Five New Polyoxypregnane Glycosides from the Vines of Aspidopterysobcordata and Their Antinephrolithiasis Activity
Abstract
:1. Introduction
2. Results
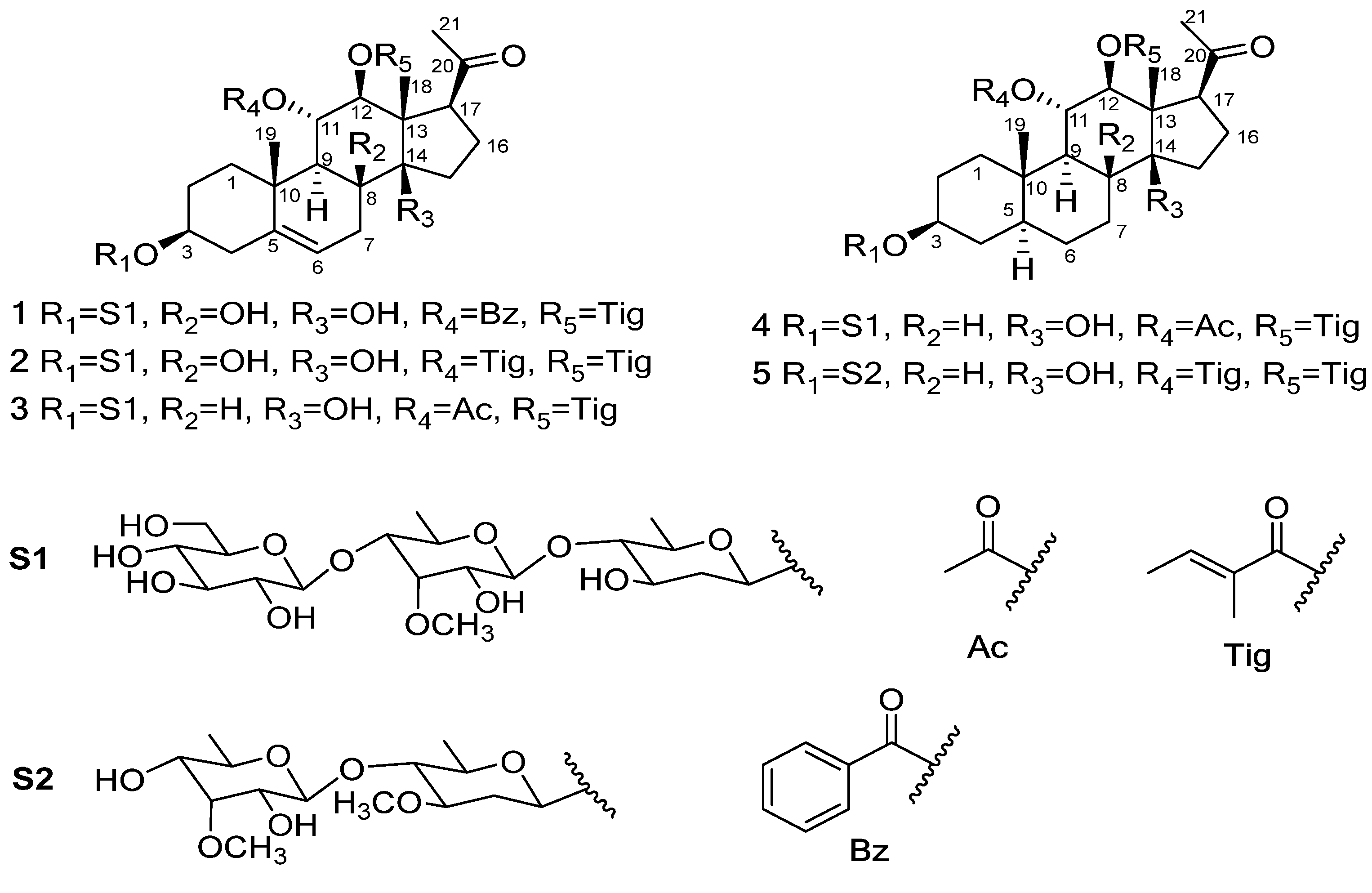
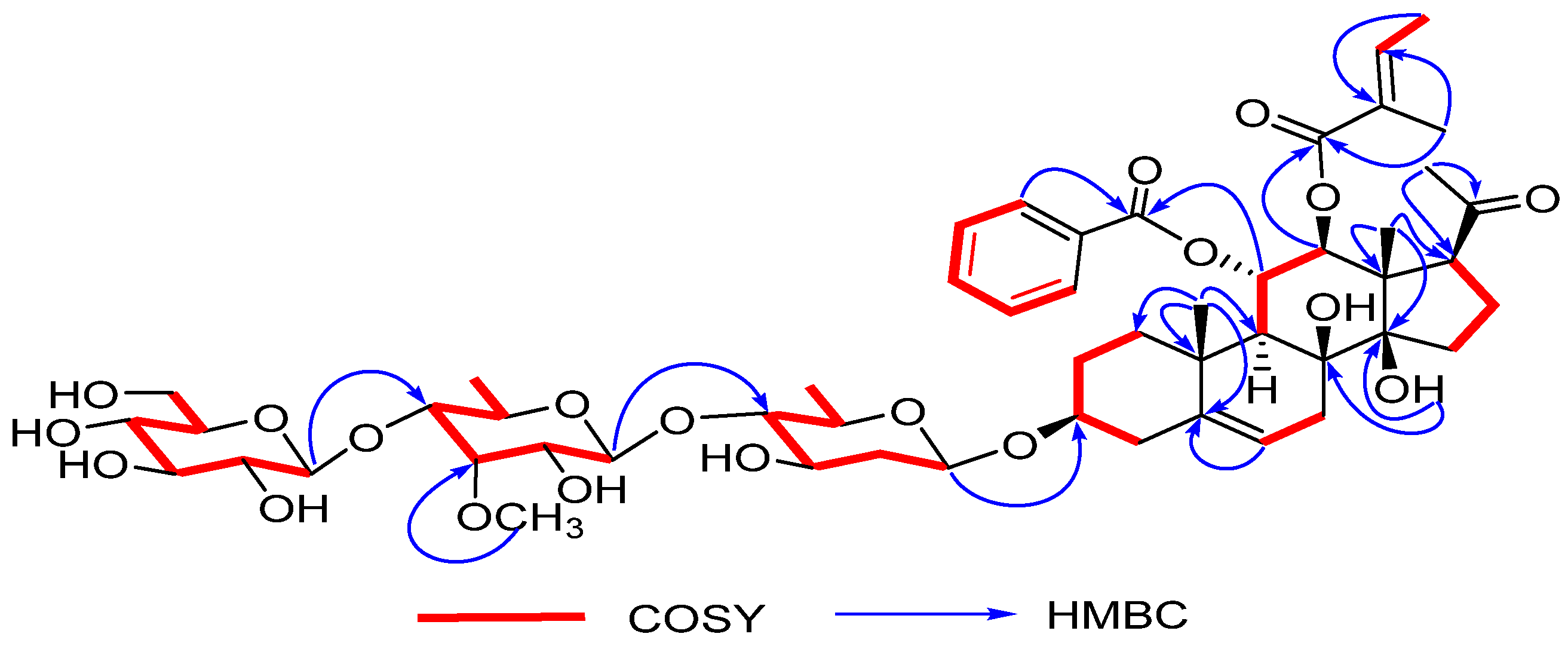
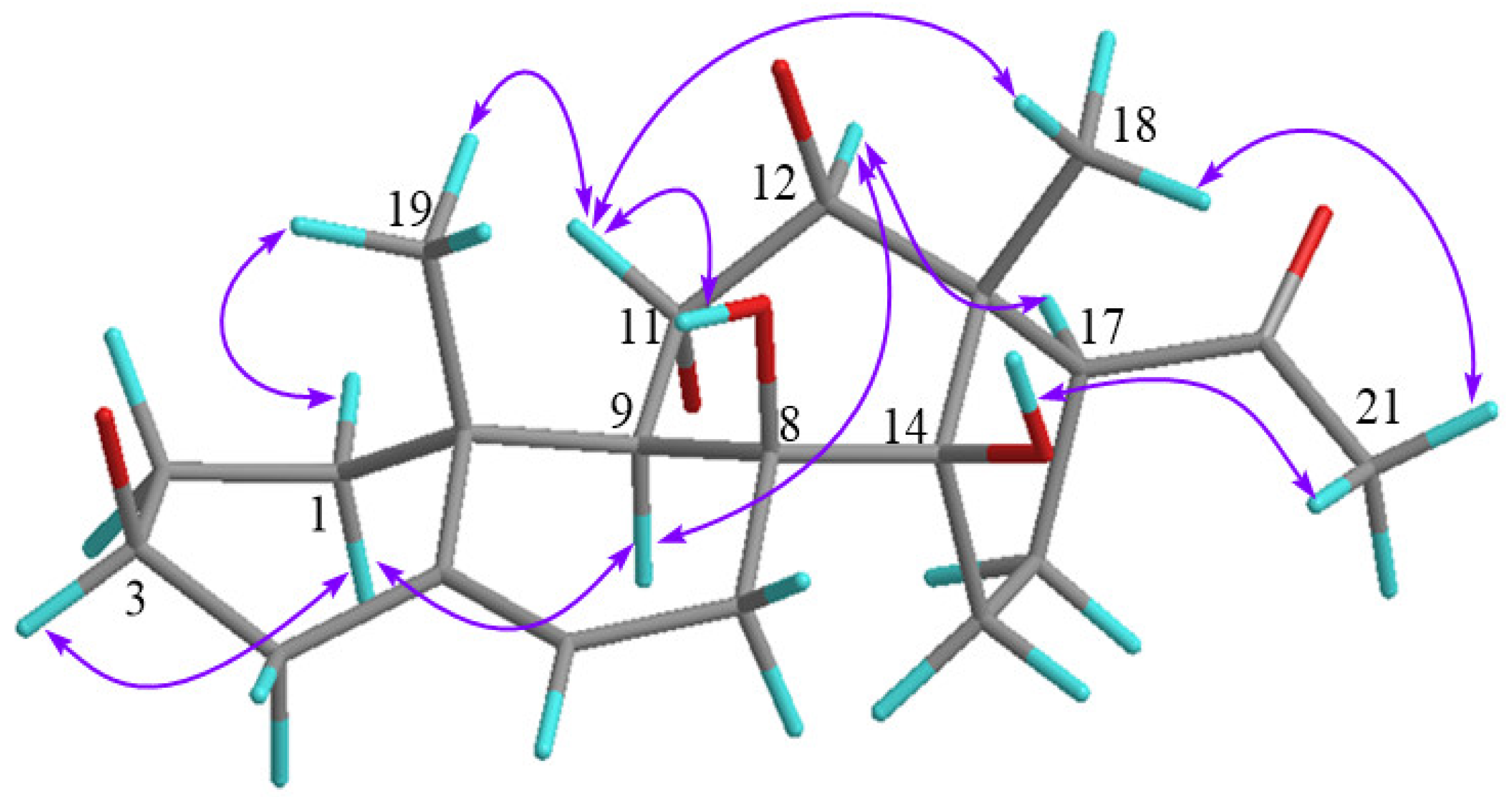
2.1. Structure Determination
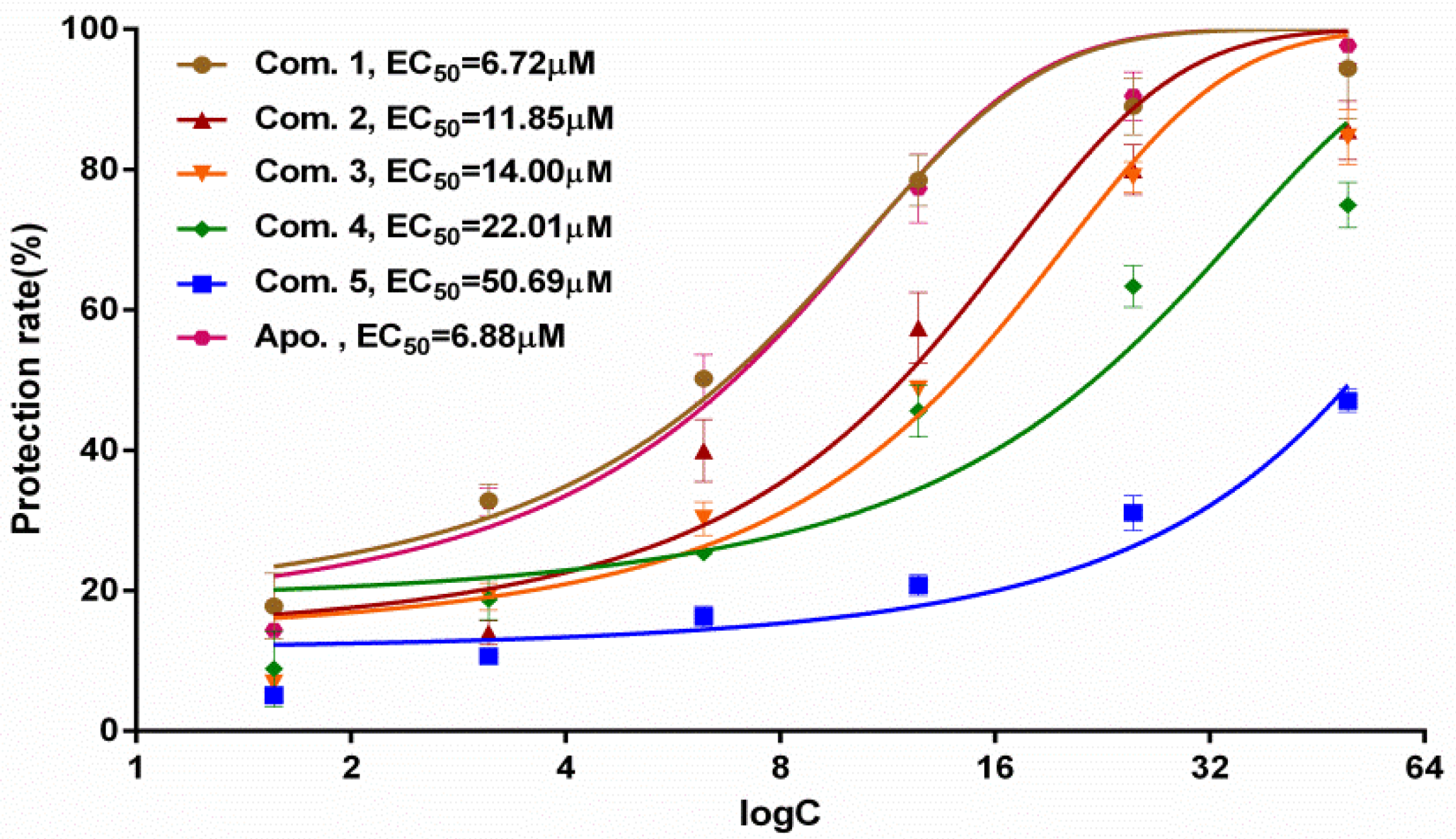
2.2. Antinephrolithiasis Activity
3. Materials and Methods
3.1. General Experimental Materials
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Characterization of Compounds 1–5
3.5. Compound Hydrolysis
3.6. Cytotoxicity Assay
3.7. Antinephrolithiasis Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Reichstein, T. Cardenolid-und pregnanglykoside. Naturwissenwdhaftern 1967, 54, 53–67. [Google Scholar]
- Gupta, M.; Gupta, V.; Khare, N.N. Structural studies of biologically importantsteroidal pregnane glycosides from medicinal plants. Trends Carbohydr. Res. 2015, 7, 53–97. [Google Scholar]
- Si, Y.; Sha, X.S.; Shi, L.L.; Wei, H.Y.; Jin, Y.X.; Ma, G.X.; Zhang, J. Review on pregnaneglycosides and their biological activities. Phytochem. Lett. 2022, 47, 1–17. [Google Scholar]
- Yan, Y.; Zhang, J.X.; Liu, K.X.; Huang, T.; Yan, C.; Huang, L.J.; Liu, S.; Mu, S.Z.; Hao, X.J. Seco-pregnane steroidal glycosides from the roots of Cynanchumatratum andtheir anti-TMV activity. Fitoterapia 2014, 97, 50–63. [Google Scholar]
- Lee, C.L.; Hwang, T.L.; He, W.J.; Tsai, Y.H.; Yen, C.T.; Yen, H.F.; Chen, C.J.; Chang, W.Y.; Wu, Y.C. Anti-neutrophilic inflammatory steroidal glycosides from Solanumtorvum. Phytochemistry 2013, 95, 315–321. [Google Scholar]
- Plaza, A.; Perrone, A.; Balestrieri, M.L.; Felice, F.; Balestrieri, C.; Hamed, A.I.; Pizza, C.; Piacente, S. New unusual pregnane glycosides with antiproliferative activity from Solenostemma argel. Steroids 2005, 70, 594–603. [Google Scholar]
