Anisotropic Thermal Expansion and Electronic Structure of LiInSe2
Abstract
1. Introduction
2. Experimental Methods
3. Computation Methods
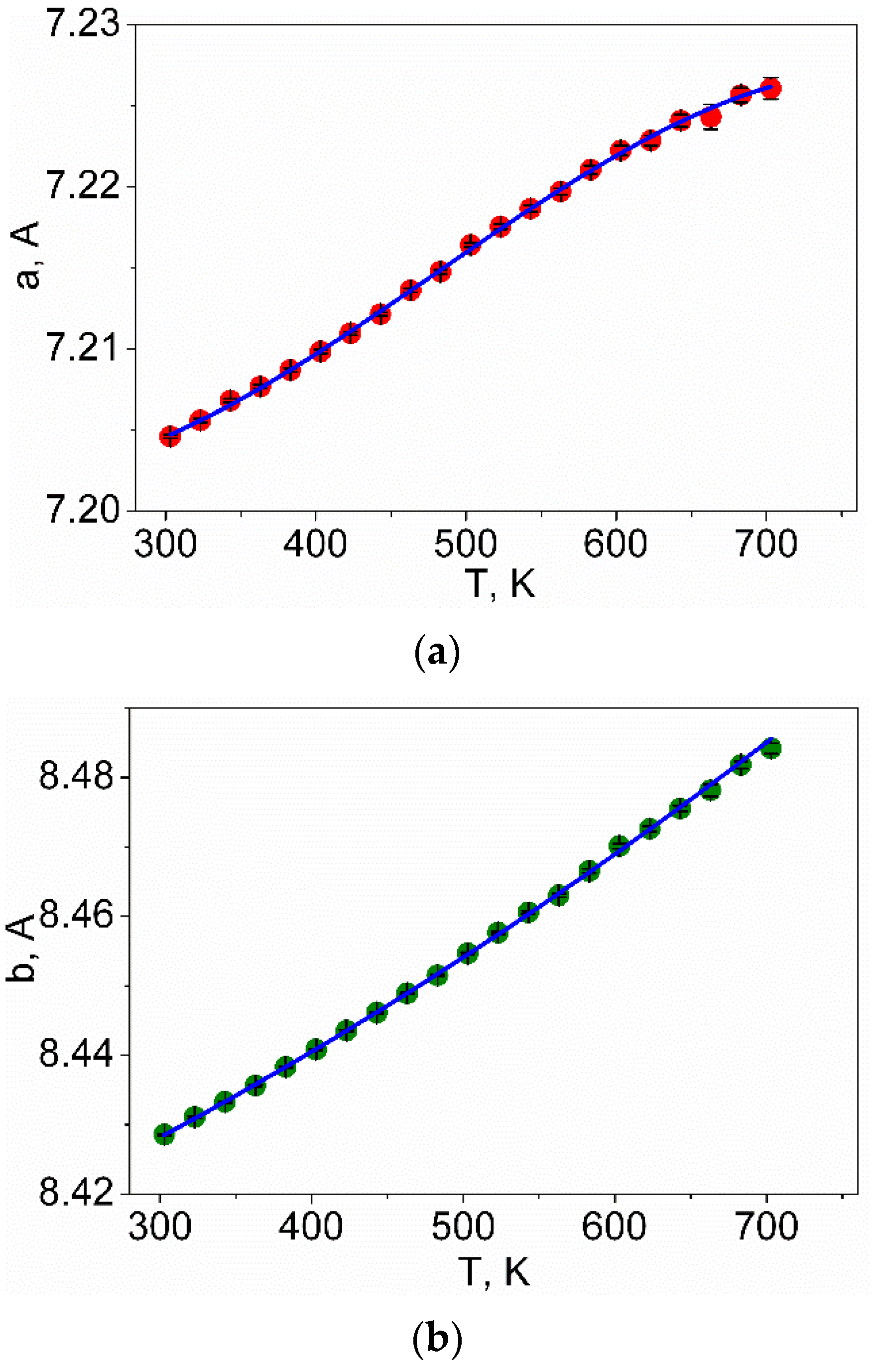
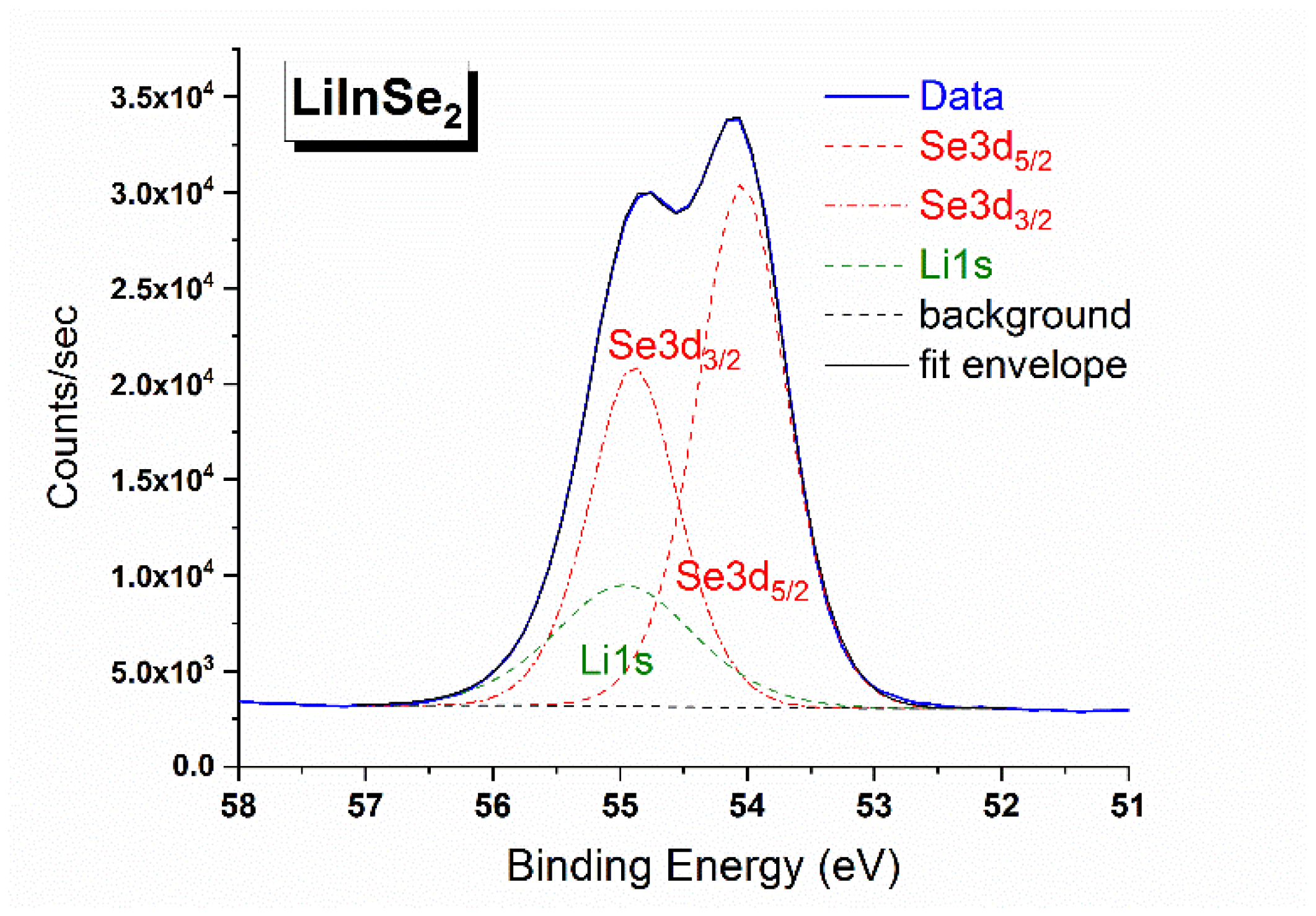
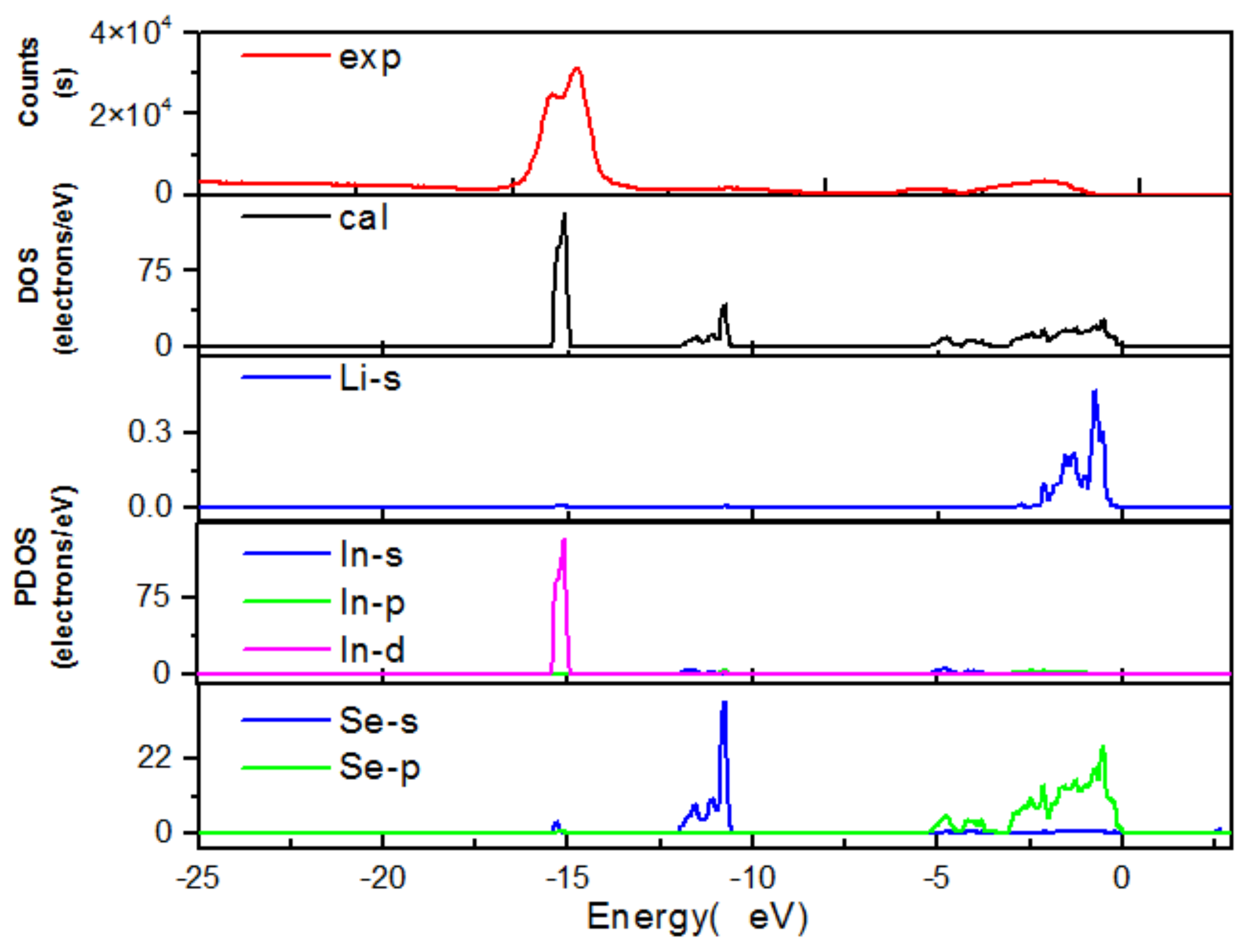
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ohmer, M.C.; Goldstein, J.T.; Zelmon, D.E.; Saxler, A.W.; Hegde, S.M.; Wolf, J.D.; Schunemann, P.G.; Pollak, T.M. Infrared properties of AgGaTe2, a nonlinear optical chalcopyrite semiconductor. J. Appl. Phys. 1999, 86, 94–99. [Google Scholar] [CrossRef]
- Fossier, S.; Salaün, S.; Mangin, J.; Bidault, O.; Thénot, I.; Zondy, J.-J.; Chen, W.; Rotermund, F.; Petrov, V.; Petrov, P.; et al. Optical, vibrational, thermal, electrical, damage, and phase-matching properties of lithium thioindate. J. Opt. Soc. Am. B 2004, 21, 1981–2007. [Google Scholar] [CrossRef]
- Yin, W.; Feng, K.; He, R.; Mei, D.; Lin, Z.; Yao, J.; Wu, Y. BaGa2MQ6 (M = Si, Ge; Q = S, Se): A new series of promising IR nonlinear optical materials. Dalton Trans. 2012, 41, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Reshak, A.H.; Kityk, I.V.; Parasyuk, O.V.; Kamarudin, H.; Auluck, S. Influence of replacing Si by Ge in the chalcogenide quaternary sulfides Ag2In2Si(Ge)S6 on the chemical bonding, linear and nonlinear optical susceptibilities, and hyperpolarizability. J. Phys. Chem. B 2013, 117, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound Copper Chalcogenide Nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Sengar, B.S.; Garg, V.; Kumar, A.; Awasthi, V.; Kumar, S.; Atuchin, V.V.; Mukherjee, S. Band alignment of Cd-free (Zn, Mg)O layer with Cu2ZnSn(S,Se)4 and its effect on the photovoltaic properties. Opt. Mater. 2018, 84, 748–756. [Google Scholar] [CrossRef]
- Yelisseyev, A.P.; Molokeev, M.S.; Jiang, X.; Krinitsin, P.G.; Isaenko, L.I.; Lin, Z. Structure and Optical Properties of the Li2In2GeSe6 Crystal. J. Phys. Chem. C 2018, 122, 17413–17422. [Google Scholar] [CrossRef]
