The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Study
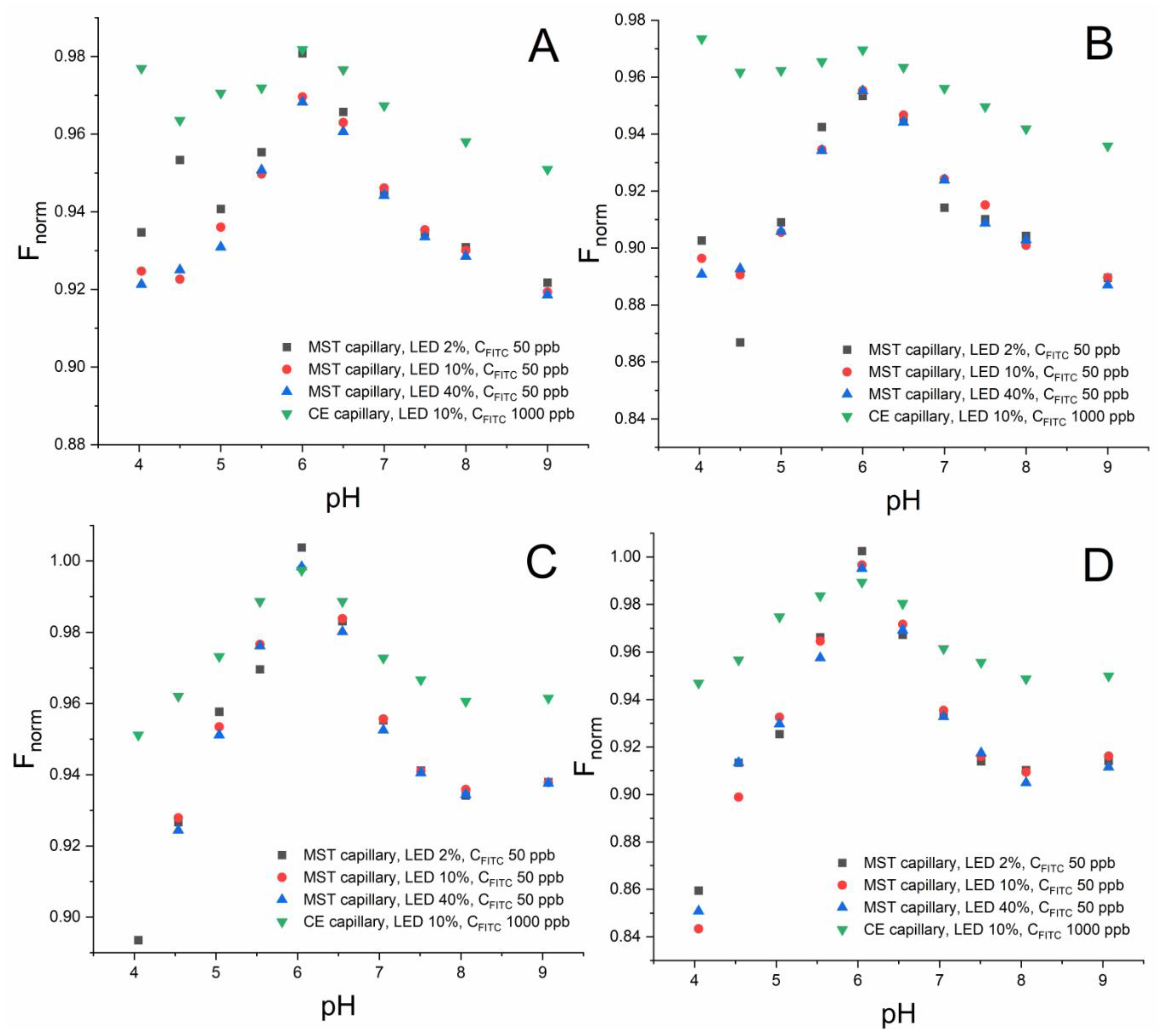
2.1.1. Sensitivity in the CE and MST Capillaries
2.1.2. MST Responses in the CE and MST Capillaries
2.1.3. Repeatability of MST Responses in the CE and MST Capillaries
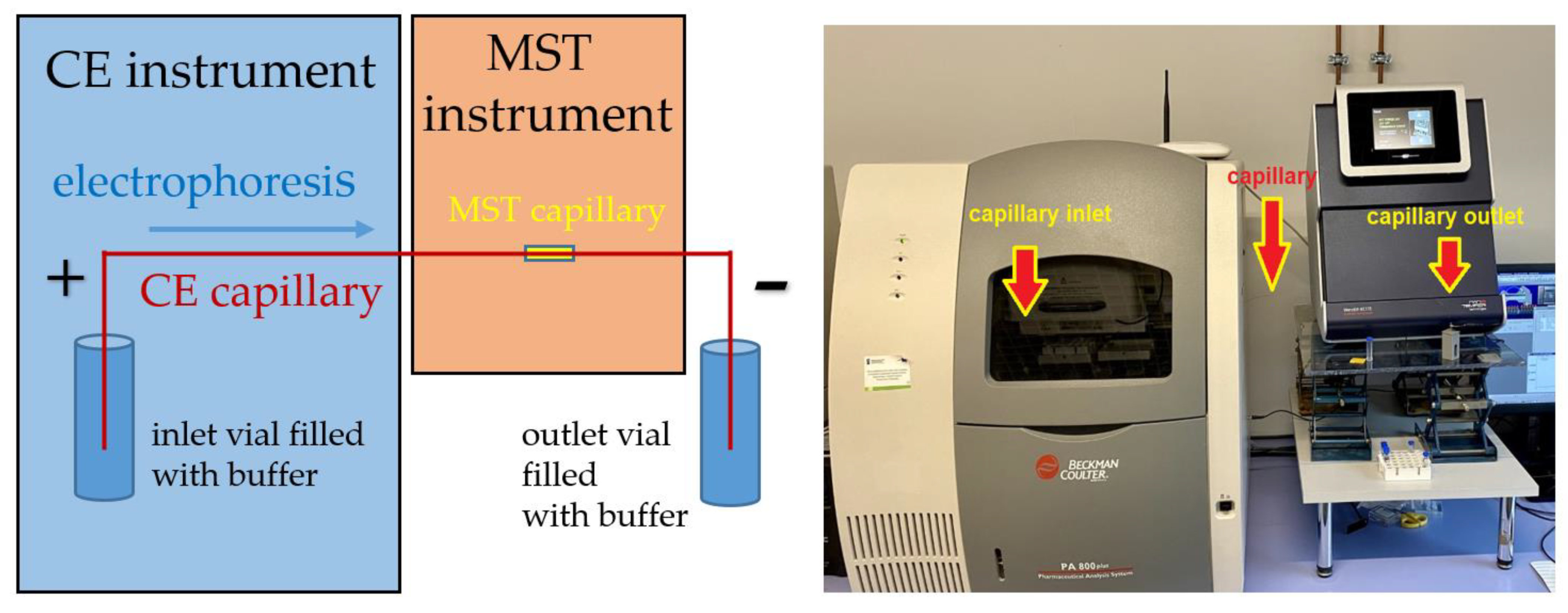
2.2. Full Integration of the CE and MST Instruments
2.3. Integration of the Low-Cost and Green CE Setup with MST
3. Materials and Methods
3.1. Materials and Reagents
3.2. MST Instrumentation
3.3. CE Instrumentation
3.4. CE-MST Interface
3.5. Home-Made Portable CE
3.6. Methodology of pKa Determination
3.6.1. MST Method
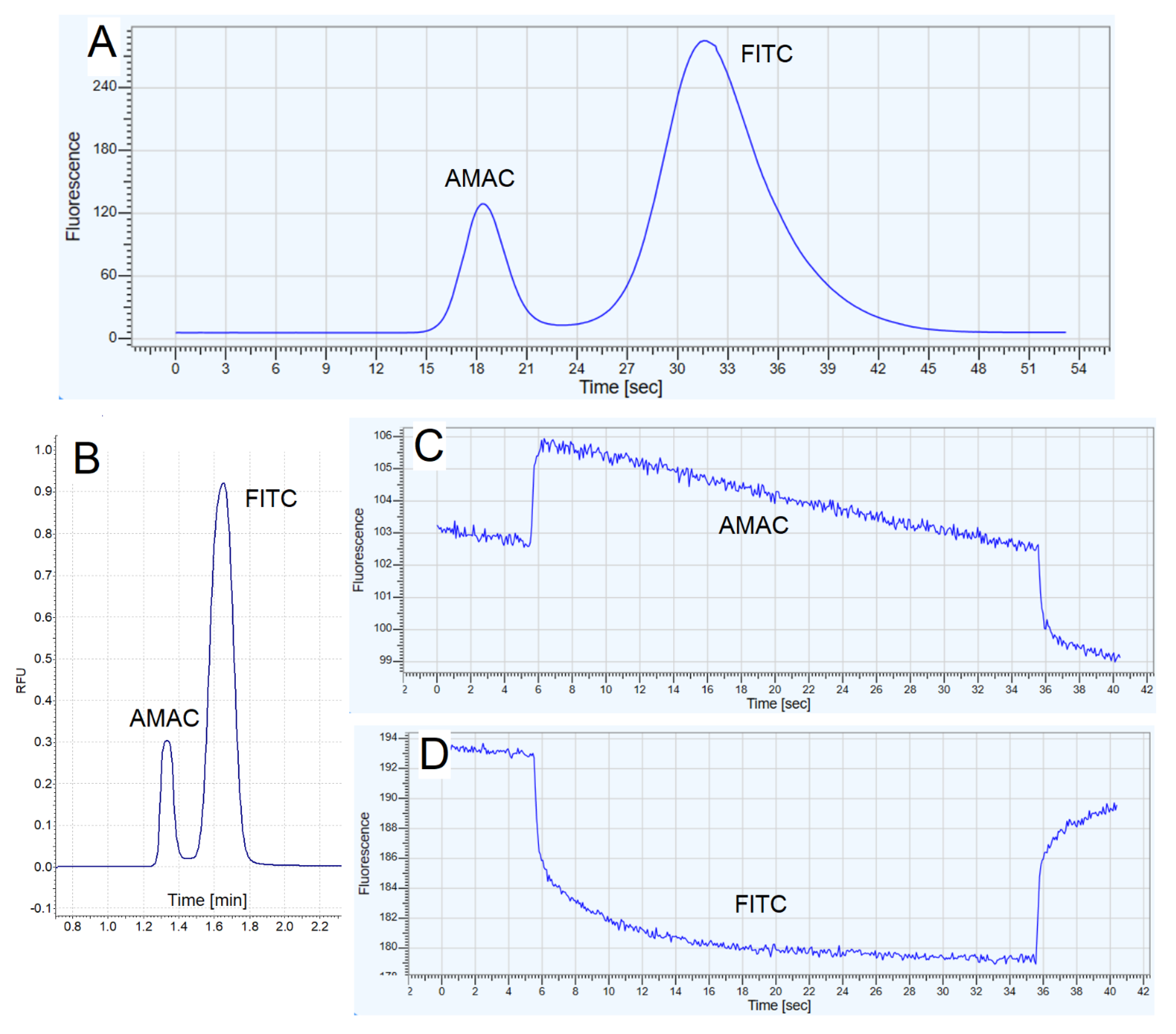
3.6.2. CE Electrophoretic Method
3.6.3. CE Fluorescent Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Jerabek-Willemsen, M.; Wienken, C.J.; Braun, D.; Baaske, P.; Duhr, S. Molecular Interaction Studies Using Microscale Thermophoresis. ASSAY Drug Dev. Technol. 2011, 9, 342–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, J.A.C.; Ianeselli, A.; Braun, D. Kinetic Microscale Thermophoresis for Simultaneous Measurement of Binding Affinity and Kinetics. Angew. Chem. Int. Ed. 2021, 60, 13988–13995. [Google Scholar] [CrossRef] [PubMed]
- López-Méndez, B.; Uebel, S.; Lundgren, L.P.; Sedivy, A. Microscale Thermophoresis and Additional Effects Measured in NanoTemper Monolith Instruments. Eur. Biophys. J. 2021, 50, 653–660. [Google Scholar] [CrossRef]
- Sedivy, A. Standard Operating Procedure for NanoTemper Monolith Measurements. Eur. Biophys. J. 2021, 50, 381–387. [Google Scholar] [CrossRef]
- Nasreddine, R.; Nehmé, R. Microscale Thermophoresis for Studying Protein-Small Molecule Affinity: Application to Hyaluronidase. Microchem. J. 2021, 170, 106763. [Google Scholar] [CrossRef]
