Abstract
3,4-Dihydro-2(1H)-pyridones (3,4-DHPo) and their derivatives are privileged structures, which has increased their relevance due to their biological activity in front of a broad range of targets, but especially for their importance as synthetic precursors of a variety of compounds with marked biological activity. Taking into account the large number of contributions published over the years regarding this kind of heterocycle, here, we presented a current view of 3,4-dihydro-2(1H)-pyridones (3,4-DHPo). The review includes general aspects such as those related to nomenclature, synthesis, and biological activity, but also highlights the importance of DHPos as building blocks of other relevant structures. Additional to the conventional multicomponent synthesis of the mentioned heterocycle, nonconventional procedures are revised, demonstrating the increasing efficiency and allowing reactions to be carried out in the absence of the solvent, becoming an important contribution to green chemistry. Biological activities of 3,4-DHPo, such as vasorelaxant, anti-HIV, antitumor, antibacterial, and antifungal, have demonstrated this heterocycle’s potential in medicinal chemistry.
1. Introduction
The study of determinate structures such as small molecules in drug discovery has increased, engaging most in medicinal chemistry. These structures, particularly those based on N-heterocycles, represent a class of molecules capable of binding to multiple receptors with high affinity, showing a broad range of biological activity.
In this regard, the 3,4-dihydro-2(1H)-pyridones (3,4-DHPo) are biologically active N-heterocycles analogs of the well-known 1,4-dihydropyridines (1,4-DHPs) and dihydropyrimidines (DHPMs) [1], which have been introduced in the scientific landscape. In this context, DHPo’s as the Milrinone and the Amrinone are drugs with cardiotonic activity successfully used to treat heart failure (Figure 1) [2]. In addition, they and their derivatives have also been reported to possess antitumor [3], antibacterial [4], anti-HIV [5], and other biological activities [6,7,8,9]. These results encourage many research groups to search for potentially active DHPo’s analogs.
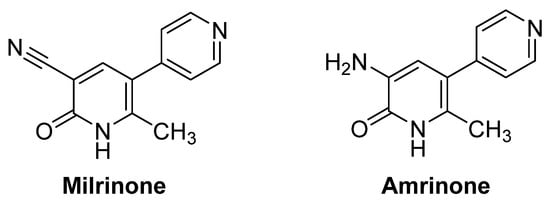
Figure 1.
Milrinone and Amrinone structures increase cardiac contractility vasodilators.
At the same time, the 3,4-Dihydro-2(1H)-pyridones (3,4-DHPo) and their derivatives are extensively used as precursors in the synthesis of bioactive molecules such as (±)-Andranginine (Figure 2) [10], elective α1a adrenergic receptors [11], Rho-kinase inhibitors [12], and P2X7 receptor antagonists [13].
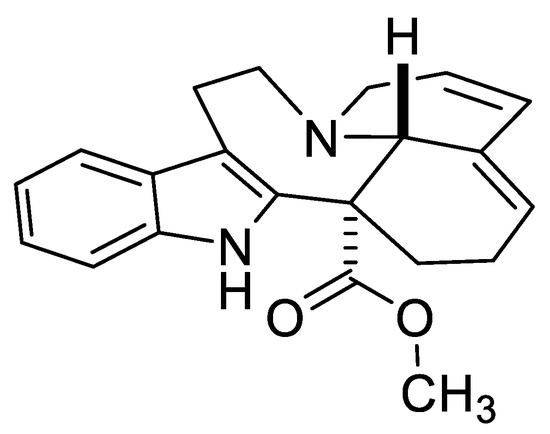
Figure 2.
(±)-Andranginine structure.
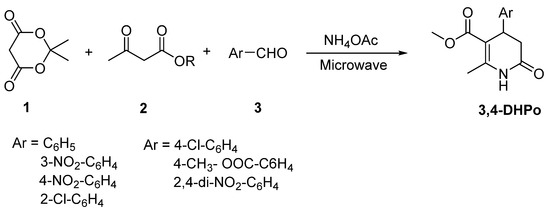
The synthesis of 3,4-DHPo derivatives has been important with the passing of time. Different synthetic procedures to synthesize them have taken place in the last years, although the most reported uses are the multicomponent reaction (MCR-4CR) of Meldrum’s acid (1), a β-keto-ester derivative (2), and aromatic aldehydes (3) in the presence of ammonium acetate. Additionally, a broad range of techniques have been covered, from the conventional synthesis, through energy resources such as microwave, ultrasound, and infrared assisted to solid and liquid phase synthesis, and recently the chemoenzymatic assisted methodology applied to their asymmetric synthesis (Scheme 1).
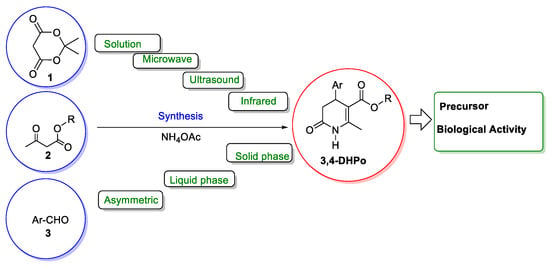
Scheme 1.
3,4-Dihydro-2(1H)-pyridone (3,4-DHPo) synthesis procedures and applications, Meldrum’s acid (1), β-keto-ester derivative (2), aldehyde derivative (3).
Due to the importance of this N-heterocycle, this review aims to present the principal characteristics of the 3,4-DHPo structures, the different procedures to synthesize them developed over time, the main biological application, and their crucial importance as precursors to molecules with high relevance in medicinal chemistry. A critical overview of the advantages and disadvantages of the conventional and nonconventional synthetic methods to prepare 3,4-DHPo are highlighted.
2. Nomenclature, Structure, and General Synthesis
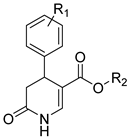
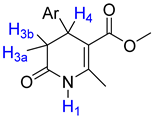
The heterocyclic 3,4-dihydro-2(1H)-pyridones (3,4-DHPo) (Figure 3) are commonly known 4-aryl substituted 5-alkoxycarbonyl-6-methyl-3,4-dihydropyridones (Figure 3a), although the correct IUPAC name is 4-arylsubstituted-2-methyl-6-oxo-1,4,5,6-tetrahydro-3-pyridinecarboxylates (Figure 3b). The calculated tridimensional structure (Figure 3c) shows the atoms’ corroborated disposition in the DHPo [14,15,16].
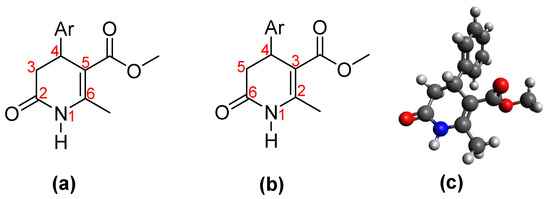
Figure 3.
3,4-DHPo structures. (a) Common name, 4-aryl substituted 5-alkoxycarbonyl-6-methyl-3,4-dihydropyridones; (b) IUPAC name, 4-arylsubstituted-2-methyl-6-oxo-1,4,5,6-tetrahydro-3-pyridinecarboxylates; and (c) 3D structure model, whose substituent purple color represents the aryl (Ar) group.
The effort to synthesize 1,4-DHP derivatives by modifying its ring gave rise to the first accidental synthesis of 3,4-DHPo, as novel heterocycles that showed interesting biological activity [17]. The general synthesis of 3,4-DHPo as a racemic mixture involves a multicomponent reaction (MCR 4-CR) of Meldrum’s acid (1), a β-keto-ester derivative (2), and an aromatic aldehyde (3) in the presence of ammonium acetate and with or without solvent (Scheme 2). Responsible for blocking the formation of the 1,4-dihydropyridines ring is the acidic character of Meldrum’s acid (pKa = 9.97), which is higher than the β-keto-ester (pKa = 11.0) [18].
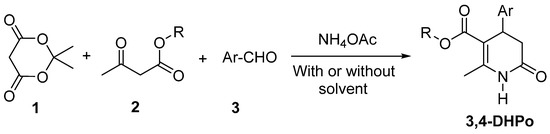
Scheme 2.
General synthesis of 3,4-Dihydro-2(1H)-pyridones (3,4-DHPo).
These kinds of molecules present a stereogenic center at C-4. Its absolute configuration (R- versus S-enantiomer) was a critical factor for the biological activity as an antagonist or agonist of calcium ions [19]. The mechanism reported in Scheme 3 has been demonstrated in synthesizing intermediaries and their characterization [20].
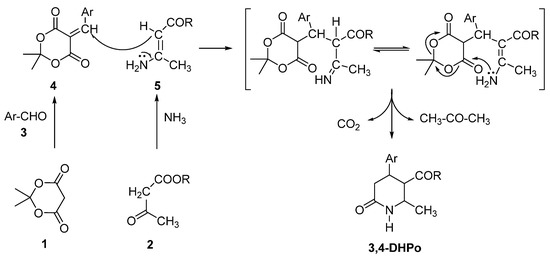
Scheme 3.
Proposed reaction mechanism for 3,4-DHPo synthesis.
The generally accepted mechanism follows a Hantzsch-like pathway with the previous formation of two intermediates; four from Knoevenagel condensation of Meldrum’s acid (1) with the corresponding aromatic aldehyde (3), and five from the reaction involving β-keto-ester (2) and ammonia. A Michael-type addition of the enamine 5 on ylidene compound 5 gives rise to DHPo’s (Scheme 3) [20].
3. Synthetic Strategies
Different strategies have been reported to obtain 3,4-DHPo, conventional synthesis with or without solvent, using different energy resources such as microwave, ultrasound, and infrared to improve the yield and develop green chemistry reactions. Solid-phase organic synthesis (SPOS) and liquid-phase synthesis (LPS) on soluble polymers as supports have also been developed. Finally, chemoenzymatic to asymmetric synthesis has also been established.
3.1. Conventional Synthesis
The first strategy used to obtain the 3,4-DHPo involved the Multicomponent Reaction (MCR) using equimolecular amounts of starting compounds under ethanol reflux for six hours, allowing the synthesis of 3,4-DHPo derivatives with a 15–26% yield [21]. One year later [22], the synthesis of two 3-CN-DHPo with 51–70% yield from pyran derivatives previously synthesized was reported.
The Suárez group has improved the efficiency of this MCR procedure, replaced the ethanol with acetic acid, and allowed moderate to high yields (Scheme 3) [17,18,19,23,24,25,26,27,28,29]. The best results are due to the catalytic effect and the higher boiling point of acetic acid, which improve the decarboxylation step and increase the yields compared with previously reported methods [21,30].
In 2011, Sun et al. developed a protocol to synthesize structurally diverse 3,4-DHPo via MCR, similar to those previously described by the Suárez group but using arylamines, acetylenedicarboxylate, aromatic aldehydes, and Meldrum acid as starting reagents. The proposed mechanism involves a Michael addition of the enamino ester formed in situ from the reaction of arylamine with dimethyl acetylenedicarboxylate to arylidine cyclic 1,3-diketones [31].
An efficient one-pot synthesis of polysubstituted dihydropyridones derivatives was reported by Khazaei and Anary-Abbasinejad [32]. The reaction was achieved using cyanoacetamide (6), aryl aldehydes derivatives (3), ethyl acetoacetate (2), and ammonium acetate and using pyridine as the catalyst, in ethanol as solvent, and under reflux conditions obtaining 54–68% of yields (Scheme 4). The advantage of this method is the use of neutral conditions and the facility of mixing reagents without any previous activation or modification.
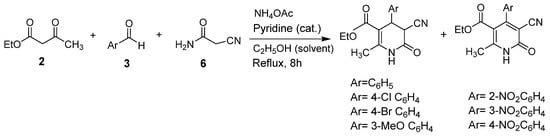
Scheme 4.
Synthesis of polysubstituted dihydropyridones derivatives.
An improvement of this method was reported by Dehghan et al. They used similar general conditions, introducing some variation [33]. The researchers changed ammonium acetate to ammonium carbonate and did not use pyridine. Additionally, they also used and compared ethanol and water as reaction solvents. A significant yield improvement was obtained in water (90–96%) compared with ethanol (55–75%).
