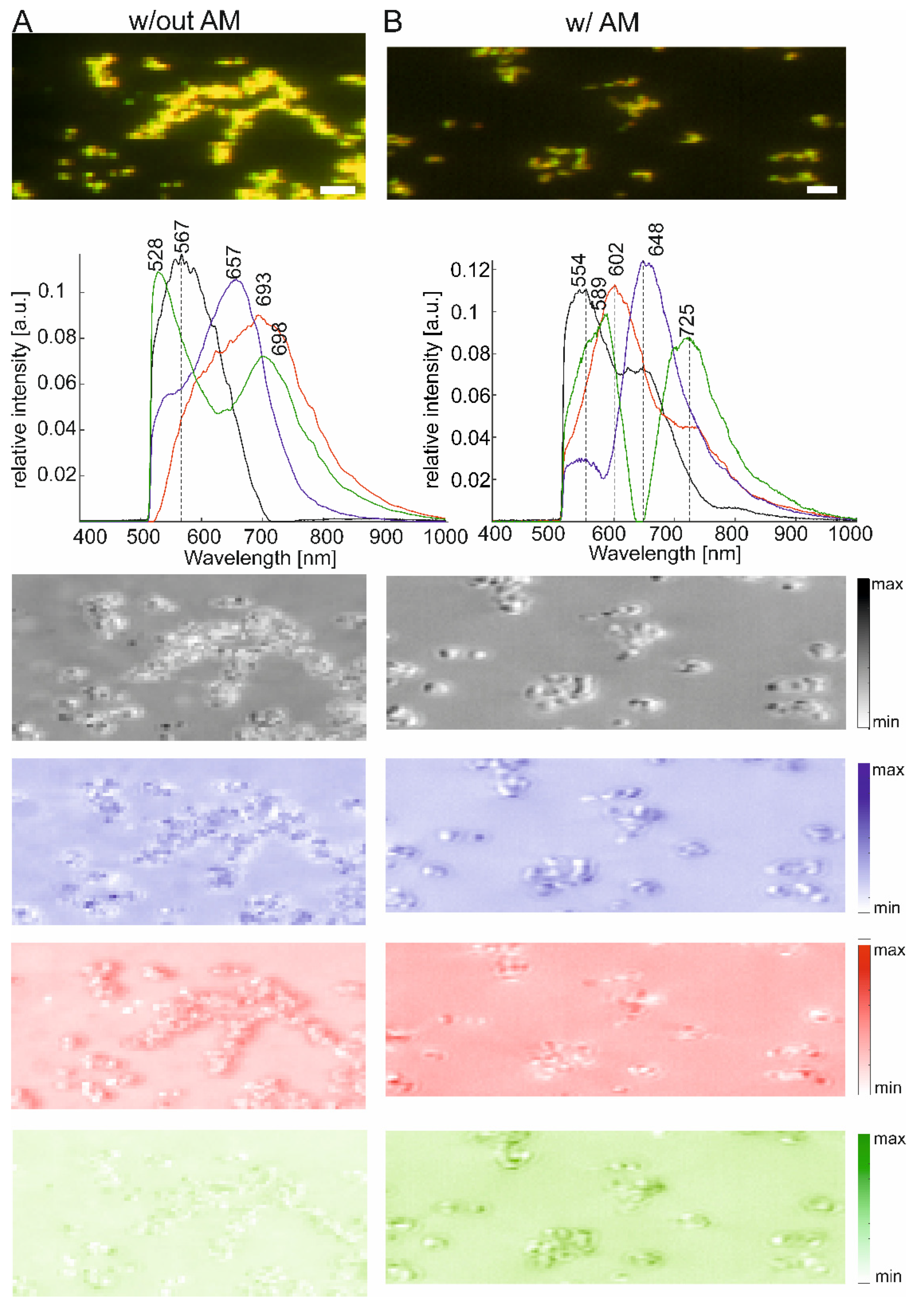
2.2. Agglomerates of Gold Nanoparticles with and without Protein
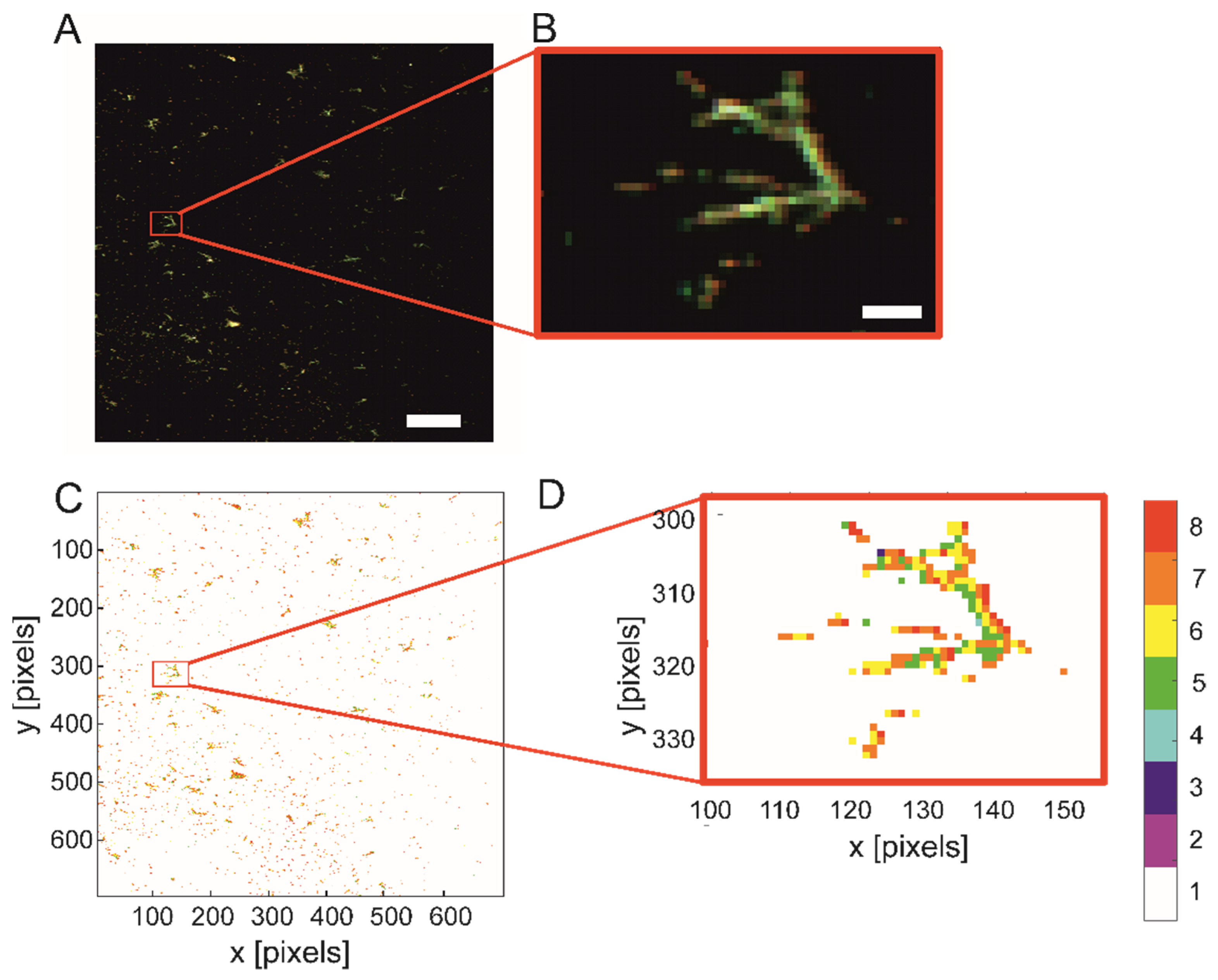
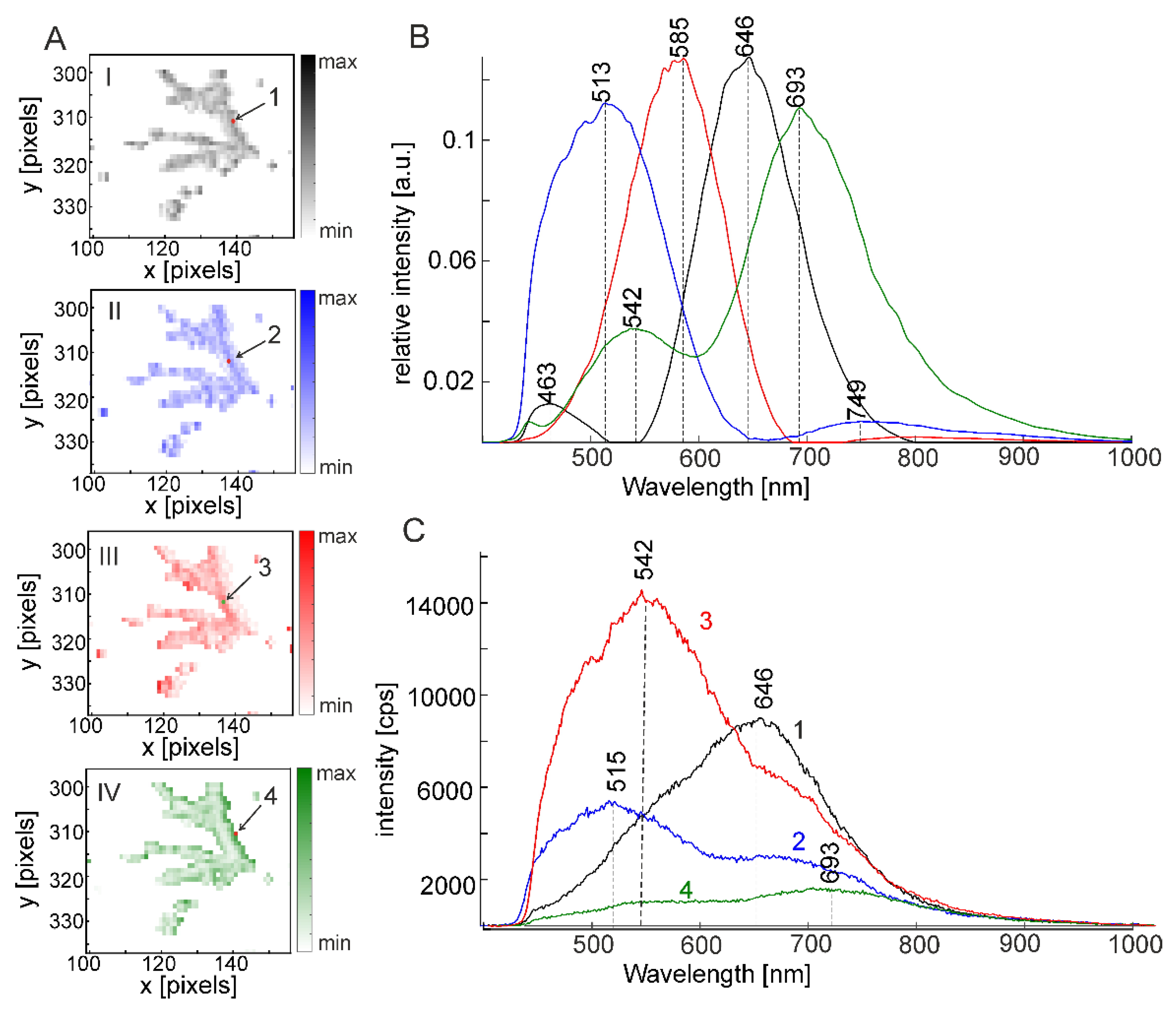
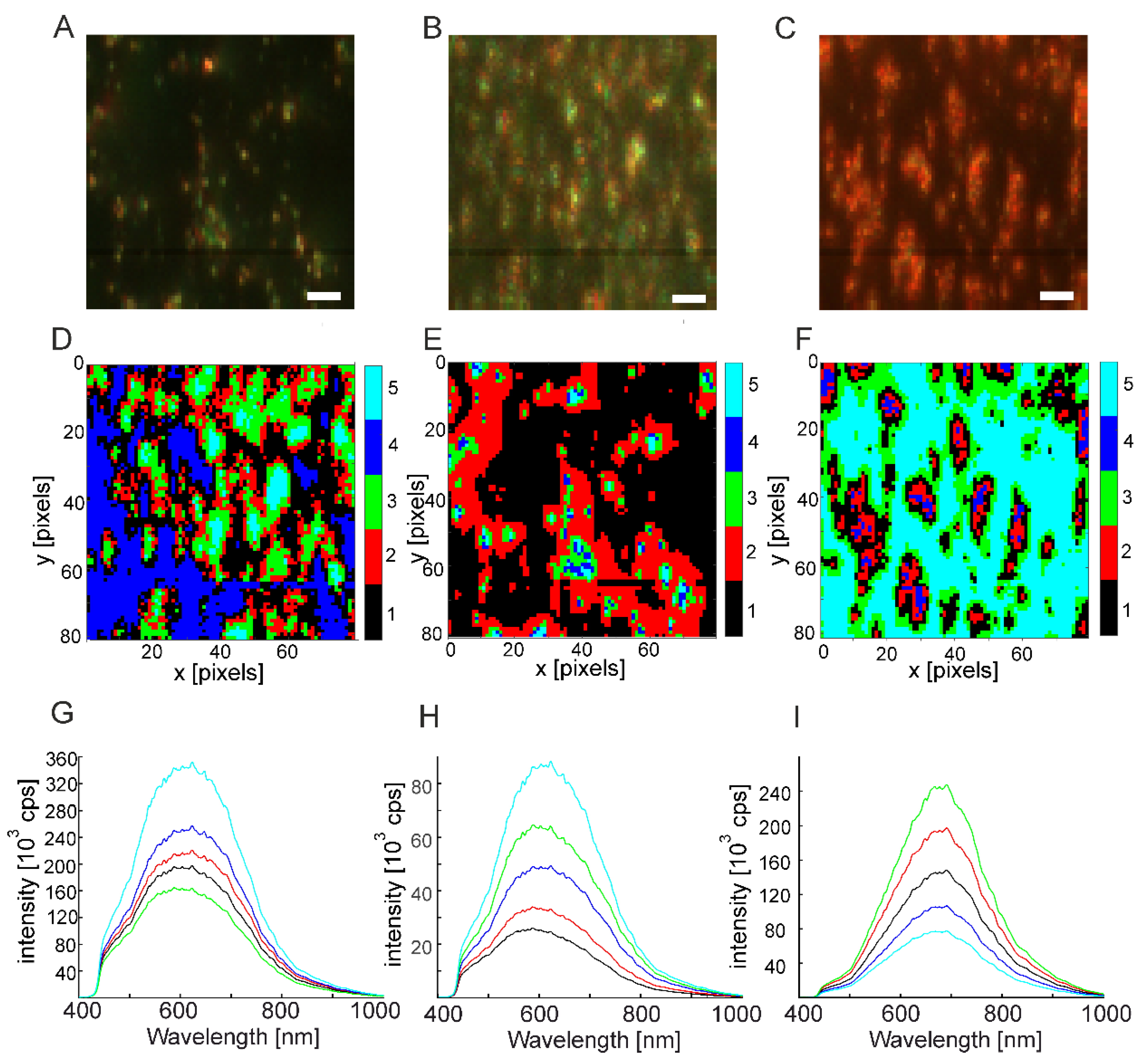
2.3. Silver Nanoparticles in Polymerizing Samples
3. Materials and Methods
3.1. Sample Preparation
3.2. Dark Field Scattering Microscopy
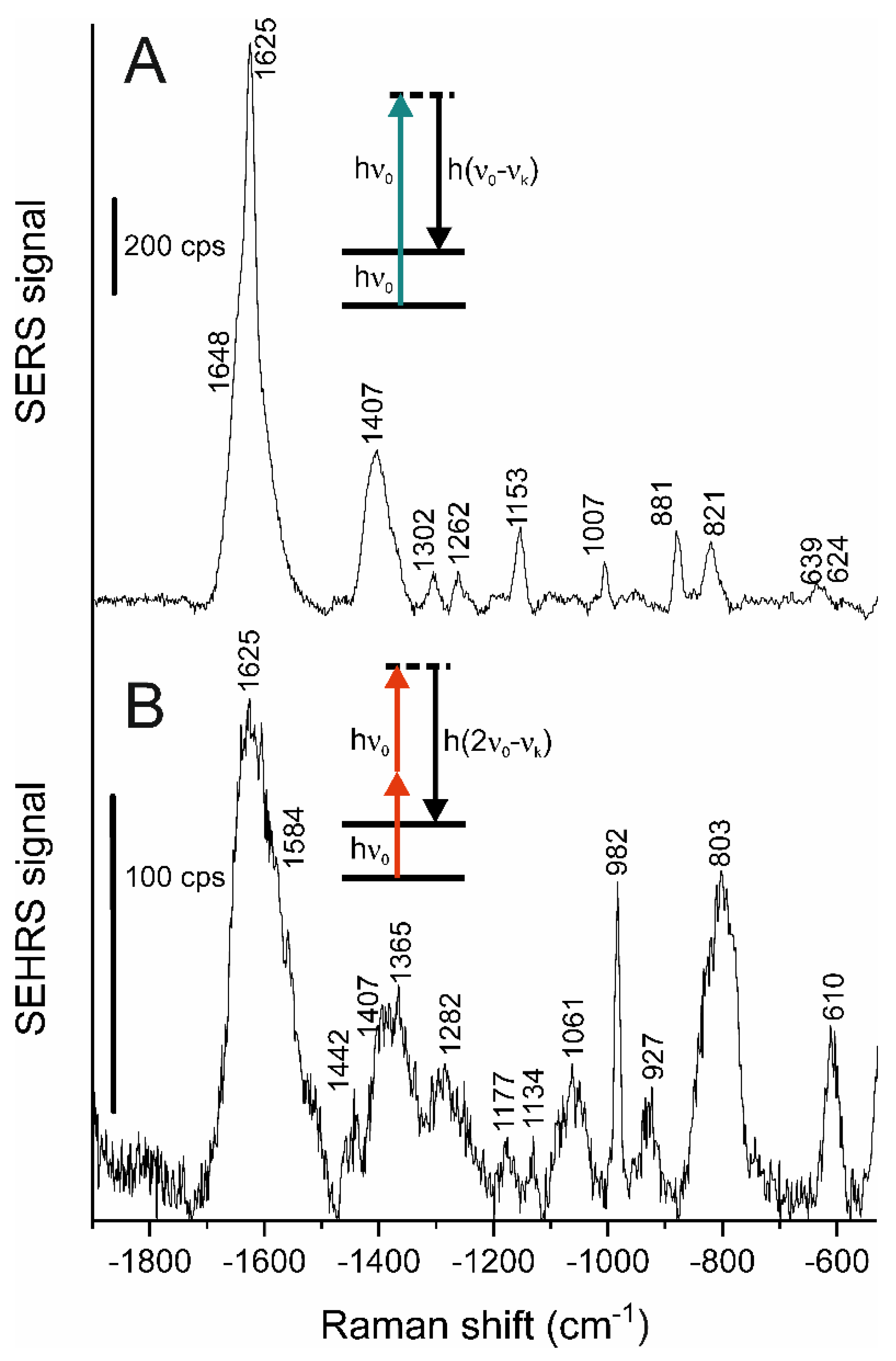
3.3. SERS and SEHRS Experiments
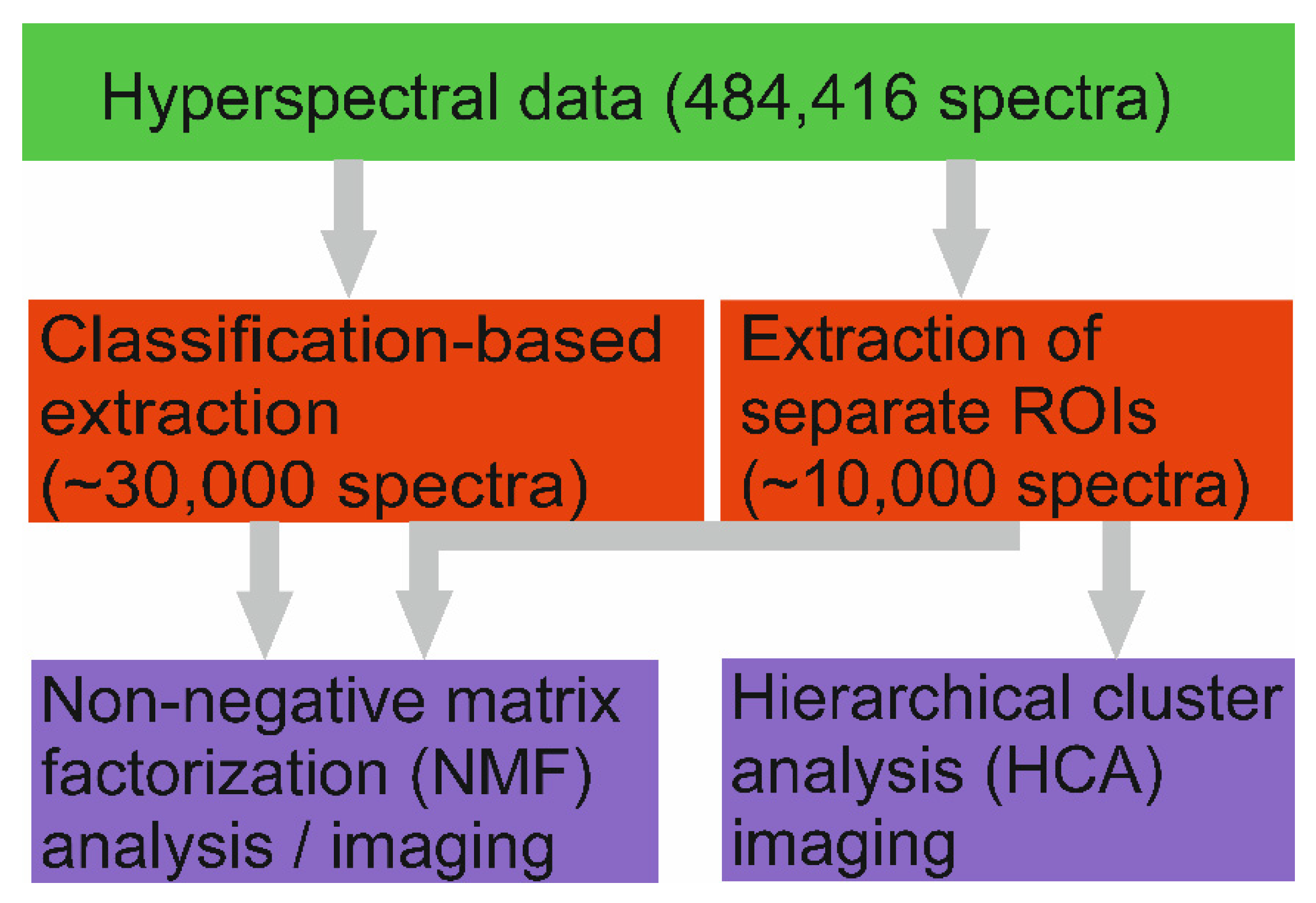
3.4. Multivariate Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sönnichsen, C.; Alivisatos, A.P. Gold Nanorods as Novel Nonbleaching Plasmon-Based Orientation Sensors for Polarized Single-Particle Microscopy. Nano Lett. 2005, 5, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Novo, C.; Funston, A.; Wang, H.; Staleva, H.; Zou, S.; Mulvaney, P.; Xia, Y.; Hartland, G.V. Dark-field microscopy studies of single metal nanoparticles: Understanding the factors that influence the linewidth of the localized surface plasmon resonance. J. Mater. Chem. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Wei, H.; Reyes-Coronado, A.; Nordlander, P.; Aizpurua, J.; Xu, H. Multipolar plasmon resonances in individual Ag nanorice. ACS Nano. 2010, 4, 2649–2654. [Google Scholar] [CrossRef]
- Ringe, E.; Zhang, J.; Langille, M.R.; Mirkin, C.A.; Marks, L.D.; Van Duyne, R.P. Correlating the structure and localized surface plasmon resonance of single silver right bipyramids. Nanotechnology. 2012, 23, 444005. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, C.; Gollmer, D.A.; Horrer, A.; Fulmes, J.; Weber-Bargioni, A.; Cabrini, S.; Schuck, P.J.; Kern, D.P.; Fleischer, M. A single particle plasmon resonance study of 3D conical nanoantennas. Nanoscale 2013, 5, 7861–7866. [Google Scholar] [CrossRef]