- Ounaissia, K.; Pertuit, D.; Mitaine-offer, A.C.; Miyamoto, T.; Tanaka, C.; Delemasure, S.P.; Dutartre, P.; Smati, D.; Lacaille-dubois, M.A. New pregnane and phenolic glycosides from Solenostemmaargel. Fitoterapia 2016, 114, 98–104. [Google Scholar]
- Song, C.W.; Lung, P.K.; Qin, X.J.; Cheng, G.G.; Gu, J.L.; Liu, Y.P.; Luo, X.D. New antimicrobial pregnane glycosides from the stem of Ecdysantherarosea. Fitoterapia 2014, 99, 267–275. [Google Scholar]
- Zhao, D.; Feng, B.; Chen, S.; Chen, G.; Li, Z.; Li, X.; Sang, X.; An, X.; Wang, H.; Pei, Y. C21 steroidal glycosides from the roots of Cynanchum paniculatum. Fitoterapia 2016, 113, 51–57. [Google Scholar]
- Huang, L.J.; Wang, B.; Zhang, J.X.; Yan, C.; Mu, S.Z.; Hao, X.J. Studies oncytotoxic pregnane sapogenins from Cynanchumwilfordii. Fitoterapia 2015, 101, 107–116. [Google Scholar]
- Liu, X.; Zhang, Y.; Huang, W.; Luo, J.; Li, Y.; Tan, W.; Zhang, A. Development of high potent and selective Bcl-2 inhibitors bearing the structural elements of natural product artemisinin. Eur. J. Med. Chem. 2018, 159, 149–165. [Google Scholar]
- Song, J.; Dai, R.; Deng, Y.; Lv, F. Rapid structure prediction by HPLC-ESI-MS of twenty-five polyoxypregnane tetraglycosides from Dregea Sinensis with NMR confirmation of eight structures. Phytochemistry 2018, 147, 147–157. [Google Scholar]
- Zhang, X.; Zhou, Y.; Zuo, J.; Yu, B. Total synthesis of periploside A, aunique pregnane hexasaccharide with potent immunosuppressive effects. Nat. Commun. 2015, 6, 5879. [Google Scholar]
- Yao, S.; To, K.K.-W.; Wang, Y.-Z.; Yin, C.; Chai, S.; Ke, C.Q.; Lin, G.; Ye, Y. Polyoxypregnane steroids from the stems of Marsdenia tenacissima. J. Nat. Prod. 2014, 77, 2044–2053. [Google Scholar]