- Entzian, C.; Schubert, T. Studying Small Molecule–Aptamer Interactions Using MicroScale Thermophoresis (MST). Methods 2016, 97, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Lippok, S.; Seidel, S.A.I.; Duhr, S.; Uhland, K.; Holthoff, H.-P.; Jenne, D.; Braun, D. Direct Detection of Antibody Concentration and Affinity in Human Serum Using Microscale Thermophoresis. Anal. Chem. 2012, 84, 3523–3530. [Google Scholar] [CrossRef] [PubMed]
- Wienken, C.J.; Baaske, P.; Rothbauer, U.; Braun, D.; Duhr, S. Protein-Binding Assays in Biological Liquids Using Microscale Thermophoresis. Nat. Commun. 2010, 1, 100. [Google Scholar] [CrossRef] [Green Version]
- Duhr, S.; Braun, D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. USA 2006, 103, 19678–19682. [Google Scholar] [CrossRef] [Green Version]
- Schmitt-Kopplin, P.; Fekete, A. The CE-way of thinking: “All is Relative!”. Method. Mol. Biol. 2016, 1483, 3–19. [Google Scholar] [CrossRef]
- Galbusera, C.; Thachuk, M.; Lorenzi, E.; Chen, D. Affinity capillary electrophoresis using a low-concentration additive with the consideration of relative mobilities. Anal. Chem. 2002, 74, 1903–1914. [Google Scholar] [CrossRef] [PubMed]
- Faller, T.; Engelhardt, H. How to achieve higher repeatability and reproducibility in capillary electrophoresis. J. Chromatogr. A 1999, 853, 83–94. [Google Scholar] [CrossRef]
- Mayer, B.X. How to increase precision in capillary electrophoresis. J. Chromatogr. A 2001, 907, 21–37. [Google Scholar] [CrossRef]
- Ali, I.; Aboul-Enein, H.Y.; Gupta, V.K. Precision in Capillary Electrophoresis. Anal. Lett. 2006, 39, 2345–2357. [Google Scholar] [CrossRef]