- Celiksoy, S.; Ye, W.; Wandner, K.; Kaefer, K.; Sönnichsen, C. Intensity-Based Single Particle Plasmon Sensing. Nano Lett. 2021, 21, 2053–2058. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Jakab, A.; Khalavka, Y.; Sönnichsen, C. Light-controlled one-sided growth of large plasmonic gold domains on quantum rods observed on the single particle level. Nano Lett. 2009, 9, 3710–3714. [Google Scholar] [CrossRef]
- Fang, C.; Lee, Y.H.; Shao, L.; Jiang, R.; Wang, J.; Xu, Q.H. Correlating the plasmonic and structural evolutions during the sulfidation of silver nanocubes. ACS Nano. 2013, 7, 9354–9365. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Banjac, K.; Verlekar, S.S.; Cometto, F.P.; Lingenfelder, M.; Galland, C. Structural Order of the Molecular Adlayer Impacts the Stability of Nanoparticle-on-Mirror Plasmonic Cavities. ACS Photon. 2021, 8, 1863–1872. [Google Scholar] [CrossRef]
- Oh, H.; Park, Y.; Song, H. Tracking Underpotential Deposition of Copper on Individual Silver Nanocubes by Real-Time Single-Particle Plasmon Scattering Imaging. J. Phys. Chem. C 2020, 124, 20398–20409. [Google Scholar] [CrossRef]
- McFarland, A.D.; Van Duyne, R.P. Single Silver Nanoparticles as Real-Time Optical Sensors with Zeptomole Sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Collins, S.S.E.; Wei, X.; McKenzie, T.G.; Funston, A.M.; Mulvaney, P. Single Gold Nanorod Charge Modulation in an Ion Gel Device. Nano Lett. 2016, 16, 6863–6869. [Google Scholar] [CrossRef]
- Zhou, J.; Panikkanvalappil, S.R.; Kang, S.; Yu, S.; Zhang, S.; El-Sayed, M.; Tsukruk, V.V. Enhanced Electrochemical Dark-Field Scattering Modulation on a Single Hybrid Core-Shell Nanostructure. J. Phys. Chem. C. 2019, 123, 28343–28352. [Google Scholar] [CrossRef]
