Synergistic Effects of Plant Growth Regulators and Elicitors on α-Humulene and Zerumbone Production in Zingiber zerumbet Smith Adventitious Root Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Explant Sterilization
2.3. Initiation of Adventitious Root Culture of Z. zerumbet
2.4. Combined Effects of Auxin and Cytokinin on Adventitious Root Growth and Secondary Metabolite Production of Z. zerumbet Root Suspension Culture
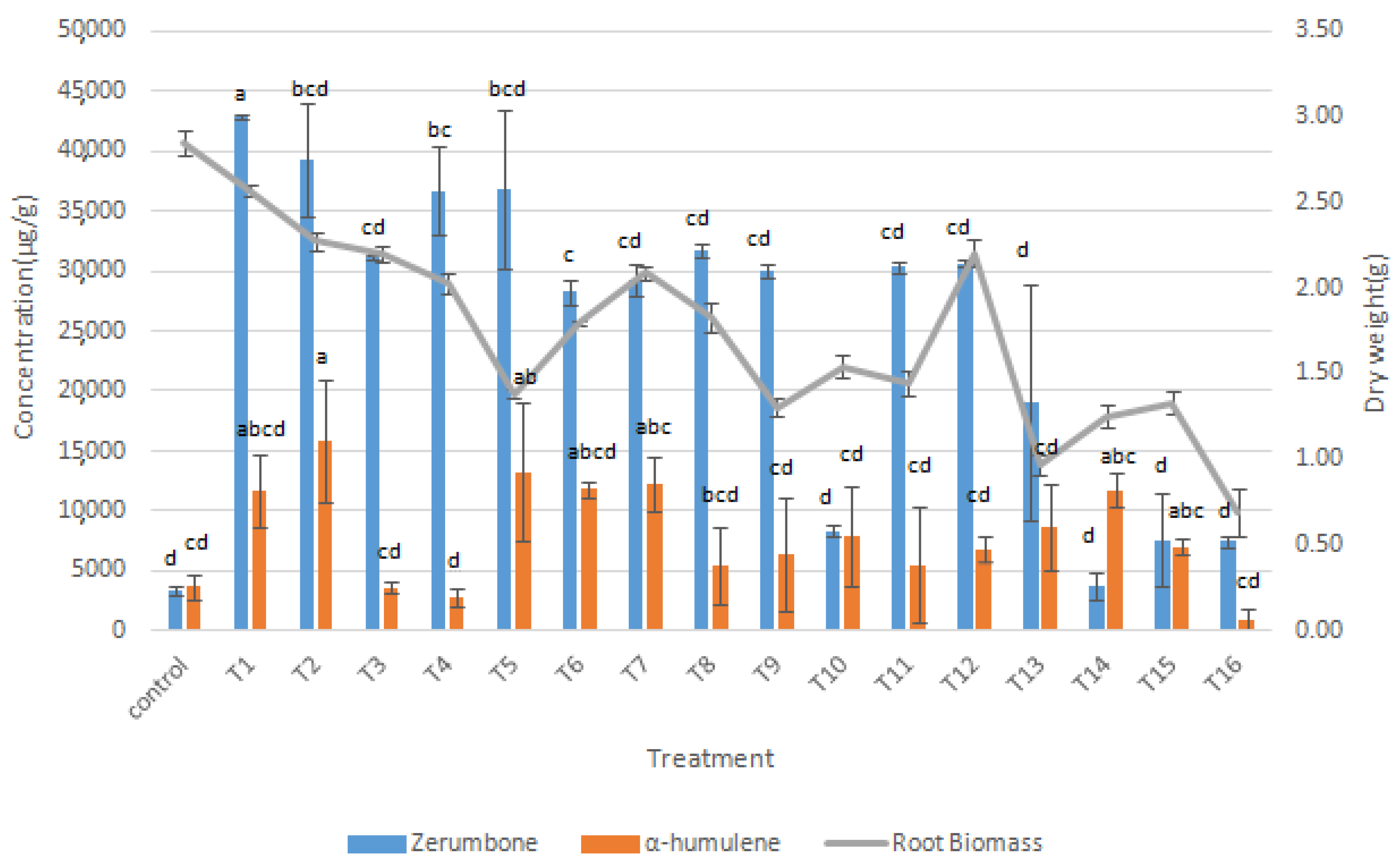
2.5. Synergistic Effects of Elicitors on Root Growth and Bioactive Compounds Production
2.6. Bioactive Compound Extraction
2.7. Measurement of Zerumbone and α-Humulene
2.8. Statistical Analysis
3. Results and Discussion
3.1. Initiation of Adventitious Root Culture (AdRC) of Z. zerumbet
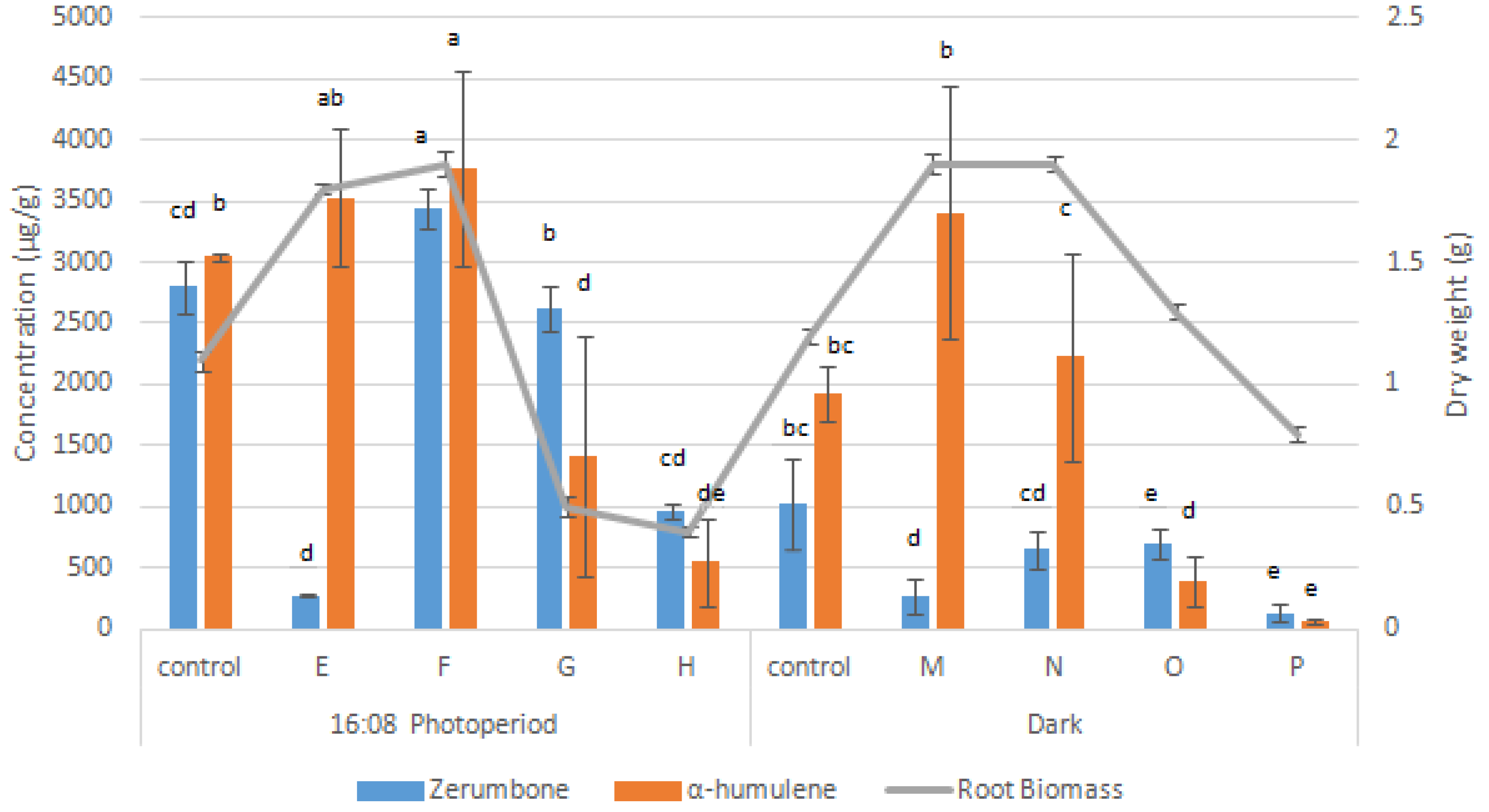
3.2. Combined Effects of Auxin and Cytokinin on Adventitious Root Growth and Secondary Metabolite Production of Z. zerumbet Root Suspension Culture
3.3. Synergistic Effects of Elicitors on Root Growth and Bioactive Compounds Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Umar, M.I.; Asmawi, M.Z.; Sadikun, A.; Altaf, R.; Iqbal, M.A. Phytochemistry and medicinal properties of Kaempferia galanga L. (Zingiberaceae) extracts. Afr. J. Pharm. Pharmacol. 2011, 5, 1638–1647. [Google Scholar] [CrossRef]
- Labban, L. Medicinal and pharmacological properties of turmeric (Curcuma longa): A review. Pharma. Biomed. Sci. J. 2014, 5, 17–23. [Google Scholar]
