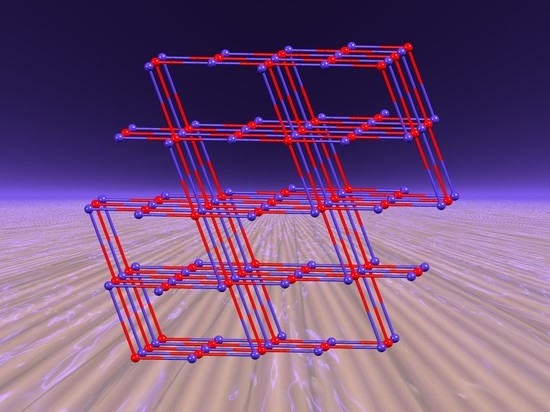
A Tail Does Not Always Make a Difference: Assembly of cds Nets from Co(NCS)2 and 1,4-bis(n-Alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands
Abstract
:1. Introduction
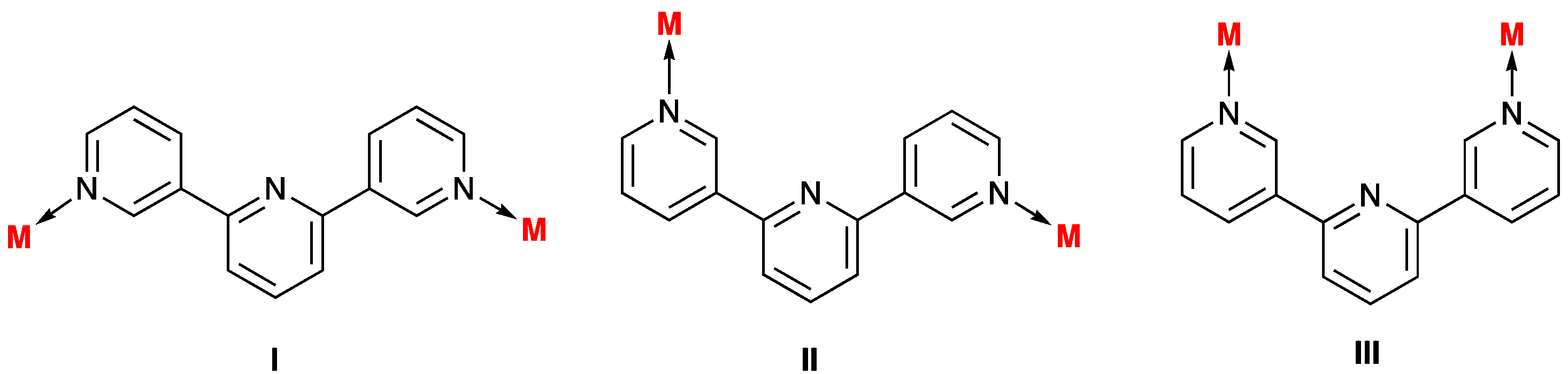
2. Results and Discussion
2.1. Crystal Growth Conditions
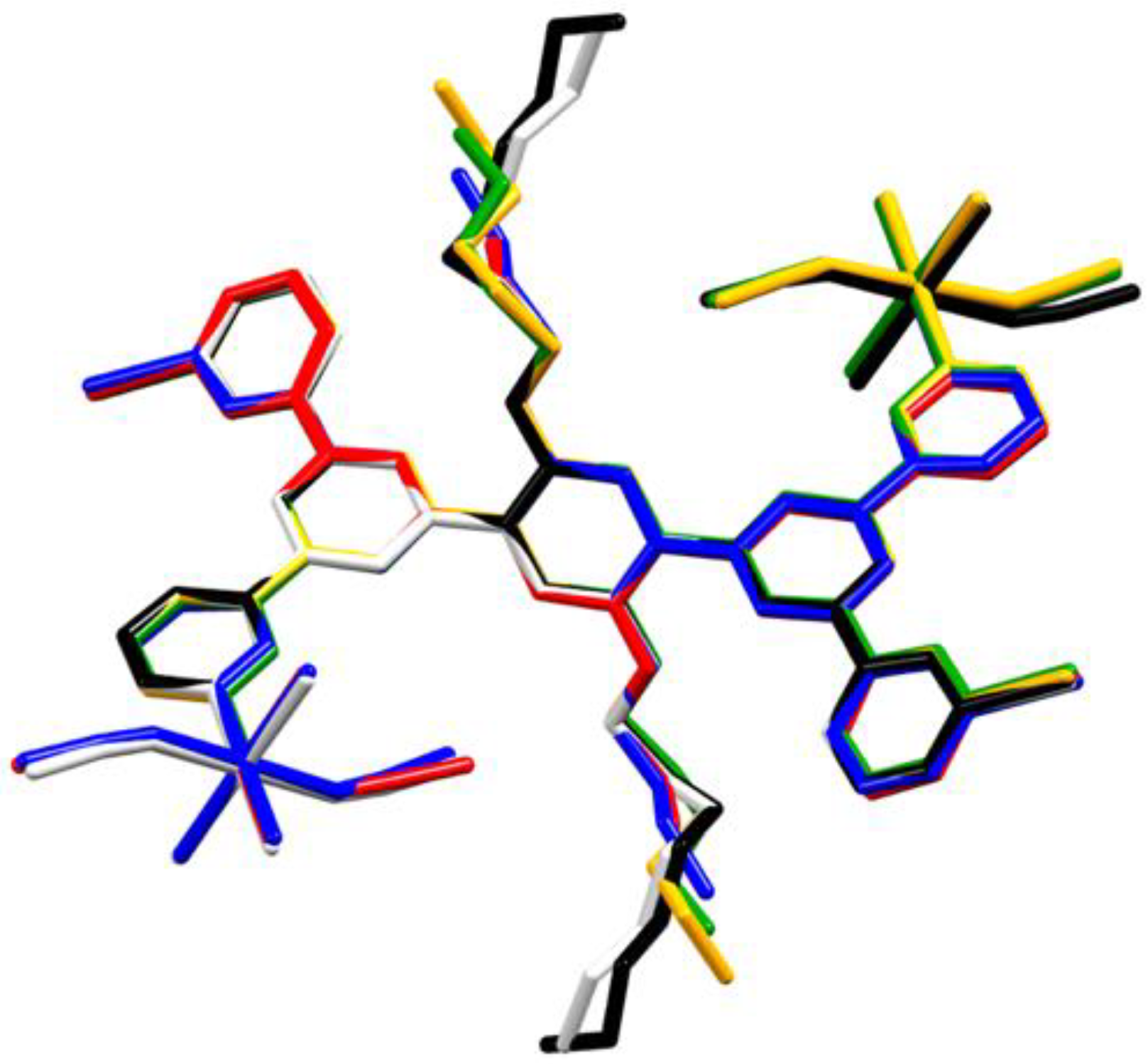
2.2. Single Crystal Structures
2.3. Bulk Sample Analysis
2.4. Role of Solvents
3. Materials and Methods
3.1. General
3.2. [Co(NCS)2(3)]n.3.5nC6H4Cl2
3.3. [Co(NCS)2(4)]n.5.5nC6H4Cl2
3.4. [Co(NCS)2(5)]n.4nC6H4Cl2
3.5. [Co(NCS)2(6)]n.3.8nC6H4Cl2
3.6. [Co(NCS)2(7)]n.3.1nC6H4Cl2
3.7. [Co(NCS)2(8)]n.1.6nC6H4Cl2.2nMeOH
3.8. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grafino, J.; Vargas, M.; Garland, M.T.; Ibáñez, A.; Gaviño, R.; Baggio, R. The novel ligand 4′-phenyl-3,2′:6′,3″-terpyridine (L) and the supramolecular structure of the dinuclear complex [Zn2(μ-L)(acac)4].H2O (acac = acetylacetonato). Inorg. Chem. Comm. 2008, 11, 1388–1391. [Google Scholar] [CrossRef]
- Yang, P.; Wang, M.-S.; Shen, J.-J.; Li, M.-X.; Wang, Z.-X.; Shaob, M.; He, X. Seven novel coordination polymers constructed by rigid 4-(4-carboxyphenyl)-terpyridine ligands: Synthesis, structural diversity, luminescence and magnetic properties. Dalton Trans. 2014, 43, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Luo, F.; Tang, G.; Zhang, J. A 4-(4-carboxphenyl)-3,2′:6′,3″-terpyridine-based luminescent cadmium(II) coordination polymer for the detection of 2,4,6-trinitrophenol. Acta Crystallogr. 2019, C75, 508–513. [Google Scholar] [CrossRef]
- Li, N.; Zhu, Q.-E.; Hu, H.-M.; Guo, H.-L.; Xie, J.; Wang, F.; Dong, F.-X.; Yang, M.-L.; Xue, G.-L. Hydrothermal syntheses, crystal structures and luminescence properties of zinc(II) coordination polymers constructed by bifunctional 4′-(4-carboxyphenyl)-3,2′:6′,3″-terpyridine. Polyhedron 2013, 49, 207–215. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, M.-L.; Hu, H.-M.; Xu, B.; Wang, X.; Xue, G. Syntheses, structures and luminescence for zinc coordination polymers based on a multifunctional 4′-(3-carboxyphenyl)-3,2′:6′,3″-terpyridine ligand. J. Solid State Chem. 2016, 239, 121–130. [Google Scholar] [CrossRef]