- Yelisseyev, A.; Lobanov, S.; Molokeev, M.; Zhang, S.; Pugachev, A.; Lin, Z.; Vedenyapin, V.; Kurus, A.; Khamoyam, A.; Isaenko, L. A new nonlinear optical selenide crystal AgLiGa2Se4 with good comprehensive performance in mid-infrared region. Adv. Opt. Mater. 2020, 9, 2001856. [Google Scholar] [CrossRef]
- Azarapin, N.O.; Aleksandrovsky, A.S.; Atuchin, V.V.; Gavrilova, T.A.; Krylov, A.S.; Molokeev, M.S.; Mukherjee, S.; Oreshonkov, A.S.; Andreev, O.V. Synthesis, structural and spectroscopic properties of orthorhombic compounds BaLnCuS3 (Ln = Pr, Sm). J. Alloy. Compd. 2020, 832, 153134. [Google Scholar] [CrossRef]
- Garg, V.; Sengar, B.S.; Siddharth, G.; Kumar, S.; Atuchin, V.V.; Mukherjee, S. Insights into the sputter-instigated valence plasmon oscillations in CIGSe thin films. Surf. Interfaces 2021, 25, 101146. [Google Scholar] [CrossRef]
- Petrov, V.; Yelisseyev, A.; Isaenko, L.; Lobanov, S.; Titov, A.; Zondy, J.-J. Second harmonic generation and optical parametric amplification in the mid-IR with orthorhombic biaxial crystals LiGaS2 and LiGaSe2. Appl. Phys. B 2004, 78, 543–546. [Google Scholar] [CrossRef]
- Isaenko, L.; Vasilyeva, I.; Merkulov, A.; Yelisseyev, A.; Lobanov, S. Growth of new nonlinear crystals LiMX2 (M = Al, In, Ga; X = S, Se, Te) for the mid-IR optics. J. Cryst. Growth 2005, 275, 217–223. [Google Scholar] [CrossRef]
- Andreev, Y.; Atuchin, V.; Lanskii, G.; Pervukhina, N.; Popov, V.; Trocenco, N. Linear optical properties of LiIn(S1–xSex)2 crystals and tuning of phase matching conditions. Solid State Sci. 2005, 7, 1188–1193. [Google Scholar] [CrossRef]
- Isaenko, L.; Krinitsin, P.; Vedenyapin, V.; Yelisseyev, A.; Merkulov, A.; Zondy, A.J.-J.; Petrov, V. LiGaTe2: A New Highly Nonlinear Chalcopyrite Optical Crystal for the Mid-IR. Cryst. Growth Des. 2005, 5, 1325–1329. [Google Scholar] [CrossRef]
- Petrov, V.; Zondy, J.; Bidault, O.; Isaenko, L.; Vedenyapin, V.; Yelisseyev, A.; Chen, W.; Tyazhev, A.; Lobanov, S.; Marchev, G.; et al. Optical, thermal, electrical, damage, andphase-matching properties of lithium selenoindate. J. Opt. Soc. Am. B 2010, 27, 1902–1927. [Google Scholar] [CrossRef]
- Kato, K.; Petrov, V.; Umemura, N. Sellmeier and thermo-optic dispersion formulas for LiInSe2. Appl. Opt. 2014, 53, 1063. [Google Scholar] [CrossRef]