- Sun, D.F.; Sun, J.Y.; Fan, H.X.; Yao, Q.Q. Advances in studies on C21 steroidal glycosides of plants in Asclepiadaceae. Chin. Trad. Herbal Drugs 2014, 45, 1491–1495. [Google Scholar]
- Liu, S.Z.; Chen, Z.H.; Wu, J.; Wang, L.Y.; Wang, H.M.; Zhao, W.M. Appetite suppressing pregnane glycosides from the roots of Cynanchum auriculatum. Phytochemistry 2013, 93, 144–153. [Google Scholar]
- Tatsuno, S.; Yokosuka, A.; Hatauma, F.; Mashiko, Y.; Mimaki, Y. Pregnaneglycosides from the bark of Marsdenia cundurango and their cytotoxic activity. J. Nat. Med. 2019, 73, 93–103. [Google Scholar]
- Guo, H.W.; Tian, Y.G.; Liu, Y.H.; Huang, J.; Wang, J.X.; Long, H.; Wei, H. Discovery of polyoxypregnane derivatives from Aspidopterys obcordata with their potential antitumor activity. Front. Chem. 2021, 9, 799911. [Google Scholar]
- Minpei, K.; Satoshi, K.; Daichi, M.; Yukiko, M.; Hiroshi, S.; Yoshihiro, M. Aestivalosides A–L, twelve pregnane glycosides from the seeds of Adonis aestivalis. Phytochemistry 2018, 150, 75–84. [Google Scholar]
- Zhao, D.; Su, S.S.; Chen, S.F.; Lu, X.; Chen, G.; Wang, Y.B.; Su, G.Y.; Pei, Y.H. Two new C21 steroidal glycosides isolated from Cynanchum komarovii. Chin. J. Nat. Med. 2018, 16, 610–614. [Google Scholar]
- Wu, R.; Ye, Q.; Chen, N.; Zhang, G. Study on the chemical constituents of Aspidopterys obcordata Hemsl. Nat. Prod. Rsc. Dev. 2001, 13, 14–16. [Google Scholar]
- Li, H.; Peng, C.Z.; Guan, Y.H.; Niu, Y.F.; Zhang, L.X. Resources investigation on Aspidopterys obcordata. Shi Zhen Guo Yi Guo Yao 2011, 22, 2999–3000. [Google Scholar]