- Jamal, A.; Javed, K.; Aslam, M.; Jafri, M.A. Gastroprotective effect of cardamom, Elettaria cardamomum Maton. Fruits in rats. J. Ethnopharmacol. 2006, 103, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Archana, C.P.; Geetha, S.P.; Balachandran, I. In vitro microrhizome induction in three high yielding cultivars of Zingiber officinale Rosc. and their phytopathological analysis. Int. J. Adv. Biotechnol. Res. 2013, 4, 296–300. [Google Scholar]
- Jalil, M.; Annuar, M.S.M.; Tan, B.C.; Khalid, N. Effect of selected physicochemical parameters on zerumbone production of Zingiber zerumbet Smith cell suspension culture. Evident-Based Complementary Altern. Med. 2015, 2015, 757514. [Google Scholar]
- Yob, N.J.; Jofrry, S.; Meor Mohd Affandi, M.M.R.; Teh, L.K.; Salleh, M.Z.; Zakaria, Z.A. Zingiber zerumbet (L.) Smith: A Review of Its Ethnomedical, Chemical and Pharmalogical Uses. Evident-Based Complementary Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef]
- Akhtar, N.; Jantan, I.; Arshad, L.; Haque, M.A. Standardized ethanol extract, essential oil and zerumbone of Zingiber zerumbet rhizome suppress phagocytic activity of human neutrophils. BMC Complementary Altern. Med. 2019, 19, 331. [Google Scholar] [CrossRef]
- Girisa, S.; Shabnam, B.; Monisha, J.; Fan, L.; Halim, C.E.; Arfuso, F.; Ahn, K.S.; Sethi, G.; Kunnumakkara, A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules 2019, 24, 734. [Google Scholar] [CrossRef]
- Nalawade, S.M.; Sagare, A.P.; Lee, C.Y.; Kao, C.L.; Tsay, H.S. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003, 44, 79–98. [Google Scholar]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2013, 23, 762. [Google Scholar] [CrossRef]
