Abstract
Traditional Chinese medicine (TCM) has been used to treat diabetes for a long time, but its application has not been widely accepted due to unstandardized product quality and complex pharmacological mechanisms. The modernization of TCM is crucial for its further development, and in recent years the metabolomics technique has largely driven its modernization. This review focuses on the application of NMR-based metabolomics in diabetic therapy using TCM. We identified a series of metabolic pathways that altered significantly after TCM treatment, providing a better understanding of the metabolic mechanisms of TCM for diabetes care.
1. Introduction
Diabetes is a common metabolic disease characterized by hyperglycemia owing to insulin secretion deficiency for type 1 diabetes (T1D) or insulin resistance for type 2 diabetes (T2D), which has become a global health problem [1]. In 2021, approximately 537 million adults between 20 and 79 years of age suffered from diabetes worldwide, and this number is projected to increase to 783 million by 2045 [1]. More than three out of every four diabetic patients were living in low- and middle-income countries. Moreover, diabetes caused 6.7 million deaths in 2021 [1]. Currently, T2D can be treated by a number of different medications such as metformin, sulfonylureas, glinides, thiazolidinediones, DPP-4 inhibitors, GLP-1 receptor agonists and SGLT2 inhibitors. However, there are fewer treatment methods for T1D, so all T1D patients require daily insulin injections to maintain normal blood glucose levels. Therefore, there is an urgent need to discover novel therapeutic strategies, especially for T1D. Traditional Chinese medicine (TCM) is a system of healing that originated thousands of years ago that has also been used to treat diabetes for a long time [2,3,4]. However, several problems including unstandardized product quality and complex pharmacological mechanisms have restricted its wide acceptance and application [5]. Therefore, TCM modernization is crucial for its further development [6]. This review aims to provide the currently available information on potential metabolic mechanisms of TCM on the management and treatment of diabetes for diabetic patients, pharmacologists, drug developers and endocrinologists.
2. Metabolomics as a Powerful Tool for the Modernization of TCM
In recent years, omics technologies have largely driven the modernization of TCM [7]. Metabolomics is the apogee of the omics cascade that attempts to analyze a comprehensive set of metabolites in biological samples and explore changes in metabolic pathways related to genomic and proteomic perturbations [8]. TCM possesses several typical characteristics such as being multi-component, multi-target and multi-pathway, resulting in great difficulty when attempting to explore its pharmacological mechanisms [9]. Notably, metabolomics, especially untargeted metabolomics, can detect a global set of metabolites without bias in living organisms after TCM treatment, which provides the possibility of exploring the metabolic mechanisms of TCM in disease prevention and treatment [10]. Currently, two analytical platforms are mainly employed to acquire metabolomic data, including mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy [11]. These techniques have their advantages and disadvantages, as listed in Table 1. For example, the MS-based method has a higher sensitivity and more metabolites can be detected even using a minimal sample size. Moreover, the MS-based method is also a flexible technique, which can couple liquid or gas chromatography to achieve the selection and separation of different metabolites. However, it also has a number of disadvantages including low reproducibility, complex sample preparation, non-recyclable samples, relatively poor quantitative analysis and difficult metabolite identification. The NMR-based method possesses several strengths, such as high reproducibility, simple sample preparation, non-destructive, fast analysis, good quantitative analysis and straightforward identification, although this method cannot analyze non-protonated metabolites. In addition, compared with the MS-based method, NMR analysis needs a larger sample size and has a relatively low sensitivity. Figure 1 shows the typical 1H NMR-based metabolomics profiling obtained from serum, liver and feces samples in healthy mice [12], and the detailed metabolite assignments are listed in Table 2, where a series of metabolites can be identified involving amino acid metabolism, energy metabolism, fatty acid metabolism, ketone body metabolism and others. NMR metabolomics profiling is tissue-specific due to different metabolite compositions. Therefore, different analytical sequences have been developed for NMR analysis. For example, a Carr–Purcell–Meiboom–Gill (CPMG) sequence is usually conducted for serum samples in order to minimize the line-broadening effect of blood macromolecules including proteins and lipids. However, for samples with a high water content such as urine, a standard single-pulse sequence (ZGPR) can be used to reduce the impact of water signals on metabolomics profiling. In addition, there is a greater likelihood of overlapping peaks from multiple metabolites with NMR analysis, resulting in difficult identification and quantification. One way to solve this problem is to perform NMR experiments under higher magnetic fields. Moreover, 2D J-resolved spectroscopy and spectral deconvolution have also been used to address the peak overlap of metabolites.

Table 1.
Summary of the main advantages and disadvantages of nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) in metabolomics.
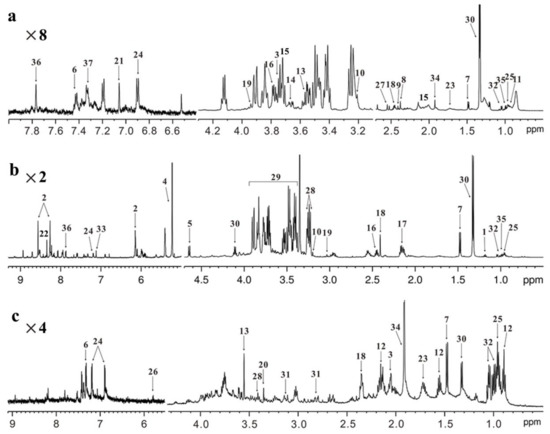
Figure 1.