- Al-Zubeidi, A.; McCarthy, L.A.; Rafiei-Miandashti, A.; Heiderscheit, T.S.; Link, S. Single-particle scattering spectroscopy: Fundamentals and applications. Nanophotonics. 2021, 10, 1621–1655. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Torreggiani, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Hollow Au/Ag nanostars displaying broad plasmonic resonance and high surface-enhanced Raman sensitivity. Nanoscale 2015, 7, 13629–13637. [Google Scholar] [CrossRef]
- Asiala, S.M.; Marr, J.M.; Gervinskas, G.; Juodkazis, S.; Schultz, Z.D. Plasmonic color analysis of Ag-coated black-Si SERS substrate. Phys. Chem. Chem. Phys. 2015, 17, 30461–30467. [Google Scholar] [CrossRef]
- Yoon, J.H.; Selbach, F.; Langolf, L.; Schlücker, S. Ideal Dimers of Gold Nanospheres for Precision Plasmonics: Synthesis and Characterization at the Single-Particle Level for Identification of Higher Order Modes. Small 2018, 14, 1702754. [Google Scholar] [CrossRef]
- Kitahama, Y.; Uemura, S.; Katayama, R.; Suzuki, T.; Itoh, T.; Ozaki, Y. Polarization dependence of tip-enhanced Raman and plasmon-resonance Rayleigh scattering spectra. Appl. Phys. Lett. 2017, 110, 233104. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Shih, T.M.; Yang, W.; Hu, S.; Hu, X.; Li, J.; Ren, B.; Mao, B.; Yang, Z.; et al. Plasmon-Induced Magnetic Resonance Enhanced Raman Spectroscopy. Nano Lett. 2018, 18, 2209–2216. [Google Scholar] [CrossRef]
- Jassim, N.M.; Wang, K.; Han, X.; Long, H.; Wang, B.; Lu, P. Plasmon assisted enhanced second-harmonic generation in single hybrid Au/ZnS nanowires. Opt. Mater. 2017, 64, 257–261. [Google Scholar] [CrossRef]
- Wang, X.; Liu, C.; Gao, C.; Yao, K.; Masouleh, S.S.M.; Berté, R.; Ren, H.; Menezes, L.D.S.; Cortés, E.; Bicket, I.C.; et al. Self-Constructed Multiple Plasmonic Hotspots on an Individual Fractal to Amplify Broadband Hot Electron Generation. ACS Nano. 2021, 15, 10553–10564. [Google Scholar] [CrossRef]