- Baque, M.A.; Elgirban, A.; Lee, E.J.; Paek, K.Y. Sucrose regulated enhanced induction of anthraquinone, phenolics, flavonoids biosynthesis and activities of antioxidant enzymes in adventitious root suspension cultures of Morinda citrifolia (L.). Acta Physiol. Plant. 2011, 34, 405–415. [Google Scholar] [CrossRef]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G.; Mckhann, H.; Garcion, C.; Vaucheret, H.; Sandberg, G. Auxin and light control of adventitious rooting in Arabidopsis require argonaute1. Plant Cell 2005, 17, 1343–1359. [Google Scholar] [CrossRef]
- Bienaimé, C.; Melin, A.; Bensaddek, L.; Attoumbré, J.; Nava-Saucedo, E.; Baltora-Rosset, S. Effects of plant growth regulators on cell growth and alkaloids production by cell cultures of Lycopodiella inundata. Plant Cell Tissue Organ Cult. (PCTOC) 2015, 123, 523–533. [Google Scholar] [CrossRef]
- George, E.; Hall, M.; Klerk, G.J. The components of plant tissue culture media. II. Organic additions, osmotic and pH effects and support systems. In Plant Progation by Tissue Culture; George, E., Hall, M., Klerk, G.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 115–173. [Google Scholar]
- Kannan, N.; Manokari, M.; Shekhawat, S.M. Enhanced production of anthraquinones and phenolic compounds using chitosan from the adventitious roots of Morinda coreia Buck. and Ham. Ind. Crops Prod. 2020, 148, 112321. [Google Scholar] [CrossRef]