Furthermore, the Hakimi research group reported a new one-pot, four-component synthesis of 3,4-dihydro-2-pyridone derivatives (3,4-DHPo). The reaction of Meldrum’s acid (1), methyl acetoacetate (2), benzaldehyde derivatives (3), and ammonium acetate and using SiO2-Pr-SO3H as an efficient catalyst under solvent-free conditions was reported (Scheme 5) [34]. The advantages of this methodology are high product yields (78–93%), being environmentally benign, short reaction times, and easy handling.
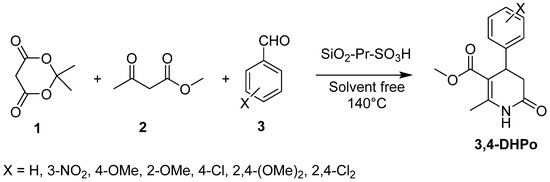
Scheme 5.
Synthesis of 3,4-DHPo derivatives using SiO2-Pr-SO3H as the catalyst.
Moreover, the preparation of 3,4-dihydro-2H-chromeno[4,3-b]pyridine-2,5(1H)-dione derivatives using 4-hydroxycoumarin, an aromatic aldehyde, ammonia, and Meldrum’s acid under refluxing with 1-propanol has also been published [35].
Li et al. reported the synthesis of 1,4 DHPs derivatives with the precursor ethyl 4,4,4-trifluoro-3-oxobutanoate and a short report with the brominating of 3,4 DHPo using N-bromosuccinimide [36,37]. The reaction of α-hydroxyketene-(S,S)-acetals and active methylenes to obtain 3,4 DHPo derivatives was also reported [38]. Razdan and coworkers reported the synthesis of 3,4-dihydro-2-pyridones, using Bi(III) nitrate immobilized on neutral alumina as the catalyst, in the presence of co-catalyst of Zn (II) chloride with 79–88% yield [39].
On the other hand, fluorinated DHPo derivatives synthesis has also been developed. For example, Song et al. reported the synthesis of 3-aryl-4-unsubstituted-6-CF3-pyridin-2-ones and ethyl 2-hydroxy-6-oxo-4-aryl-2-(trifluoromethyl)-piperidine-3-carboxylate as essential building blocks for the construction of trifluoromethylated heterocycles, and studying the effect of base and solvents in the reaction obtained 0–93% of yields [40,41]. Further, Smits et al. published the formation of fluorous 3,4-dihydro-2(1H)-pyridone-5-carboxylate as a cationic amphiphile. The 3,4-DHPo moiety plays a key role as a scaffold for attaching cationic head groups [9].
Instead, Dostanic et al. published about synthesizing (substituted phenylazo)-pyridones in the presence of KOH and acetone to obtain 11–61% yield [42]. These were used dyeing polyester fabrics as yellow dyes. Another report described the synthesis of 3,4-dihydropyridones derivatives in the presence of Cs2CO3 and toluene with 53–66% yield [43]. The synthesis of aza- analogs of 3,4-DHPo with anticancer activity was reported by Bariwal et al. using benzoylacetone, substituted aldehyde, urea or thiourea with HCl, and ethanol as a solvent with 55–77% yield [44].
Besides, the conventional reaction has been improved by using different catalysts. Zhiqiang et al. reported a three-component cascade reaction to achieve 3,4-DHPo derivatives using imidazole as a catalyst with ethylene glycol as solvent [45]. Later, Bhattacharyya et al. reported a greener method to obtain 3,4-DHPo derivatives using a one-pot multicomponent reaction in aqueous media catalyzed by nanostructured ZnO [46]. Additionally, Khazaei et al. used ZnO nanoparticles to give 3,4-DHPo derivatives under ethanol as solvent [44]. Ziarani et al. published the synthesis of 3,4-DHPo derivatives by sulphonic-acid-functionalized ordered nanoporous SBA-15 as a nano heterogeneous catalyst via one-pot, four-component reaction under solvent-free conditions [47]. Further, Pradhan et al. presented green protocols to achieve 3,4-DHPo derivatives using two catalysts such as the vitamin B1 or PEG–SO3H in water as solvent [48]. All these described reactions showed moderate to good yields. Besides, those reactions where a catalyst was attached to solid or polymeric supports showed better results due to the possibility of the most efficient purification procedures.
Zhang et al. reported the synthesis of 5-cyano-2-pyridinone catalyzed by Zn-SSA. The silica sulfuric acid (SSA) was modified with zinc chloride to form the novel catalyst (Zn-SSA), which improved the chemo-selectivity in the reaction [49]. The synthesis was developed using 3-dicarbonyl compounds (7), malononitrile (8), arylaldehyde (3), and solvent-free conditions (Scheme 6).
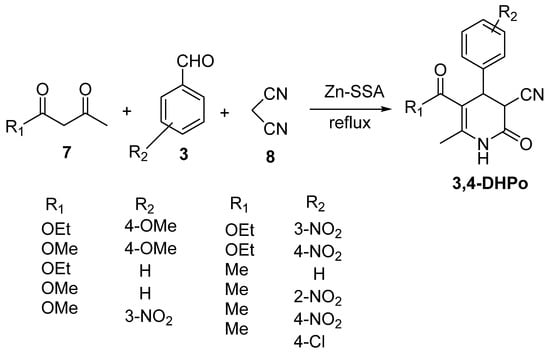
Scheme 6.
Synthesis of 5-cyano-2-pyridinone using Zn-SSA as catalyst.
The synthesis of 3,4-dihydropyridine-2(1H)-ones catalyzed by ZnBr2, FeCl3, AlCl3, BF3, Cu(OTf)2, In(OTf)2, and BF3 OEt2 was reported by [50]. This method was developed via Blaise reaction forming a cyclic intermediate (9) from benzonitrile (10) and Reformatsky reagents, which was generated in situ from ethyl bromoacetate (11) and zinc power in ethyl acrylate (12) and tetrahydrofuran with 0–81% yield (Scheme 7).
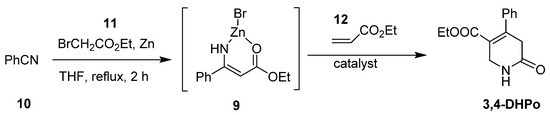
Scheme 7.
Synthesis of 3,4-dihydropyridin-2-ones catalyzed by ZnBr2, FeCl3, AlCl3, BF3, Cu(OTf)2, In(OTf)2, and BF3 OEt2.
Paravidino et al. developed a novel four-multicomponent reaction (4CR) of phosphonate (13), aldehydes derivatives (14), nitriles (15), and α-acidic isonitriles (16) to obtain 3,4-DHPo derivatives in 53–88% of yield and with complete diastereoselectivity in favor of the cis-diastereomer (Scheme 8) [51,52].
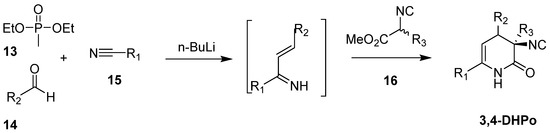
Scheme 8.
Multicomponent Reaction (4CR) of phosphonate (13), aldehydes derivatives (14), nitriles (15), and α-acidic isonitriles (16) to obtain 3,4-DHPo.
Another report showed the synthesis of 3,4-DHPo derivatives via tandem olefins isomerization–RCM reaction, through the in situ generated intermediate 18 from readily available N-Allyl amines type 17 as dienes catalyzed by second-generation Grubbs catalyst (ruthenium catalysts) and heated to obtain 3,4-DHPo derivatives with 57–85% yield (Scheme 9) [53].
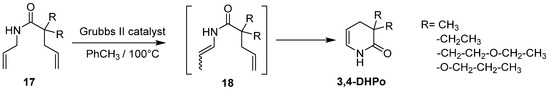
Scheme 9.
Tandem olefins isomerization–RCM to obtain 3,4-DHPo.
The conventional synthesis has been offered a broad possibility to obtain the desired product; however, nonconventional energy sources as microwave, infrared, and ultraviolet have also been incorporated to improve the efficiency of this MCR.
3.2. Nonconventional SYNTHESIS
- -
- Microwave-Assisted Synthesis
In the last decades, microwave-assisted organic synthesis has been used as a tool for many known and new organic reactions. In general, its application allowed to reduce reaction times; increase efficiency; selective heating; excellent reproducibility; and, in some cases, avoid or minimize the use of solvents, contributing to green procedure development [54].
In 2003, our group reported the first solvent-free and accessible one-pot condensation reaction of Meldrum’s acid (1) in the presence of methyl acetate (2), aldehyde derivatives (3), and ammonium acetate to obtained 4-aryl substituted 5-alkoxycarbonyl- 6-methyl-3,4-dihydropyridones (3,4-DHPo) [20]. The mixtures were irradiated at controlled temperatures and times with continuous mechanical stirring, which provided a good homogeneity of materials and 81–91% yields (Scheme 10).
Scheme 10.
Microwave-Assisted Multicomponent preparation (4CR) of 3,4-Dihydro-2(1H)-pyridones (3,4-DHPo).
Afterward, another author reported the obtention of 3,4-DHPos derivatives with a similar technique and 70–92% yields [55]. Jaques et al. reported the quantitative MW-assisted synthesis of 3,4-dihydro-2(1H)-pyridones without solvents but in the presence of solid support as a catalyst [56].
Besides, Hernandez et al. published the oxidation reaction of 4H-pyrans derivatives (19) to obtain 3-cyano-2-pyridones (20) in ethanol, using H2SO4 catalyst source and MW irradiation (Scheme 11) [57]. These compounds are hybrid milrinone–enifedipine analogs.
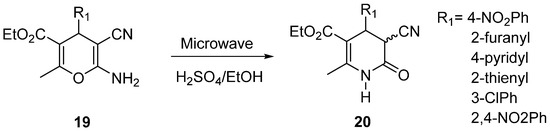
Scheme 11.
Microwave-Assisted oxidation of 4H-pyrans derivatives (19) to 3,4-Dihydro-2(1H)-pyridones (20).
This research group compared the different energy sources for oxidation; carried out the reaction at room temperature, at ethanol reflux, under infrared and microwave irradiation; and obtained 8, 72, 80, and 86% yields, respectively. Furthermore, the reaction times decreased from seven hours at room temperature until seven and five minutes for IR and MW irradiation, respectively. These comparisons show the importance of using different energy resources and their potential. Besides, ultrasound has been the other nonconventional energy source used to efficiently synthesize 3,4-DHPo.
- -
- Ultrasound-Assisted Synthesis
The ultrasonic activation is based on cavitation effects, allowing this technique to improve the mass transfer in several organic reactions reported. In 2011, our group published the synthesis of 4-aryl 3,4-dihydropyridone derivatives (3,4-DHPo) by ultrasound-assisted technique, through the one-step condensation of Meldrum’s acid (1), alkyl acetoacetates (2), aromatic aldehydes (3), and ammonium acetate, using glacial acetic acid as solvent, at room temperature, and obtained high yields (Scheme 12) [58].
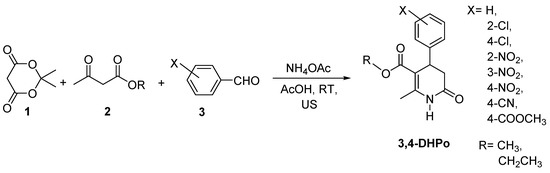
Scheme 12.
Ultrasound-Assisted Multicomponent preparation (4CR) of 3,4-Dihydro-2(1H)-pyridones (3,4-DHPo).
The main advantages of ultrasound-assisted synthesis compared with conventional procedures are the milder conditions, the shorter reaction times, and the higher yields that improve the efficiency of the organic synthesis of these heterocycles. In addition, another energy source, such as the infrared-assisted technique, has also been explored.
- -
- Infrared-Assisted Synthesis
Parallel to the MW-assisted synthesis design of 3,4-DHPo derivatives, our group also reported the preparation of 3,4-Dihydro-2(1H)-pyridones derivatives (3,4-DHPo) by infrared-assisted method of the same multicomponent reaction under solvent-free conditions and similar reagents with moderate yields (Scheme 13) [59].
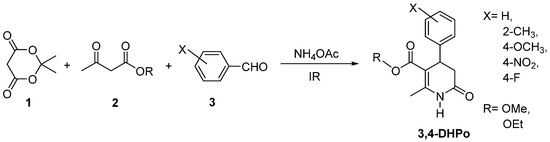
Scheme 13.
