Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Headspace Profiles of the Investigated Mead and the Corresponding Honey Samples
2.2. DHLLE Extract Profiles of the Investigated Mead and the Corresponding Honey Samples
2.3. Traceability of Chemical Markers of Botanical Origin and Comparison with Control
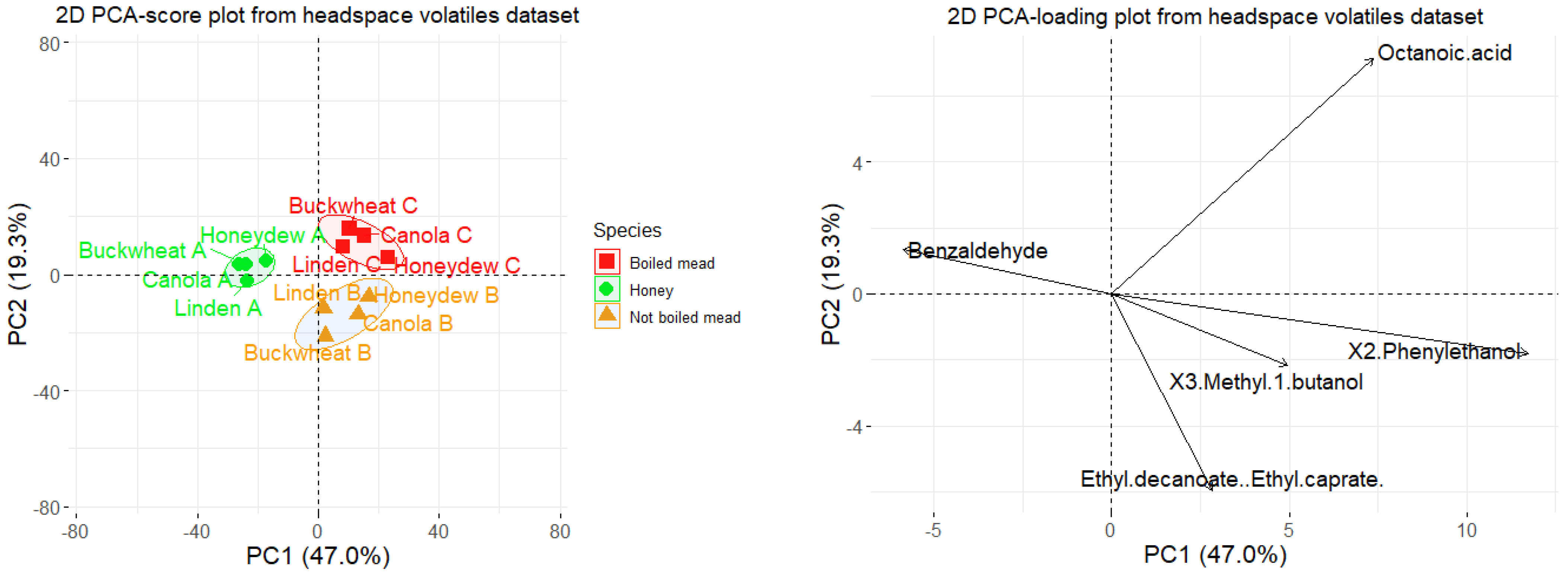
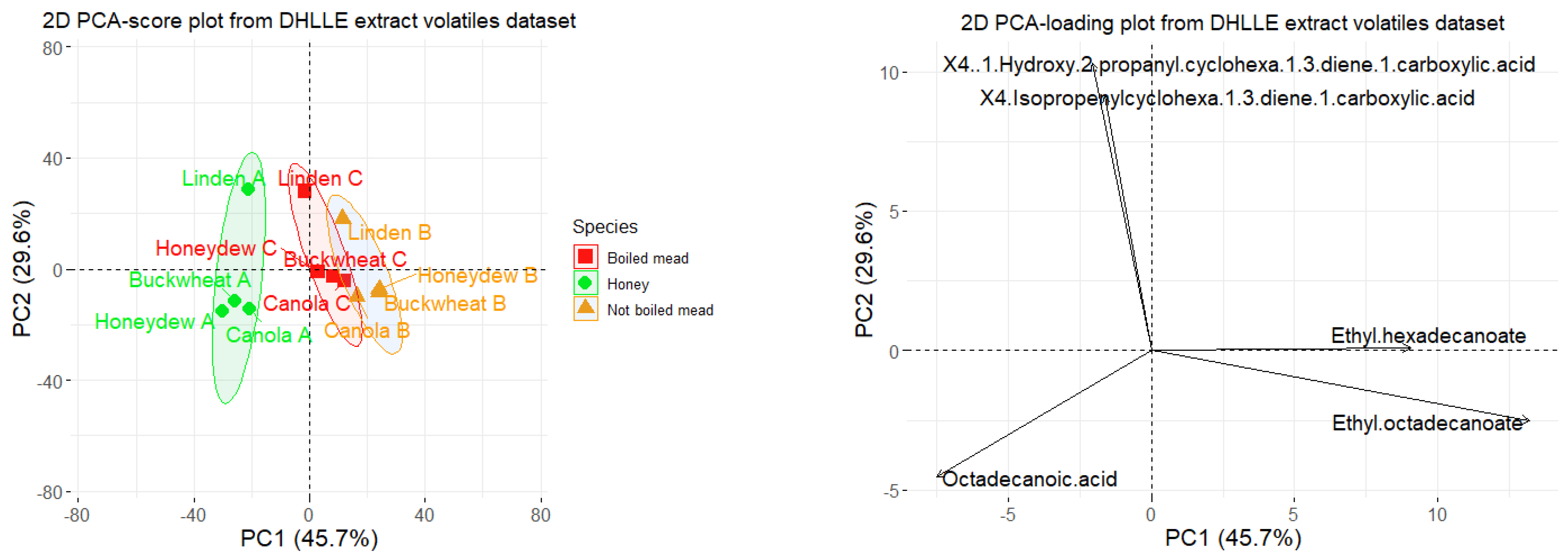
2.4. Comparison between Volatile Profiles of Honey, Boiled and Not Boiled Meads–Principal Component Analysis (PCA)
3. Materials and Methods
3.1. Materials and Samples
3.2. Headspace Solid-Phase Microextraction (HS-SPME)
3.3. Dehydration Homogenous Liquid–Liquid Extraction Method
3.4. Chromatographic Conditions
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef] [PubMed]
- European Commission Application for Registration of a TSG Council Regulation (EC) No 509/2006 ‘Trójniak’ EC No PL/TSG/007/0033/06.09.2005. Off. J. Eur. Union 2007, 265, 29–34.
- Gustaitytė, S. Kaunas Genius Loci through the Eyes of Travelers from the Middle Ages to the Middle of the 20th Century; Vytautas Magnus University: Kaunas, Lithuania, 2016. [Google Scholar]
- Wintersteen, C.L.; Andrae, L.M.; Engeseth, N.J. Effect of Heat Treatment on Antioxidant Capacity and Flavor Volatiles of Mead. J. Food Sci. C Food Chem. Toxicol. 2005, 70, C119–C126. [Google Scholar] [CrossRef]
- Bednarek, M.; Szwengiel, A. Distinguishing between saturated and unsaturated meads based on their chemical characteristics. Lwt 2020, 133, 109962. [Google Scholar] [CrossRef]
- Starowicz, M.; Granvogl, M. Effect of Wort Boiling on Volatiles Formation and Sensory Properties of Mead. Molecules 2022, 27, 710. [Google Scholar] [CrossRef] [PubMed]
- Kuś, P.M.; Jerković, I. New Sample Preparation Method for Honey Volatiles Fingerprinting Based on Dehydration Homogeneous Liquid–Liquid Extraction (DHLLE). Molecules 2018, 23, 1769. [Google Scholar] [CrossRef] [Green Version]