NMR-based metabolomics profiling. Typical 600 MHz 1H NMR spectra obtained from (a) serum, (b) liver and (c) feces in healthy mice. Metabolite assignment: 1, 3-hydroxybutyrate; 2, AMP; 3, NAG; 4, α-glucose; 5, β-glucose; 6, phenylalanine; 7, alanine; 8, acetone; 9, pyruvate; 10, choline; 11, LDL/VLDL; 12, butyrate; 13, glycine; 14, glycerol; 15, glutamate; 16, glutamine; 17, glutathione; 18, succinate; 19, creatine; 20, methanol; 21, methylhistidine; 22, formate; 23, lysine; 24, tyrosine; 25, leucine; 26, uracil; 27, citrate; 28, taurine; 29, glucose/amino acid region; 30, lactate; 31, aspartate; 32, valine; 33, fumarate; 34, acetate; 35, isoleucine; 36, histidine; 37, tryptophan. Amplification: ×2, 2 times; ×4, 4 times; ×8, 8 times.

Table 2.
Metabolite assignment in 1H NMR-based metabolomics profiling.
In this review, we focus on the application of NMR metabolomics in diabetes therapy using TCM, providing a better understanding of the metabolic mechanisms of TCM. Figure 2 illustrates the flowchart of the NMR-based metabolomics method for elucidating the metabolic mechanisms of TCM for diabetes treatment. In brief, diabetic rodent models are treated with TCM after a period of time and then biological samples are collected for NMR-based metabolomics analysis, such as serum, plasma, urine, feces and tissue samples. Metabolomics data are subjected to multivariate and univariate analyses to identify important metabolites that are significantly altered after TCM treatment. Finally, metabolic pathway analysis is performed to elucidate potential therapeutic mechanisms of TCM for diabetes.
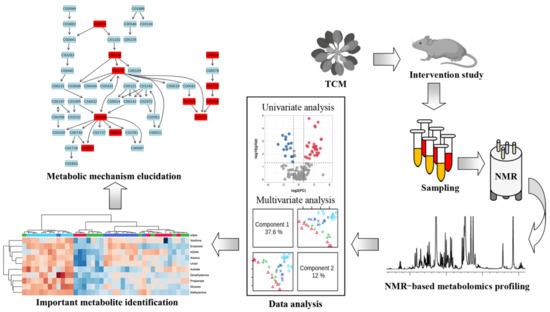
Figure 2.
Flowchart depicting NMR-based metabolomics method to elucidate metabolic mechanisms of traditional Chinese medicine for the treatment of diseases.
3. Potential Metabolic Mechanisms of TCM on Diabetes Care
A systematic search of the PubMed, SCOPUS and Web of Science databases was conducted for relevant studies from 2012 to 2022. Different possible combinations of the following search terms were used: “traditional Chinese medicine”, “Chinese medicine”, “TCM”, “diabetes”, “diabetic”, “nuclear magnetic spectroscopy”, “NMR”, “metabolomic”, “metabonomic” and “metabolic”. We independently searched the literature to minimize bias and firstly screened studies according to titles and abstracts. Inclusion and exclusion criteria were discussed and defined for literature selection. The following inclusion criteria were used: Firstly, studies should be related to diabetes, NMR-based metabolomic analysis, TCM treatment and rodents. Secondly, studies need to measure a specific metabolite level and compare the metabolic differences in diabetes with and without TCM treatment. The exclusion criteria included drug structure analysis, in vitro studies, biomarker discovery or pathological studies. Moreover, reviews, meta-analyses, abstracts and case reports were also excluded. The detailed procedure of literature selection is illustrated in Figure 3, and the information of 25references included in this review are listed in Table 3, of which there are 17 references on T2D and 8 references on T1D. In animal studies, T1D models were developed by streptozotocin or alloxan induction, but lacked non-obese diabetic (NOD) mouse models. Several T2D animal models were used including high-fat diet-fed and low-dose streptozotocin-treated models, KKay mice and Zucker diabetic rats, whereas the use of db/db mice as a widely used preclinical model of T2D also needs to be considered for future studies.
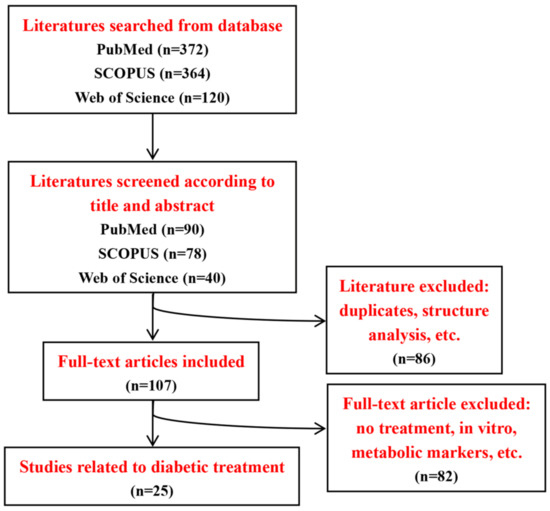
Figure 3.
Flowchart of literature search and selection.

Table 3.
Summary of main metabolic changes after TCM treatment.
Subsequently, metabolic pathway analysis was carried out on the basis of the metabolites included in this review by the MetaboAnalyst 5.0 [38]. The result of pathway analysis was presented according to −log (p) values from the pathway enrichment analysis and pathway impact values from the pathway topology analysis. A metabolic pathway with high values of these two parameters was identified as the important pathway in the response to TCM treatment, as shown in Figure 4.