Infrared-Assisted Multicomponent preparation (4CR) of 3,4-Dihydro-2(1H)-pyridones (3,4-DHPo).
To summarize, different energy sources have been used to prepare 3,4-DHPo, allowing a broad range of products with varying substituent patterns. Particularly, nonconventional techniques such as MW and IR lead to high yields, short reaction times, and safe and straightforward work-up, constituting a notable improvement and involving green chemistry to synthesize these organic molecules. The support (insoluble or soluble)-assisted synthesis of 3,4-DHPo has also been a successful development.
3.3. Solid-Phase Organic Synthesis (SPOS)
One of the most commonly used techniques in combinatorial chemistry is Solid-Phase Organic Synthesis (SPOS), because it allows the rapid synthesis of many structurally diverse molecules in a short time. In 2006, our group published the first SPOS of 3,4-DHPo derivatives following a solid-support assisted synthetic strategy (Scheme 14) [60]. The immobilized acetoacetate (21) was obtained by reaction of 2,2,6-trimethyl-1,3-dioxin-4-one (1) and Wang resin (0.92 mmol OH/g); the further reaction of 21 in the presence of NH4OAc and HOAc led to the corresponding immobilized enamine (22), which reacted with the Knovenagel derivatives (23) to afford the expected immobilized 3,4-DHPo (24). The heterocycle was cleaved from the resin with 71–85% overall yield (Scheme 14) [60].
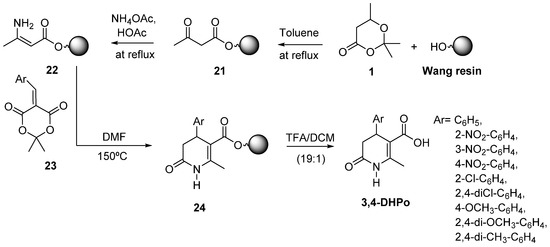
Scheme 14.
SPOS of 3,4-Dihydro-2(1H)-pyridones derivatives (3,4-DHPo).
This technique has been used in response to the increment of target molecules synthesis to combinatorial chemistry. SPOS of 3,4-DHPo derivatives presents good results and opens the way to synthesize other molecules with biological activity. Additionally, the synthesis of 3,4-DHPo derivatives using soluble polymers as support has also been studied [61].
3.4. Liquid-Phase Organic Synthesis (LPOS)
The employment of soluble polymers as supports in organic synthesis is known as liquid-phase synthesis (LPS). In 2008, Fu et al. reported the LPS of 4-substituted-5-methoxycarbonyl-6-methyl-3,4-dihydropyridones on polyethylene glycol (PEG) 4000 assisted by MW irradiation (Scheme 15) [62]. First, the acetoacetylation of PEG was realized to obtain the immobilized acetoacetate (21); further, condensation of 21 with aldehyde derivative (3), Meldrum’s acid in the presence of ammonium acetate, and solvent-free assisted by microwave irradiation allowed to obtain the immobilized 3,4-DHPo (24). The target compound 3,4-DHPo was obtained after cleavage using NaOMe in MeOH with 88–95% yield.
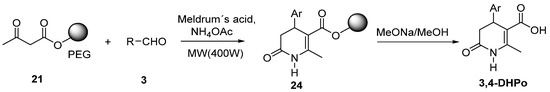
Scheme 15.
LP-MW assisted of 3,4-Dihydro-2(1H)-pyridones derivatives (3,4-DHPo).
The LPS of 3,4-DHPo derivatives showed excellent results and improved the overall results with the solid phase, allowing the one-step condensation, which was not possible in the SPOS procedure. On the other hand, all synthesized 3,4-DHPo showed at least one chiral center at C4, and all previously reported procedures allowed to obtain the corresponding racemic mixtures. Hence, efforts have been made to search for the chemo-selective synthesis of these derivatives.
3.5. Asymmetric Synthesis
The 3,4-DHPo structure is closely related to the configuration of its chiral center at C4, bringing biological activity. The chemoenzymatic synthesis can search the specific chiral center configuration and, at the same time, use an ecofriendly procedure. Torres et al. reported the chemoenzymatic preparation of a series of racemic 4-aryl-5-(tert-butoxycarbonyl)-6-methyl-3,4-dihydro-2(1H)-pyridones (25) using several combinations of lipases (PSL, CRL, CAL-A, and CAL-B) and organic solvents such as 1,4-dioxane, DIPE, and TMBE [63]. The authors improved the enzymatic hydrolysis of R-diesters (27) derivatives, making subsequent S-enantiomer (26) separation viable by acid–base extraction procedures (Scheme 16). An improvement to this methodology was made by the chemoenzymatic preparation of optically active phenolic 3,4-dihydropyridin-2-ones [64].
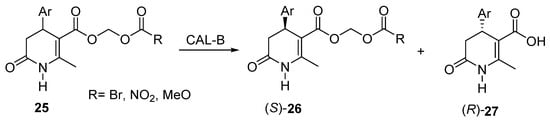
Scheme 16.
Chemoenzymatic preparation of optically active phenolic 3,4-dihydropyridin-2-ones (3,4-DHPo) through hydrolysis of diesters.
Another enzymatic multicomponent reaction (EMCR) was reported using benzaldehyde, cyanoacetamide, ketone, and Acylaze Amano (AA)-catalyzed. This single enzymatic catalyzed reaction is attractive due to its high atom economy, easy work-up process, tolerance to a wide range of substituted reagents (benzaldehyde and ketones), and all mild conditions [65]. The best result was shown in the enzymatic hydrolysis of 4-aryl-5-(tert-butoxycarbonyl)-6-methyl-3,4-dihydro-2(1H)-pyridones with CAL-B enzyme and TMBE as a solvent to obtain high ee (93–95%) and moderate yields (30–31%) of (S)-derivative (26).
On the other side, Huang et al. reported the first asymmetric synthesis of 3,4-DHPo derivatives [66]. The formation of monoacid (R)-30 was carried out by desymmetrization or asymmetric methanolysis of prochiral anhydride (29) using the organocatalyst 28, 2-Me-THF, and MeOH as solvents (Scheme 17). The conversion was 100%, and the best-observed ee of 80%. The next step was the selective formylation to obtain the intermediate 31, and finally, the cyclization of 31 with ammonium acetate using acetic acid achieved the final product (R)-32, with an overall yield of three steps 48% and >95% ee (Scheme 17) [66]. The same group also reported the pilot-scale enantioselective synthesis of 32 and kilogram-scale production of N-methyl derivative of 32 in an excellent overall yield of ~22% with excellent stereochemical purity (97% ee) [13].
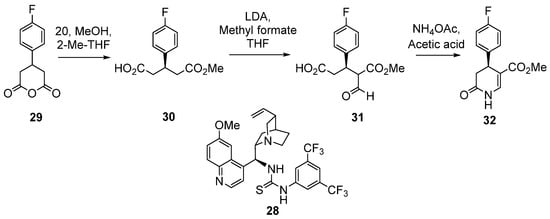
Scheme 17.
Asymmetric synthesis of (R)-3,4-DHPo derivatives (3,4-DHPo, 32).
Wanner et al. published the enantioselective synthesis of (R)-3,4-DHPo through N-Heterocyclic carbene (NHC)-catalyzed aza-Claisen reaction of enal (35) and enamine (36) in the presence of N-mesityl catalyst (33) and oxidant (34); the better bases were DBU, NMM, and i-Pr2Net, showing higher enantioselectivity with 60% to quantitative yields, and 79–96% enantiomeric excess (Scheme 18) [13].
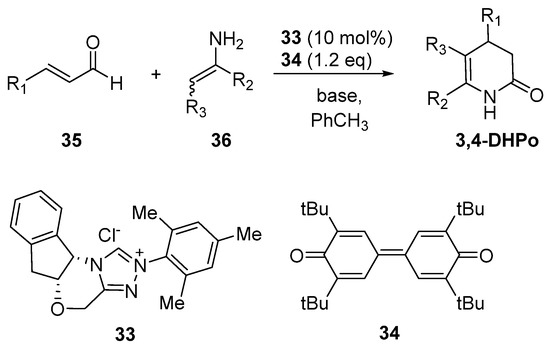
Scheme 18.
Asymmetric synthesis of optically active 3,4-dihydropyridin-2-ones ((R)-3,4-DHPo) through N-Heterocyclic carbene (NHC)-catalyzed aza-Claisen reaction.
Vellalath et al. described the enantioselective nucleophile catalyzed Michael/proton transfer/lactamization cascade with 3,4-difluorocinnamoyl chloride (37) and the enamine (39) in the presence of 37 as a catalyst with a nonpolar solvent and LiCl as an additive, which affected enantioselectivity; this mild process delivered 3,4-DHPo derivative in 78% yield and 92% ee (Scheme 19) [67].
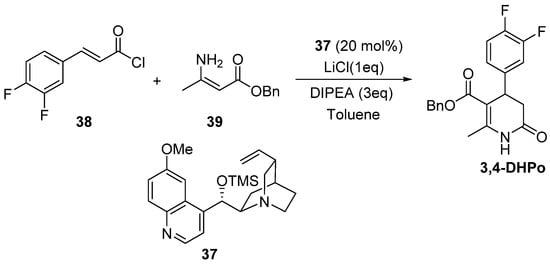
Scheme 19.
Asymmetric synthesis of optically active 3,4-dihydropyridin-2-ones ((R)-3,4-DHPo) through Michael/proton transfer/lactamization cascade.
As time went on, different strategies were developed to obtain 3,4-DHPo derivatives in higher yields. Using nonconventional sources such as microwave and infrared radiation increased the yield; reduced the reaction time; and, in many cases, avoided the use of solvent, approaching green chemistry. However, access to the equipment can become a handicap. On the other hand, the main disadvantage of described conventional and nonconventional methodologies is the lack of stereoselectivity, which only shows in the asymmetrical synthesis. Nevertheless, a broad range of procedures described shows the importance of these heterocycles, not only because of their biological activity but also because they are crucial starting materials for synthesizing other more complex entities.
4. Structural Characterization
The structure of 3,4-Dihydro-2(1H)-pyridones has been determined for physical and analytical techniques such as melting point (Table 1), 1H- and 13C-NMR spectroscopy, mass spectrometry (electron impact (EI) and electrospray ionization (ESI)), and X-ray. These techniques allowed to obtain a broad range of databases of physical properties to synthesize 3,4-DHPo [20,28,59].

Table 1.
Melting Point of 3,4-DHPo derivatives.
The technique most used in the characterization of 3,4-DHPo derivatives has been 1H-NMR spectroscopy, allowing us to corroborate the ring formation through the ABX pattern, as was explained by our group [68], which showed the protons H1, H3a, H3b, and H4 of the heterocycle ring (Table 2 and Figure 4).

Table 2.
1H-NMR spectroscopy, ABX pattern of 3,4-DHPo derivatives (δ, ppm; J, Hz).
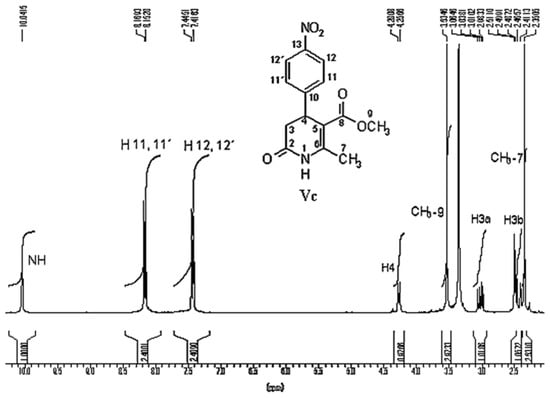
Figure 4.
1H-NMR spectrum of 6-methyl-5-methoxycarbonyl-4-(4′-nitrophenyl)-3,4-dihydro-2(1H)-pyridone.
Electron impact (EI) and electrospray ionization (ESI) have also been used to characterize the 3,4-DHPo. Our group reported the fragmentation patterns of the even-electron ions formed under ESI conditions and the odd-electron ions generated under EI conditions from substituted 3,4-DHPo [20]. The characteristic fragmentation pattern under EI conditions was also established (Scheme 20) [65].