- Kuś, P.M.; Jerković, I. Application of the dehydration homogeneous liquid–liquid extraction (Dhlle) sample preparation method for fingerprinting of honey volatiles. Molecules 2021, 26, 2277. [Google Scholar] [CrossRef] [PubMed]
- Morakul, S.; Mouret, J.R.; Nicolle, P.; Trelea, I.C.; Sablayrolles, J.M.; Athes, V. Modelling of the gas-liquid partitioning of aroma compounds during wine alcoholic fermentation and prediction of aroma losses. Process. Biochem. 2011, 46, 1125–1131. [Google Scholar] [CrossRef]
- Mitri, S.; Koubaa, M.; Maroun, R.G.; Rossignol, T.; Nicaud, J.M.; Louka, N. Bioproduction of 2-Phenylethanol through Yeast Fermentation on Synthetic Media and on Agro-Industrial Waste and By-Products: A Review. Foods 2022, 11, 109. [Google Scholar] [CrossRef]
- Eshkol, N.; Sendovski, M.; Bahalul, M.; Katz-Ezov, T.; Kashi, Y.; Fishman, A. Production of 2-phenylethanol from L-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain. J. Appl. Microbiol. 2009, 106, 534–542. [Google Scholar] [CrossRef]
- Antonelli, A.; Castellari, L.; Zambonelli, C.; Carnacini, A. Yeast influence on volatile composition of wines. J. Agric. Food Chem. 1999, 47, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, E.; Cabaroglu, T.; Selli, S.; Bozdoǧan, A.; Kürkçüoǧlu, M.; Paydaş, S.; Başer, K.H.C. Identification of volatile aroma compounds of strawberry wine using solid-phase microex-traction techniques coupled with gas chromatography-mass spectrometry. Flavour Fragr. J. 2006, 21, 68–71. [Google Scholar] [CrossRef]
- De Mello Castanho Amboni, R.D.; Da Silva Junkes, B.; Yunes, R.A.; Fonseca Heinzen, V.E. Quantitative structure—Odor relationships of aliphatic esters using topological indices. J. Agric. Food Chem. 2000, 48, 3517–3521. [Google Scholar] [CrossRef]
- Leffingwell & Associates Odor Properties & Molecular Visualization. Available online: http://www.leffingwell.com/esters.htm (accessed on 5 April 2022).