- Li, Y.; Ma, G.; Lv, Y.; Su, J.; Li, G.; Chen, X. Efficacy of Obcordata A from Aspidopterys obcordata on Kidney Stones by Inhibiting NOX4 Expression. Molecules 2019, 24, 1957. [Google Scholar]
- Hu, M.G.; Li, Y.H.; Sun, Z.C.; Huo, X.W.; Zhu, N.L.; Sun, Z.H.; Liu, Y.Y.; Wu, H.F.; Xu, X.D.; Ma, G.X.; et al. New polyoxypregnane glycosides from Aspidopterys obcordata vines with antitumor activity. Fitoterapia 2018, 129, 203–209. [Google Scholar]
- Li, S.; Liu, Q.-Q. A study on the mechanism of the protective effect of GuangeFang on sepsis-associated acute kidney injury. World J. Tradit. Chin. Med. 2021, 7, 414–418. [Google Scholar]
- Wiessner, J.H.; Hung, L.Y.; Mandel, N.S. Crystal attachment to injured renal collecting duct cells: Influence of urine proteins and pH. Kidney Int. 2003, 63, 1313–1320. [Google Scholar]
- Song, M.F.; Li, Y.H.; Zhang, Z.L.; Lv, Y.N.; Li, X.L.; Li, G. Inhibiting effect of Aspidopterys obcordata Hemsl on renal calculus. Chin. J. New Drugs 2015, 24, 1047–1052. [Google Scholar]
- Han, Y.; Hao, M.M.; Ruan, J.Y.; Bai, Y.; Wang, T.; Zhang, Y. Research Progress on Chemical Constituents and Pharmacological Effects of Aspidopterys Obcordata Hemsl. Chin. J. Ethomed. Ethnopharm. 2020, 29, 68–72. [Google Scholar]
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.17 (m), 1.81 (m) | 1.17 (m), 1.81 (m) | 1.17 (m), 1.81 (m) | 1.20 (m), 1.71 (m) | 0.92 (m), 1.90 (m) |
| 2 | 1.71 (m), 1.99 (m) | 1.71 (m), 2.00 (m) | 1.61 (m), 2.01 (m) | 1.75 (m), 1.99 (m) | 1.59 (m), 2.03 (m) |
| 3 | 3.01 (m) | 3.06 (m) | 3.06 (m) | 3.01 (m) | 3.26 (m) |
| 4 | 2.21 (m), 2.43 (m) | 2.21 (m), 2.42 (m) | 2.21 (m), 2.31 (m) | 1.59 (m), 1.62 (m) | 1.44 (m), 2.09 (m) |
| 5 | - | - | - | 1.18 (m) | 1.90 (m) |
| 6 | 5.29 (d, J = 5.4 Hz) | 5.17 (d, J = 5.4 Hz) | 5.45 (d, J = 5.4 Hz) | 1.07 (m), 1.61 (m) | 1.04 (m), 1.66 (m) |