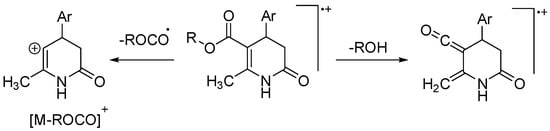
Scheme 20.
Fragmentation pattern of 3,4-DHPo derivatives established under EI conditions.
Under ESI conditions, molecular ions [M + H]+ and [M-H]− were observed, corresponding with the positive and negative modes. Additionally, some structures were proposed to explain the fragment ions found in the spectra (Scheme 21 and Scheme 22).
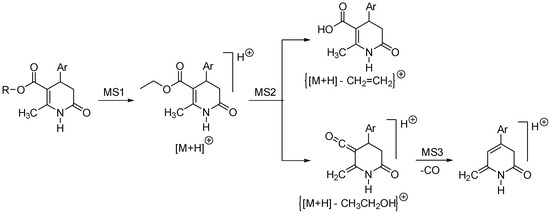
Scheme 21.
Fragmentation pattern of 3,4-DHPo derivatives established under ESI positive mode.
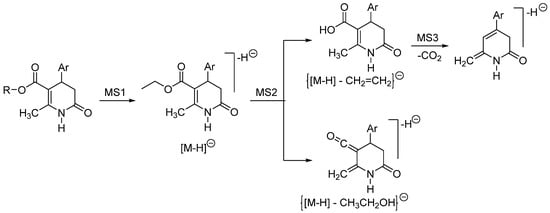
Scheme 22.
Fragmentation pattern of 3,4-DHPo derivatives established under ESI negative mode.
Different authors have confirmed the structure of 3,4-DHPo with different substituents by X-ray diffraction [17,29,30,69,70,71,72,73], semiempirical (AM1) calculations [25], NMR spectroscopic including NOE experiments, and coupling constants to determinate the structural conformation in solution [17].
There are some structural requirements to the 3,4-DHPo derivatives conformation, such as the absolute configuration at C-4 (R-versus S-enantiomer) acting as a molecular switch, the substituted phenyl ring occupies an axial position perpendicularly bisecting the boatlike DHPo ring in a synperiplanar orientation, and the cis-carbonyl ester orientation concerning the olefinic double bond [17]. The semiempirical (AM1) calculations and NOE experiments defined two conformational structures; a first presents the aryl substituent at C-4 extended in a pseudoaxial position, and a second in which the aryl substituent is in a pseudoequatorial position; besides, the first conformation was 2–4 kcal/mol more stable than second structure. In both arrangements, the pyridone ring presented a twisted boat [17,19,23,24]. The X-ray studies confirmed the pseudoaxial disposition (Figure 5), which was stable in a solid state after the crystallization of ethanol [17].
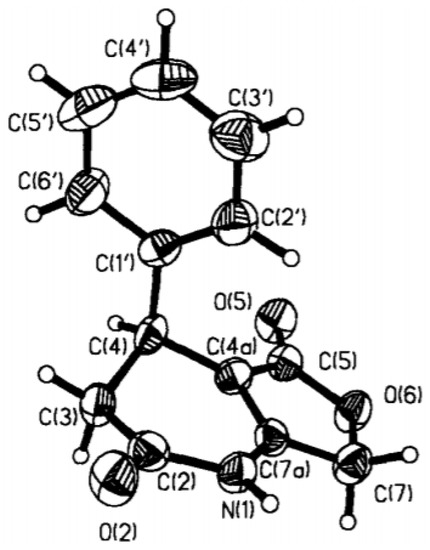
Figure 5.
Crystallographic structure of 3,4-DHPo [Reprinted/adapted with permission from Ref. [17]. 1998, Elsevier].
5. 3,4-Dihydro-2(1H)-Pyridones (3,4-DHPo) as Synthetic Precursors
Over time, the 1,4-DHP and 3,4-DHPo cores have served as scaffolds for the relevant design of more complex entities with various biological activities.
The effort to improve the synthesis of 1,4-DHP with a broad range of substitution patterns gave rise to 3,4-DHPo as crucial intermediaries. For example, 3,4-DHPo were converted to the aromatics 4-(3-nitrophenyl) pyridines (Figure 6a) [74], and to alkyl 4-aryl-6-chloro-5-formyl-2-methyl-1,4-dihydropyridine-3-carboxylate derivatives (Figure 6b) by reaction with the Vilsmeier–Haack reagent. 3,4-DHPo and 6-chloro-5-formyl-1,4-DHPs (Figure 6b) have become versatile intermediaries of other compounds (Figure 6) [26,27,28,75,76,77,78,79,80,81].
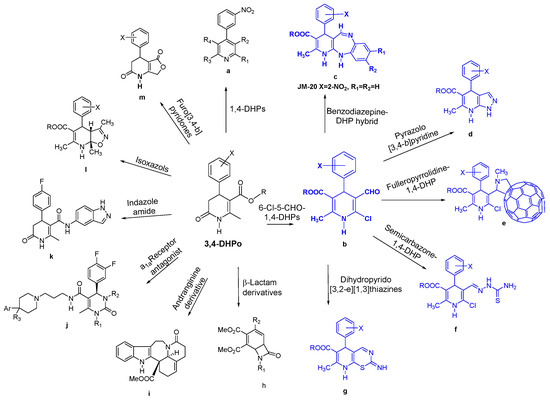
Figure 6.
3,4-Dihydro-2(1H)-pyridones (3,4-DHPo) as synthetic precursors.
Starting from b, more complex structures have been synthesized. The 1,5-benzodiazepine fused to a dihydropyridine moiety (Figure 6c) have been prepared, and one derivative (JM-20, Figure 6c) has shown promising neuroprotective and antioxidant properties [82,83,84,85]. Besides, [3,4-b]pyridines derivatives (Figure 6d) were obtained by treatment of b with hydrazine hydrate. Fulleropyrrolidines endowed with chlorine-containing biologically active 1,4-dihydropyridines (1,4-DHPs) (Figure 6e) were also prepared using Prato’s procedure [75,80]. The chloro-formyl derivative b also allowed to obtain 1,4-dihydropyridines (1,4-DHPs) bearing a semicarbazone moiety on C5 (Figure 6f) [86] and iminium salts of dihydropyrido[3,2-e] [1,3]thiazines (Figure 6g) [27].
3,4-DHPo has been used as an intermediate for the formation of β-lactams derivatives through photochemical cycloaddition (Figure 6h) [87]. Besides, this heterocycle has been incorporated in the diastereoselective synthesis of 3-Oxo-14,15-dihydroandranginine, a unique indole alkaloid with an unusual ring system that includes a tetrahydroazepine unit condensed with an hexahydroquinoline entity in a trans–trans fashion (Figure 6i) [34]. The α1a receptor antagonist from 3,4-DHPo was also synthesized, and its efficacy was demonstrated in a screen of prostate contraction model in rats (Figure 6j) [11].
Indazole amide (Figure 6k) [11] was also prepared through saponification of the ester provided by 3,4-DHPo, which smoothly combined with an indazole to give close pyridine analogs derivatives with different aryl groups. Thus, imidazole amide is an interesting structure with a selective ROCK1 inhibition. Moreover, a series of isoxazolo[5,4-b]pyridin-6(7H)-ones (Figure 6l) [19] have been synthesized by the reaction of novel 3,4-dihydro-2(1H)-pyridones with hydroxylamine hydrochloride and following 5-endo-trig cyclization. Additionally, 3,4-DHPos is an intermediate for the synthesis of Furo[3,4-b]-2(1H)-pyridones (Figure 6m) [17,25,88], which act as potential Calcium channel modulators.
Sadhu et al. report an efficient way for the photochemical dehydrogenation of various substituted 3,4-DHPo to obtain 2-Pyridone derivatives in excellent yields (83–97%) using different photoinduced electron transfer (PET) sensitizers (Scheme 23) [89].
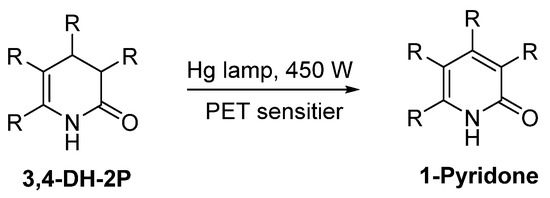
Scheme 23.
Photochemical dehydrogenation of substituted 3,4-DHPo.
Lawesson’s reagent has been widely used as a powerful, mild, and versatile reagent for transforming carbonyl functionalities into their thio analogs. Our group reported the synthesis of 4-aryl substituted alkyl 2-methyl-6-thioxo-1,4,5,6-tetrahydropyridine-3-carboxylates (40) with a 29–93% yield. The thionation of 3,4-DHPo was carried out in a one-step procedure of the 3,4-DHPo derivatives (41) by exposure to microwave irradiation under solvent and solvent-free conditions (Scheme 24) [90].
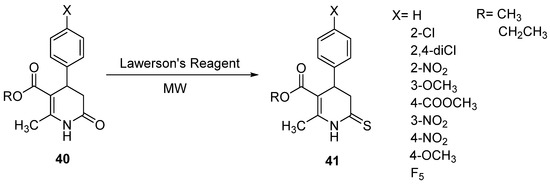
Scheme 24.
Transforming of 3,4-DHPo carbonyl group into their thio analogs by MW irradiation.
In 2011, our group reported the preparation of N-heterocycles using nonconventional synthesis as an ecofriendly approach to producing heterocyclic nitrogen compounds starting with DHPo. MW-assisted synthesis (MWAS) of alkyl 4-arylsubstituted-6-chloro-5-formyl-2-methyl-1,4-dihydropyridine-3-carboxylates (43) and 4-arylsubstituted-4,7-dihydrofuro[3,4-b]pyridine-2,5(1H,3H)-diones (44) from 3,4-DHPo’s (42) were reported (Figure 7) [91]. US-assisted synthesis (USAS) was also used to obtain chloro-formyl derivatives from 3,4-DHPo’s as starting materials (Figure 7) [27]. MWAS and USAS showed higher yields in shorter reaction times and milder conditions.
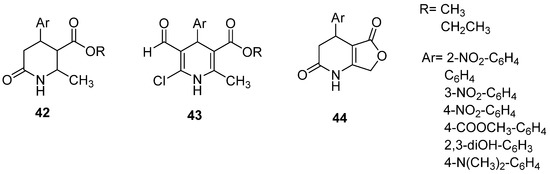
Figure 7.
N-Heterocycles were prepared using nonconventional synthesis, MWAS, and USAS.
Ueyama et al. identified the 3,4-DHPo derivative 45 as a degradation product of Azelnidipine (46) after radical initiator-based oxidative conditions (Figure 8) [92].
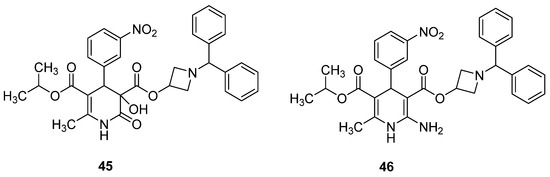
Figure 8.
3,4-DHPo derivative (45) obtained from the degradation of Azelnidipine (46).
3,4-DHPo has been used as an enamine precursor to obtain nitrogen heterocycles such as dihydropyridinones by applying the nucleophile-catalyzed Michael/proton transfer/lactamization (NCMPL) cascade, allowing the total synthesis of α1a adrenergic receptor antagonist (47) (Scheme 25) [67].
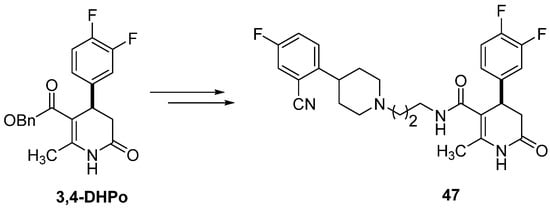
Scheme 25.
3,4-DHPo as the synthetic precursor of dihydropyridinone (47).
Quinolizin-4-ones (49) have been prepared from 6-ciano-3,4-DHPo’s derivatives (48) with low to high yields (20–90%) through allylation/intramolecular Heck reaction sequence (Scheme 26). Quinolizin-4-ones showed attractive biological activities related to CNS diseases, including Alzheimer’s [44].
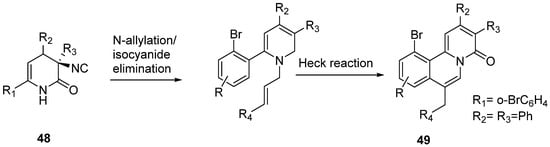
Scheme 26.