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid-phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Dereń, M.; Kafarski, P. Phenolic compounds and abscisic acid as potential markers for the floral origin of two Polish unifloral honeys. Food Chem. 2012, 131, 1149–1156. [Google Scholar] [CrossRef]
- Jerković, I. Volatile Benzene Derivatives as Honey Biomarkers. Synlett 2013, 24, 2331–2334. [Google Scholar] [CrossRef]
- Naef, R.; Jaquier, A.; Velluz, A.; Bachofen, B. From the linden flower to linden honey—Volatile constituents of linden nectar, the extract of bee-stomach and ripe honey. Chem. Biodivers. 2004, 1, 1870–1879. [Google Scholar] [CrossRef]
- Frérot, E.; Velluz, A.; Decorzant, E.; Naef, R. From Linden Flower to Linden. Part 2: Honey Glycosidic Precursors of Cyclohexa-1,3-diene-1-carboxylic Acids. Chem. Biodivers. 2006, 3, 94–100. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Czyżewska, U.; Jankowska, E.; Bakier, S. Determination of royal jelly acids in honey. Food Chem. 2011, 124, 387–391. [Google Scholar] [CrossRef]
- Kuś, P.M.; Jerković, I.; Marijanović, Z.; Tuberoso, C.I.G. Screening of Polish Fir Honeydew Honey Using GC/MS, HPLC-DAD, and Physical-Chemical Parameters: Benzene Deriva-tives and Terpenes as Chemical Markers. Chem. Biodivers. 2017, 14, e1700179. [Google Scholar] [CrossRef]
- Bordiga, M.; Lorenzo, C.; Pardo, F.; Salinas, M.R.; Travaglia, F.; Arlorio, M.; Coïsson, J.D.; Garde-Cerdán, T. Factors influencing the formation of histaminol, hydroxytyrosol, tyrosol, and tryptophol in wine: Temperature, alcoholic degree, and amino acids concentration. Food Chem. 2016, 197, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Torrea, D.; Fraile, P.; Garde, T.; Ancín, C. Production of volatile compounds in the fermentation of chardonnay musts inoculated with two strains of Saccharomyces cerevisiae with different nitrogen demands. Food Control 2003, 14, 565–571. [Google Scholar] [CrossRef]
- Miller, A.C.; Wolff, S.R.; Bisson, L.F.; Ebeler, S.E. Yeast Strain and Nitrogen Supplementation: Dynamics of Volatile Ester Production in Chardonnay Juice Fermentations. Am. J. Enol. Vitic. 2007, 58, 470–483. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com/database/compound/compounds-index.php (accessed on 17 February 2021).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 7 June 2022).