- Drebushchak, V.A.; Isaenko, L.I.; Lobanov, S.I.; Krinitsin, P.G.; Grazhdannikov, S.A. Experimental heat capacity of LiInS2, LiInSe2, LiGaS2, LiGaSe2, and LiGaTe2 from 180 to 460 K. J. Therm. Anal. 2017, 129, 103–108. [Google Scholar] [CrossRef]
- Kurus, A.; Yelisseyev, A.; Lobanov, S.; Plyusnin, P.; Molokeev, M.; Solovyev, L.; Samoshkin, D.; Stankus, S.; Melnikova, S.; Isaenko, L. Thermophysical properties of lithium thiogallate that are important for optical applications. RSC Adv. 2021, 11, 39177–39187. [Google Scholar] [CrossRef]
- Kish, Z.Z.; Kanishcheva, A.S.; Mikhailov, Y.N.; Lazarev, V.B.; Semrad, E.E.; Peresh, E.Y. Synthesis and crystal structure of lithium thioindate. Dokl. Akad. Nauk. SSSR 1985, 280, 398–401. [Google Scholar]
- Leal-Gonzalez, J.; Melibary, S.; Smith, A. Structure of lithium gallium sulfide, LiGaS2. Acta Cryst. 1990, 46, 2017–2019. [Google Scholar] [CrossRef]
- Isaenko, L.; Yelisseyev, A.; Lobanov, S.; Titov, A.; Petrov, V.; Zondy, J.-J.; Krinitsin, P.; Merkulov, A.; Vedenyapin, V.; Smirnova, J. Growth and properties of LiGaX2 (X = S, Se, Te) single crystals for nonlinear optical applications in the mid-IR. Cryst. Res. Technol. 2003, 38, 379–387. [Google Scholar] [CrossRef]
- Kuriyama, K.; Nozaki, T. Single-crystal growth and characterization of LiGaSe2. J. Appl. Phys. 1981, 52, 6441–6443. [Google Scholar] [CrossRef]
- Neumann, H.; Hönle, W.; Kuhn, G. Die Kristallstruktur von LiInSe2. Z. Anorg. Allg. Chem. 1986, 543, 161–168. [Google Scholar]
- Kühn, G.; Schumann, B.; Oppermann, D.; Neumann, H.; Sobotta, H. Preparation, structure, and infrared lattice vibrations of LilnTe2. Z. Anorg. Allg. Chem. 1985, 531, 61–66. [Google Scholar] [CrossRef]
- Kim, J.; Hughbanks, T. ChemInform Abstract: Synthesis and Structures of New Ternary Aluminum Chalcogenides: LiAlSe2, α-LiAlTe2, and β-LiAlTe2. ChemInform 2000, 39, 3092–3097. [Google Scholar] [CrossRef]
- Kidyarov, B.I.; Atuchin, V.V. Universal crystal classification system “Point symmetry—Physical property”. Ferroelectrics 2007, 360, 96–99. [Google Scholar] [CrossRef]