Quinolizin-4-ones prepared from 6-ciano-3,4-DHPo’s derivatives.
Additionally, in 2019, simple 2-pyridones were applied in synthesizing alkaloids and alkaloid-inspired compounds based on the piperidine or pyridine framework [93], demonstrating the versatility of the revised scaffold.
6. Biological Activity
DHPos and its derivatives possess a wide variety of biological activities such as vasorelaxant [57], reverse transcriptase inhibition of human immunodeficiency virus-1 [5,83,94], Rho-kinase inhibitors [12], anticancer [3], antibacterial [4], human rhinovirus 3C protease inhibitors [6,7], urease inhibitors [34], antifungal activity [95], glycine/NMDA receptor inhibitor [8], and as cellular transport [9].
- -
- Vasorelaxant activity
The regulation of blood pressure depends on vascular tone. In addition, nitric oxide (NO) is an excellent vasodilator molecule, but a low production of endothelium-derived NO causes a diminished vasodilator tone. This increases vascular resistance, which contributes to elevated blood pressure [96]. Therefore, various research groups are focused on finding compounds with vasorelaxant activity. A recent study demonstrated that 3-cyano-pyridin-2-ones (Figure 9) show a significant vasorelaxant. Three of them are the most potent and revealed an endothelium-independent effect.
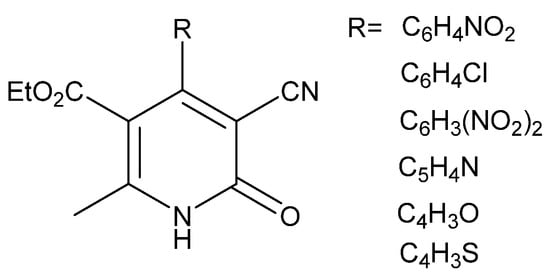
Figure 9.
5-cyano-pyridin-2-ones with vasorelaxant activity.
In addition, 3-cyano-2-pyridone derivatives were synthesized as calcium channel blockers and probable PD3 and PD4 inhibitors, taken as comparison; nifedipine for L-type calcium channel blocker, milrinone and amrinone for PD3 and PD4 inhibition. These compounds also have antihypertensive and vasorelaxant activity [57].
- -
- HIV-1 inhibitors
Non-nucleoside reverse transcriptase inhibitors (NNRTIs) play an essential role in treating HIV infections. They have been used as the main target in the attack against this virus, and most of them present butterfly-like conformation [94,97]. This conformation facilitates the intramolecular interactions between receptor and ligand. The NNRTI interacts specifically with the HIV reverse transcriptase (RT) substrate-binding site and inhibits its replication. Pyridone derivatives act in this way due to their favorable conformation. Hence, they are highly active against HIV-1. Parreira et al. reported a series of 22 DHPo derivatives such as 50 (Figure 10), which are inhibitors of HIV-1 [94]. In 2001, they synthesized 32 DHPo and proved their HIV-1 inhibitor activity; one of these compounds (51) (Figure 10) showed higher activity [90]. Additionally, another research group described the use of pyridine cocktails to attack viral variants that exhibit drug resistance [98].
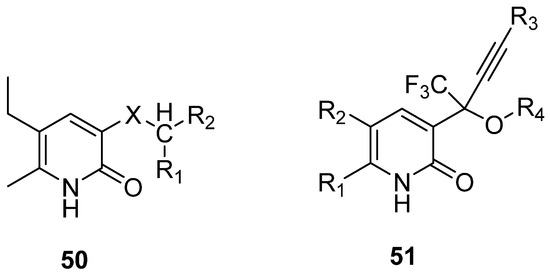
Figure 10.
3,4-DHPo with HIV-1 inhibitor activity.
- -
- Antitumor activity
Several DHPos have emerged during the last twenty years with potent antitumor activity. They have been mainly tested against P388 lymphocytic leukemia cells, demonstrating potential antitumor activity [99,100]. Oxygen-containing functional groups play an essential role in P388 activity; at least two groups are required. Hwang group reported a series of compounds (52) with high activity (Figure 11) [99]. Additionally, 3-Hydroxy-2- pyridone Nucleosides (53) [100] and series 2-pyridones (54) were reported (Figure 11) [3]. These studies demonstrate the potential utility of 3,4-Dihydro-2(1H)-pyridones as building blocks in drug design.
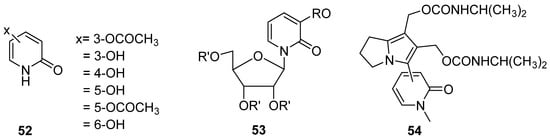
Figure 11.
3,4-DHPo with antitumor activity.
- -
- Antibacterial and antifungal activity
DHPos derivatives present favorable properties as antibacterial agents against multidrug-resistant bacteria such as streptococci and anaerobic microorganisms [4]. In 2018, in vitro antimicrobial activity was reported in some 4-(biphenyl-4-yl)-1,4-dihydropyridine and 4-(biphenyl-4-yl)pyridine derivatives, followed by molecular docking and DFT studies [101]. Ahamed et al. have tested in vitro antibacterial activity of some 1,4-dihydropyridine derivatives against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus epidermidis, Staphylococcus aureus, and Klebsiella pneumoniae. Most of these compounds were highly active against E. coli, and some even showed antifungal activity [102].
- -
- Other biological activities
Although the DHPos have been exceptionally well-explored as a vasorelaxant, they have a privileged scaffold that could act as human rhinovirus 3C protease inhibitors, urease inhibitors, and Rho-Kinase N-methyl-D-aspartate (NMDA) inhibitors. Recent studies have reported a series of 3,4-dihydro-2-pyridone derivatives, from which 4-(4-nitrophenyl)-5-methoxycarbonyl-6-methyl-3,4-dihydropyridone (3,4-DHPo) exhibited the most potent activity (IC50 = 29.12 μM). This inhibitory activity grows with the increase in the electron-withdrawing ability of the groups [34].
NMDA inhibitors are highly interesting in pharmaceutical research due to their application in treating moderate to severe Alzheimer’s disease [103]. A series of 122 DHPos derivatives have been proven to be NMDA inhibitors through QSAR methodology [8]. Five of them, whose structures have a DHPo ring fused to a substituted aromatic ring (55 to 59), showed the highest inhibitory capacity (Figure 12).
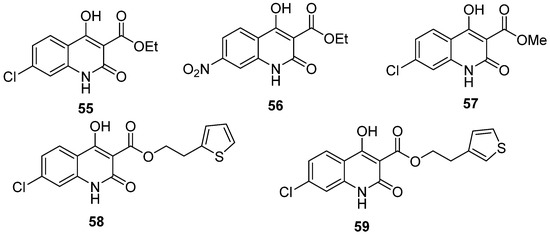
Figure 12.
3,4-DHPo with NMDA inhibitor activity.
Additionally, some dihydropyridines act as calcium channel blockers, potential candidates for schizophrenia and antihypertensive treatment [104,105].
7. Conclusions
Since the first accidental 3,4-DHPo synthesis, the most extensive method reported to obtain these derivatives proceeded via a multicomponent reaction (MCR-4CR) of Meldrum’s acid, a β-keto-ester, and an aromatic aldehyde in the presence of ammonium acetate. This experimental procedure is simple and allows a wealth of molecular diversity depending on substituents in the starting reagents. Besides, this base strategy has been extended to nonconventional methods such as Microwave-, Ultrasound-, or Infrared-assisted reactions, allowing us to increase the efficiency by reducing the reaction time; increasing the yields; and, for some of them, eliminating the reaction solvents, as an important contribution to Green Chemistry. SPOS and LPOS have also been applied, allowing for obtaining a combinatorial library of these structures.
All 3,4-DHPo synthesized showed a broad range of biological activity highlighted as vasorelaxants and antihypertensives due to its structural similarity with 1,4-DHPs. Many of them also showed activity as antitumors, HIV-inhibitors, or antibacterial and antifungal, among others. On the other hand, 3,4-DHPo have become excellent synthetic precursors of many different complex structures with exciting applications.
High expectations still surround the next generation and further evolution of 3,4-DHPo. The improvement of asymmetric synthesis methodologies will allow the enantioselective preparation of this heterocycle, opening up the possibilities of enhancing biological activities. In the short and long term, more complex structures can be synthesized using DHPo as base molecular skeletons. In addition, the multicomponent platforms that characterize DHPo synthesis will promote the use of virtual screening tools and combinatorial chemistry, allowing us access to leading compounds based on this heterocycle. All described advancements contribute to an in-depth understanding of the potential of this scaffold and pave the way to apply novel 3,4-DHPo-based derivatives for further rational development in drug discovery.