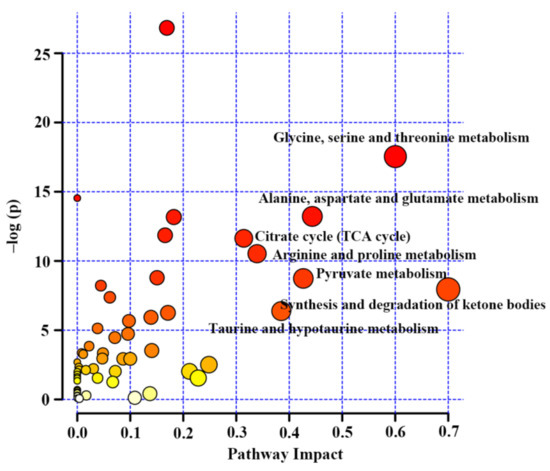
Figure 4.
Metabolic pathway analysis based on differentiated metabolites from NMR metabolomics studies on diabetic treatment using traditional Chinese medicine.
3.1. Amino Acid Metabolism
Amino acid metabolism has been reported to play a key role in insulin secretion and thereby affect the onset and development of diabetes [39]. In this review, most studies reported a reduced amino acid metabolism in diabetic rodents, including glycine, serine and threonine metabolism (Figure 5), alanine, aspartate and glutamate metabolism (Figure 6) and arginine and proline metabolism (Figure 7). We found that the changes in these amino acids might be associated with the regulation of insulin signaling. For example, Wang-Sattler et al. revealed that a lower glycine level could be a predictor for impaired glucose tolerance and T2D [40]. Glycine can increase insulin sensitivity by suppressing oxidative stress in sucrose-fed rats [41]. In addition, serine/threonine phosphorylation plays an essential role in the regulation of pancreatic β-cell growth/survival and insulin signaling [42,43]. Brennan et al. revealed that alanine increased insulin secretion via the physiological regulation of β-cell electrical activity [44]. Alanine can oxidize to glutamate in β-cells [44], and glutamate serves as an intracellular messenger in the regulation of insulin secretion in response to glucose [45]. In a supplementation trial, glutamate has also been evidenced to improve glucose metabolism by increasing insulin secretion in healthy males [46]. Moreover, Gheni et al. elucidated that glutamate derived from the malate-aspartate shuttle is a key signal between glucose metabolism and cAMP action in incretin-induced insulin secretion [47]. Monti et al. conducted a human intervention study for 18 months and found that arginine supplementation significantly increased regression to normal glucose tolerance, although there was no significant effect on the incidence of diabetes [48]. In addition, arginine has also been reported to perform an essential role in pancreatic β-cell functional integrity [49] and improve insulin sensitivity [50]. In this review, we found that the metabolism of these aminoacids was up-regulated after TCM treatment in most studies, suggesting that TCM may improve insulin action and glycemic control via the regulation of amino acid metabolism.
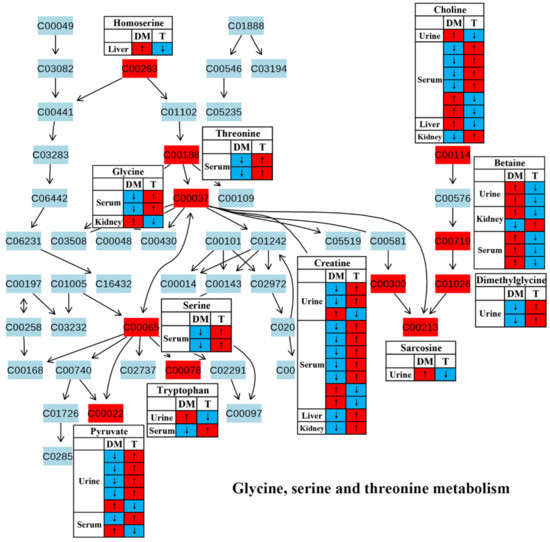
Figure 5.
The effect of traditional Chinese medicine onglycine, serine and threonine metabolism during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00037, glycine; C00065, serine; C00078, tryptophan; C00114, choline; C00188, threonine; C00213, sarcosine; C00263, homoserine; C00300, creatine; C00719, betaine; C01026, dimethylglycine.
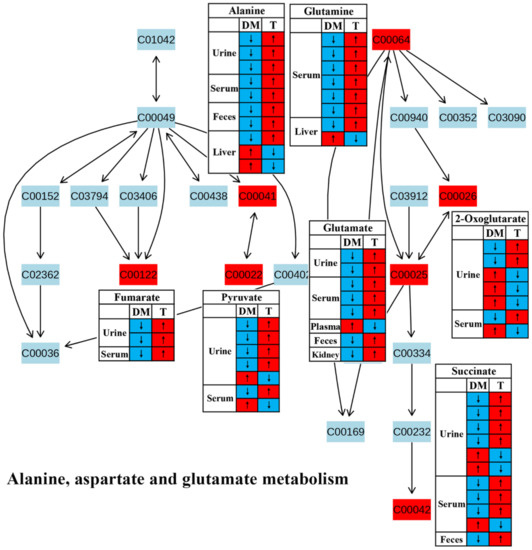
Figure 6.
The effect of traditional Chinese medicine on alanine, aspartate and glutamate metabolism during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00025, glutamate; C00026, 2-oxoglutarate; C00041, alanine; C00042, succinate; C00064, glutamine; C00122, fumarate.
