MOFs-Derived Three-Phase Microspheres: Morphology Preservation and Electromagnetic Wave Absorption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Ni/Co-MOFs and Ni-MOFs
2.3. Preparation of NMO and NCMO
2.4. Characterization
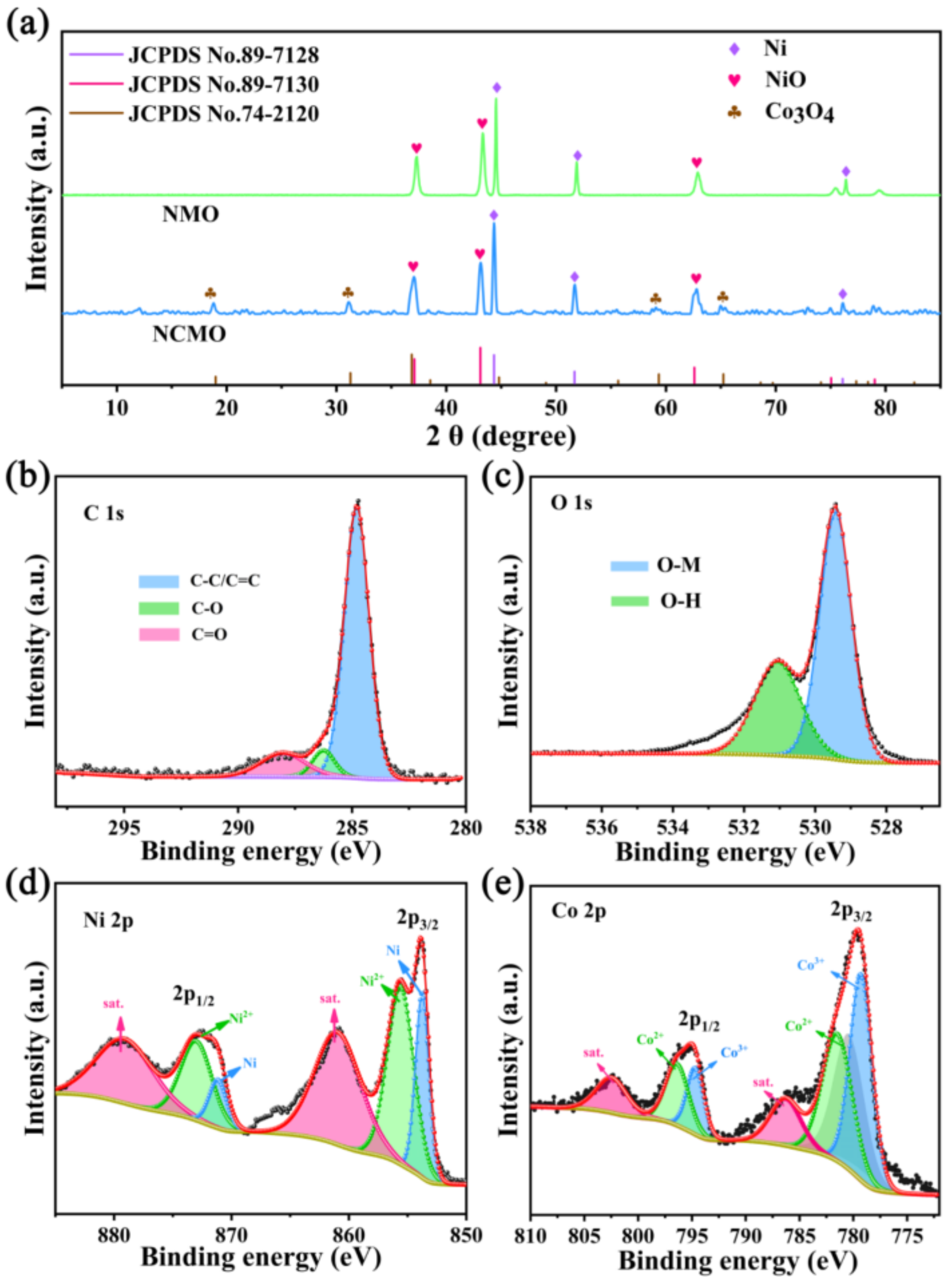
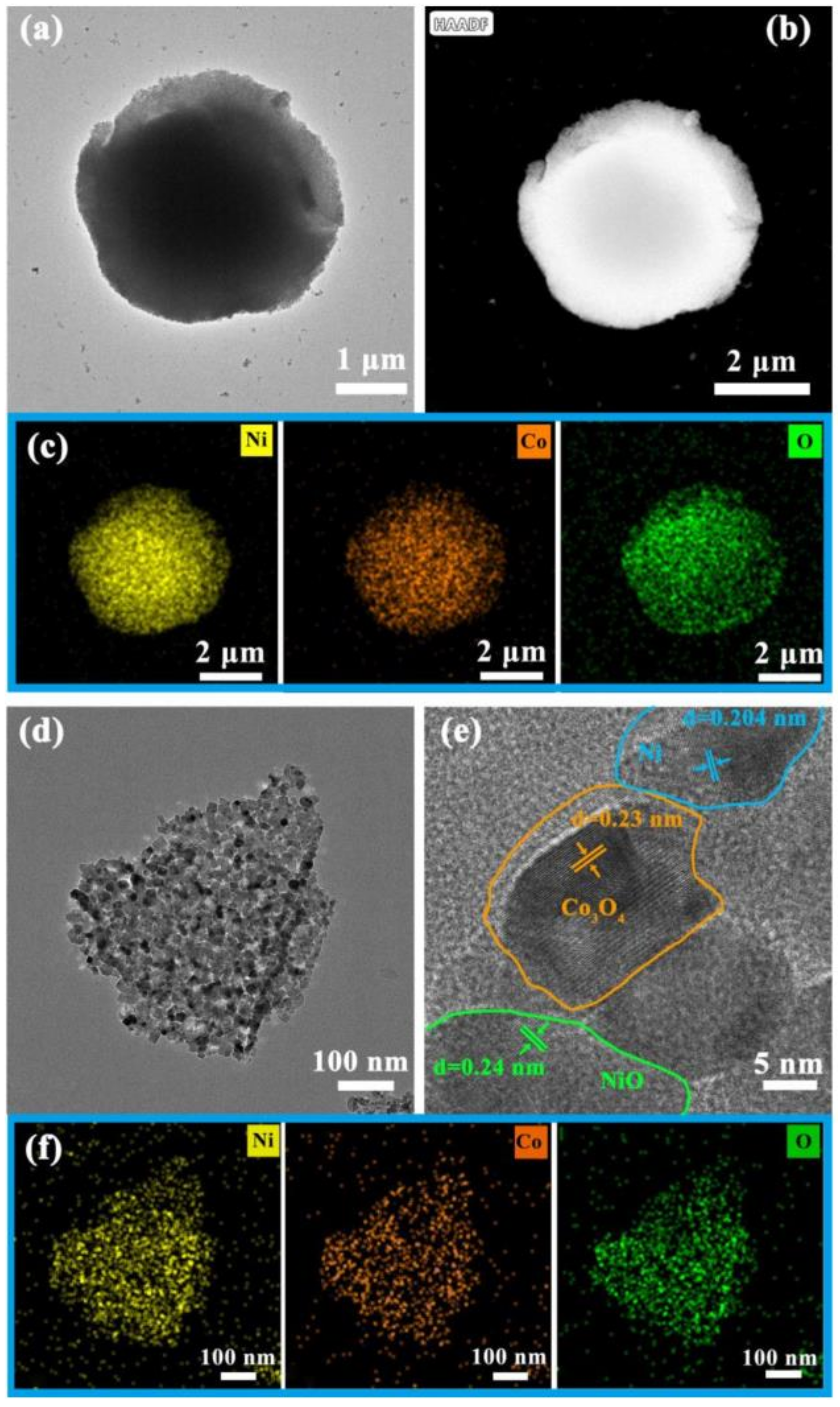
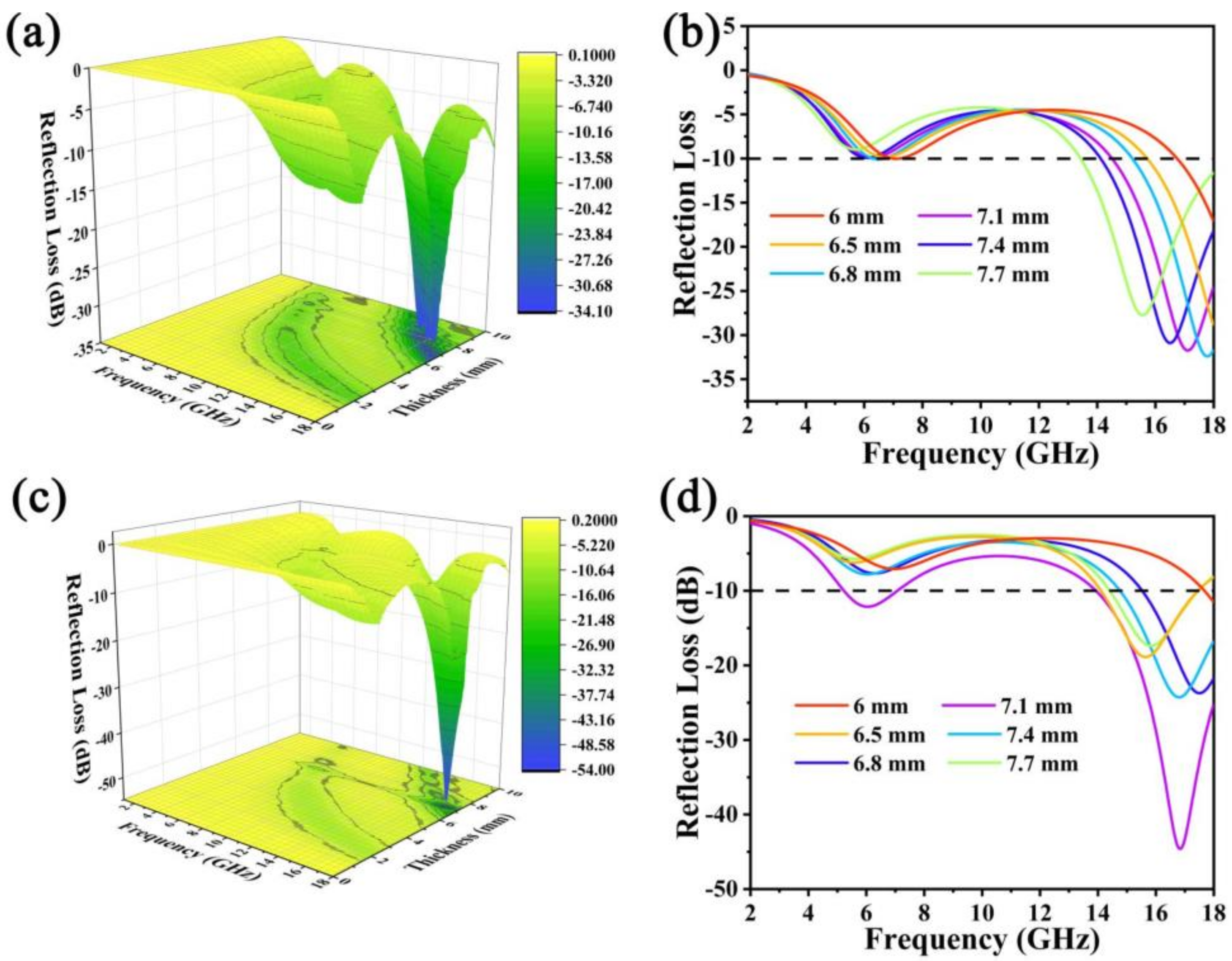
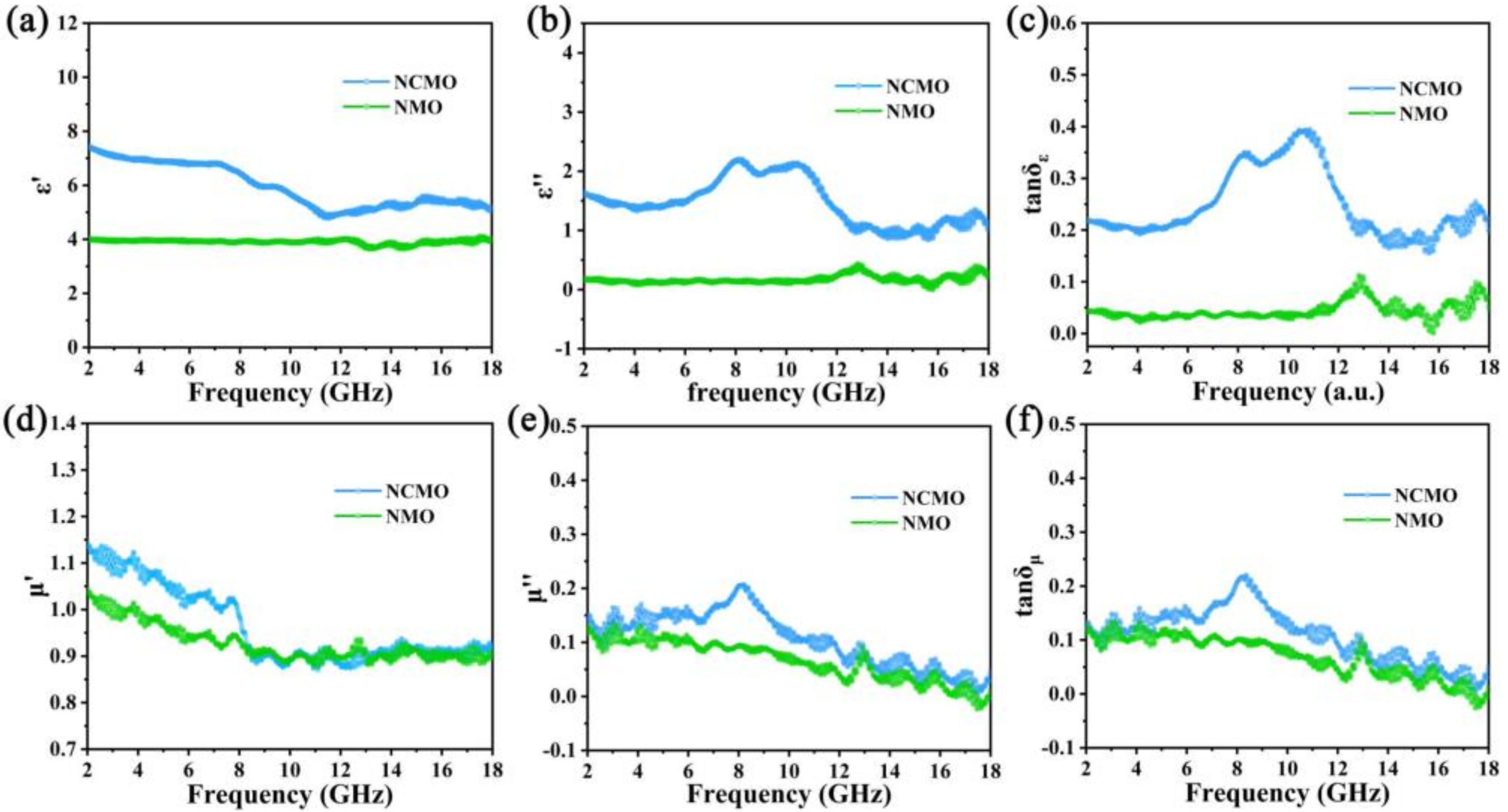
3. Results Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Y.; Cai, R.; Wang, D.; Li, K.; Sun, Q.; Xiao, Y.; Teng, H.; Huang, X.; Sun, T.; Liu, Z. Lightweight, Low-Cost Co2SiO4@diatomite Core-Shell Composite Material for High-Efficiency Microwave Absorption. Molecules 2022, 27, 1055. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ma, M.; Liao, Z.; Tong, Z.; Chen, Y.; Wang, R.; Ma, Y.; Wu, G. One-dimensional Ni@Co/C@PPy composites for superior electromagnetic wave absorption. J. Colloid Interface Sci. 2022, 605, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Di, X.; Fu, Y.; Wu, X.; Cao, J. Facile synthesis of the three-dimensional flower-like ZnFe2O4@MoS2 composite with heterogeneous interfaces as a high-efficiency absorber. J. Colloid Interface Sci. 2021, 587, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Plyushch, A.; Macutkevic, J.; Svirskas, S.; Banys, J.; Plausinaitiene, V.; Bychanok, D.; Maksimenko, S.A.; Selskis, A.; Sokal, A.; Lapko, K.N.; et al. Silicon carbide/phosphate ceramics composite for electromagnetic shielding applications: Whiskers vs. particles. Appl. Phys. Lett. 2019, 114, 183105. [Google Scholar] [CrossRef]