Author Contributions
Conceptualization, H.R., M.S. and F.A.; formal analysis, S.C.-G.; investigation, S.C.-G., R.O. and V.C.; writing—original draft preparation, S.C.-G. and V.C.; writing—review and editing, M.S., H.R. and F.A.; supervision, H.R. and F.A.; project administration, H.R. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Blue Skies National Research Foundation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
H.R. and S.C.-G. appreciate the support through the Yachay Tech Internal Project (CHEM19-11) “New linkers for bioconjugations and its therapeutics uses”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaur, R.; Chaudhary, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Recent Synthetic and Medicinal Perspectives of Dihydropyrimidinones: A Review. Eur. J. Med. Chem. 2017, 132, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Kalme, Z.A.; Zhalubovskis, R.A.; Shmidlers, A.; Celmins, J.; Duburs, G. Synthesis of 6-Bromomethyl-Substituted D Derivatives of Pyridin-2(1H)-Ones and Their Reaction with Nucleophiles. Chem. Heterocycl. Compd. 2004, 40, 862–868. [Google Scholar] [CrossRef]
- Anderson, W.K.; Dean, D.C.; Endo, T. Synthesis, Chemistry, and Antineoplastic Activity of α-Halopyridinium Salts: Potential Pyridone Prodrugs of Acylated Vinylogous Carbinolamine Tumor Inhibitors. J. Med. Chem. 1990, 33, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Qun, L.; Mitscher, L.A.; Shen, L.L. The 2-Pyridone Antibacterial Agents: Bacterial Topoisomerase Inhibitors. Med. Res. Rev. 2000, 20, 231–293. [Google Scholar]
- Sayahi, M.H.; Gorjizadeh, M.; Meheiseni, M.; Sayyahi, S. One-Pot Multi-Component Process for the Synthesis of 4-Azaphenanthrene-3,10-Dione, 1,8-Dioxo-Octahydroxanthene and Tetrahydrobenzo[b]Pyran Derivatives Catalyzed by the Deep Eutectic Solvent Choline Chloride-Oxalic Acid. Z. Fur Nat. Sect. B J. Chem. Sci. 2020, 75, 269–279. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Prins, T.J.; Zhou, R.; Brown, E.L.; Maldonado, F.C.; Fuhrman, S.A.; Zalman, L.S.; Tuntland, T.; Lee, C.A.; Patick, A.K.; et al. Structure-Based Design, Synthesis, and Biological Evaluation of Irreversible Human Rhinovirus 3C Protease Inhibitors. 6. Structure-Activity Studies of Orally Bioavailable, 2-Pyridone-Containing Peptidomimetics. J. Med. Chem. 2002, 45, 1607–1623. [Google Scholar] [CrossRef]
- Dragovich, P.S.; Webber, S.E.; Babine, R.E.; Fuhrman, S.A.; Patick, A.K.; Matthews, D.A.; Reich, S.H.; Marakovits, J.T.; Prins, T.J.; Zhou, R.; et al. Structure-Based Design, Synthesis, and Biological Evaluation of Irreversible Human Rhinovirus 3C Protease Inhibitors. 2. Peptide Structure-Activity Studies. J. Med. Chem. 1998, 41, 2819–2834. [Google Scholar] [CrossRef]
- Patankar, S.J.; Jurs, P.C. Prediction of Glycine/NMDA Receptor Antagonist Inhibition from Molecular Structure. J. Chem. Inf. Comput. Sci. 2002, 42, 1053–1068. [Google Scholar] [CrossRef]
- Smits, R.; Goncharenko, Y.; Vesere, I.; Skrivele, B.; Petrichenko, O.; Vigante, B.; Petrova, M.; Plotniece, A.; Duburs, G. Synthesis and Self-Assembly of Novel Fluorous Cationic Amphiphiles with a 3,4-dihydro-2(1H)-pyridone spacer. J. Fluor. Chem. 2011, 132, 414–419. [Google Scholar] [CrossRef]
- Spanu, P.; Rassu, G.; Pinna, L.; Battistini, L.; Casiraghi, G. Diastereoselective Synthesis of 3-Oxo-14, 15-Dihydroandranginine. Tetrahedron Asymmetry 1997, 8, 3237–3243. [Google Scholar] [CrossRef]
- Nantermet, P.G.; Barrow, J.C.; Selnick, H.G.; Homnick, C.F.; Freidinger, R.M.; Chang, R.S.L.; O’Malley, S.S.; Reiss, D.R.; Broten, T.P.; Ransom, R.W.; et al. Selective α(1a) Adrenergic Receptor Antagonists Based on 4-Aryl-3,4-Dihydropyridine-2-Ones. Bioorganic Med. Chem. Lett. 2000, 10, 1625–1628. [Google Scholar] [CrossRef]
- Goodman, K.B.; Cui, H.; Dowdell, S.E.; Gaitanopoulos, D.E.; Ivy, R.L.; Sehon, C.A.; Stavenger, R.A.; Wang, G.Z.; Viet, A.Q.; Xu, W.; et al. Development of Dihydropyridone Indazole Amides as Selective Rho-Kinase Inhibitors. J. Med. Chem. 2007, 50, 6–9. [Google Scholar] [CrossRef]
- Huang, X.; Broadbent, S.; Dvorak, C.; Zhao, S.H. Pilot-Plant Preparation of 3,4-Dihydropyridin-2-One Derivatives, the Core Structures of P2 × 7 Receptor Antagonists. Org. Process Res. Dev. 2010, 14, 612–616. [Google Scholar] [CrossRef]
- Smits, R.; Turovska, B.; Belyakov, S.; Plotniece, A.; Duburs, G. Synthesis of 5-Carboxy-6-Methyl-3,4-Dihydro-2(1H)-Pyridone Derivatives and Their Electrochemical Oxidation to 2-Pyridones. Chem. Phys. Lett. 2016, 649, 84–87. [Google Scholar] [CrossRef]
- Memarian, H.R.; Kalantari, M.; Rudbari, H.A.; Sabzyan, H.; Bruno, G. DFT and Structural Studies of 2-Oxo-1,2,3,4-Tetrahydropyridines. Comput. Theor. Chem. 2017, 1099, 75–86. [Google Scholar] [CrossRef]
- Memarian, H.R.; Kalantari, M.; Sabzyan, H. NMR and DFT Studies of 2-Oxo-1,2,3,4-Tetrahydropyridines: Solvent and Temperature Effects. Aust. J. Chem 2018, 71, 380–388. [Google Scholar] [CrossRef]
- Ochoa, E.; Suárez, M.; Verdecia, Y.; Pita, B.; Martín, N.; Quinteiro, M.; Seoane, C.; Soto, J.; Duque, J.; Pomés, R. Structural Study of 3,4-Dihydropyridones and Furo [3,4-b]-2(1H)-Pyridones as Potential Calcium Channel Modulators. Tetrahedron 1998, 54, 12409–12420. [Google Scholar] [CrossRef]
- Vinet, L.; Zhedanov, A. A “Missing” Family of Classical Orthogonal Polynomials. J. Phys. A Math. Theor. 2011, 44, 085201. [Google Scholar] [CrossRef]
- Suarez, M.; Verdecia, Y.; Estael, O.; Salfrán, E.; Morán, L.; Martín, N.; Martínez, R.; Quinteiro, M.; Seoane, C.; Soto, J.L.; et al. Synthesis and Structural Study of 3,4-Dihydro-2(1H)-Pyridones and Isoxazolo[5,4-b]Pyridin-6(7H)-Ones. Eur. J. Org. Chem. 2000, 2, 2–11. [Google Scholar] [CrossRef]
- Rodríguez, H.; Suarez, M.; Pérez, R.; Petit, A.; Loupy, A. Solvent-Free Synthesis of 4-Aryl Substituted 5-Alkoxycarbonyl-6-Methyl-3,4-Dihydropyridones under Microwave Irradiation. Tetrahedron Lett. 2003, 44, 3709–3712. [Google Scholar] [CrossRef]
- Světlík, J.; Goljer, I.; Tureček, F. Oxygen-Bridged Tetrahydropyridines, Hexahydropyridines, and Dihydropyridones via a Hantzsch-like Synthesis with 4-(2-Hydroxyphenyl)but-3-En-2-One. J. Chem. Soc. Perkin Trans. 1990, 1, 1315–1318. [Google Scholar] [CrossRef]
- Zayed, S.E.; Abou Elmaged, E.I.; Metwally, S.A.; Elnagdi, M.H. Reactions of Six-Membered Heterocyclic β-Enaminonitriles with Electrophilic Reagents. Collect. Czechoslov. Chem. Commun. 1991, 56, 2175–2182. [Google Scholar] [CrossRef]
- Suárez, M.; Martínez-Alvarez, R.; Martín, N.; Verdecia, Y.; Ochoa, E.; Alba, L.; Seoane, C.; Kayali, N. Electrospray Ionisation and Ion-Trap Fragmentation of Substituted 3,4-Dihydro-2(1H)-Pyridin-2-Ones. Rapid Commun. Mass Spectrom. 2002, 16, 749–754. [Google Scholar] [CrossRef]
- Suárez, M.; Salfrán, E.; Ochoa, E.; Verdecia, Y.; Alba, L.; Martín, N.; Seoane, C.; Martínez-Alvarez, R.; De Armas, H.N.; Blaton, N.M.; et al. Structural Study of Highly Halogenated Dihydropyridine Derivatives as Potential Calcium Channel Modulators. J. Heterocycl. Chem. 2003, 40, 269–275. [Google Scholar] [CrossRef]
- Morales, A.; Ochoa, E.; Suárez, M.; Verdecia, Y.; González, L.; Martín, N.; Quinteiro, M.; Seoane, C.; Soto, J.L. Novel Hexahydrofuro [3,4-b]-2(1H)-Pyridones from 4-Aryl Substituted 5-Alkoxycarbonyl-6-Methyl-3,4-Dihydropyridones. J. Heterocycl. Chem. 1996, 33, 103–107. [Google Scholar] [CrossRef]
- Novoa De Armas, H.; Blaton, N.M.; Peeters, O.M.; De Ranter, C.J.; Suárez, M.; Ochoa, E.; Verdecia, Y.; Salfrán, E. Synthesis, Crystal Structure, and Molecular Modeling (AM1) of Methyl 6-Chloro-4-(2-Chlorophenyl)-5-Formyl-2-Methyl-1, 4-Dihydropyridine-3-Carboxylate. J. Chem. Crystallogr. 2000, 30, 237–243. [Google Scholar] [CrossRef]
- Suárez, M.; Novoa, H.; Verdecia, Y.; Ochoa, E.; Alvarez, A.; Pérez, R.; Martínez-Alvarez, R.; Molero, D.; Seoane, C.; Blaton, N.M.; et al. A Straightforward Synthesis and Structure of Unprecedented Iminium Salts of Dihydropyrido [3,2-e][1,3]Thiazines. Tetrahedron 2006, 62, 1365–1371. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez, H.; Coro, J.; Niebla, V.; Rodríguez, A.; Martínez-Alvarez, R.; de Armas, H.N.; Suárez, M.; Martín, N. Efficient Sonochemical Synthesis of Alkyl 4-Aryl-6-Chloro-5-Formyl-2-Methyl-1,4-Dihydropyridine-3-Carboxylate Derivatives. Ultrason. Sonochem. 2012, 19, 221–226. [Google Scholar] [CrossRef]
- Rodríguez, J.D.; Hernández, R.P.; González, A.G.; Navarro, M.S.; Reyes, Y.V.; Rodríguez, E.O.; Mascarenhas, Y. 5-Methoxycarbonyl-6-Methyl-4-Phenyl-3,4-Dihydro-2(1H)-Pyridone. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 1644–1645. [Google Scholar] [CrossRef]
- De Armas, H.N.; Peeters, O.M.; Blaton, N.M.; De Ranter, C.J.; Navarro, M.S.; Reyes, Y.V.; Rodríguez, E.O.; Salfrán, E. Ethyl 5-Ethoxymethylene-2-Methyl-6-Oxo-4-Phenyl-1,4,5,6-Tetrahydropyridine- 3carboxyl Ate. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, 230–231. [Google Scholar] [CrossRef]
- Sun, J.; Xia, E.-Y.; Wu, Q.; Yan, C.-G. Synthesis of 3,4-Dihydropyridin-2(1H)-Ones and 3,4-Dihydro-2H-Pyrans via Four-Component Reactions of Aromatic Aldehydes, Cyclic 1,3-Carbonyls, Arylamines, and Dimethyl Acetylenedicarboxylate. ACS Comb. Sci. 2011, 13, 421–426. [Google Scholar] [CrossRef]
- Researchers, Y.; Club, E.; Branch, Q. Four-Component Reaction Between Cyanoacetamide, Aryl Aldehydes and Ethyl Acetoacetate with Ammonium Acetate. Chem. Sci. Trans. 2014, 3, 977–982. [Google Scholar] [CrossRef][Green Version]
- Dehghan, A.; Singh, S.; Anary-Abbasinejad, M.; Hassanabadi, A. Four-Component Reaction between Cyanoacetamide, Aryl Aldehydes, and Ethyl Acetoacetate with Ammonium Carbonate. Res. Chem. Intermed. 2015, 41, 1001–1009. [Google Scholar] [CrossRef]