- Schaeper, J.P.; Sepaniak, M.J. Parameters affecting reproducibility in capillary electrophoresis. Electrophoresis 2000, 21, 1421–1429. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Garmash, A.; Kudryavtsev, A.V.; Menzinger, F.; Perminova, I.; Hertkorn, N.; Freitag, D.; Petrosyan, V.; Kettrup, A. Quantitative and qualitative precision improvements by effective mobility-scale data transformation in capillary electrophoresis analysis. Electrophoresis 2001, 22, 77–87. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Menzinger, F.; Freitag, D.; Kettrup, A. Improving the Use of CE in a Chromatographer’s World. LC•GC Eur. 2001, 14, 384–388. [Google Scholar] [CrossRef]
- Nowak, P.M.; Woźniakiewicz, M. On-line coupling between capillary electrophoresis and microscale thermophoresis (CE–MST); the proof-of-concept. Analyst 2018, 143, 4854–4859. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Woźniakiewicz, M. The Acid-Base/Deprotonation Equilibrium Can Be Studied with a MicroScale Thermophoresis (MST). Molecules 2022, 27, 685. [Google Scholar] [CrossRef]
- Nowak, P.; Woźniakiewicz, M.; Kościelniak, P. Application of capillary electrophoresis in determination of acid dissociation constant values. J. Chromatogr. A 2015, 1377, 1–12. [Google Scholar] [CrossRef]
- Simpson, S.L., Jr.; Quirino, J.P.; Terabe, S. On-line sample preconcentration in capillary electrophoresis. Fundamentals and applications. J. Chromatogr. A 2008, 1184, 504–541. [Google Scholar] [CrossRef]