- Wang, T.-T.; Zhang, J.-L.; Hu, H.-M.; Cheng, Y.; Xue, L.-L.; Wang, X.; Wang, B.-Z. Syntheses, structures and luminescent properties of Zn/Cd coordination polymers based on 4′-(2-carboxyphenyl)-3,2′:6′,3″-terpyridine. Polyhedron 2018, 151, 43–50. [Google Scholar] [CrossRef]
- Zheng, L.-N.; Cheng, Y.; Hu, H.-M.; Bai, C.; Wang, X.; Xue, G. Syntheses, structures and magnetic properties for transition metal coordination polymers based on polycarboxylate and isomeric terpyridyl carboxylate ligands. J. Solid State Chem. 2019, 272, 210–220. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, F.; Hu, H.-M.; Bai, C.; Xue, G.-L. Nitro explosive and cation sensing by a luminescent 2D Cu(I) coordination polymer with multiple Lewis basic sites. Inorg. Chem. Commun. 2016, 73, 37–40. [Google Scholar] [CrossRef]
- Toledo, D.; Pena, O.; Roisnel, T.; Pivan, J.-Y.; Moreno, Y. New cobalt(II) coordination polymer based on carboxyphenyl-tpy ligand: Photoluminescence, crystal structures and magnetic properties, without orbital contribution. J. Coord. Chem. 2018, 71, 22–34. [Google Scholar] [CrossRef]
- Zhang, T.-H.; Bai, C.; Hu, H.-M.; Zhang, J.-L.; Li, X.-Y.; Wang, X.; Wang, B.-Z. Cadmium(II) and cobalt(II) coordination compounds based on a benzenesulfonic terpyridine ligand: Syntheses, structures, luminescent sensing and magnetic properties. J. Solid State Chem. 2021, 298, 122148. [Google Scholar] [CrossRef]
- Li, N.; Guo, H.-L.; Hu, H.-M.; Song, J.; Xu, B.; Yang, M.-L.; Dong, F.-X.; Xue, G.-L. Hydrothermal syntheses, crystal structures and luminescence properties of zinc(II) and cadmium(II) coordination polymers based on bifunctional 3,2′:6′,3″-terpyridine-4′-carboxylic acid. J. Solid State Chem. 2013, 198, 416–423. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.-J.; He, J.-E.; Chen, Y.-Y.; Zheng, S.-R.; Fan, J.; Zhang, W.-G. Construction of New Coordination Polymers from 4′-(2,4-disulfophenyl)-3,2′:6′,3″-terpyridine: Polymorphism, pH-dependent syntheses, structures, and properties. J. Solid State Chem. 2016, 233, 444–454. [Google Scholar] [CrossRef]