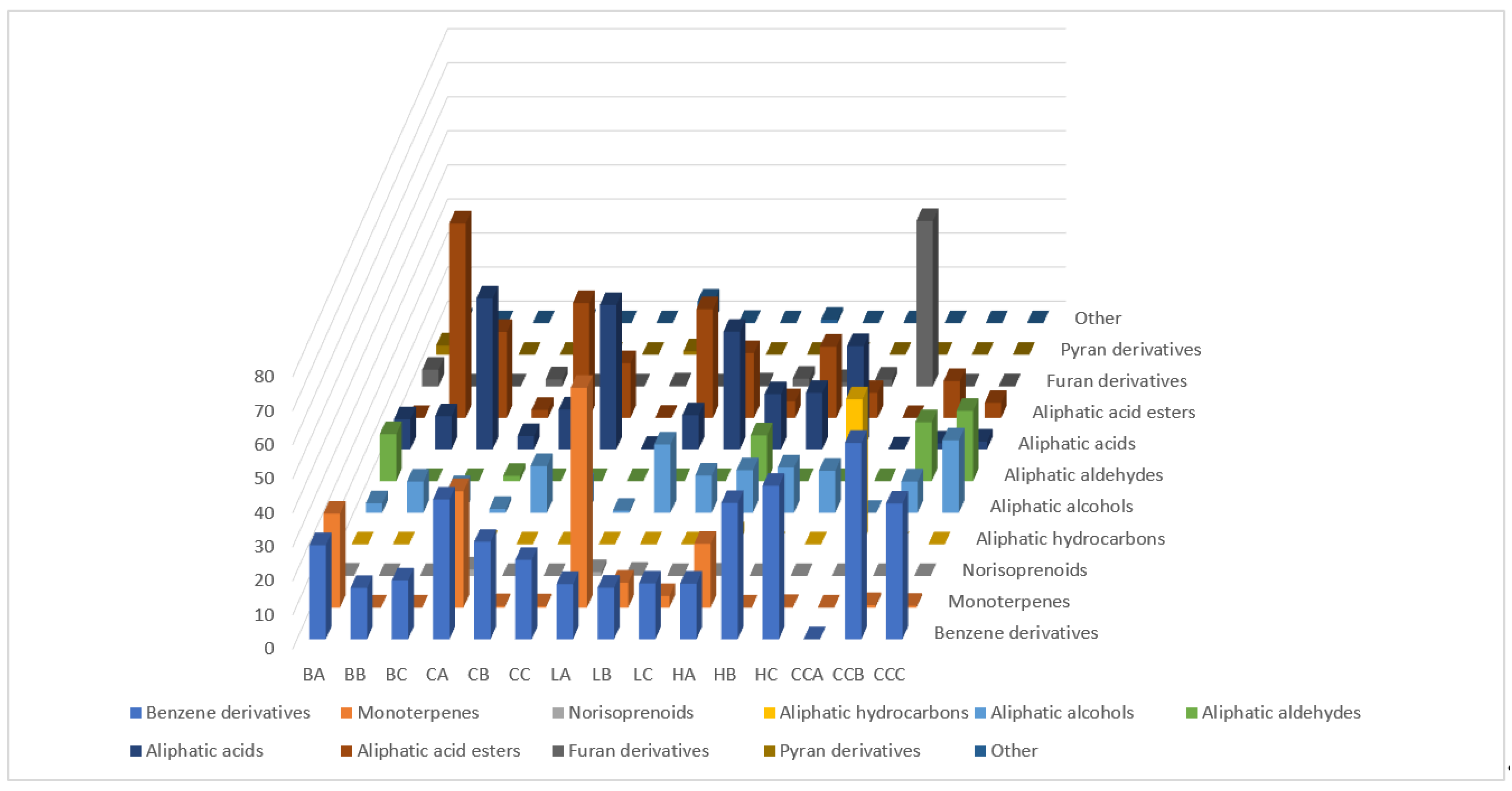
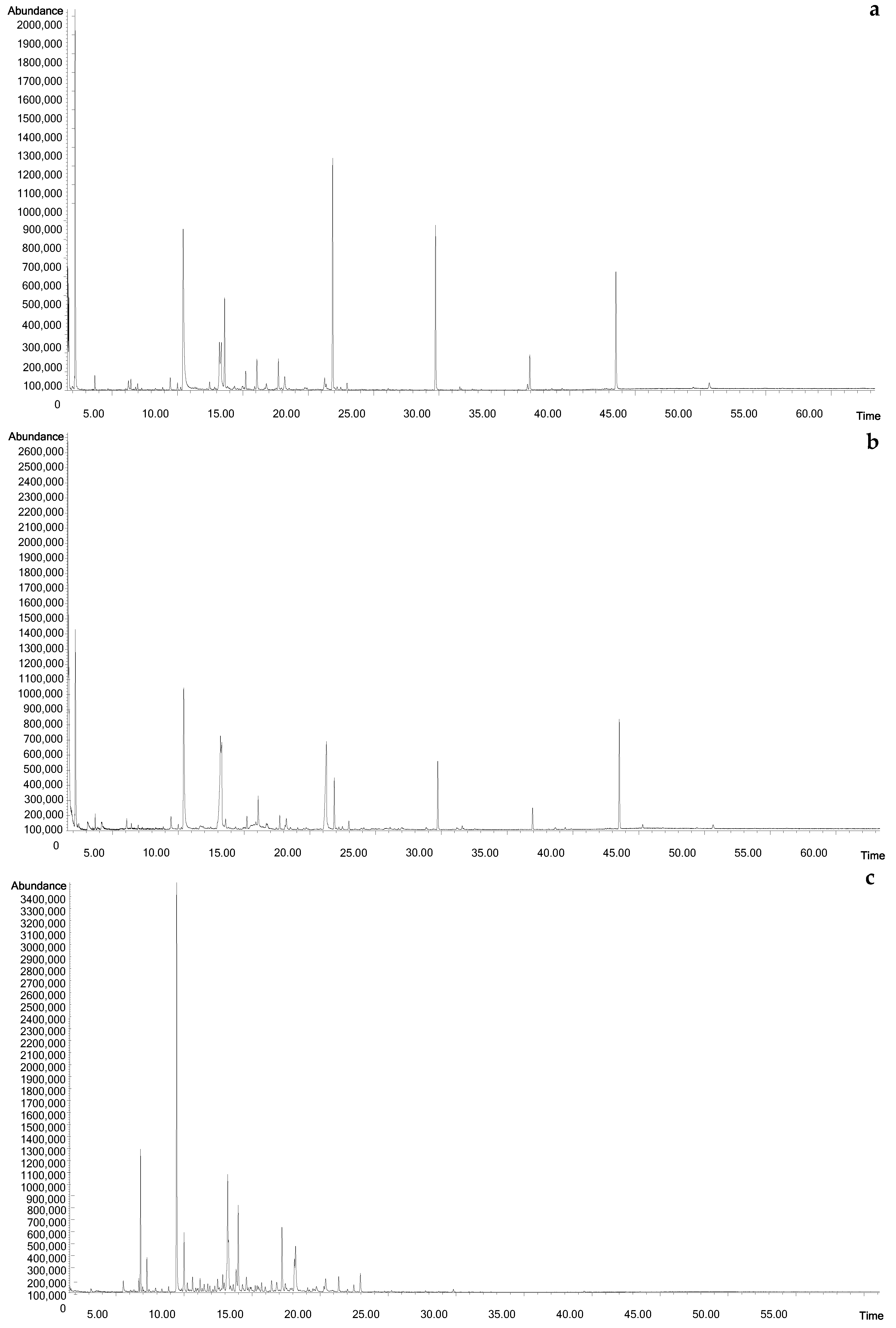
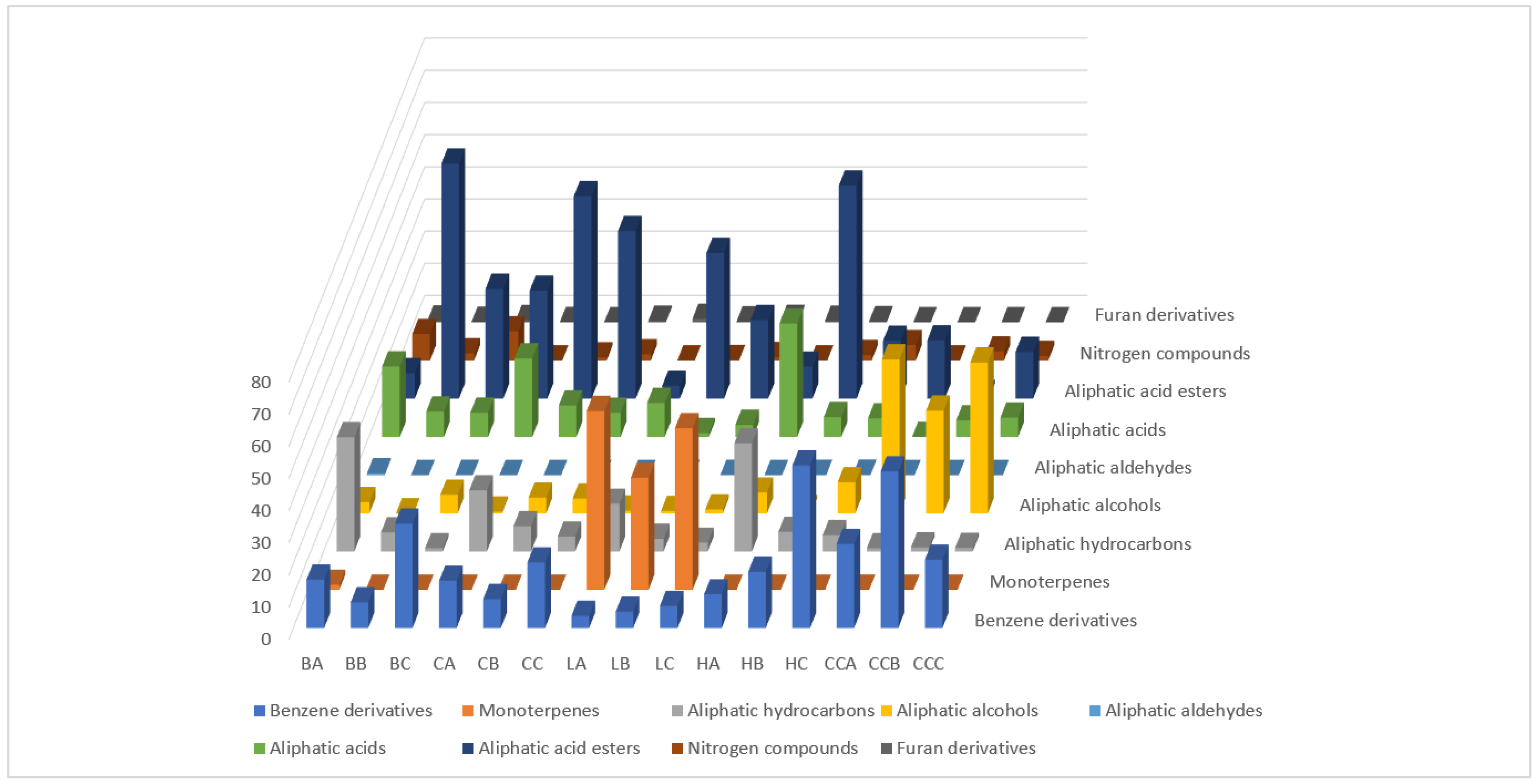
| No. | Compound | RI 1 | Buckwheat | Canola | Linden | Honeydew | Control | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |||
| Area [%] | |||||||||||||||||
| Benzene derivatives | |||||||||||||||||
| 1 | Isopropylbenzene | 932 | - | - | - | - | - | - | 0.20 | 0.15 | 0.18 | - | - | - | - | - | - |
| 2 | Benzaldehyde | 967 | 13.11 | 0.10 | 0.10 | 27.34 | - | - | 0.90 | 0.05 | - | 6.64 | 0.34 | 0.16 | - | 0.93 | 2.11 |
| 3 | 2-Phenylpropene | 987 | - | - | - | - | - | - | - | 0.08 | - | - | - | - | - | - | - |
| 4 | Benzyl alcohol | 1043 | 3.02 | - | - | 1.61 | 0.44 | 0.20 | - | 0.06 | - | 1.65 | 0.05 | 0.02 | - | - | - |
| 5 | Phenylacetaldehyde | 1049 | 5.11 | - | - | 1.09 | - | - | 0.28 | - | - | 2.19 | 0.25 | 0.15 | - | 0.34 | 0.36 |
| 6 | Acetophenone (1-Phenylethanone) | 1072 | - | - | - | - | - | - | - | - | - | - | 0.13 | 0.23 | - | - | - |
| 7 | 4-Methylphenol | 1086 | 2.98 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 8 | 2-Phenylethanol | 1120 | 0.02 | 13.37 | 11.77 | 4.13 | 26.57 | 20.94 | 0.17 | 12.73 | 13.40 | 0.70 | 35.13 | 38.32 | - | 50.93 | 31.98 |
| 9 | Ethyl benzoate | 1175 | - | - | - | 1.48 | - | - | - | - | - | - | - | - | - | - | - |
| 10 | 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran | 1176 | - | - | - | - | - | - | 0.60 | - | - | - | - | - | - | - | - |
| 11 | Benzoic acid | 1185 | - | - | - | 1.34 | - | - | - | - | - | - | - | - | - | - | - |
| 12 | Methyl salicylate | 1196 | - | - | - | - | - | - | - | - | - | 0.85 | - | - | - | - | - |
| 13 | 2-(4′-Methylphenyl)propanal | 1207 | - | - | - | - | - | - | 6.05 | - | - | - | - | - | - | - | - |