- Ma, Y.P.; Li, Q.; Luo, J.B.; Huang, C.Z.; Zhou, J. Weak Reaction Scatterometry of Plasmonic Resonance Light Scattering with Machine Learning. Anal. Chem. 2021, 93, 12131–12138. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Chen, S.X.; Hu, P.P.; Huang, C.Z.; Zhou, J. Automated Plasmonic Resonance Scattering Imaging Analysis via Deep Learning. Anal. Chem. 2021, 93, 2619–2626. [Google Scholar] [CrossRef]
- Iakovlev, I.A.; Deviatov, A.Y.; Lvov, Y.; Fakhrullina, G.; Fakhrullin, R.F.; Mazurenko, V.V. Probing Diffusive Dynamics of Natural Tubule Nanoclays with Machine Learning. ACS Nano. 2022, 16, 5867–5873. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wo, X.; Wang, Y.; Li, M.; Zhou, C.; Wang, W. Postrecording Pixel-Reconstruction Approach for Correcting the Lateral Drifts in Surface Plasmon Resonance Microscope. Anal. Chem. 2019, 91, 13620–13626. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Irudayaraj, J. Single-Cell Quantification of Cytosine Modifications by Hyperspectral Dark-Field Imaging. ACS Nano. 2015, 9, 11924–11932. [Google Scholar] [CrossRef]
- El-Khoury, P.Z.; Joly, A.G.; Hess, W.P. Hyperspectral Dark Field Optical Microscopy of Single Silver Nanospheres. J. Phys. Chem. C. 2016, 120, 7295–7298. [Google Scholar] [CrossRef]
- De O. Pereira, M.L.; de Souza Paiva, R.; Vasconcelos, T.L.; Oliveira, A.G.; Oliveira Salles, M.; Toma, H.E.; Grasseschi, D. Photoinduced electron transfer dynamics of AuNPs and Au@PdNPs supported on graphene oxide probed by dark-field hyperspectral microscopy. Dalton Trans. 2020, 49, 16296–16304. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Perez, P.; Pelaz, B.; Tsoutsi, D.; Soliman, M.G.; Parak, W.J.; Rivera-Gil, P. Hyperspectral-enhanced dark field analysis of individual and collective photo-responsive gold-copper sulfide nanoparticles. Nanoscale 2021, 13, 13256–13272. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lei, G.; Zheng, L.L.; Gao, P.F.; Huang, C.Z. HSI colour-coded analysis of scattered light of single plasmonic nanoparticles. Nanoscale 2016, 8, 11467–11471. [Google Scholar] [CrossRef]
- Zhang, Z.; Gernert, U.; Gerhardt, R.F.; Höhn, E.-M.; Belder, D.; Kneipp, J. Catalysis by Metal Nanoparticles in a Plug-In Optofluidic Platform: Redox Reactions of p-Nitrobenzenethiol and p-Aminothiophenol. ACS Catal. 2018, 8, 2443–2449. [Google Scholar] [CrossRef]
- Quaroni, L.; Chumanov, G. Preparation of Polymer-Coated Functionalized Silver Nanoparticles. J. Am. Chem. Soc. 1999, 121, 10642–10643. [Google Scholar] [CrossRef]
- Büchner, T.; Drescher, D.; Traub, H.; Schrade, P.; Bachmann, S.; Jakubowski, N.; Kneipp, J. Relating surface-enhanced Raman scattering signals of cells to gold nanoparticle aggregation as determined by LA-ICP-MS micromapping. Anal. Bioanal. Chem. 2014, 406, 7003–7014. [Google Scholar] [CrossRef]
- Drescher, D.; Büchner, T.; Schrade, P.; Traub, H.; Werner, S.; Guttmann, P.; Bachmann, S.; Kneipp, J. Influence of Nuclear Localization Sequences on the Intracellular Fate of Gold Nanoparticles. ACS Nano. 2021, 15, 14838–14849. [Google Scholar] [CrossRef]
- Zhang, Z.; Merk, V.; Hermanns, A.; Unger, W.E.S.; Kneipp, J. Role of Metal Cations in Plasmon-Catalyzed Oxidation: A Case Study of p-Aminothiophenol Dimerization. ACS Catal. 2017, 7, 7803–7809. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface-Enhanced Hyper Raman Spectra of Aromatic Thiols on Gold and Silver Nanoparticles. J. Phys. Chem. C 2020, 124, 6233–6241. [Google Scholar] [CrossRef]