- Li, C.; Chen, G.; Jiang, W.; Jiang, X.; Yan, X. High-performance electromagnetic wave absorption of FeNi/N, S-codoped carbon composites in 2–40 GHz. Carbon 2021, 174, 201–213. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevič, J.; Kuzhir, P.; Sokal, A.; Lapko, K.; Selskis, A.; Banys, J. Synergy Effects in Electromagnetic Properties of Phosphate Ceramics with Silicon Carbide Whiskers and Carbon Nanotubes. Appl. Sci. 2019, 9, 4388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, X.; Quan, B.; Ji, G.; Liang, X.; Liu, W.; Du, Y. A facile self-template strategy for synthesizing 1D porous Ni@C nanorods towards efficient microwave absorption. Nanotechnology 2017, 28, 115704. [Google Scholar] [CrossRef]
- Zhao, Z.; Kou, K.; Zhang, L.; Wu, H. High efficiency electromagnetic wave absorber derived from transition metal layered double hydroxides. J. Colloid Interface Sci. 2020, 579, 733–740. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Zhang, Y.; Wu, G.; Wu, H. Facile synthesis of ellipsoid-like MgCo2O4/Co3O4 composites for strong wideband microwave absorption application. Compos. Part B Eng. 2019, 176, 107240. [Google Scholar] [CrossRef]
- Liu, X.; Hao, C.; Jiang, H.; Zeng, M.; Yu, R. Hierarchical NiCo2O4/Co3O4/NiO porous composite: A lightweight electromagnetic wave absorber with tunable absorbing performance. J. Mater. Chem. C 2017, 5, 3770–3778. [Google Scholar] [CrossRef]