- Hakimi, A.; Lashgari, N.; Mahernia, S.; Ziarani, G.; Amanlou, M. Facile One-Pot Four-Component Synthesis of 3,4-Dihydro-2-Pyridone Derivatives: Novel Urease Inhibitor Scaffold. Res. Pharm. Sci. 2017, 12, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Firoozpour, L.; Nikookar, H.; Moghimi, S.; Mahdavi, M.; Asadipour, A.; Ranjbar, P.R.; Foroumadi, A. An Efficient Approach to the Synthesis of Coumarin-Fused Dihydropyridinones. Heterocycl. Commun. 2017, 23, 305–308. [Google Scholar] [CrossRef]
- Li, X.; Song, L.; Xing, C.; Zhao, J.; Zhu, S. Study on the Reactions of Ethyl 4,4,4-Trifluoro-3-Oxobutanoate with Arylidenemalononitriles. Tetrahedron 2006, 62, 2255–2263. [Google Scholar] [CrossRef]
- Kalme, Z.; Šmidlers, A.; Celminš, J.; Liepinsh, E.; Krauze, A.; Duburs, G. Unexpected Reaction on Bromination of 3,4-Dihydropyridin-2(1H)-Ones with N-Bromosuccinimide. Chem. Heterocycl. Compd. 2007, 43, 662–664. [Google Scholar] [CrossRef]
- Liu, J.; Liang, F.; Liu, Q.; Li, B. BF3·OEt2-Mediated C-C Bond-Forming Reaction of α-Hydroxyketene-(S,S)-Acetals with Active Methylene Compounds and Its Application in the Synthesis of Substituted 3,4-Dihydro-2-Pyridones. Synlett 2007, 1, 0156–0160. [Google Scholar] [CrossRef]
- Singh, J.; Koul, S.; Pannu, A.P.S.; Sharma, R.L.; Razdan, T.K. Simple and Efficient One-Pot Synthesis of 3,4-Dihydro-2-Pyridones via Solid-Supported Bi(III)Nitrate-Catalyzed Double Michael Addition-Azaannulation. ChemInform 2008, 39, 349–357. [Google Scholar] [CrossRef]
- Yi, H.; Song, L.; Wang, W.; Liu, J.; Zhu, S.; Deng, H.; Shao, M. First Synthesis of 3-Aryl-4-Unsubstituted-6-CF3-Pyridin-2-Ones via Aryl Migration Reaction in the Presence of PhI(OAc)2/NaOH. Chem. Commun. 2010, 46, 6941–6943. [Google Scholar] [CrossRef]
- Wang, P.; Song, L.; Yi, H.; Zhang, M.; Zhu, S.; Deng, H.; Shao, M. Convenient One-Pot Synthesis of Fluorinated DHPs Derivatives and Their Further Transformations. Tetrahedron Lett. 2010, 51, 3975–3977. [Google Scholar] [CrossRef]
- Dostanic, J.; Valentic, N.; Uscumlic, G.; Mijin, D. Synthesis of 5-(Substituted Phenylazo)-6-Hydroxy-4-Methyl-3- Cyano-2-Pyridones from Ethyl 3-Oxo-2-(Substituted Phenyldiazenyl)Butanoates. J. Serb. Chem. Soc. 2011, 76, 499–504. [Google Scholar] [CrossRef]
- Yao, C.; Wang, D.; Lu, J.; Li, T.; Jiao, W.; Yu, C. N-Heterocyclic Carbene Catalyzed Reactions of α-Bromo-α,β-Unsaturated Aldehydes/α,β-Dibromoaldehydes with 1,3-Dinucleophilic Reagents. Chem.-A Eur. J. 2012, 18, 1914–1917. [Google Scholar] [CrossRef]
- Khazaei, M.; Anary-Abbasinejad, M.; Hassanabadi, A.; Sadeghi, B. ZnO Nanoparticles: An Efficient Reagent, Simple and One-Pot Procedure for Synthesis of Highly Functionalized Dihydropyridine Derivatives. E-J. Chem. 2012, 9, 615–620. [Google Scholar] [CrossRef]
- Liu, Z.; Tan, L.; Wu, Q.; Lin, X. Imidazole-Catalyzed Three-Component Cascade Reaction for the Facile Synthesis of Highly Substituted 3,4-Dihydropyridin-2-One Derivatives. Chin. J. Chem. 2012, 30, 2343–2348. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Pradhan, K.; Paul, S.; Das, A.R. Nano Crystalline ZnO Catalyzed One Pot Multicomponent Reaction for an Easy Access of Fully Decorated 4H-Pyran Scaffolds and Its Rearrangement to 2-Pyridone Nucleus in Aqueous Media. Tetrahedron Lett. 2012, 53, 4687–4691. [Google Scholar] [CrossRef]
- Mohammandi Ziarani, G.; Mousavi, S.; Lashgari, N.; Badiei, A. Mesostructured SBA-15-Pr-SO3H: An Efficient Solid Acid Catalyst for One-Pot and Solvent-Free Synthesis of 3,4-Dihydro-2-Pyridone Derivatives. J. Chem. Sci. 2013, 125, 1359–1364. [Google Scholar] [CrossRef]
- Pradhan, K.; Bhattacharyya, P.; Paul, S.; Das, A.R. Synthesis of 3,4-Dihydropyridin-2-One Derivatives in Convergent Mode Applying Bio Catalyst Vitamin B1 and Polymer Supported Catalyst PEG–SO3 H from Two Different Sets of Building Blocks. Tetrahedron Lett. 2012, 53, 5840–5844. [Google Scholar] [CrossRef]
- Zhang, L.J.; Zhang, X.; You, Z.S.; Li, H.; Feng, T.; Wang, W.L. Chem-Grafted Zn-SSA as an Efficient Heterogeneous Catalyst to Synthesize 2-Pyridinones. Catal. Lett. 2016, 146, 2081–2086. [Google Scholar] [CrossRef]
- Meng, T.; Liu, L.; Jia, H.; Ren, L.; Feng, C.; Wang, X.; Zhao, W. One-Pot Synthesis of 3,4-Dihydropyridin-2-Ones via Tandem Reaction of Blaise Reaction Intermediate and Acrylic Ester. Appl. Organomet. Chem. 2016, 30, 47–50. [Google Scholar] [CrossRef]
- Paravidino, M.; Bon, R.S.; Scheffelaar, R.; Vugts, D.J.; Znabet, A.; Schmitz, R.F.; de Kanter, F.J.J.; Lutz, M.; Spek, A.L.; Groen, M.B.; et al. Diastereoselective Multicomponent Synthesis of Dihydropyridones with an Isocyanide Functionality. Org. Lett. 2006, 8, 5369–5372. [Google Scholar] [CrossRef]
- Den Heeten, R.; Van Der Boon, L.J.P.; Broere, D.L.J.; Janssen, E.; De Kanter, F.J.J.; Ruijter, E.; Orru, R.V.A. Concise Synthesis of Highly Substituted Benzo[a]Quinolizines by a Multicomponent Reaction/Allylation/Heck Reaction Sequence. Eur. J. Org. Chem. 2012, 6, 275–280. [Google Scholar] [CrossRef]
- Si, C.; Fales, K.R.; Boyer, R.D.; Njoroge, F.G. An Efficient Synthesis of 3,4-Dihydropyridone via a Tandem Olefin Isomerization-Ring-Closing Metathesis Reaction. Tetrahedron Lett. 2014, 55, 5529–5532. [Google Scholar] [CrossRef]
- Maru, M.S.; Sudhadevi Antharjanam, P.K.; Khan, N.U.H. Catalyst-Free Solid Phase Microwave-Assisted Synthesis of 1,4-Dihydropyridine Derivatives and Their Single Crystal Structure Determination. ChemistrySelect 2019, 4, 774–782. [Google Scholar] [CrossRef]
- Tu, S.; Fang, F.; Zhu, S.; Li, T.; Zhang, X.; Zhuang, Q.; Ji, S.; Zhang, Y. Microwave-Assisted Synthesis of Phenylenedi(4′,4″-Tetrahydroquinoline) and Di(4′,4″-Tetrahydropyridine) Derivatives. J. Heterocycl. Chem. 2005, 42, 29–32. [Google Scholar] [CrossRef]
- Eynde, J.J.V.; Labuche, N.; Van Haverbeke, Y. Microwave-Mediated Domino Reactions in Dry Medium. Preparation of Dihydropyridinones and Pyridinones Structurally Related to Hantzsch Esters. Synth. Commun. 1997, 27, 3683–3690. [Google Scholar] [CrossRef]
- Hernández, F.; Sánchez, A.; Rendón-Vallejo, P.; Millán-Pacheco, C.; Alcaraz, Y.; Delgado, F.; Vázquez, M.A.; Estrada-Soto, S. Synthesis, Ex Vivo and in Silico Studies of 3-Cyano-2-Pyridone Derivatives with Vasorelaxant Activity. Eur. J. Med. Chem. 2013, 70, 669–676. [Google Scholar] [CrossRef]
- Ruiz, E.; Rodríguez, H.; Coro, J.; Salfrán, E.; Suárez, M.; Martínez-alvarez, R.; Martín, N. Ultrasonics Sonochemistry Ultrasound-Assisted One-Pot, Four Component Synthesis of 4-Aryl 3,4-Dihydropyridone Derivatives. Ultrason. Sonochem. 2011, 18, 32–36. [Google Scholar] [CrossRef]
- Noguez, M.O.; Marcelino, V.; Rodríguez, H.; Martín, O.; Martínez, J.O.; Arroyo, G.A.; Pérez, F.J.; Suárez, M.; Miranda, R. Infrared Assisted Production of 3,4-Dihydro-2(1H)-Pyridones in Solvent-Free Conditions. Int. J. Mol. Sci. 2011, 12, 2641–2649. [Google Scholar] [CrossRef]
- Rodríguez, H.; Martín, O.; Ochoa, E.; Suárez, M.; Reyes, O.; Garay, H.; Albericio, F.; Martín, N. Solid-Phase Synthesis and Structural Study of Substituted 1,4,5,6-Tetrahydro-6-Oxopyridine-3-Carboxylic Acids. QSAR Comb. Sci. 2006, 25, 921–927. [Google Scholar] [CrossRef]
- Rodríguez, H.; Reyes, O.; Suarez, M.; Garay, H.E.; Pérez, R.; Cruz, L.J.; Verdecia, Y.; Martín, N.; Seoane, C. Solid-Phase Synthesis of 4-Aryl Substituted 5-Carboxy-6-Methyl-3,4-Dihydropyridones. Tetrahedron Lett. 2002, 43, 439–441. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, X.; Sheng, S.; Wei, M.; Liu, X. Rapid Microwave-Assisted Liquid-Phase Synthesis of 4-Substituted-5-methoxycarbonyl-6-methyl-3,4-dihydropyridones on Poly(Ethylene Glycol) Support. Synth. Commun. 2008, 38, 1249–1258. [Google Scholar] [CrossRef]
- Torres, S.Y.; Ochoa, E.; Verdecia, Y.; Rebolledo, F. Chemoenzymatic Preparation of Optically Active 4-Aryl-5-Carboxy-6-Methyl-3,4-Dihydro-2(1H)-Pyridone Derivatives. Tetrahedron 2014, 70, 4675–4684. [Google Scholar] [CrossRef]
- Torres, S.Y.; Brieva, R.; Rebolledo, F. Chemoenzymatic Synthesis of Optically Active Phenolic 3,4-Dihydropyridin-2-Ones: A Way to Access Enantioenriched 1,4-Dihydropyridine and Benzodiazepine Derivatives. Org. Biomol. Chem. 2017, 15, 5171–5181. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Hu, Y.J.; Chen, X.Y.; Wu, Q.; Lin, X.F. Enzymatic Multicomponent Reaction for Simultaneous Synthesis of Two Important Scaffolds, Pyridin-2-Ones and α-Alkylated Nitriles. Tetrahedron 2015, 71, 663–668. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Broadbent, S. The First Asymmetric Synthesis of a 4-Aryl-Substituted 5-Carboxy-3,4-Dihydropyridin-2-One Derivative. Tetrahedron Lett. 2010, 51, 1554–1557. [Google Scholar] [CrossRef]
- Vellalath, S.; Van, K.N.; Romo, D. Direct Catalytic Asymmetric Synthesis of N-Heterocycles from Commodity Acid Chlorides by Employing α,β-Unsaturated Acylammonium Salts. Angew. Chem. Int. Ed. 2013, 52, 13688–13693. [Google Scholar] [CrossRef]
- Molero, D.; Suárez, M.; Martínez-Álvarez, R.; Verdecia, Y.; Martín, N.; Seoane, C.; Ochoa, E. 1H and 13C Spectral Assignment of 2(1H)-Pyridone Derivatives. Magn. Reson. Chem. 2004, 42, 704–708. [Google Scholar] [CrossRef]
- O’Callaghan, C.N.; McMurry, T.B.H.; Draper, S.M.; Champeil, E. Nitrile and Non-Nitrile Pyridine Products from the Reaction of 2-Cyano-3-(x-Nitrophenyl)Prop-2-Enamides with Methyl 3-Oxobutanoate. J. Chem. Res. 1999, 6, 690–691. [Google Scholar] [CrossRef]
- Pendleton, M.D.; Beddoes, R.L.; Andrew, N.J.; Joule, J.A.; Butters, M. Syntheses of Some 1,2- and 1,4-Dihydropyridines and X-Ray Crystal Structures of 1-Dimethylamino-5-Ethoxycarbonyl-1,4-Dihydro-3-Methoxycarbonyl-2- Methyl-4-Phenylpyridine, 3-Cyano-3,4-Dihydro-5-Methoxycarbonyl-6-Methyl-4-Phenylpyridin-2(1H)-One and 5-Etho. J. Chem. Res. 1997, 86–87. [Google Scholar] [CrossRef]