| 7 | 1.81 (m), 2.24 (m) | 1.88 (m), 2.21 (m) | 1.17 (m), 2.11 (m) | 1.42 (m), 1.60 (m) | 1.36 (m), 1.37 (m) |
| 8 | - | - | 1.80 (m) | 2.06 (m) | 2.06 (m) |
| 9 | 2.01 (d, J = 10.8 Hz) | 1.96 (d, J = 10.2 Hz) | 1.63(d, J = 10.2 Hz) | 1.65 (d, J = 10.8 Hz) | 1.66 (d, J = 10.2 Hz) |
| 10 | - | - | - | - | - |
| 11 | 5.89 (t, J = 10.8 Hz) | 5.70 (t, J = 10.2 Hz) | 5.25 (t, J = 10.2 Hz) | 5.16 (t, J = 10.2 Hz) | 5.21 (t, J = 10.8 Hz) |
| 12 | 5.02 (d, J = 10.8 Hz) | 4.89 (d, J = 10.2 Hz) | 4.78 (d, J = 10.2 Hz) | 4.97 (d, J = 10.2 Hz) | 4.75 (d, J = 10.8 Hz) |
| 13 | - | - | - | - | - |
| 14 | - | - | - | - | - |
| 15 | 1.81 (m), 2.21 (m) | 1.85 (m), 2.21 (m) | 1.21 (m), 1.31 (m) | 1.41 (m), 1.53 (m) | 1.49 (m), 1.56 (m) |
| 16 | 1.80 (m), 2.11 (m) | 1.79 (m), 2.11 (m) | 1.39 (m), 2.41 (m) | 1.81 (m), 2.19 (m) | 1.81 (m), 2.12 (m) |
| 17 | 2.70 (m) | 2.89 (m) | 3.34 (m) | 3.35 (m) | 3.34 (m) |
| 18 | 1.14 (s) | 1.22 (s) | 0.96 (s) | 0.81(s) | 0.82 (s) |
| 19 | 1.35 (s) | 1.43 (s) | 1.01 (s) | 0.91 (s) | 0.92 (s) |
| 20 | - | - | - | - | - |
| 21 | 2.13 (s) | 2.15 (s) | 2.06 (s) | 2.10 (s) | 2.07 (s) |
| 11-O | Bz | Tig | Ac | Ac | Tig |
| 2 | - | - | 1.80 (s) | 1.78 (s) | - |
| 3 | 7.80 (dd, J = 7.2, 1.2 Hz) | 6.61 (q, J = 7.2 Hz) | - | - | 6.58 (q, J = 7.2 Hz) |
| 4 | 7.49 (t, J = 7.2 Hz) | 1.73 (d, J = 7.2 Hz) | - | - | 1.69 (d, J = 7.2 Hz) |
| 5 | 7.62 (t, J = 7.2 Hz) | 1.61 (s) | - | - | 1.60 (s) |
| 6 | 7.49 (t, J = 7.2 Hz) | - | - | - | - |
| 7 | 7.80 (dd, J = 7.2, 1.2 Hz) | - | - | - | - |
| 12-O | Tig | Tig | Tig | Tig | Tig |
| 2 | - | - | - | - | - |
| 3 | 6.49 (q, J = 7.2 Hz) | 6.69 (q, J = 7.2 Hz) | 6.83 (q, J = 7.2 Hz) | 6.81 (q, J = 7.2 Hz) | 6.68 (q, J = 7.2 Hz) |