| 14 | 4,7-Dimethylbenzofuran | 1216 | - | - | - | - | - | - | 0.56 | - | - | - | - | - | - | - | - |
| 15 | Ethyl phenylacetate | 1248 | - | - | - | 1.62 | - | - | - | - | - | - | 0.24 | 0.10 | - | - | - |
| 16 | 2-Phenethyl acetate | 1260 | - | 1.59 | 4.14 | - | 1.31 | 1.61 | - | 1.74 | 2.31 | - | 3.01 | 4.25 | - | - | - |
| 17 | 2-Phenylbut-2-enal * | 1268 | - | - | - | - | - | - | 0.96 | - | - | - | - | - | - | - | - |
| 18 | 4-Propylbenzaldehyde * | 1275 | - | - | 0.72 | - | - | - | - | - | - | - | 0.41 | 0.70 | - | 1.88 | 1.07 |
| 19 | 1-(2-Aminophenyl)ethanone | 1303 | 0.68 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 20 | 4-Vinyl-2-methoxyphenol | 1317 | - | - | - | - | 0.03 | - | - | - | 0.29 | - | - | - | - | - | - |
| 21 | Ethyl benzenepropanoate | 1351 | - | - | - | - | 0.20 | 0.33 | - | - | - | - | - | - | - | - | - |
| 22 | 3,5-Dimethoxybenzaldehyde (Syringaldehyde) | 1440 | - | - | - | 1.07 | - | - | - | - | - | - | - | - | - | - | - |
| 23 | 2,4-Bis(1,1-dimethylethyl)phenol (BHT) | 1518 | - | - | 0.22 | - | - | - | - | 0.05 | 0.08 | - | 0.12 | 0.42 | - | 1.58 | 1.25 |
| 24 | Diisobutyl phthalate | 1896 | 2.06 | - | 0.28 | 1.01 | 0.03 | - | 0.09 | 0.13 | 0.11 | 2.49 | 0.32 | 0.51 | - | 1.42 | 2.23 |
| 25 | Dibutyl phthalate | 1962 | 0.62 | - | - | 0.34 | - | 0.20 | - | - | - | 0.95 | - | 0.19 | - | 0.56 | 0.82 |
| Monoterpenes | |||||||||||||||||
| 26 | 2,3-Dimethylbicyclo [2.2.1]hept-2-ene (Santene) | <900 | - | - | - | - | - | - | - | - | - | 0.69 | - | - | - | - | - |
| 27 | α-Pinene | 941 | - | - | - | - | - | - | - | - | - | 0.45 | - | - | - | - | - |
| 28 | (E)-3,3-Dimethyl-δ1,α-cyclohexaneacetaldehyde * | 1013 | - | - | - | - | - | - | 6.37 | 0.12 | - | 0.84 | - | - | - | - | - |