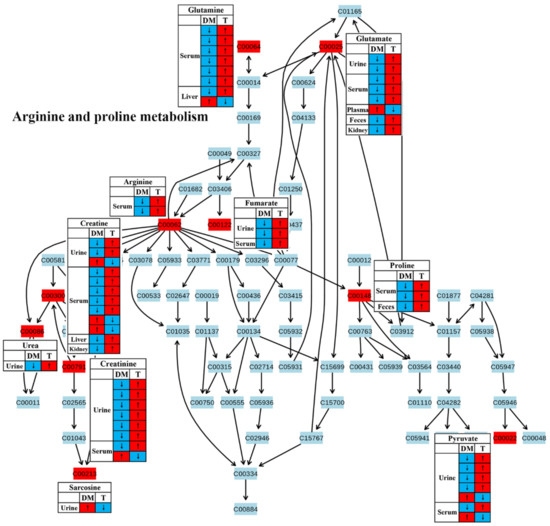
Figure 7.
The effect of traditional Chinese medicine on arginine and proline metabolism during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00025, glutamate; C00062, arginine; C00064, glutamine; C00086, urea; C00122, fumarate; C00148, proline; C00213, sarcosine; C00300, creatine; C00791, creatinine.
3.2. Energy Metabolism
In this review, decreases in pyruvate metabolism (Figure 8) and the TCA cycle (Figure 9) in diabetic rodents were reported by most studies, which confirm that mitochondrial dysfunction occurs in diabetes. Mitochondrial damage has been associated with pancreatic β-cell dysfunction and insulin resistance, resulting in the abnormal glucose metabolism of diabetes [51,52,53,54]. However, notably, treatment with TCM can up-regulate these two metabolic pathways in diabetic rodents, suggesting that TCM may alleviate the mitochondrial dysfunction induced by diabetes. Moreover, glucose as a main source for energy production can also be converted to pyruvate, and then pyruvate oxidized to CO2 and H2O via the tricarboxylic acid cycle (TCA cycle). Therefore, increased energy metabolism boosts glucose depletion, which might be a possible mechanism of the glucose-lowering effect of TCM treatment.
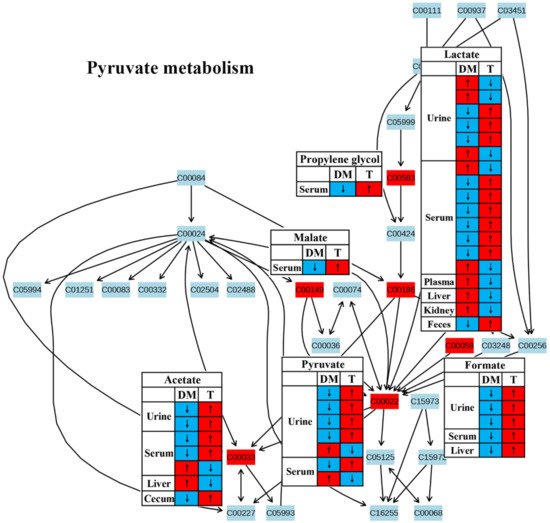
Figure 8.
The effect of traditional Chinese medicine on pyruvate metabolism during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00033, acetate; C00058, formate; C00149, malate; C00186, lactate; C00583, propylene glycol.
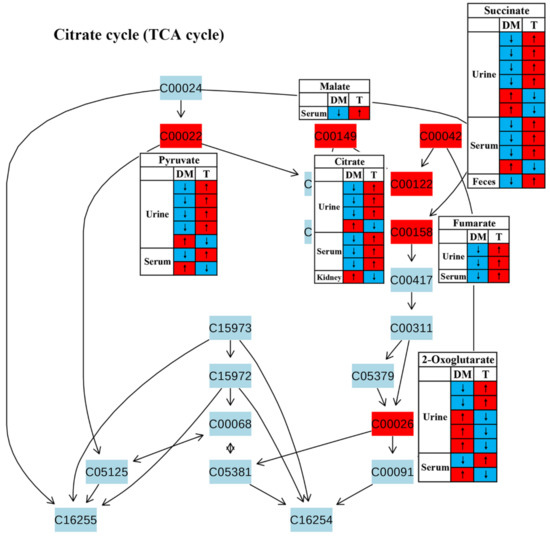
Figure 9.
The effect of traditional Chinese medicine on TCA cycle during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00149, malate; C00042, succinate; C00122, fumarate; C00158, citrate; C00026, 2-oxoglutarate.
3.3. Synthesis and Degradation of Ketone Bodies
We also identified significant changes in the synthesis and degradation of ketone bodies in response to TCM treatment (Figure 4). Ketone bodies are derived from fatty acid metabolism and mostly generated in the liver as an alternative source of energy [55]. Their homeostasis is maintained by the balance of synthesis (ketogenesis) and degradation (ketolysis) of ketone bodies. Ketogenesis is the process of converting fatty acids into two major ketonebodies, acetoacetate and β-hydroxybutyrate [56]. However, changes in acetoacetate and β-hydroxybutyrate in diabetic rodents after TCM treatment were inconsistent based on the current findings using NMR metabolomics (Figure 10). The levels of ketone bodies can also be regulated by insulin; for example, an elevated insulin level promotes ketone body clearance by increasing their catabolic pathway in extrahepatic tissues [57]. Thus, enhanced ketone body production was observed in diabetes patients owing to insulin insufficiency or resistance [58]. Notably, the level of acetone in the urine and serum was significantly reduced in diabetic rodents but increased after TCM treatment in all studies included in this review (Figure 10), suggesting an enhanced ketone body degradation since acetone is produced via the decarboxylation of acetoacetate [56]. Nevertheless, the causal relationship between ketone body degradation and insulin signaling after TCM treatment still needs further confirmation.