- Huang, J.-J.; Atuchin, V.; Andreev, Y.M.; Lanskii, G.; Pervukhina, N. Potentials of LiGa(S1−xSex)2 mixed crystals for optical frequency conversion. J. Cryst. Growth 2006, 292, 500–504. [Google Scholar] [CrossRef]
- Tyazhev, A.; Marchev, G.; Vedenyapin, V.; Kolker, D.; Yelisseyev, A.; Lobanov, S.; Isaenko, L.; Zondy, J.; Petrov, V. LiInSe2 nanosecond optical parametric oscillator tunable from 4.7 to 8.7 μm. Proc. SPIE 2010, 7582, 75820E. [Google Scholar]
- Takeya, K.; Takemoto, Y.; Kawayama, I.; Murakami, H.; Matsukawa, T.; Yoshimura, M.; Mori, Y.; Tonouchi, M. Terahertz emission from coherent phonons in lithium ternary chalcopyrite crystals illuminated by 1560 nm femtosecond laser pulses, EPL 91. Eur. Lett. 2010, 91, 20004. [Google Scholar] [CrossRef]
- Beutler, M.; Rimke, I.; Büttner, E.; Petrov, V.; Isaenko, L. Femtosecond mid-IR difference-frequency generation in LiInSe2. Opt. Mater. Express 2013, 3, 1834–1838. [Google Scholar] [CrossRef]
- Isaenko, L.; Yelisseyev, A.; Lobanov, S.; Vedenyapin, V.; Krinitsyn, P.; Petrov, V. Properties of LiGa0.5In0.5Se2: A Quaternary Chalcogenide Crystal for Nonlinear Optical Applications in the Mid-IR. Crystals 2016, 6, 85. [Google Scholar] [CrossRef]
- Wang, S.; Dai, S.; Jia, N.; Zong, N.; Li, C.; Shen, Y.; Yu, T.; Qiao, J.; Gao, Z.; Peng, Q.; et al. Tunable 7–12 μm picosecond optical parametric amplifier based on a LiInSe2 mid-infrared crystal. Opt. Lett. 2017, 42, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.-B.; Jia, N.; Chen, J.-K.; Shen, Y.; Yang, S.; Li, Y.-J.; Liu, Q.; Yang, F.; Zong, N.; Wang, Z.-M.; et al. Picosecond mid-infrared optical parametric amplifier based on LiInSe2 with tenability extending from 3.6 to 4.8 μm. Opt. Express 2017, 25, 12860–12866. [Google Scholar] [CrossRef]
- Kato, K.; Petrov, V.; Miyata, K. Phase-matching properties of LiIn(SxSe1-x)2. Proc. SPIE 2020, 11264, 112641W. [Google Scholar]
- Tupitsyn, E.; Bhattacharya, P.; Rowe, E.; Matei, L.; Groza, M.; Wiggins, B.; Burger, A.; Stowe, A. Single crystal of LiInSe2 semiconductor for neutron detector. Appl. Phys. Lett. 2012, 101, 202101. [Google Scholar] [CrossRef]