- Breadmore, M.C.; Grochocki, W.; Kalsoom, U.; Alves, M.N.; Phung, S.C.; Rokh, M.T.; Cabot, J.M.; Ghiasvand, A.; Li, F.; Shallan, A.I.; et al. Recent advances in enhancing the sensitivity of electrophoresis and electrochromatography in capillaries and microchips (2016–2018). Electrophoresis 2019, 40, 17–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gładysz, M.; Król, M.; Woźniakiewicz, M.; Kościelniak, P. The increase of detection sensitivity of micellar electrokinetic capillary chromatography method of stamp pad inks components by applying a sample stacking mode for the purpose of questioned document examination. Talanta 2018, 184, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Wozźniakiewicz, M.; Kosścielniak, P. Seven approaches to elimination of the inherent systematic errors in determination of electrophoretic mobility by capillary electrophoresis. Anal. Chem. 2017, 89, 3630–3638. [Google Scholar] [CrossRef]
- Nowak, P.M.; Biel, I.; Kózka, G.; Klag, M.; Woźniakiewicz, M. Influence of pH measurement inaccuracy on the values of acidity constant determined on the basis of electrophoretic and thermophoretic data. Microchem. J. 2022, 181, 107689. [Google Scholar] [CrossRef]
- Gołąb, M.; Przybyłowska, M.; Kubáň, P.; Itterheimová, P.; Woźniakiewicz, M. Development of CE-C4D method for determination tropane alkaloids. Molecules 2021, 26, 5749. [Google Scholar] [CrossRef]
- Koel, M.; Kaljurand, M. Application of the principles of green chemistry in analytical chemistry. Pure Appl. Chem. 2006, 78, 1993–2002. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC—Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Nowak, P.M.; Kościelniak, P. What color is your method? Adaptation of the RGB additive color model to analytical method evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC—Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]

| pH | MST Capillary (wide) | CE Capillary (narrow) | NSR | ||