| 29 | p-Cymene | 1029 | - | - | - | 0.30 | - | - | 1.74 | 0.10 | 0.28 | 0.26 | - | - | - | 0.15 | 0.05 |
| 30 | Limonene | 1034 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.20 | 0.11 |
| 31 | 1,8-Cineole | 1037 | - | - | - | - | - | - | - | - | - | 0.93 | - | - | - | - | - |
| 32 | γ-Terpinene | 1063 | - | - | - | - | 0.33 | 0.20 | - | - | - | - | - | - | - | - | - |
| 33 | trans-Linalool oxide | 1077 | 5.52 | - | - | 5.86 | - | - | - | 0.13 | 0.32 | 1.54 | - | - | - | - | - |
| 34 | cis-Linalool oxide | 1091 | 3.51 | - | - | 5.17 | - | - | - | - | - | 0.21 | - | - | - | - | - |
| 35 | 1-Methyl-4-(1-methylethenyl)-benzene (p-Cymenene) | 1092 | - | - | - | - | - | - | 24.27 | 0.73 | 0.80 | - | - | 0.16 | - | 0.38 | 0.38 |
| 36 | Linalool | 1102 | - | - | - | 0.47 | - | - | 0.25 | - | - | 3.80 | - | - | - | - | - |
| 37 | Hotrienol | 1106 | 9.79 | - | - | 14.15 | 0.11 | 0.20 | 3.91 | 0.39 | 0.39 | - | - | - | - | - | - |
| 38 | trans-p-Mentha-2,8-dien-1-ol | 1125 | - | - | - | - | - | - | 1.10 | - | - | - | - | - | - | - | - |
| 39 | cis-p-Mentha-2,8-dien-1-ol | 1140 | - | - | - | - | - | - | 0.94 | 0.23 | 0.10 | - | - | - | - | - | - |
| 40 | Lilac aldehyde A | 1146 | - | - | - | 2.12 | - | - | - | - | - | - | - | - | - | - | - |
| 41 | 1-(1,4-Dimethyl-3-cyclohexen-1-yl)ethanone | 1154 | - | - | - | - | - | - | 0.62 | - | - | - | - | - | - | - | - |
| 42 | Lilac aldehyde B | 1155 | 1.43 | - | - | 4.20 | - | - | - | - | - | - | - | - | - | - | - |
| 43 | Menthofuran | 1167 | - | - | - | - | - | - | 0.41 | - | - | - | - | - | - | - | - |
| 44 | Lilac aldehyde D | 1169 | 1.43 | - | - | 1.60 | - | - | - | - | - | - | - | - | - | - | - |