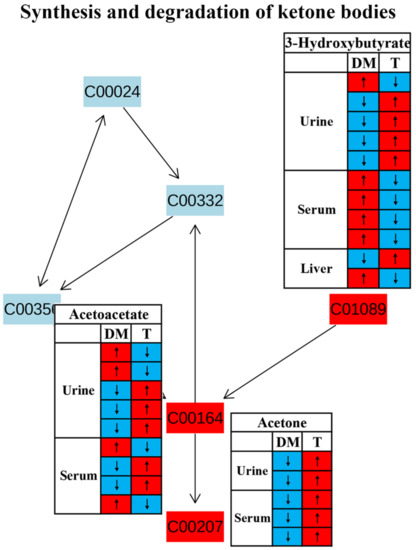
Figure 10.
The effect of traditional Chinese medicine on synthesis and degradation of ketone bodies during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00207, acetone; C00164, acetoacetate; C01089, 3-hydroxybutyrate.
3.4. Taurine and Hypotaurine Metabolism
Metabolic pathway analysis suggested that taurine and hypotaurine metabolism was affected after TCM treatment (Figure 4).Taurine has been reported to restore insulin secretion and exert an antidiabetic effect [59,60,61].In this review, the level of taurine in the urine was increased in diabetic rodents but reduced after TCM treatment in most studies, as shown in Figure 11.However, the level of taurine was decreased in the livers of diabetic rodents and increased by the administration of TCM such as Dendrobium officinale water extract [20] and the Qijian mixture [26], which could be beneficial to improve insulin signaling in the liver [62]. Carneiro et al. revealed that taurine facilitated glucose homeostasis by regulating the expression of genes for glucose-stimulated insulin secretion [63]. Additionally, taurine can also affect the electrogenic response and calcium homeostasis in β-cells and then result in insulin secretion [64]. Although the current findings on taurine metabolism are inconsistent, we speculate that an increased taurine level after TCM treatment should have a positive effect on diabetes therapy.
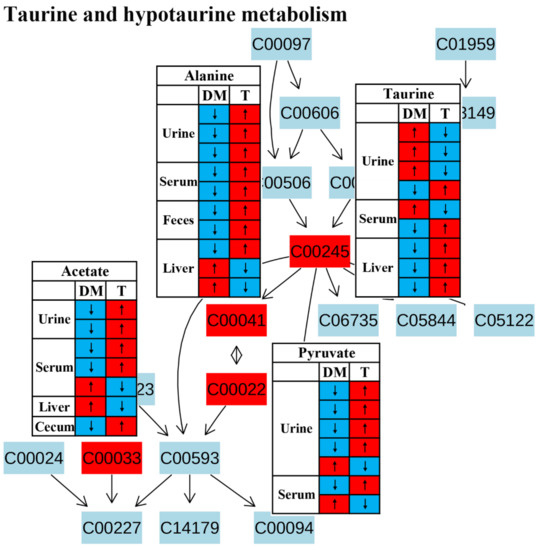
Figure 11.
The effect of traditional Chinese medicine on taurine and hypotaurine metabolism during diabetic treatment. Each row in the table represents one study and arrow indicates relative change tendency of metabolite. Red and wathet blue colors indicate the increase and decrease in metabolite level in DM relative to normal controls or in DM after TCM treatment, respectively. DM, diabetes mellitus; T, TCM treatment. Metabolite: C00022, pyruvate; C00033, acetate; C00041, alanine; C00245, taurine.
4. Conclusions and Perspectives
This review has focused mostly on NMR metabolomics and provides a panoramic view of the metabolic responses to diabetic treatment using TCM. Treatment with TCM up-regulates energy metabolism, amino acid metabolism, ketone body degradation and taurine metabolism and then increases insulin secretion and reduces blood glucose levels (Figure 12). However, the relevant studies are still inadequate to make a final conclusion. Moreover, the real knowledge for a complex biological system needs to integrate genes, proteins and metabolites, suggesting that a multi-omics analysis could be one of the avenues to explore in the future. In this review, most studies focused on analyses of biofluids, such as urine and serum, but we suggest that attention should also be paid to metabolic changes in organs and tissues in order to explore potential mechanisms underlying the treatment of diabetic complications using TCM. Notably, the fecal metabolome also needs to be paid more attention in order to explore the role of gut microbiota in diabetes therapy via TCM treatment [65].
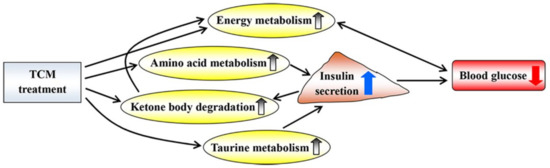
Figure 12.
Potential metabolic mechanisms of traditional Chinese medicine on diabetic treatment. Up and down arrows indicate increase and decrease after TCM treatment, respectively.
Thus far, the clinical studies of TCM in diabetes have mostly focused on glycemic control, but with no metabolomics investigations. Thus, metabolic pathways affected by TCM treatment in rodents still need to be validated in human intervention studies in order to uncover potential pharmacological mechanisms of TCM for its clinical translational application. We also recommend using a multi-omics analysis for elucidating whether these metabolic changes have a causative role in diabetes therapy. Such metabolomics information could then be used to evaluate TCM interventions and discover new targets for diabetic treatment. Relative to T2D, there are fewer therapeutic methods for T1D, but there are few TCM studies on T1D. We appeal for the need to discover novel therapeutic strategies for T1D patients from TCM in the future.
Author Contributions
Y.H., J.L., Q.Z., J.C., W.D. and M.L. contributed to the literature search and selection; H.Z., Y.H., J.L. and Q.Z. contributed to the result’s interpretation and writing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Medical and Health Science and Technology Project of Zhejiang Province (No.: 2022KY1215).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 11 March 2022).