| 4 | 1.53 (d, J = 7.2 Hz) | 1.74 (d, J = 7.2 Hz) | 1.80 (d, J = 7.2 Hz) | 1.79 (d, J = 7.2 Hz) | 1.73 (d, J = 7.2 Hz) |
| 5 | 1.35 (s) | 1.69 (s) | 1.78 (s) | 1.76 (s) | 1.60 (s) |
| Oli/Ole-1 | 4.60 (d, J = 9.6 Hz) | 4.62 (d, J = 9.6 Hz) | 4.78 (d, J = 9.6 Hz) | 4.65 (t, J = 9.6 Hz) | 4.56 (t, J = 9.6 Hz) |
| 2 | 1.82 (m), 2.12 (m) | 1.82 (m), 2.23 (m) | 1.82 (m), 2.21 (m) | 1.42 (m), 2.21 (m) | 1.91 (m), 2.25 (m) |
| 3 | 3.01 (m) | 3.42 (m) | 3.52 (m) | 3.02 (m) | 3.47 (m) |
| 4 | 3.85 (m) | 3.86 (m) | 3.21 (m) | 3.82 (m) | 3.37 (m) |
| 5 | 3.14 (m) | 3.01 (m) | 3.01 (m) | 3.00 (m) | 3.45 (m) |
| 6 | 1.22 (d, J = 6.0 Hz) | 1.19 (d, J = 6.0 Hz) | 1.24 (d, J = 6.0 Hz) | 1.19 (d, J = 6.0 Hz) | 1.09 (d, J = 6.0 Hz) |
| -OCH3 | - | - | - | - | 3.26 (s) |
| Allo-1 | 4.42 (d, J = 7.8 Hz) | 4.45 (d, J = 8.4 Hz) | 4.48 (d, J = 7.8 Hz) | 4.46 (d, J = 7.8 Hz) | 4.55 (d, J = 7.8 Hz) |
| 2 | 3.21 (m) | 3.22 (m) | 3.01 (m) | 3.24 (m) | 3.15 (m) |
| 3 | 3.82 (m) | 3.81 (m) | 3.81 (m) | 3.85 (m) | 3.04 (m) |
| 4 | 3.38 (m) | 3.61 (m) | 3.33 (m) | 3.25 (m) | 3.34 (m) |
| 5 | 3.61 (m) | 3.61 (m) | 3.61 (m) | 3.27 (m) | 3.26 (m) |
| 6 | 1.21 (d, J = 6.0 Hz) | 1.23 (d, J = 6.0 Hz) | 1.20 (d, J = 6.0 Hz) | 1.23 (d, J = 6.0 Hz) | 1.23 (d, J = 6.6 Hz) |
| 3-OCH3 | 3.74 (s) | 3.74 (s) | 3.46 (s) | 3.46 (s) | 3.47 (s) |
| Glc-1 | 4.21(d, J = 7.8 Hz) | 4.22(d, J = 7.8 Hz) | 4.22(d, J = 7.8 Hz) | 4.22(d, J = 7.8 Hz) | - |
| 2 | 2.89 (m) | 2.89 (m) | 2.99 (m) | 3.24 (m) | - |
| 3 | 3.05 (m) | 3.02 (m) | 3.15 (m) | 3.05 (m) | - |
| 4 | 3.40 (m) | 3.41 (m) | 3.01 (m) | 3.04 (m) | - |
| 5 | 3.31 (m) | 3.24 (m) | 3.71 (m) | 3.19 (m) | - |
| 6 | 3.51 (m) | 3.74 (m) | 3.50 (m) | 3.46 (m) | - |