- Krajewski, J.W.; Urbanczyk-Lipkowska, Z.; Gluzinski, P. Ethyl 3-Cyano-6-Methyl-2-Oxo-4-(3-Pyridyl)-3,4-Dihydro-5-Pyridinecarboxylate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1978, 34, 2863–2865. [Google Scholar] [CrossRef]
- Kettmann, V.; Svetlík, J. Methyl 1,4,5,6-Tetrahydro-2-Methyl-4-(2-Nitrophenyl)-6-Oxopyridine-3-Carboxylate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1999, 55, 661–663. [Google Scholar] [CrossRef]
- Smits, R.; Belyakov, S.; Vigante, B.; Duburs, G. Methyl 6-Oxo-4-Phenyl-2-[(Z)-2-(Pyridin-2-Yl)Ethenyl]-1,4,5,6- Tetrahydropyridine-3-Carboxylate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o3489. [Google Scholar] [CrossRef]
- Carabateas, P.M.; Brundage, R.P.; Gelotte, K.O.; Gruett, M.D.; Lorenz, R.R.; Opalka, C.J.; Singh, B.; Thielking, W.H.; Williams, G.L.; Lesher, G.Y. 1-Ethyl-1,4-Dihydro-4-Oxo-7-(Pyridinyl)-3-Quinolinecarboxylic Acids. I. Synthesis of 3- and 4-(3-Aminophenyl)Pyridine Intermediates. J. Heterocycl. Chem. 1984, 21, 1849–1856. [Google Scholar] [CrossRef]
- Alvarez, A.; Ochoa, E.; Verdecia, Y.; Suárez, M.; Solá, M.; Martín, N. Theoretical Study of the Highly Diastereoselective 1,3-Dipolar Cycloaddition of 1,4-Dihydropyridine-Containing Azomethine Ylides to [60]Fullerene (Prato’s Reaction). J. Org. Chem. 2005, 70, 3256–3262. [Google Scholar] [CrossRef]
- Novoa de Armas, H.; Peeters, O.M.; Blaton, N.M.; De Ranter, C.J.; Suárez Navarro, M.; Salfrán Solano, E.; Verdecia Reyes, Y.; Ochoa Rodríguez, E. 6-Methyl-4-Phenylthieno [2,3-b]Pyridine-2,5-Dicarboxylic Acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o450–o452. [Google Scholar] [CrossRef]
- Novoa de Armas, H.; Peeters, O.M.; Blaton, N.M.; De Ranter, C.J.; Suárez Navarro, M.; Salfrán Solano, E.; Verdecia Reyes, Y.; Ochoa Rodríguez, E. 4-(4-Bromophenyl)-2,5-Diethoxycarbonyl-6-Methylthieno [2,3-b]Pyridine. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, o384–o386. [Google Scholar] [CrossRef]
- De Armas, H.N.; Peeters, O.M.; Blaton, N.M.; De Ranter, C.J.; Salfrán, E.; Navarro, M.S.; Rodríguez, E.O.; Reyes, Y.V. 2,5-Diethoxycarbonyl-6-Methyl-4-Phenylthieno [2,3-6]Pyridine. Acta Crystallogr. Sect. E Struct. Rep. Online 2003, 59, 321–323. [Google Scholar] [CrossRef]
- Chinnakali, K.; Fun, H.-K.; Sriraghavan, K.; Ramakrishnan, V.T. 8-(2-Bromo-4-Methylanilino)-9-Hydroxy-4-Methyl-7,8,9,10-Tetrahydro-7,8-Benzocoumarin. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, IUC9800003. [Google Scholar] [CrossRef]
- Suárez, M.; Verdecia, Y.; Illescas, B.; Martínez-Alvarez, R.; Alvarez, A.; Ochoa, E.; Seoane, C.; Kayali, N.; Martín, N. Synthesis and Study of Novel Fulleropyrrolidines Bearing Biologically Active 1,4-Dihydropyridines. Tetrahedron 2003, 59, 9179–9186. [Google Scholar] [CrossRef]
- Marín-Prida, J.; Pardo Andreu, G.L.; Rossignoli, C.P.; Durruthy, M.G.; Rodríguez, E.O.; Reyes, Y.V.; Acosta, R.F.; Uyemura, S.A.; Alberici, L.C. The Cytotoxic Effects of VE-3N, a Novel 1,4-Dihydropyridine Derivative, Involve the Mitochondrial Bioenergetic Disruption via Uncoupling Mechanisms. Toxicol. In Vitro 2017, 42, 21–30. [Google Scholar] [CrossRef]
- Garrido-Suárez, B.B.; Garrido, G.; Castro-Labrada, M.; Merino, N.; Valdés, O.; Pardo, Z.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; Delgado-Hernández, R.; Godoy-Figueiredo, J.; et al. Anti-Hypernociceptive and Anti-Inflammatory Effects of JM-20: A Novel Hybrid Neuroprotective Compound. Brain Res. Bull. 2020, 165, 185–197. [Google Scholar] [CrossRef]
- Fonseca-Fonseca, L.A.; da Silva, V.D.A.; Wong-Guerra, M.; Ramírez-Sánchez, J.; Yaquis, A.S.P.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; de Araújo, F.M.; Santana, R.C.; Outeiro, T.F.; et al. JM-20 Protects against 6-Hydroxydopamine-Induced Neurotoxicity in Models of Parkinson’s Disease: Mitochondrial Protection and Antioxidant Properties. NeuroToxicology 2021, 82, 89–98. [Google Scholar] [CrossRef]
- Garrido-Suárez, B.B.; Garrido, G.; Menéndez, A.B.; Aparicio, G.; Valdés, O.; Ochoa-Rodríguez, E.; Verdecia-Reyes, Y.; Delgado-Hernández, R. Preventive and Therapeutic Effects of JM-20 on Paclitaxel-Evoked Painful Peripheral Neuropathy in Rats. J. Pharm. Pharmacogn. Res. 2021, 9, 165–174. [Google Scholar]
- Torres, S.Y.; Rebolledo, F. A Highly Efficient Synthesis of Optically Active Hybrid 1 H -1,5-Benzodiazepine-1,4-Dihydropyridines. Synthesis 2016, 48, 1414–1420. [Google Scholar] [CrossRef]
- Martín, N.; Verdecia, Y.; Ochoa, E.; Barried, B.; Molero, D.; Seoane, C.; Novoa, H.; Blaton, N.M.; Peetersd, O.M.; Alvarez, A.; et al. Synthesis and Structural Study of Semicarbazone-Containing 1,4-Dihydropyridine. Heterocycles 2006, 68, 1631. [Google Scholar] [CrossRef]
- Somekawa, K.; Okumura, Y.; Uchida, K.; Shimo, T. Preparations of 2-Azabicyclo [2.2.2]Octa-5,7-Dien-3-Ones and 7-Azabicyclo [4.2.0]Octa-2,4-Dien-8-Ones from Addition Reactions of 2-Pyridones. J. Heterocycl. Chem. 1988, 25, 731–734. [Google Scholar] [CrossRef]
- Duque Rodríguez, J.; Pomés Hernández, R.; Diáz de Delgado, G.; Roque, E.; Suárez Navarro, M.; Verdecia Reyes, Y.; Ochoa Rodríguez, E.; Pita Mieres, B.; Espinosa, R.; Alba Gutierrez, L. 2-Ethoxycarbonyl-5-Methoxycarbonyl-6-Methyl-4-Phenyl-4,7-Dihydrothieno [2,3-b]Pyridine. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, IUC9800002. [Google Scholar] [CrossRef]
- Sadhu, P.S.; Ravinder, M.; Kumar, P.A.; Rao, V.J. Photochemical Dehydrogenation of 3,4-Dihydro-2-Pyridones. Photochem. Photobiol. Sci. 2009, 8, 513. [Google Scholar] [CrossRef]
- Rodríguez, H.; Coro, J.; Lam, A.; Salfrán, E.; Rodríguez-Salarichs, J.; Suárez, M.; Albericio, F.; Martin, N. High-Throughput Preparation of Alkyl 4-Aryl Substituted-2-Methyl-6-Thioxo-1,4,5,6-Tetrahydropyridine-3-Carboxylates under Microwave Irradiation. Arkivoc 2011, 2011, 125–141. [Google Scholar] [CrossRef]
- Rodríguez, H.; Martin, O.; Suarez, M.; Martín, N.; Albericio, F. Eco-Friendly Methodology to Prepare N-Heterocycles Related to Dihydropyridines: Microwave-Assisted Synthesis of Alkyl 4-Arylsubstituted-6-Chloro-5-Formyl-2-Methyl-1,4-Dihydropyridine-3-Carboxylate and 4-Arylsubstituted-4,7-Dihydrofuro [3,4-b]Pyridine-2,5. Molecules 2011, 16, 9620–9635. [Google Scholar] [CrossRef] [PubMed]
- Ueyama, E.; Takahashi, F.; Ohashi, J.; Konse, T.; Kishi, N.; Kano, K. Mechanistic Study on Degradation of Azelnidipine Solution under Radical Initiator-Based Oxidative Conditions. J. Pharm. Biomed. Anal. 2012, 61, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Sośnicki, J.G.; Idzik, T.J. Pyridone–Powerful Precursors for the Synthesis of Alkaloids, Their Derivatives, and Alkaloid-Inspired Compounds. Synthesis 2019, 51, 3369–3396. [Google Scholar] [CrossRef]
- De Clercq, E. Perspectives of Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs) in the Therapy of HIV-1 Infection. Il Farm. 1999, 54, 26–45. [Google Scholar] [CrossRef]
- Xuan, T.D.; Minh, T.N.; Khanh, T.D. Isolation and Biological Activities of 3-Hydroxy-4(1H)-Pyridone. J. Plant Interact. 2016, 11, 94–100. [Google Scholar] [CrossRef]
- Yin, M.H.; Kang, D.G.; Choi, D.H.; Kwon, T.O.; Lee, H.S. Screening of Vasorelaxant Activity of Some Medicinal Plants Used in Oriental Medicines. J. Ethnopharmacol. 2005, 99, 113–117. [Google Scholar] [CrossRef]
- Parreira, R.L.T.; Abrahão, O.; Galembeck, S.E. Conformational Preferences of Non-Nucleoside HIV-1 Reverse Transcriptase Inhibitors. Tetrahedron 2001, 57, 3243–3253. [Google Scholar] [CrossRef]
- Corbett, J.W.; Kresge, K.J.; Pan, S.; Cordova, B.C.; Klabe, R.M.; Rodgers, J.D.; Erickson-Viitanen, S.K. Trifluoromethyl-Containing 3-Alkoxymethyl- and 3-Aryloxymethyl-2-Pyridinones Are Potent Inhibitors of HIV-1 Non-Nucleoside Reverse Transcriptase. Bioorg. Med. Chem. Lett. 2001, 11, 309–312. [Google Scholar] [CrossRef]
- Hwang, D.R.; Driscoll, J.S. Pyridones as Potential Antitumor Agents. J. Pharm. Sci. 1979, 68, 816–819. [Google Scholar] [CrossRef]
- Mao, D.T.; Driscoll, J.S.; Marquez, V.E. Synthesis of 3-Hydroxy-2-and-4-Pyridone Nucleosides as Potential Antitumor Agents. J. Med. Chem. 1984, 27, 160–164. [Google Scholar] [CrossRef]
- Malani, A.; Makwana, A.; Monapara, J.; Ahmad, I.; Patel, H.; Desai, N. Synthesis, Molecular Docking, DFT Study, and in Vitro Antimicrobial Activity of Some 4-(Biphenyl-4-Yl)-1,4-Dihydropyridine and 4-(Biphenyl-4-Yl)Pyridine Derivatives. J. Biochem. Mol. Toxicol. 2021, 35, e22903. [Google Scholar] [CrossRef]
- Ahamed, A.; Arif, I.A.; Mateen, M.; Surendra Kumar, R.; Idhayadhulla, A. Antimicrobial, Anticoagulant, and Cytotoxic Evaluation of Multidrug Resistance of New 1,4-Dihydropyridine Derivatives. Saudi J. Biol. Sci. 2018, 25, 1227–1235. [Google Scholar] [CrossRef]
- Lin, C.H.; Lane, H.Y. The Role of N-Methyl-D-Aspartate Receptor Neurotransmission and Precision Medicine in Behavioral and Psychological Symptoms of Dementia. Front. Pharmacol. 2019, 10, 540. [Google Scholar] [CrossRef]
- Lintunen, J.; Lähteenvuo, M.; Tiihonen, J.; Tanskanen, A.; Taipale, H. Adenosine Modulators and Calcium Channel Blockers as Add-on Treatment for Schizophrenia. NPJ Schizophrenia 2021, 7, 1. [Google Scholar] [CrossRef]
- Jeong, H.S.; Lim, H.S.; Park, H.J.; Lee, W.S.; Choi, J.O.; Lee, H.S.; Jo, S.H.; Hong, S.J. Clinical Outcomes between Calcium Channel Blockers and Angiotensin Receptor Blockers in Hypertensive Patients without Established Cardiovascular Diseases during a 3-Year Follow-Up. Sci. Rep. 2021, 11, 1783. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).