- Miao, P.; Cao, J.; Kong, J.; Li, J.; Wang, T.; Chen, K.-J. Bimetallic MOF-derived hollow ZnNiC nano-boxes for efficient microwave absorption. Nanoscale 2020, 12, 13311–13315. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Li, X.; Chen, G.; Wang, Z.; Zhou, X.; Wu, H. Controllable adjustment of cavity of core-shelled Co3O4@NiCo2O4 composites via facile etching and deposition for electromagnetic wave absorption. J. Colloid Interface Sci. 2021, 594, 424–434. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Y.; Song, Y.; Li, J.; Yin, F.; Yuan, Y. Periodic Three-Dimensional Nitrogen-Doped Mesoporous Carbon Spheres Embedded with Co/Co3O4 Nanoparticles toward Microwave Absorption. ACS Appl. Mater. Interfaces 2020, 12, 24102–24111. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Wang, H.; Li, Q.; Feng, Z.; Wei, F.; Yao, K.X.; Sun, Z.; Qi, J.; Sui, Y. Highly stable Co3O4 nanoparticles/carbon nanosheets array derived from flake-like ZIF-67 as an advanced electrode for supercapacacitor. Chem. Eng. J. 2021, 419, 129631. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, Y.; Yang, H.; Wang, L.; Wang, M.; Wen, B. Hollow Ni/C microspheres derived from Ni-metal organic framework for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 123207. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Z.; Li, X.; Ren, J.; Wang, Y.; Zhang, Q.; Zhang, B. MOF-derived yolk-shell Co@ ZnO/Ni@ NC nanocage: Structure control and electromagnetic wave absorption performance. J. Colloid Interface Sci. 2021, 600, 99–110. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xiao, F.; Huang, Z.; Wang, Y.; Richardson, C.; Chen, Z.; Jiao, L.; Yuan, H. Facile synthesis of nanocage Co3O4 for advanced lithium-ion batteries. J. Power Source 2015, 298, 203–208. [Google Scholar] [CrossRef]