| 45 | Borneol | 1172 | 0.60 | - | - | - | - | - | 1.06 | 0.42 | - | 2.01 | - | - | - | - | - |
| 46 | Terpinen-4-ol | 1181 | - | - | - | - | - | - | - | 0.35 | 0.10 | 1.38 | - | - | - | - | - |
| 47 | trans-Car-2-en-4-ol * | 1184 | - | - | - | - | - | - | 0.80 | - | - | - | - | - | - | - | - |
| 48 | p-Cymen-8-ol | 1189 | 2.07 | - | - | - | - | - | 13.21 | 2.97 | - | 1.73 | - | - | - | - | - |
| 49 | α-Terpineol | 1194 | - | - | - | - | - | - | - | - | - | 5.71 | - | - | - | - | - |
| 50 | trans-Isopiperitenol | 1204 | - | - | - | - | - | - | 2.10 | - | - | - | - | - | - | - | - |
| 51 | p-Menth-1-en-9-al (isomer I) | 1218 | 3.24 | - | - | 0.31 | - | - | - | - | - | - | - | - | - | - | - |
| 52 | cis-Isopiperitenol | 1223 | - | - | - | - | - | - | 1.29 | - | - | - | - | - | - | - | - |
| 53 | trans-Carveol | 1224 | - | - | - | - | - | - | - | 0.29 | - | - | - | - | - | - | - |
| 54 | 4-(1-Methylethyl)benzaldehyde (Cuminal) | 1243 | - | - | - | - | - | - | 0.49 | - | - | - | - | - | - | - | - |
| 55 | Carvotanacetone | 1251 | - | - | - | - | - | - | 0.70 | - | - | - | - | - | - | - | - |
| 56 | Piperitone | 1258 | - | - | - | - | - | - | 0.42 | - | - | - | - | - | - | - | - |
| 57 | 4-Isopropylcyclohexa-1,3-dienecarbaldehyde | 1286 | - | - | - | - | - | - | 4.72 | - | - | - | - | - | - | - | - |
| 58 | 2-Methyl-3-phenylprop-2-enal * | 1292 | - | - | - | - | - | - | 0.64 | - | - | - | - | - | - | - | - |
| 59 | Thymol | 1293 | - | - | - | - | - | - | 0.65 | 0.22 | - | - | - | - | - | - | - |
| 60 | Carvacrol | 1308 | - | - | - | - | - | - | 4.32 | 0.89 | 1.04 | - | - | - | - | - | - |
| 61 | Limonene-1.2-diol | 1347 | - | - | - | - | - | - | 0.84 | 0.59 | 0.35 | - | - | - | - | - | - |
| Norisoprenoids | |||||||||||||||||