- Wang, J.; Lu, L.; Ding, Q.; Zhang, S.-L.; Wang, J.; Singh, A.; Kumar, A.; Ma, A. Multi-responsive luminescent 2D Zn(II)-based coordination polymer for detection of trinitrophenol and Fe3+. J. Coord. Chem. 2020, 73, 307–316. [Google Scholar] [CrossRef]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Manipulating the Conformation of 3,2′:6′,3″-Terpyridine in [Cu2(μ-OAc)4(3,2′:6′,3″-tpy)]n 1D-Polymers. Chemistry 2021, 3, 182–198. [Google Scholar] [CrossRef]
- Yoshida, J.; Nishikiori, S.; Yuge, H. Bis(3-cyano-pentane-2,4-dionato) Co(II) as a linear building block for coordination polymers: Combinations with two polypyridines. J. Coord. Chem. 2013, 66, 2191–2200. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Tetratopic bis(4,2′:6′,4″-terpyridine) and bis(3,2′:6′,3″-terpyridine) Ligands as 4-Connecting Nodes in 2D-Coordination Networks and 3D-Frameworks. J. Inorg. Organomet. Polym. Mater. 2018, 28, 414–427. [Google Scholar] [CrossRef] [Green Version]
- Capomolla, S.S.; Manfroni, G.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Versatility within (4,4) networks assembled from 1,4-bis(n-alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene and [Cu(hfacac)2] (Hhfacac = 1,1,1,5,5,5-hexafluoropentane-2,4-dione). Polyhedron 2022, 224, 116005. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Crystallogr. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Karpacheva, M.; Constable, E.C.; Housecroft, C.E. Sometimes the Same, Sometimes Different: Understanding Self-Assembly Algorithms in Coordination Networks. Polymers 2018, 10, 1369. [Google Scholar] [CrossRef] [Green Version]
- Manfroni, G.; Prescimone, A.; Batten, S.R.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Trinodal Self-Penetrating Nets from Reactions of 1,4-Bis(alkoxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands with Cobalt(II) Thiocyanate. Crystals 2019, 9, 529. [Google Scholar] [CrossRef] [Green Version]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Prescimone, A. A 3-dimensional {42.84} lvt net built from a ditopic bis(3,2′:6′,3″-terpyridine) tecton bearing long alkyl tails. CrystEngComm 2015, 17, 2070–2073. [Google Scholar] [CrossRef] [Green Version]
- Vujovic, S.; Constable, E.C.; Housecroft, C.E.; Morris, C.D.; Neuburger, M.; Prescimone, A. Engineering 2D→2D parallel interpenetration using long alkoxy-chain substituents. Polyhedron 2015, 92, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef] [Green Version]