- Liao, Q.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Hu, S.; Yang, H.; Li, H.; Tong, Y. Highly cuboid-shaped heterobimetallic metal–organic frameworks derived from porous Co/ZnO/C microrods with improved electromagnetic wave absorption capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef]
- Ji, J.; Huang, Y.; Yin, J.; Zhao, X.; Cheng, X.; He, S.; Li, X.; He, J.; Liu, J. Synthesis and Electromagnetic and Microwave Absorption Properties of Monodispersive Fe3O4/α-Fe2O3 Composites. ACS Appl. Nano Mater. 2018, 1, 3935–3944. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, D.; Tian, K.; Hu, W.; Wang, J.; Su, M.; Li, L. Facile preparation of core–shell Fe3O4@Polypyrrole composites with superior electromagnetic wave absorption properties. J. Phys. Chem. C 2017, 121, 15784–15792. [Google Scholar] [CrossRef]
- Lestari, W.; Winarni, I.; Rahmawati, F. Electrosynthesis of Metal-Organic Frameworks (MOFs) Based on Nickel (II) and Benzene 1,3,5-tri Carboxylic Acid (H3BTC): An Optimization Reaction Condition; IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012064. [Google Scholar]
- Yaghi, O.M.; Li, H.; Groy, T.L. Construction of Porous Solids from Hydrogen-Bonded Metal Complexes of 1,3,5-Benzenetricarboxylic Acid. J. Am. Chem. Soc. 1996, 118, 9096–9101. [Google Scholar] [CrossRef]
- Yu, L.; Wan, G.; Qin, Y.; Wang, G. Atomic layer deposition assisted fabrication of high-purity carbon nanocoil for electrochemical energy storage. Electrochim. Acta 2018, 268, 283–294. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, Y.; Wang, X.; Chen, S.; Xu, F.; Zuo, L.; Wu, J.; Sun, L.; Li, Z.; Hou, H. Nitrogen-doped porous carbon/Co3O4 nanocomposites as anode materials for lithium-ion batteries. ACS Appl. Mater. Interfaces 2014, 6, 7117–7125. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Yang, L.; Hou, L.; Li, J.; Sun, Y.; Zhang, X.; Shen, L.; Lu, X.; Xiong, S.; Lou, X.W. Flexible hybrid paper made of monolayer Co3O4 microsphere arrays on rGO/CNTs and their application in electrochemical capacitors. Adv. Funct. Mater. 2012, 22, 2560–2566. [Google Scholar] [CrossRef]
- Lei, L.; Yao, Z.; Zhou, J.; Zheng, W.; Wei, B.; Zu, J.; Yan, K. Hydrangea-like Ni/NiO/C composites derived from metal-organic frameworks with superior microwave absorption. Carbon 2021, 173, 69–79. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. Ni/NiO nanosheets for alkaline hydrogen evolution reaction: In situ electrochemical-Raman study. Electrochim. Acta 2020, 361, 137040. [Google Scholar] [CrossRef]
- Chandra Sekhar, S.; Nagaraju, G.; Yu, J.S. High-performance pouch-type hybrid supercapacitor based on hierarchical NiO-Co3O4-NiO composite nanoarchitectures as an advanced electrode material. Nano Energy 2018, 48, 81–92. [Google Scholar] [CrossRef]
- Deng, X.; Li, J.; Zhu, S.; He, F.; He, C.; Liu, E.; Shi, C.; Li, Q.; Zhao, N. Metal–organic frameworks-derived honeycomb-like Co3O4/three-dimensional graphene networks/Ni foam hybrid as a binder-free electrode for supercapacitors. J. Alloys Compd. 2017, 693, 16–24. [Google Scholar] [CrossRef]
- Wu, F.; Liu, P.; Wang, J.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of magnetic tubular fiber with multi-layer heterostructure and its microwave absorbing properties. J. Colloid Interface Sci. 2020, 577, 242–255. [Google Scholar] [CrossRef]