- Tavakoli, F.; Rafieiolhossaini, M.; Ravash, R.; Ebrahimi, M. Subject: UV-B radiation and low temperature promoted hypericin biosynthesis in adventitious root culture of Hypericum perforatum. Plant Signal. Behav. 2020, 15, 7. [Google Scholar] [CrossRef]
- Cui, X.; Murthy, H.N.; Zhang, J. Effect of nutritional factors on the accretion of secondary metabolites in Malaysian ginseng adventitious root cultures. Plant Biotechnol. Rep. 2020, 14, 381–386. [Google Scholar] [CrossRef]
- Krishnan, S.; Siril, E. Elicitor mediated adventitious root culture for the large-scale production of anthraquinones from Oldenlandia umbellata L. Ind. Crops Prod. 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Jiang, X.; Piao, X.C.; Gao, R.; Jin, M.; Jiang, J.; Jin, X.; Lian, M.L. Improvement of bioactive compound accumulation in adventitious root cultures of an endangered plant species, Oplopanax elatus. Acta Physiol. Plant. 2017, 39, 226. [Google Scholar] [CrossRef]
- Baque, M.A.; Shiragi, M.H.K.; Moh, S.H.; Lee, E.J.; Paek, K.Y. Production of biomass and bioactive compounds by adventitious root suspension cultures of Morinda citrifolia (L.) in a liquid-phase airlift balloon-type bioreactor. Cell Dev. Biol. Plant 2013, 49, 737–749. [Google Scholar] [CrossRef]
- Cui, X.H.; Chakrabarty, D.; Lee, E.J.; Paek, K.Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef]