- Klein, Y.M.; Prescimone, A.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. What a difference a tail makes: 2D→2D parallel interpenetration of sheets to interpenetrated nbo networks using ditopic-4,2′:6′,4″-terpyridine ligands. CrystEngComm 2017, 19, 2894–2902. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2D-coordination networks: Combined effects of a conformationally flexible 3,2′:6′,3″-terpyridine and chain length variation in 4′-(4-n-alkyloxyphenyl) substituents. Molecules 2020, 25, 1663. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-S.; Wu, Y.-P.; Zhao, J.; Zhang, J.; Lu, J.Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014, 261, 1–27. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; RSC Publishing: Cambridge, UK, 2009; ISBN 978-0-85404-837-3. [Google Scholar]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 4,2′6′,4″- and 3,2′:6′,3″-terpyridines: The conflict between well-defined vectorial properties and serendipity in the assembly of 1D-, 2D- and 3D-architectures. Materials 2017, 10, 728. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Chapuis, G. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A computer program for topological analysis of discrete electron densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2019, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar] [CrossRef]
- LeBail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis. In Materials Science Forum; Transtec Publications: Zurich, Switzerland, 2000; pp. 118–123. [Google Scholar]






| Compound | Co–NNCS/Å | Co–Npy/Å | Range of N–Co–N Bond Angles a/deg |
|---|---|---|---|
| [Co(NCS)2(3)]n.3.5nC6H4Cl2 | 2.077(4) | 2.151(4), 2.245(4) | 87.82(15)–92.18(15) |
| [Co(NCS)2(4)]n.5.5nC6H4Cl2 | 2.069(5), 2.051(5), 2.069(5) | 2.149(5), 2.262(5), 2.175(5), 2.288(5), 2.176(5), 2.253(5) | 87.60(18)–91.64(18) |
| [Co(NCS)2(5)]n.4nC6H4Cl2 | 2.062(3) | 2.249(3), 2.182(3) | 89.29(12)–90.71(12) |
| [Co(NCS)2(6)]n.3.8nC6H4Cl2 | 2.0633(19) | 2.1854(19), 2.2469(19) | 89.12(7)–90.88(7) |
| [Co(NCS)2(7)]n.3.1nC6H4Cl2 | 2.059(3) | 2.181(3), 2.255(3) | 88.62(12)–91.38(12) |