- Agrawal, V.V.; Kulkarni, G.U.; Rao, C.N.R. Surfactant-promoted formation of fractal and dendritic nanostructures of gold and silver at the organic-aqueous interface. J. Colloid Interface Sci. 2008, 318, 501–506. [Google Scholar] [CrossRef]
- Brennan, M.E.; Whelan, A.M.; Kelly, J.M.; Blau, W.J. Silver nanoparticle self-organization into dendritic fractals. Synth. Met. 2005, 154, 205–208. [Google Scholar] [CrossRef]
- Karpov, S.V.; Gerasimov, V.S.; Isaev, I.L.; Markel, V.A. Spectroscopic studies of fractal aggregates of silver nanospheres undergoing local restructuring. J. Chem. Phys. 2006, 125, 111101. [Google Scholar] [CrossRef]
- Szekeres, G.P.; Kneipp, J. SERS Probing of Proteins in Gold Nanoparticle Agglomerates. Front. Chem. 2019, 7, 30. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Gühlke, M.; Kneipp, J. Surface-Enhanced Hyper-Raman Spectra of Adenine, Guanine, Cytosine, Thymine, and Uracil. J. Phys. Chem. C. 2016, 120, 15415–15423. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; Sirimuthu, N.M.S. Surface-Enhanced Raman Spectroscopy (SERS) for Sub-Micromolar Detection of DNA/RNA Mononucleotides. J. Am. Chem. Soc. 2006, 128, 15580–15581. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Stefancu, A.; Herman, K.; Tódor, I.S.; Iancu, S.D.; Moisoiu, V.; Leopold, L.F. The role of adatoms in chloride-activated colloidal silver nanoparticles for surface-enhanced Raman scattering enhancement. Beilstein J. Nanotechnol. 2018, 9, 2236–2247. [Google Scholar] [CrossRef] [PubMed]
- Stefancu, A.; Nan, L.; Zhu, L.; Chiș, V.; Bald, I.; Liu, M.; Leopold, N.; Maier, S.A.; Cortes, E. Controlling Plasmonic Chemistry Pathways through Specific Ion Effects. Adv. Opt. Mater. 2022, 10, 2200397. [Google Scholar] [CrossRef]