- Saiman, M.Z.; Mustafa, N.R.; Schulte, A.E.; Verpoorte, R.; Choi, Y.H. Induction, characterization, and NMR-based metabolic profiling of adventitious root cultures from leaf explants of Gynura procumbens. Plant Cell Tissue Organ Cult. 2012, 109, 465–475. [Google Scholar] [CrossRef]
- Ling, A.P.K.; Kok, K.M.; Hussein, S. Adventitious rooting of Orthosiphon stamineus in response to sucrose concentrations andmedium pH. Am. Eurasian J. Sustain. Agric. 2009, 3, 93–100. [Google Scholar]
- Mona, M.I.; Mohamed, E.B.; Rady, K.; Mohamed, R. In-vitro adventitious root production of Cichorium endivia L. and antioxidants, total phenolic, and total flavonoids assessments. Egypt Pharm. J. 2019, 18, 216–227. [Google Scholar]
- Sevik, H.; Guney, K. Effects of IAA, IBA, NAA, and GA3 on rooting and morphological features of Melissa officinalis L. stem cuttings. Sci. World J. 2013, 2013, 909507. [Google Scholar] [CrossRef]
- Naseem, M.; Kaltdorf, M.; Dandekar, T. The nexus between growth and defence signalling: Auxin and cytokinin modulate plant immune response pathways. J. Exp. Bot. 2015, 66, 4885–4896. [Google Scholar] [CrossRef] [PubMed]
- Lulu, T.; Park, S.Y.; Ibrahim, R.; Paek, K.Y. Production of biomass and bioactive compounds from adventitious roots by optimization of culturing conditions of Eurycoma longifolia in balloon-type bubble bioreactor system. J. Biosci. Bioeng. 2015, 119, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Silja, P.K.; Satheeshkumar, K. Establishment of adventitious root cultures from leaf explants of Plumbago rosea and enhanced plumbagin production through elicitation. Ind. Crop Prod. 2015, 76, 479–486. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, M.; Wen, W.; Yu, R. Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn. Rev. 2015, 9, 24–28. [Google Scholar]