| [Co(NCS)2(8)]n.1.6nC6H4Cl2.2nMeOH | 2.068(5) | 2.182(5), 2.243(4) | 88.58(18)–91.42(18) |
| Compound | py-py/° | pycentral-Arene Spacer/° |
|---|---|---|
| [Co(NCS)2(3)]n.3.5nC6H4Cl2 | 12.9, 28.7 | 48.4 |
| [Co(NCS)2(4)]n.5.5nC6H4Cl2 | 16.0, 24.6; a 10.6, 24.9; b 17.5. 22.8 c | 46.8 a; 46.8 b; 50.0 c |
| [Co(NCS)2(5)]n.4nC6H4Cl2 | 10.0, 28.1 | 52.3 |
| [Co(NCS)2(6)]n.3.8nC6H4Cl2 | 8.1, 28.3 | 52.8 |
| [Co(NCS)2(7)]n.3.1nC6H4Cl2 | 11.1, 24.7 | 50.3 |
| [Co(NCS)2(8)]n.1.6nC6H4Cl2.2nMeOH | 10.8, 25.7 | 51.4 |
| Ligand, L | 3 | 4 | 5 | 6 | 7 | 8 |
| Void space/% | 49.3 | 44.5 | 43.1 | 39.8 | 35.6 | 34.4 |
| Compound | Initial Weight /mg | Weight Loss /mg | Weight Loss /% | Calculated Solvent Molecules a |
|---|---|---|---|---|
| [Co(NCS)2(3)]n.3.5nC6H4Cl2 | 1.14 | 0.45 | 39.5 | 3.7 mol C6H4Cl2 |
| [Co(NCS)2(4)]n.5.5nC6H4Cl2 | 0.76 | 0.30 | 39.5 | 3.8 mol C6H4Cl2 |
| [Co(NCS)2(6)]n.3.8nC6H4Cl2 | 1.74 | 0.67 | 38.5 | 3.9 mol C6H4Cl2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capomolla, S.S.; Manfroni, G.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. A Tail Does Not Always Make a Difference: Assembly of cds Nets from Co(NCS)2 and 1,4-bis(n-Alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands. Molecules 2022, 27, 4995. https://doi.org/10.3390/molecules27154995
Capomolla SS, Manfroni G, Prescimone A, Constable EC, Housecroft CE. A Tail Does Not Always Make a Difference: Assembly of cds Nets from Co(NCS)2 and 1,4-bis(n-Alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands. Molecules. 2022; 27(15):4995. https://doi.org/10.3390/molecules27154995
Chicago/Turabian StyleCapomolla, Simona S., Giacomo Manfroni, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2022. "A Tail Does Not Always Make a Difference: Assembly of cds Nets from Co(NCS)2 and 1,4-bis(n-Alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands" Molecules 27, no. 15: 4995. https://doi.org/10.3390/molecules27154995
APA StyleCapomolla, S. S., Manfroni, G., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2022). A Tail Does Not Always Make a Difference: Assembly of cds Nets from Co(NCS)2 and 1,4-bis(n-Alkyloxy)-2,5-bis(3,2′:6′,3″-terpyridin-4′-yl)benzene Ligands. Molecules, 27(15), 4995. https://doi.org/10.3390/molecules27154995