- Ma, M.; Li, W.; Tong, Z.; Ma, Y.; Bi, Y.; Liao, Z.; Zhou, J.; Wu, G.; Li, M.; Yue, J. NiCo2O4 nanosheets decorated on one-dimensional ZnFe2O4@SiO2@C nanochains with high-performance microwave absorption. J. Colloid Interface Sci. 2020, 578, 58–68. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.W.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Zhang, H.; Yang, Z.; Che, R. Dimensional design and core–shell engineering of nanomaterials for electromagnetic wave absorption. Adv. Mater. 2022, 34, 2107538. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, G.-S.; Yin, P.-G. Designed fabrication of reduced graphene oxides/Ni hybrids for effective electromagnetic absorption and shielding. Carbon 2018, 139, 759–767. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Ji, G.; Li, D.; Zhang, Y.; Ma, J.; Du, Y. Composition design and structural characterization of MOF-derived composites with controllable electromagnetic properties. ACS Sustain. Chem. Eng. 2017, 5, 7961–7971. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Yu, R. An efficient Co/C microwave absorber with tunable Co nanoparticles derived from a ZnCo bimetallic zeolitic imidazolate framework. Part. Part. Syst. Charact. 2018, 35, 1800107. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Lv, H.; Ji, G.; Du, Y. A novel hierarchically porous magnetic carbon derived from biomass for strong lightweight microwave absorption. Carbon 2019, 142, 245–253. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, H.; Ji, G.; Xu, Z.J. Interface strategy to achieve tunable high frequency attenuation. ACS Appl. Mater. Interfaces 2016, 8, 6529–6538. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Liang, L.; Li, Q.; Yan, X.; Feng, Y.; Wang, Y.; Zhang, H.-B.; Zhou, X.; Liu, C.; Shen, C.; Xie, X. Multifunctional Magnetic Ti3C2Tx MXene/Graphene Aerogel with Superior Electromagnetic Wave Absorption Performance. ACS Nano 2021, 15, 6622–6632. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Shu, T.; Yang, X.; Qiao, M.; Wang, D.; Li, X.; Rao, J.; Liu, Z.; Zhang, Y.; Yang, P.; et al. MOFs-Derived Three-Phase Microspheres: Morphology Preservation and Electromagnetic Wave Absorption. Molecules 2022, 27, 4773. https://doi.org/10.3390/molecules27154773
Yang X, Shu T, Yang X, Qiao M, Wang D, Li X, Rao J, Liu Z, Zhang Y, Yang P, et al. MOFs-Derived Three-Phase Microspheres: Morphology Preservation and Electromagnetic Wave Absorption. Molecules. 2022; 27(15):4773. https://doi.org/10.3390/molecules27154773
Chicago/Turabian StyleYang, Xin, Tie Shu, Xianfeng Yang, Min Qiao, Dashuang Wang, Xinghua Li, Jinsong Rao, Zhaohui Liu, Yuxin Zhang, Pingan Yang, and et al. 2022. "MOFs-Derived Three-Phase Microspheres: Morphology Preservation and Electromagnetic Wave Absorption" Molecules 27, no. 15: 4773. https://doi.org/10.3390/molecules27154773
APA StyleYang, X., Shu, T., Yang, X., Qiao, M., Wang, D., Li, X., Rao, J., Liu, Z., Zhang, Y., Yang, P., & Yao, K. (2022). MOFs-Derived Three-Phase Microspheres: Morphology Preservation and Electromagnetic Wave Absorption. Molecules, 27(15), 4773. https://doi.org/10.3390/molecules27154773