- Egner, J.C.; Groza, M.; Burger, A.; Stassun, K.G.; Buliga, V.; Matei, L.; Bodnarik, J.G.; Stowe, A.C.; Prettymane, T.H. Integration of a 6LilnSe2 thermal neutron detector into a CubeSat instrument. J. Astronom. Telescopes Instrum. Syst. 2016, 2, 046001. [Google Scholar] [CrossRef]
- Belushkin, A.V.; Bogdzel, A.A.; Goloshumova, A.A.; Isaenko, L.I.; Lobanov, S.I.; Milkov, V.M.; Tarasova, A.Y.; Yelisseyev, A.P. Study of LiInSe2 Single Crystals for the Thermal Neutron Detection. Synchrotron Neutron Tech. 2020, 14 (Suppl. S1), S15–S18. [Google Scholar] [CrossRef]
- Lukosi, E.; Hamm, D.; Preston, J.; Hausladen, P.; Brune, C.; Massey, T.; Jacobs, D.; Burger, A.; Stowe, A. First evaluation of fast neutron imaging with LiInSe2 semiconductors. Nucl. Instrum. Methods Phys. Res. Sect. A 2020, 976, 164254. [Google Scholar] [CrossRef]
- Matei, L.; Hawrami, R.; Buliga, V.; Babalola, S.; Duff, M.C.; Inabinet, L.; Baldwin, T.; Jandeska, A.; Burger, A. Lithium indium diselenide—An advanced material for neutron detection. Nucl. Instrum. Methods Phys. Res. Sect. A 2021, 1020, 165898. [Google Scholar] [CrossRef]
- Jia, N.; Wang, S.; Gao, Z.; Wu, Q.; Li, C.; Zhang, X.; Yu, T.; Lu, Q.; Tao, X. Optimized Growth of Large-Sized LiInSe2 Crystals and the Electric–Elastic Properties. Cryst. Growth Des. 2017, 17, 5875–5880. [Google Scholar] [CrossRef]
- Grazhdannikov, S.A.; Krinitsyn, P.G.; Kurus’, A.F.; Isaenko, L.I.; Yelisseyev, A.P.; Molokeev, M.S. LiGaTe2 (LGT) nonlinear crystal: Synthesis and crystal growth processes exploration. Mater. Sci. Semicond. Process. 2017, 72, 52–59. [Google Scholar] [CrossRef]
- Jia, N.; Xiong, X.; Wang, S.; Yu, T.; Han, B.; Qiao, J.; Li, C.; Tao, X. Optimized oriented seed growth and optical properties of high-quality LiInSe2 crystals. CrystEngComm 2018, 48, 7802–7808. [Google Scholar] [CrossRef]
- Yelisseyev, A.P.; Drebushchak, V.; Titov, A.S.; Isaenko, L.I.; Lobanov, S.I.; Lyapunov, K.M.; Gruzdev, V.A.; Komarov, S.G.; Petrov, V.; Zondy, J.-J. Thermal properties of the midinfrared nonlinear crystal LiInSe2. J. Appl. Phys. 2004, 96, 3659–3665. [Google Scholar] [CrossRef]