- Anaya, F.; Fghire, R.; Wahbi, S.; Loutfi, K. Antioxidant enzymes and physiological traits of Vicia faba L. as affected by salicylic acid under salt stress. J. Mater. Environ. Sci. 2017, 8, 2549–2563. [Google Scholar]
- Faizal, A.; Sari, A.V. Enhancement of saponin accumulation in adventitious root culture of Javanese ginseng (Talinum paniculatum Gaertn.) through methyl jasmonate and salicylic acid elicitation. Afr. J. Biotechnol. 2019, 18, 130–135. [Google Scholar]
- Baque, M.A.; Humayun, M.; Shiragi, M.; Lee, E.J.; Paek, K.Y. Elicitor effect of chitosan and pectin on the biosynthesis of anthraquinones, phenolics and flavonoids in adventitious root suspension cultures of Morinda citrifolia (L.). Aust. J. Crop Sci. 2012, 6, 1349–1355. [Google Scholar]
- Sukito, A.; Tachibana, S. Effect of methyl jasmonate and salycilic acid synergism on enhancement of bilobalide and ginkgolide production by immobilized cell cultures of Ginkgo biloba. Bioresour. Bioprocess. 2016, 3, 24. [Google Scholar] [CrossRef][Green Version]
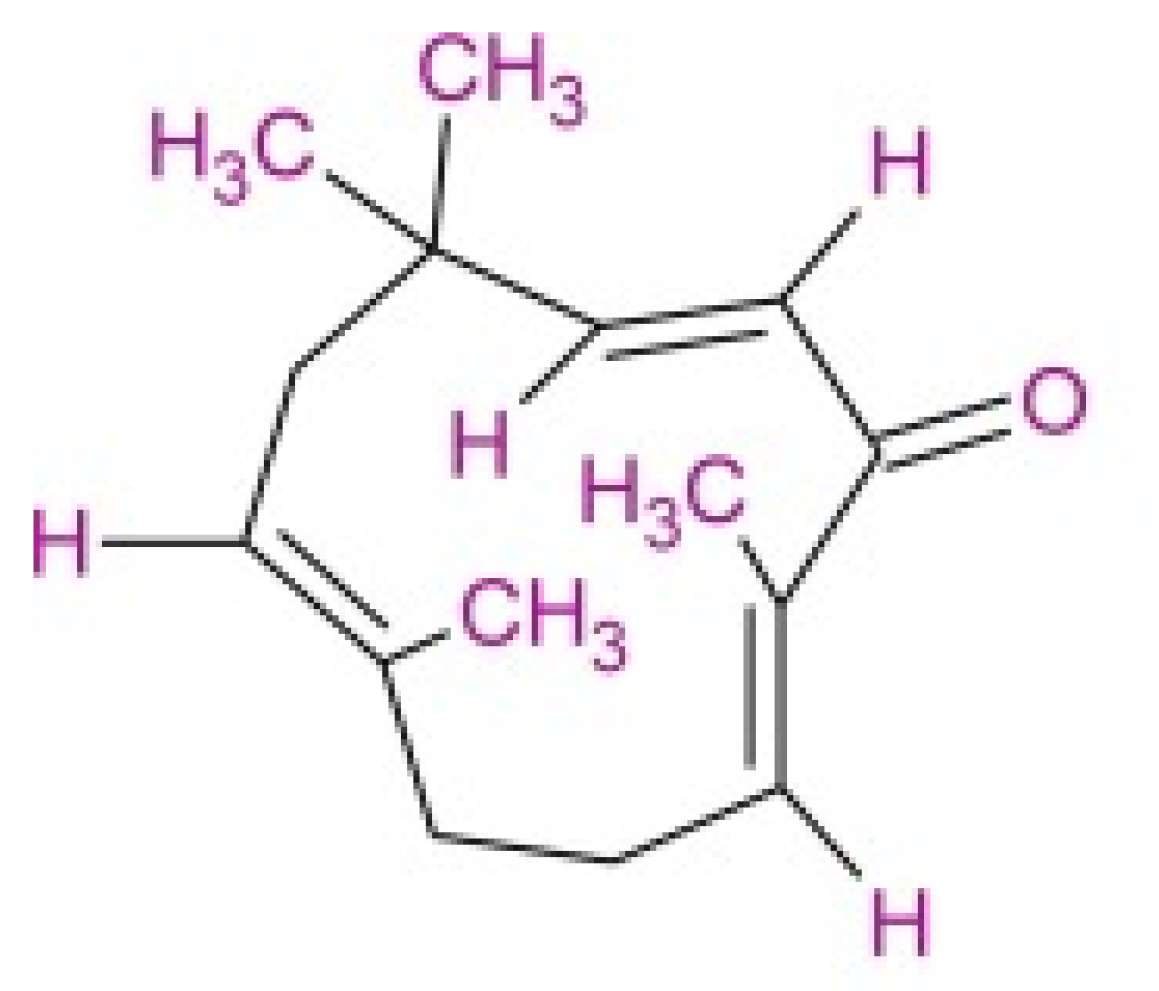
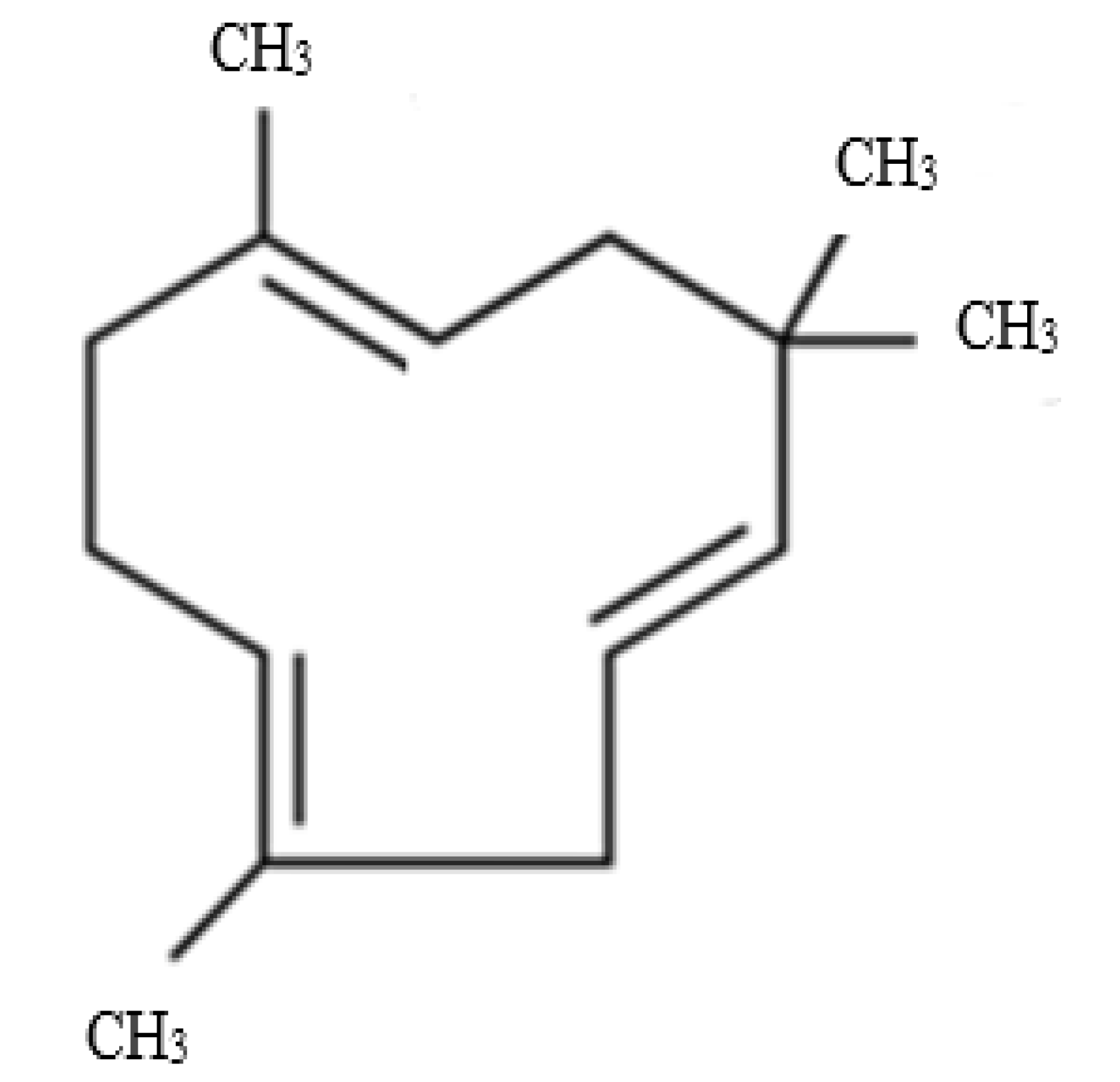
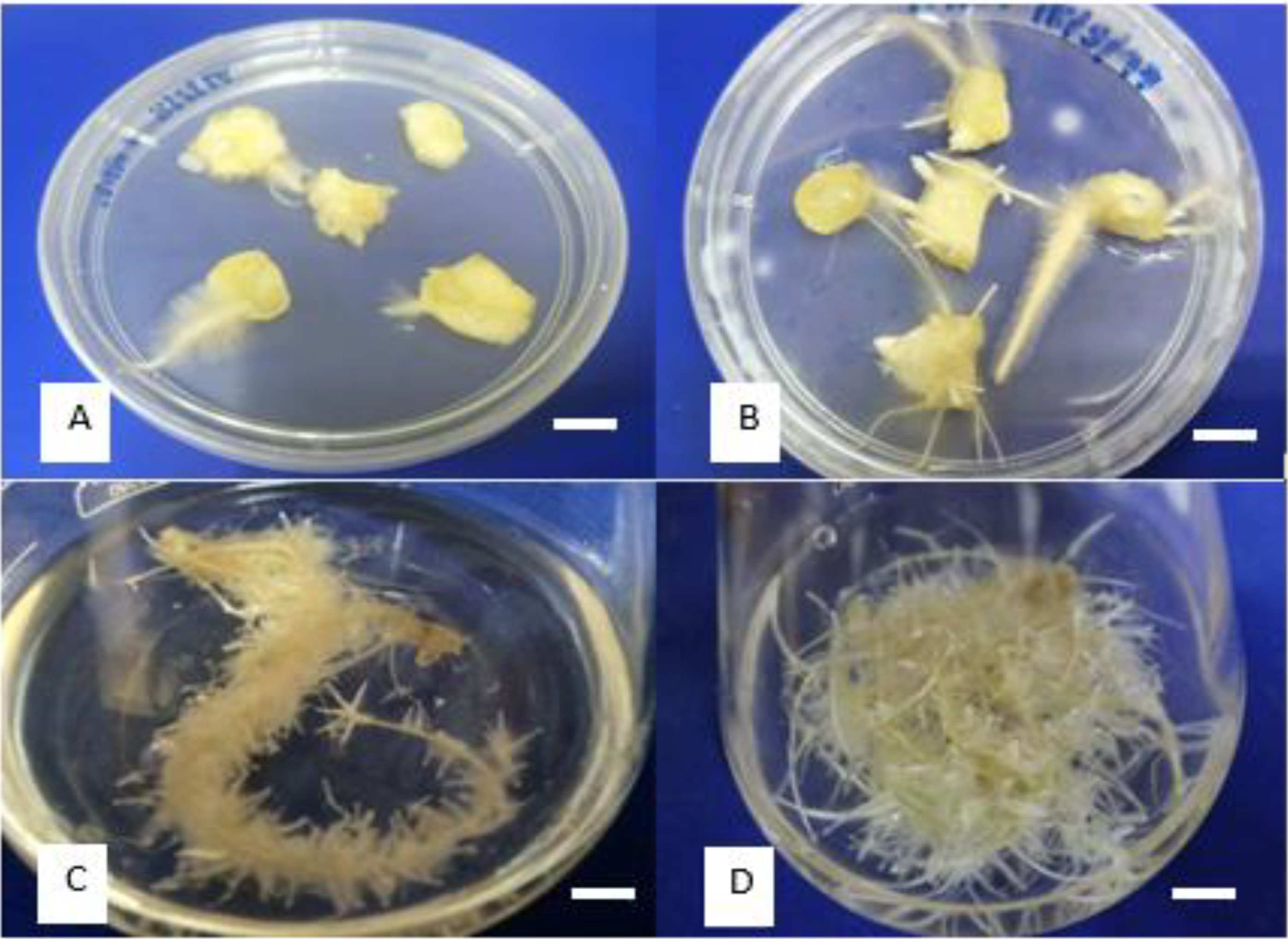
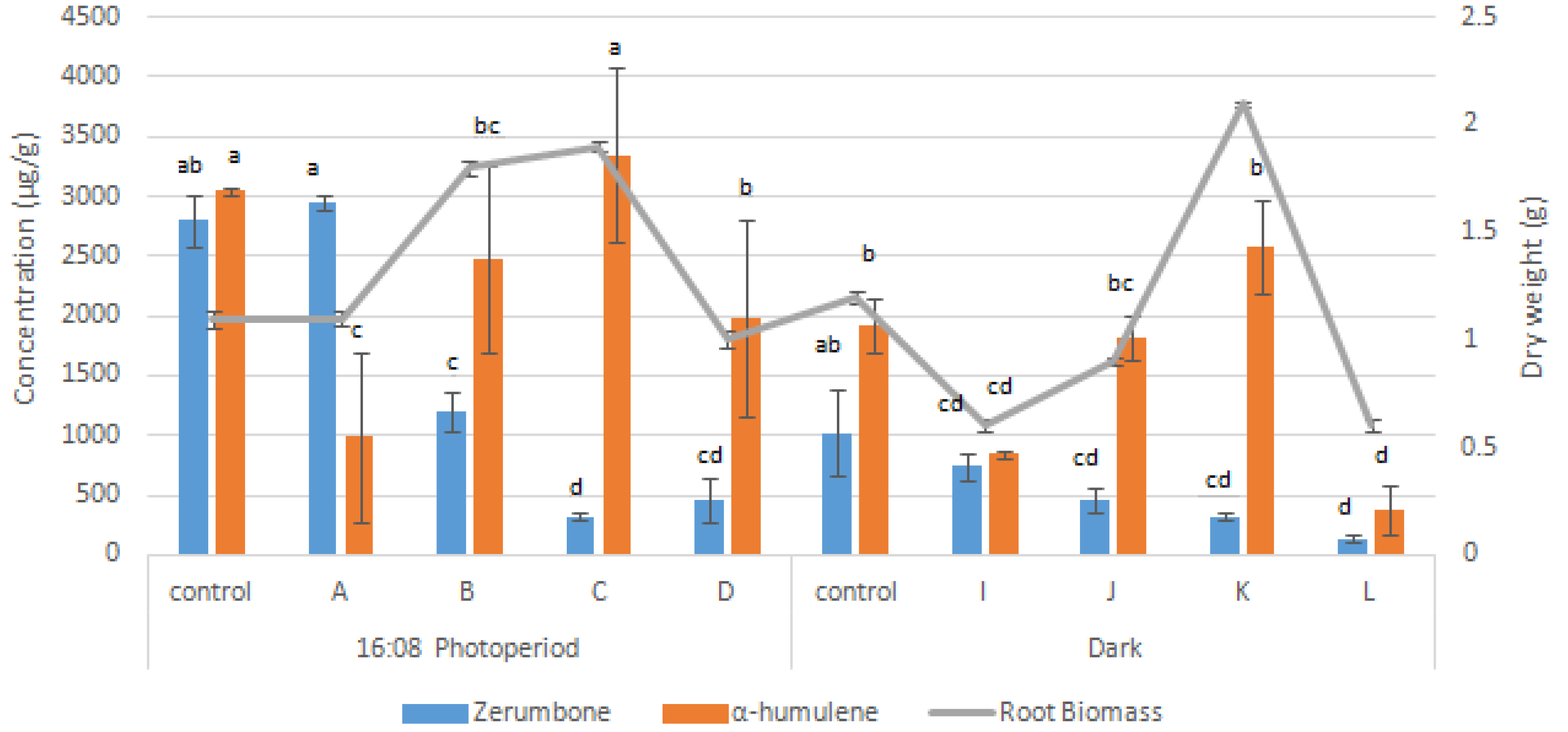

| Plant Growth Regulators (mg/L) | Percentage (%) | Root Length (cm) | Roots per Explant |
|---|---|---|---|
| Control | 13 de | 0.4 ± 0.1 e | 2.0 ± 0.6 e |