| 8-OH/14-OH | 3.98 (s)/4.75 (s) | 3.90 (s)/4.71 (s) | -/4.48 (s) | -/4.42 (s) | -/4.40 (s) |
| Position | 1 | 2 | 3 | 4 | 5 | Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 38.0 | 38.0 | 37.8 | 37.0 | 37.0 | 7 | 129.2 | - | - | - | - |
| 2 | 29.0 | 29.1 | 29.4 | 27.5 | 27.5 | 12-O | Tig | Tig | Tig | Tig | Tig |
| 3 | 76.8 | 76.6 | 76.6 | 76.6 | 75.2 | 1 | 166.8 | 166.9 | 166.9 | 166.8 | 166.8 |
| 4 | 38.6 | 38.1 | 38.4 | 33.6 | 32.7 | 2 | 127.2 | 127.5 | 127.4 | 127.5 | 127.5 |
| 5 | 138.4 | 138.4 | 139.0 | 43.6 | 43.6 | 3 | 138.0 | 138.0 | 138.7 | 138.5 | 138.0 |
| 6 | 118.4 | 118.3 | 121.9 | 29.6 | 29.4 | 4 | 14.1 | 14.3 | 14.4 | 14.4 | 14.2 |
| 7 | 26.0 | 26.2 | 27.1 | 28.6 | 28.6 | 5 | 11.3 | 11.6 | 11.9 | 11.8 | 11.7 |
| 8 | 75.1 | 75.0 | 36.8 | 36.5 | 36.6 | Oli/Ole-1 | 96.7 | 96.8 | 96.8 | 96.5 | 96.4 |
| 9 | 47.6 | 47.6 | 46.5 | 48.7 | 48.9 | 2 | 38.9 | 38.9 | 38.9 | 38.7 | 36.6 |
| 10 | 39.2 | 39.3 | 39.0 | 37.1 | 37.1 | 3 | 68.8 | 68.8 | 68.8 | 68.8 | 78.6 |
| 11 | 71.4 | 70.5 | 70.5 | 70.8 | 70.8 | 4 | 87.2 | 87.2 | 87.2 | 87.3 | 82.3 |
| 12 | 77.6 | 77.6 | 77.0 | 77.4 | 77.5 | 5 | 69.9 | 69.9 | 70.0 | 69.9 | 70.5 |
| 13 | 54.6 | 54.6 | 54.0 | 54.0 | 53.9 | 6 | 17.2 | 17.2 | 17.2 | 17.2 | 18.0 |
| 14 | 84.5 | 84.5 | 82.9 | 82.8 | 82.9 | -OCH3 | - | - | - | - | 56.3 |
| 15 | 35.5 | 35.5 | 33.3 | 34.6 | 34.6 | Allo-1 | 101.5 | 101.6 | 101.6 | 101.6 | 100.8 |
| 16 | 23.2 | 22.8 | 22.8 | 23.0 | 23.1 | 2 | 70.5 | 70.5 | 70.5 | 70.5 | 73.1 |
| 17 | 58.6 | 58.6 | 57.8 | 57.8 | 57.8 | 3 | 81.4 | 81.4 | 81.4 | 81.4 | 82.8 |
| 18 | 12.9 | 12.9 | 11.4 | 11.5 | 11.5 | 4 | 81.6 | 81.6 | 81.6 | 81.6 | 71.6 |
| 19 | 17.6 | 17.2 | 18.6 | 13.6 | 11.7 | 5 | 68.6 | 68.6 | 68.6 | 68.6 | 69.4 |