- Yang, L.-Q.; Jiang, X.-M.; Guo, G.-C. LiGa0.54In0.46S2: A new infrared nonlinear optical material with large laser damage threshold designed by gallium substitution in LiInS2. Inorg. Chem. Commun. 2020, 115, 107852. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Liang, F.; Grazhdannikov, S.; Isaenko, L.I.; Krinitsin, P.G.; Molokeev, M.S.; Prosvirin, I.P.; Jiang, X.; Lin, Z. Negative thermal expansion and electronic structure variation of chalcopyrite type LiGaTe2. RSC Adv. 2018, 8, 9946–9955. [Google Scholar] [CrossRef] [PubMed]
- Krinitsin, P.; Yelisseyev, A.; Jiang, X.; Isaenko, L.; Molokeev, M.; Lin, Z.; Pugachev, A. Growth, structure, and optical properties of nonlinear LiGa0.55In0.45Te2 single crystals. Cryst. Growth Des. 2019, 19, 1805–1814. [Google Scholar] [CrossRef]
- Andreev, Y.M.; Atuchin, V.V.; Lanskii, G.V.; Morozov, A.N.; Pokrovsky, L.D.; Sarkisov, S.Y.; Voevodina, O.V. Growth, real structure and applications of GaSe1−xSx crystals. Mater. Sci. Eng. B 2006, 128, 205–210. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Golyashov, V.A.; Kokh, K.A.; Korolkov, I.V.; Kozhukhov, A.S.; Kruchinin, V.N.; Makarenko, S.V.; Pokrovsky, L.D.; Prosvirin, I.P.; Romanyuk, K.N.; et al. Formation of inert Bi2Se3(0001) cleaved surface. Cryst. Growth Des. 2011, 11, 5507–5514. [Google Scholar] [CrossRef]
- Kokh, K.A.; Atuchin, V.V.; Adichtchev, S.V.; Gavrilova, T.A.; Bakhadur, A.M.; Klimov, A.O.; Korolkov, I.V.; Kuratieva, N.V.; Mukherjee, S.; Pervukhina, N.V.; et al. Cu2ZnSnS4 crystal growth using an SnCl2 based flux. CrystEngComm 2021, 22, 1025–1032. [Google Scholar] [CrossRef]
- Vasilyeva, I.G.; Nikolaev, R.E. Non-stoichiometry and point native defects in non-oxide non-linear optical large single crystals: Advantages and problems. CrystEngComm 2022, 24, 1495–1506. [Google Scholar] [CrossRef]
- Siemek, K.; Yelisseyev, A.; Horodek, P.; Lobanov, S.; Goloshumova, A.; Belushkin, A.; Isaenko, L. Optical and positron annihilation studies of structural defects in LiInSe2 single crystals. Opt. Mater. 2020, 109, 110262. [Google Scholar] [CrossRef]