- Tong, X.L.; Dong, L.; Chen, L.; Zhen, Z. Treatment of diabetes using traditional Chinese medicine: Past, present and future. Am. J. Chin. Med. 2012, 40, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Covington, M.B. Traditional Chinese medicine in the treatment of diabetes. Diabetes Spectr. 2001, 14, 154–159. [Google Scholar] [CrossRef]
- Bai, L.; Li, X.; He, L.; Zheng, Y.; Lu, H.; Li, J.; Zhong, L.; Tong, R.; Jiang, Z.; Shi, J.; et al. Antidiabetic potential of flavonoids from traditional Chinese medicine: A review. Am. J. Chin. Med. 2019, 47, 933–957. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Jiang, J.G.; Chen, J. Chinese medicine and its modernization demands. Arch. Med. Res. 2008, 39, 246–251. [Google Scholar] [CrossRef]
- Qiu, J. China plans to modernize traditional medicine. Nature 2007, 446, 590–592. [Google Scholar] [CrossRef]
- Cai, F.F.; Zhou, W.J.; Wu, R.; Su, S.B. Systems biology approaches in the study of Chinese herbal formulae. Chin. Med. 2018, 13, 65. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Jiang, W.Y. Therapeutic wisdom in traditional Chinese medicine: A perspective from modern science. Trends Pharmacol. Sci. 2005, 26, 558–563. [Google Scholar] [CrossRef]
- Wang, M.; Lamers, R.-J.A.N.; Korthout, H.A.A.J.; Van Nesselrooij, J.H.J.; Witkamp, R.F.; Van Der Heijden, R.; Voshol, P.J.; Havekes, L.M.; Verpoorte, R.; Van Der Greef, J. Metabolomics in the context of systems biology: Bridging traditional Chinese medicine and molecular pharmacology. Phytother. Res. 2005, 19, 173–182. [Google Scholar] [CrossRef]
- Zheng, H.; Clausen, M.; Dalsgaard, T.; Bertram, H. Metabolomics to explore impact of dairy intake. Nutrients 2015, 7, 4875–4896. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, H.; Fan, K.; Xu, H.; Huang, Y.; Zheng, Y.; Xu, Q.; Li, C.; Zhao, L.; Li, Y.; et al. Targeting Gut microbiota and host metabolism with Dendrobium officinale dietary fiber to prevent obesity and improve glucose homeostasis in diet-induced obese mice. Mol. Nutr. Food Res. 2022, 66, 2100772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gao, H.; Zhao, Y.; Lin, D. Metabonomic analysis of the therapeutic effect of Zhibai Dihuang Pill in treatment of streptozotocin-induced diabetic nephropathy. J. Ethnopharmacol. 2012, 142, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Wang, J.; Wang, P.; Song, X.; Yang, M.; Kong, L. NMR-based metabonomic study of Chinese medicine Gegen Qinlian Decoction as an effective treatment for type 2 diabetes in rats. Metabolomics 2013, 9, 1228–1242. [Google Scholar] [CrossRef]
- Perumal, V.; Murugesu, S.; Lajis, N.H.; Khatib, A.; Saari, K.; Abdul-Hamid, A.; Khoo, W.C.; Mushtaq, M.Y.; Abas, F.; Ismail, I.S.; et al. Evaluation of antidiabetic properties of Momordica charantia in streptozotocin induced diabetic rats using metabolomics approach. Int. Food Res. J. 2015, 22, 1298–1306. [Google Scholar]
- Mediani, A.; Abas, F.; Maulidiani, M.; Khatib, A.; Tan, C.P.; Ismail, I.S.; Shaari, K.; Ismail, A.; Lajis, N.H. Metabolic and biochemical changes in streptozotocin induced obese-diabetic rats treated with Phyllanthus niruri extract. J. Pharm. Biomed. Anal. 2016, 128, 302–312. [Google Scholar] [CrossRef]
- Akhtar, M.T.; Sarib, M.S.B.M.; Ismail, I.S.; Abas, F.; Ismail, A.; Lajis, N.H.; Shaari, K. Anti-diabetic activity and metabolic changes induced by Andrographis Paniculata plant extract in obese diabetic rats. Molecules 2016, 21, 1026. [Google Scholar] [CrossRef]
- Maulidiani; Abas, F.; Khatib, A.; Perumal, V.; Suppaiah, V.; Ismail, A.; Hamid, M.; Shaari, K.; Lajis, N. Metabolic alteration in obese diabetes rats upon treatment with Centella asiatica extract. J. Ethnopharmacol. 2016, 180, 60–69. [Google Scholar] [CrossRef]
- Shen, X.L.; Liu, H.; Xiang, H.; Qin, X.M.; Du, G.H.; Tian, J.S. Combining biochemical with 1H NMR-based metabolomics approach unravels the antidiabetic activity of genipin and its possible mechanism. J. Pharm. Biomed. Anal. 2016, 129, 80–89. [Google Scholar] [CrossRef]
- Azam, A.A.; Pariyani, R.; Ismail, I.S.; Ismail, A.; Khatib, A.; Abas, F.; Shaari, K. Urinary metabolomics study on the protective role of Orthosiphon stamineus in Streptozotocin induced diabetes mellitus in rats via 1H NMR spectroscopy. BMC Complement. Altern. Med. 2017, 17, 278. [Google Scholar] [CrossRef]
- Zheng, H.; Pan, L.; Xu, P.; Zhu, J.; Wang, R.; Zhu, W.; Hu, Y.; Gao, H. An NMR-based metabolomic approach to unravel the preventive effect of water-soluble extract from Dendrobium officinale Kimura & Migo on streptozotocin-induced diabetes in mice. Molecules 2017, 22, 1543. [Google Scholar]