| 0.5 NAA + 0.5 IBA | 20 d | 0.8 ± 0.4 ed | 1.6 ± 1.2 ef |
| 0.5 NAA + 1.0 IBA | 60 b | 0.3 ± 0.1 e | 4.7 ± 1.5 cde |
| 0.5 NAA + 2.0 IBA | 67 b | 2.1 ± 0.5 bcde | 6.7 ± 2.1 c |
| 1.0 NAA + 0.5 IBA | 47 c | 1.7 ± 0.3 bcde | 4.7 ± 3.1 cde |
| 1.0 NAA + 1.0 IBA | 93 a | 3.3 ± 0.7 b | 18.0 ± 1.0 a |
| 1.0 NAA + 2.0 IBA | 100 a | 7.3 ± 1.3 a | 11.7 ± 1.5 b |
| 2.0 NAA + 0.5 IBA | 40 c | 2.6 ± 0.8 bc | 5.7 ± 2.1 cd |
| 2.0 NAA + 1.0 IBA | 73 b | 2.2 ± 0.3 bcd | 6.3 ± 1.5 c |
| 2.0 NAA + 2.0 IBA | 87 a | 1.0 ± 0.6 cde | 5.3 ± 2.1 cd |
| Light Regime | PGR | Treatment | Concentration (mg/L) | Fresh Weight (g) | Dry Weight (g) | Specific Growth Rate (µ), Day−1 |
|---|---|---|---|---|---|---|
| 16:08 | Control (NAA) | Control 16:08 | 1 | 3.9 ± 0.0 e | 1.2 ± 0.0 b | 1.4 ± 0.0 c |
| Control + IBA | A | 1 | 2.0 ± 0.1 h | 0.6 ± 0.0 c | 0.8 ± 0.0 d | |
| B | 3 | 3.0 ± 0.1 g | 0.9 ± 0.0 bc | 1.2 ± 0.0 cd | ||
| C | 5 | 6.9 ± 0.1 a | 2.1 ± 0.0 a | 2.1 ± 0.0 a | ||
| D | 7 | 1.9 ± 0.1 i | 0.6 ± 0.0 c | 0.7 ± 0.0 d | ||
| Control + BAP | E | 1 | 5.9 ± 0.1 c | 1.8 ± 0.0 ab | 1.9 ± 0.0 a | |
| F | 3 | 6.8 ± 0.1 a | 1.9 ± 0.1 a | 2.0 ± 0.1 a | ||
| G | 5 | 1.8 ± 0.1 i | 0.5 ± 0.0 c | 0.7 ± 0.0 d | ||
| H | 7 | 1.6 ± 0.1 i | 0.4 ± 0.0 c | 0.5 ± 0.0 e | ||
| Dark | Control (NAA) | Control dark | 1 | 3.6 ± 0.1 f | 1.1 ± 0.0 b | 1.4 ± 0.0 c |
| Control + IBA | I | 1 | 3.6 ± 0.1 f | 1.1 ± 0.0 b | 1.4 ± 0.0 c | |
| J | 3 | 6.3 ± 0.1 bc | 1.9 ± 0.0 a | 1.9 ± 0.0 a | ||
| K | 5 | 6.7 ± 0.1 a | 2.0 ± 0.0 a | 2.0 ± 0.0 a | ||