- Cui, Y.; Bhattacharya, P.; Buliga, V.; Tupitsyn, E.; Rowe, E.; Wiggins, B.; Johnstone, D.; Stowe, A.; Burger, A. Defects in 6LiInSe2 neutron detector investigated by photo-induced current transient spectroscopy and photoluminescence. Appl. Phys. Lett. 2013, 103, 092104. [Google Scholar] [CrossRef]
- Fairley, N.; Fernandez, V.; Richard-Plouet, M.; Guillot-Deudon, C.; Walton, J.; Smith, E.; Flahaut, D.; Greiner, M.; Biesinger, M.; Tougaard, S.; et al. Systematic and collaborative approach to problem solving using X-ray photoelectron spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces—Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494–1497. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Baroni, S.; de Gironcoli, S.; Corso, A.D.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Bruker AXS. TOPAS V4: General Profile and Structure Analysis Software for Powder Diffraction Data—User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Lavrentyev, A.; Gabrelian, B.; Vu, V.; Ananchenko, L.; Isaenko, L.; Yelisseyev, A.; Khyzhun, O. Electronic structure and optical properties of noncentrosymmetric LiGaSe2: Experimental measurements and DFT band structure calculations. Opt. Mater. 2017, 66, 149–159. [Google Scholar] [CrossRef]
- Lavrentyev, A.; Gabrelian, B.; Vu, T.V.; Isaenko, L.; Yelisseyev, A.; Khyzhun, O. Electronic structure and optical properties of LiGa0.5In0.5Se2 single crystal, a nonlinear optical mid-IR material. Opt. Mater. 2018, 80, 12–21. [Google Scholar] [CrossRef]
- Sobol, P.E.; Nelson, A.J.; Schwerdtfeger, C.R.; Stickle, W.F.; Moulder, J.F. Single crystal CulnSe2 analysis by high resolution XPS. Surf. Sci. Spectra 1992, 1, 393. [Google Scholar] [CrossRef]
- Atuchin, V.; Isaenko, L.; Kesler, V.; Lobanov, S. Core level photoelectron spectroscopy of LiGaS2 and Ga–S bonding in complex sulfides. J. Alloy. Compd. 2010, 497, 244–248. [Google Scholar] [CrossRef]








| M | S | Se | Te |
|---|---|---|---|
| In | P21nb (Pna21) [19] | Pna21 [23] | I-42d [24] |
| Ga | Pna21 [20,21] | Pna21 [21,22] | I-42d [21] |
| Al | Pna21 [25] | I-42d and P3m1 [25] |