- AL-Zuaidy, M.H.; Mumtaz, M.W.; Hamid, A.A.; Ismail, A.; Mohamed, S.; Razis, A.F.A. Biochemical characterization and 1H NMR based metabolomics revealed Melicopelunu-ankenda leaf extract a potent anti-diabetic agent in rats. BMC Complement. Altern. Med. 2017, 17, 359. [Google Scholar] [CrossRef] [PubMed]
- Sajak, A.A.B.; Mediani, A.; Maulidiani; Dom, N.S.M.; Machap, C.; Hamid, M.; Ismail, A.; Khatib, A.; Abas, F. Effect of Ipomoea aquatica ethanolic extract in streptozotocin (STZ) induced diabetic rats via 1H NMR-based metabolomics approach. Phytomedicine 2017, 36, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.S.; Zhao, L.; Shen, X.L.; Liu, H.; Qin, X.M. 1H NMR-based metabolomics approach to investigating the renal protective effects of Genipin in diabetic rats. Chin. J. Nat. Med. 2018, 16, 261–270. [Google Scholar] [CrossRef]
- Li, H.; Chu, S.; Zhao, H.; Liu, D.; Liu, X.; Qu, X.; Chen, J.; Li, Z.; Li, J. Effect of Zishen Jiangtang pill, a Chinese herbal product, on rats with diabetic osteoporosis. Evid. Based Complement. Altern. Med. 2018, 2018, 7201914. [Google Scholar] [CrossRef]
- Gao, K.; Yang, R.; Zhang, J.; Wang, Z.; Jia, C.; Zhang, F.; Li, S.; Wang, J.; Murtaza, G.; Xie, H.; et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharmacol. Res. 2018, 130, 93–109. [Google Scholar] [CrossRef]
- Singh, A.K.; Raj, V.; Keshari, A.K.; Rai, A.; Kumar, P.; Rawat, A.; Maity, B.; Kumar, D.; Prakash, A.; De, A.; et al. Isolated mangiferin and naringenin exert antidiabetic effect via PPAR γ/GLUT4 dual agonistic action with strong metabolic regulation. Chem. Biol. Interact. 2018, 280, 33–44. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Su, Z.; Ling, X.; Ji, K.; Huang, H.; Liu, X.; Yin, C.; Zhu, H.; Guo, Y.; Mo, Y.; Lu, Y.; et al. 1H NMR-based urinary metabonomic study of the antidiabetic effects of Rubus Suavissimus S. Lee in STZ-induced T1DM rats. J. Chromatogr. B 2020, 1158, 122347. [Google Scholar] [CrossRef]
- Niu, W.; Miao, J.; Li, X.; Guo, Q.; Deng, Z.; Wu, L. Metabolomics combined with systematic pharmacology reveals the therapeutic effects of Salvia miltiorrhiza and Radix Pueraria lobata herb pair on type 2 diabetes rats. J. Func. Foods 2022, 89, 104950. [Google Scholar] [CrossRef]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of anthocyanin extracts from bilberry (Vaccinium myrtillus L.) and purple potato (Solanum tuberosum L. var. ‘SynkeäSakari’) on the plasma metabolomic profile of Zucker diabetic fatty rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef]
- Du, H.; Li, Q.; Yi, H.; Xu, T.; Xu, X.M.; Kuang, T.T.; Zhang, J.; Huang, A.Q.; Fan, G. Anti-diabetic effects of Berberis kansuensis extract on type 2 diabetic rats revealed by 1H-NMR-based metabolomics and biochemistry analysis. Chem. Biodiver. 2020, 17, e2000413. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Niu, W.; Li, X.; Guo, H.; Zhang, N.; Wang, X.; Wu, L. Study on hypoglycemic effect of the drug pair of Astragalus radix and Dioscoreaerhizoma in T2DM rats by network pharmacology and metabonomics. Molecules 2019, 24, 4050. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, B.; Gong, J.; Li, L.; Chen, G.; Zhang, J.; Chen, Y.; Tian, X.; Han, B.; Guo, Y.; et al. Chickpea extract ameliorates metabolic syndrome symptoms via restoring intestinal ecology and metabolic profile in type 2 diabetic rats. Mol. Nutr. Food Res. 2021, 65, 2100007. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-R.; Yoon, D.; Kim, H.-G.; Oh, S.; Yoo, Y.; Lee, Y.-S.; Kim, K.-W.; Yi, T.-H.; Lee, D. NMR-based metabolomics approach to investigate the effects of fruits of Acanthopanax sessiliflorus in a high-fat diet induced mouse model. Metabolites 2021, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Han, M.; Lin, S.; Ye, H.; Chen, J.; Zhu, H.; Lin, W. Enteromorpha prolifera polysaccharide prevents high-fat diet-induced obesity in hamsters: A NMR-based metabolomic evaluation. J. Food Sci. 2021, 86, 3672–3685. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, C.; Zhang, Y.; Du, H.; Xu, T.; Xu, X.; Zhang, J.; Kuang, T.; Lai, X.; Fan, G.; et al. 1H NMR-based metabolomics coupled with molecular docking reveal the anti-diabetic effects and potential active components of Berberis vernae on type 2 diabetic rats. Front. Pharmacol. 2020, 11, 932. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Newsholme, P.; Bender, K.; Kiely, A.; Brennan, L. Amino acid metabolism, insulin secretion and diabetes. Biochem. Soc. Trans. 2007, 35, 1180–1186. [Google Scholar] [CrossRef]
- Wang-Sattler, R.; Yu, Z.H.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef]