- Mukherjee, M.; Saha, S.K.; Chakravorty, D. Fractal growth of silver nanoclusters in a polymer medium. Appl. Phys. Lett. 1993, 63, 42–44. [Google Scholar] [CrossRef]
- Biswas, A.; Eilers, H.; Hidden, F., Jr.; Aktas, O.C.; Kiran, C.V.S. Large broadband visible to infrared plasmonic absorption from Ag nanoparticles with a fractal structure embedded in a Teflon AF® matrix. Appl. Phys. Lett. 2006, 88, 013103. [Google Scholar] [CrossRef]
- Madzharova, F.; Heiner, Z.; Kneipp, J. Surface enhanced hyper Raman scattering (SEHRS) and its applications. Chem. Soc. Rev. 2017, 46, 3980–3999. [Google Scholar] [CrossRef] [PubMed]
- Hulteen, J.C.; Young, M.A.; Van Duyne, R.P. Surface-Enhanced Hyper-Raman Scattering (SEHRS) on Ag Film over Nanosphere (FON) Electrodes: Surface Symmetry of Centrosymmetric Adsorbates. Langmuir 2006, 22, 10354–10364. [Google Scholar] [CrossRef]
- Suh, J.S.; Michaelian, K.H. Surface-enhanced raman spectroscopy of acrylamide and polyacrylamide adsorbed on silver colloid surfaces: Polymerization of acrylamide on silver. J. Raman Spectrosc. 1987, 18, 409–414. [Google Scholar] [CrossRef]
- Gupta, M.K.; Bansil, R. Laser Raman spectroscopy of polyacrylamide. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 353–360. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Wang, S.; Chen, W.; Lei, Y. A convenient route to polyacrylonitrile/silver nanoparticle composite by simultaneous polymerization–reduction approach. Polymer. 2001, 42, 8315–8318. [Google Scholar] [CrossRef]