| 20 | 211.6 | 211.5 | 211.1 | 211.1 | 211.1 | 6 | 17.3 | 17.7 | 17.7 | 17.7 | 18.4 |
| 21 | 30.6 | 30.6 | 30.7 | 30.7 | 30.7 | 3-OCH3 | 60.9 | 61.0 | 61.0 | 61.0 | 61.4 |
| 11-O | Bz | Tig | Ac | Ac | Tig | Glc-1 | 104.8 | 104.9 | 104.9 | 104.9 | - |
| 1 | 164.8 | 165.9 | 169.6 | 169.8 | 166.4 | 2 | 76.6 | 76.6 | 76.6 | 76.6 | - |
| 2 | 129.6 | 128.0 | 21.1 | 21.2 | 127.9 | 3 | 73.7 | 73.7 | 73.7 | 73.7 | - |
| 3 | 129.2 | 138.0 | - | - | 138.0 | 4 | 70.1 | 70.2 | 70.2 | 70.2 | - |
| 4 | 128.6 | 14.3 | - | - | 14.2 | 5 | 76.9 | 76.9 | 77.0 | 76.9 | - |
| 5 | 133.4 | 11.7 | - | - | 11.6 | 6 | 61.4 | 61.4 | 61.4 | 61.4 | - |
| 6 | 128.6 | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Chen, M.; Li, Q.; Ma, G.; Wu, H.; Yang, J.; Li, Y.; Xu, X. Five New Polyoxypregnane Glycosides from the Vines of Aspidopterysobcordata and Their Antinephrolithiasis Activity. Molecules 2022, 27, 4596. https://doi.org/10.3390/molecules27144596
Sun Z, Chen M, Li Q, Ma G, Wu H, Yang J, Li Y, Xu X. Five New Polyoxypregnane Glycosides from the Vines of Aspidopterysobcordata and Their Antinephrolithiasis Activity. Molecules. 2022; 27(14):4596. https://doi.org/10.3390/molecules27144596
Chicago/Turabian StyleSun, Zhaocui, Meiying Chen, Qinglong Li, Guoxu Ma, Haifeng Wu, Junshan Yang, Yihang Li, and Xudong Xu. 2022. "Five New Polyoxypregnane Glycosides from the Vines of Aspidopterysobcordata and Their Antinephrolithiasis Activity" Molecules 27, no. 14: 4596. https://doi.org/10.3390/molecules27144596
APA StyleSun, Z., Chen, M., Li, Q., Ma, G., Wu, H., Yang, J., Li, Y., & Xu, X. (2022). Five New Polyoxypregnane Glycosides from the Vines of Aspidopterysobcordata and Their Antinephrolithiasis Activity. Molecules, 27(14), 4596. https://doi.org/10.3390/molecules27144596