- Tuttle, R.L.; Gill, N.S.; Pugh, W.; Lee, J.P.; Koeberlein, B.; Furth, E.E.; Polonsky, K.S.; Naji, A.; Birnbaum, M.J. Regulation of pancreatic β-cell growth and survival by the serine/threonine protein kinase Akt1/PKBα. Nat. Med. 2001, 7, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Shine, A.; Hewage, C.; Malthouse, J.P.; Brindle, K.M.; McClenaghan, N.; Flatt, P.R.; Newsholme, P. A nuclear magnetic resonance-based demonstration of substantial oxidative l-alanine metabolism and l-alanine-enhanced glucose metabolism in a clonal pancreatic β-cell line: Metabolism of l-alanine is important to the regulation of insulin secretion. Diabetes 2002, 51, 1714–1721. [Google Scholar] [CrossRef]
- Maechler, P.; Wollheim, C.B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature 1999, 402, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Di Sebastiano, K.M.; Bell, K.E.; Barnes, T.; Weeraratne, A.; Premji, T.; Mourtzakis, M. Glutamate supplementation is associated with improved glucose metabolism following carbohydrate ingestion in healthy males. Br. J. Nutr. 2013, 110, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Gheni, G.; Ogura, M.; Iwasaki, M.; Yokoi, N.; Minami, K.; Nakayama, Y.; Harada, K.; Hastoy, B.; Wu, X.; Takahashi, H.; et al. Glutamate acts as a key signal linking glucose metabolism to incretin/cAMP action to amplify insulin secretion. Cell Rep. 2014, 9, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.D.; Setola, E.; Lucotti, P.C.G.; Marrocco-Trischitta, M.M.; Comola, M.; Galluccio, E.; Poggi, A.; Mammì, S.; Catapano, A.L.; Comi, G.; et al. Effect of a long-term oral l-arginine supplementation on glucose metabolism: A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2012, 14, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.S.; McClenaghan, N.H.; Flatt, P.R.; de Bittencourt, P.I.H.; Murphy, C.; Newsholme, P. L-arginine is essential for pancreatic β-cell functional integrity, metabolism and defense from inflammatory challenge. J. Endocrinol. 2011, 211, 87–97. [Google Scholar] [CrossRef]
- Clemmensen, C.; Madsen, A.N.; Smajilovic, S.; Holst, B.; Bräuner-Osborne, H. L-arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids 2012, 43, 1265–1275. [Google Scholar] [CrossRef]
- Lu, H.; Koshkin, V.; Allister, E.M.; Gyulkhandanyan, A.V.; Wheeler, M.B. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 2010, 59, 448–459. [Google Scholar] [CrossRef]
- Kwak, S.H.; Park, K.S.; Lee, K.U.; Lee, H.K. Mitochondrial metabolism and diabetes. J. Diabetes Investig. 2010, 1, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Szendroedi, J.; Phielix, E.; Roden, M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011, 8, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rutter, G.A.; Georgiadou, E.; Martinez-Sanchez, A.; Pullen, T.J. Metabolic and functional specialisations of the pancreatic beta cell: Gene disallowance, mitochondrial metabolism and intercellular connectivity. Diabetologia 2020, 63, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostag. Leukotr. Essen. Fatty Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Keller, U.; Lustenberger, M.; Stauffacher, W. Effect of insulin on ketone body clearance studied by a ketone body “clamp” technique in normal man. Diabetologia 1988, 31, 24–29. [Google Scholar] [CrossRef]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stančáková, A.; Pihlajamäki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef]
- Batista, T.M.; Ribeiro, R.A.; Amaral, A.G.; de Oliveira, C.A.; Boschero, A.C.; Carneiro, E.M. Taurine supplementation restores glucose and carbachol-induced insulin secretion in islets from low-protein diet rats: Involvement of Ach-M3R, Synt 1 and SNAP-25 proteins. J. Nutr. Biochem. 2012, 23, 306–312. [Google Scholar] [CrossRef]
- Cappelli, A.P.; Zoppi, C.C.; Barbosa-Sampaio, H.C.; Costa, J.M., Jr.; Protzek, A.O.; Morato, P.N.; Boschero, A.C.; Carneiro, E.M. Taurine-induced insulin signalling improvement of obese malnourished mice is associated with redox balance and protein phosphatases activity modulation. Liver Int. 2014, 34, 771–783. [Google Scholar] [CrossRef]
- Chen, W.; Guo, J.; Zhang, Y.; Zhang, J. The beneficial effects of taurine in preventing metabolic syndrome. Food Funct. 2016, 7, 1849–1863. [Google Scholar] [CrossRef]
- Wu, N.; Lu, Y.; He, B.; Zhang, Y.Y.; Lin, J.F.; Zhao, S.; Zhang, W.; Li, Y.; Han, P. Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Res. Clin. Prac. 2010, 90, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, E.M.; Latorraca, M.Q.; Araujo, E.; Beltrá, M.; Oliveras, M.J.; Navarro, M.; Berná, G.; Bedoya, F.J.; Velloso, L.A.; Soria, B.; et al. Taurine supplementation modulates glucose homeostasis and islet function. J. Nutr. Biochem. 2009, 20, 503–511. [Google Scholar] [CrossRef] [PubMed]
- L’Amoreaux, W.J.; Cuttitta, C.; Santora, A.; Blaize, J.F.; Tachjadi, J.; El Idrissi, A. Taurine regulates insulin release from pancreatic beta cell lines. J. Biomed. Sci. 2010, 17, S11. [Google Scholar] [CrossRef] [PubMed]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).