- Sarkar, A.; Hegde, S.; Mukherjee, T.; Kapoor, S. Photo-induced formation of silver nanoparticles and polymerization of acrylamide derivatives. Res. Chem. Intermed. 2010, 36, 309–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Kneipp, J. Surface Molecular Patterning by Plasmon-Catalyzed Reactions. ACS Appl. Mater. Interfaces. 2021, 13, 43708–43714. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and Surface-Enhanced Raman of Dyes on Silver and Gold Sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Tkachenko, A.; Xie, H.; Franzen, S.; Feldheim, D.L. Assembly and Characterization of Biomolecule-Gold Nanoparticle Conjugates and Their Use in Intracellular Imaging. In NanoBiotechnology Protocols; Rosenthal, S.J., Wright, D.W., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 85–99. [Google Scholar]
- Kirchner, S.R.; Smith, K.W.; Hoener, B.S.; Collins, S.S.E.; Wang, W.; Cai, Y.Y.; Kinnear, C.; Zhang, H.; Chang, W.S.; Mulvaney, P.; et al. Snapshot Hyperspectral Imaging (SHI) for Revealing Irreversible and Heterogeneous Plasmonic Processes. J. Phys. Chem. C. 2018, 122, 6865–6875. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diehn, S.; Schlaad, H.; Kneipp, J. Multivariate Imaging for Fast Evaluation of In Situ Dark Field Microscopy Hyperspectral Data. Molecules 2022, 27, 5146. https://doi.org/10.3390/molecules27165146
Diehn S, Schlaad H, Kneipp J. Multivariate Imaging for Fast Evaluation of In Situ Dark Field Microscopy Hyperspectral Data. Molecules. 2022; 27(16):5146. https://doi.org/10.3390/molecules27165146
Chicago/Turabian StyleDiehn, Sabrina, Helmut Schlaad, and Janina Kneipp. 2022. "Multivariate Imaging for Fast Evaluation of In Situ Dark Field Microscopy Hyperspectral Data" Molecules 27, no. 16: 5146. https://doi.org/10.3390/molecules27165146
APA StyleDiehn, S., Schlaad, H., & Kneipp, J. (2022). Multivariate Imaging for Fast Evaluation of In Situ Dark Field Microscopy Hyperspectral Data. Molecules, 27(16), 5146. https://doi.org/10.3390/molecules27165146