| Compound | Type | αa MK−1 | αb MK−1 | αc MK−1 | αV MK−1 | Reference |
|---|---|---|---|---|---|---|
| LiGaTe2 | II | 19.1 | 19.1 | −8.6 | 29.4 | [45] |
| LiGa0.55In0.45Te2 | II | 18.9 | 18.9 | −5.7 | 32.3 | [46] |
| LiGa0.54In0.46S2 | I | 11.7 | 15.8 | 12.7 | [44] | |
| LiInS2 | I | 8.9 | 16.1 | 6.6 | [2] | |
| LiInSe2 | I | 11.5 ± 1.7 | 20.4 ± 2.4 | 8.9 ± 2.4 | [43] | |
| LiInSe2 | I | 8.1 (1) | 16.1 (2) | 5.64 (6) | 29.9 (3) | This work |
| Compound | LiInSe2 |
|---|---|
| Sp. Gr. | Pna21 |
| a, Å | 7.20442 (7) |
| b, Å | 8.42826 (8) |
| c, Å | 6.80491 (6) |
| V, Å3 | 413.199 (7) |
| Z | 4 |
| 2θ-range, ° | 10–140 |
| Rwp, % | 5.63 |
| Rp, % | 4.71 |
| Rexp, % | 2.67 |
| χ2 | 2.11 |
| RB, % | 2.16 |
| Line | Binding Energy, eV |
|---|---|
| In 4d5/2 | 18.06 |
| In 4d3/2 | 18.93 |
| Se 3d5/2 | 54.04 |
| Se 3d3/2 | 54.89 |
| Li 1s | 54.97 |
| Se L3M45M45 | 179.95 |
| C 1s | 285.0 (fixed) |
| O 1s | 531.46 |
| In 3d5/2 | 444.60 |
| In 3d3/2 | 452.15 |
| In M4N45N45 | 1078.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atuchin, V.V.; Isaenko, L.I.; Lobanov, S.I.; Goloshumova, A.A.; Molokeev, M.S.; Zhang, Z.; Zhang, X.; Jiang, X.; Lin, Z. Anisotropic Thermal Expansion and Electronic Structure of LiInSe2. Molecules 2022, 27, 5078. https://doi.org/10.3390/molecules27165078
Atuchin VV, Isaenko LI, Lobanov SI, Goloshumova AA, Molokeev MS, Zhang Z, Zhang X, Jiang X, Lin Z. Anisotropic Thermal Expansion and Electronic Structure of LiInSe2. Molecules. 2022; 27(16):5078. https://doi.org/10.3390/molecules27165078
Chicago/Turabian StyleAtuchin, Victor V., Ludmila I. Isaenko, Sergei I. Lobanov, Alina A. Goloshumova, Maxim S. Molokeev, Zhaoming Zhang, Xingyu Zhang, Xingxing Jiang, and Zheshuai Lin. 2022. "Anisotropic Thermal Expansion and Electronic Structure of LiInSe2" Molecules 27, no. 16: 5078. https://doi.org/10.3390/molecules27165078
APA StyleAtuchin, V. V., Isaenko, L. I., Lobanov, S. I., Goloshumova, A. A., Molokeev, M. S., Zhang, Z., Zhang, X., Jiang, X., & Lin, Z. (2022). Anisotropic Thermal Expansion and Electronic Structure of LiInSe2. Molecules, 27(16), 5078. https://doi.org/10.3390/molecules27165078