| L | 7 | 3.4 ± 0.1 fg | 1.0 ± 0.0 b | 1.3 ± 0.0 c | ||
| Control + BAP | M | 1 | 6.2 ± 0.0 bc | 1.9 ± 0.0 a | 1.9 ± 0.0 a | |
| N | 3 | 6.4 ± 0.1 b | 1.9 ± 0.0 a | 1.9 ± 0.0 a | ||
| O | 5 | 4.4 ± 0.1 d | 1.3 ± 0.0 b | 1.6 ± 0.0 b | ||
| P | 7 | 2.4 ± 0.1 h | 0.8 ± 0.0 c | 1.0 ± 0.0 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwakil, N.H.; Mohamad Annuar, M.S.; Jalil, M. Synergistic Effects of Plant Growth Regulators and Elicitors on α-Humulene and Zerumbone Production in Zingiber zerumbet Smith Adventitious Root Cultures. Molecules 2022, 27, 4744. https://doi.org/10.3390/molecules27154744
Alwakil NH, Mohamad Annuar MS, Jalil M. Synergistic Effects of Plant Growth Regulators and Elicitors on α-Humulene and Zerumbone Production in Zingiber zerumbet Smith Adventitious Root Cultures. Molecules. 2022; 27(15):4744. https://doi.org/10.3390/molecules27154744
Chicago/Turabian StyleAlwakil, Nurul Huda, Mohamad Suffian Mohamad Annuar, and Mahanom Jalil. 2022. "Synergistic Effects of Plant Growth Regulators and Elicitors on α-Humulene and Zerumbone Production in Zingiber zerumbet Smith Adventitious Root Cultures" Molecules 27, no. 15: 4744. https://doi.org/10.3390/molecules27154744
APA StyleAlwakil, N. H., Mohamad Annuar, M. S., & Jalil, M. (2022). Synergistic Effects of Plant Growth Regulators and Elicitors on α-Humulene and Zerumbone Production in Zingiber zerumbet Smith Adventitious Root Cultures. Molecules, 27(15), 4744. https://doi.org/10.3390/molecules27154744