|---|---|---|---|---|---|
| LED 2%, CFITC 50 ng∙mL−1 | LED 10%, CFITC 50 ng∙mL−1 | LED 40%, CFITC 50 ng∙mL−1 | LED 10%, CFITC 1 µg∙mL−1 | ||
| 4.03 | 0.89 | 10.62 | 49.13 | 24.19 | 8.78 |
| 4.50 | 1.10 | 13.25 | 61.80 | 43.18 | 6.14 |
| 5.00 | 1.14 | 14.39 | 67.63 | 53.62 | 5.37 |
| 5.50 | 1.28 | 16.30 | 76.00 | 70.47 | 4.63 |
| 6.00 | 2.29 | 30.97 | 142.73 | 96.60 | 6.41 |
| 6.50 | 4.71 | 65.67 | 298.17 | 138.87 | 9.46 |
| 7.00 | 10.17 | 143.00 | 642.67 | 172.50 | 16.58 |
| 7.50 | 14.83 | 212.17 | 948.33 | 208.83 | 20.32 |
| 8.00 | 17.23 | 247.33 | 1106.67 | 200.83 | 24.63 |
| 9.00 | 20.07 | 286.83 | 1281.67 | 199.57 | 28.75 |
| Mean | 7.37 | 104.05 | 467.48 | 120.87 | 13.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, P.M.; Klag, M.; Kózka, G.; Gołąb, M.; Woźniakiewicz, M. The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate. Molecules 2022, 27, 5010. https://doi.org/10.3390/molecules27155010
Nowak PM, Klag M, Kózka G, Gołąb M, Woźniakiewicz M. The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate. Molecules. 2022; 27(15):5010. https://doi.org/10.3390/molecules27155010
Chicago/Turabian StyleNowak, Paweł Mateusz, Maria Klag, Gabriela Kózka, Małgorzata Gołąb, and Michał Woźniakiewicz. 2022. "The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate" Molecules 27, no. 15: 5010. https://doi.org/10.3390/molecules27155010
APA StyleNowak, P. M., Klag, M., Kózka, G., Gołąb, M., & Woźniakiewicz, M. (2022). The First Online Capillary Electrophoresis-Microscale Thermophoresis (CE-MST) Method for the Analysis of Dynamic Equilibria—The Determination of the Acidity Constant of Fluorescein Isothiocyanate. Molecules, 27(15), 5010. https://doi.org/10.3390/molecules27155010