| 62 | Isophorone | 1126 | - | - | - | 0.93 | - | - | - | - | - | - | - | - | - | - | - |
| 63 | 4-Ketoisophorone | 1148 | 0.09 | - | - | - | - | - | - | - | - | 0.11 | - | - | - | - | - |
| 64 | trans-β-Damascenone | 1385 | - | - | - | 0.98 | - | - | 1.13 | 0.33 | - | - | - | - | - | - | - |
| Aliphatic hydrocarbons | |||||||||||||||||
| 65 | Octane | <900 | - | - | - | 0.49 | - | - | - | - | - | 2.98 | - | - | 42.61 | - | - |
| Aliphatic alcohols | |||||||||||||||||
| 66 | 2-Methylpropan-1-ol | <900 | - | - | - | - | - | - | - | 3.41 | - | - | - | 0.49 | - | 8.83 | 20.65 |
| 67 | 3-Methylbutan-1-ol | <900 | 0.92 | 9.15 | 7.26 | 0.26 | 13.70 | 8.06 | - | 16.64 | 10.93 | 0.64 | 13.31 | 11.83 | - | - | - |
| 68 | 2-Methylbutan-1-ol | <900 | 1.87 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 69 | Heptan-2-ol | 902 | - | - | - | - | - | - | - | - | - | 1.34 | - | - | - | - | - |
| 70 | 2-Ethylhexan-1-ol | 1034 | - | - | - | 0.89 | - | - | - | - | - | 2.12 | - | - | - | - | - |
| 71 | Octan-1-ol | 1076 | - | - | - | - | - | - | 0.61 | - | - | 1.69 | - | - | - | - | - |
| 72 | Nonan-1-ol | 1177 | - | - | - | - | - | - | - | - | - | 6.10 | - | - | - | - | - |
| 73 | Dodecan-1-ol | 1478 | - | - | - | - | - | - | - | - | - | 0.59 | - | - | - | 0.36 | 0.60 |
| 74 | Aliphatic aldehydes | ||||||||||||||||
| 3-Methylbutanal | <900 | 7.98 | - | - | 0.19 | - | - | - | - | - | 0.64 | - | - | - | 17.32 | 20.65 | |
| 75 | 2-Methylbutanal | <900 | 5.20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 76 | Pentanal | <900 | 0.64 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 77 | 3-Methylpentanal | <900 | - | - | - | 1.39 | - | - | - | - | - | - | - | - | - | - | - |
| 78 | Octanal | 1004 | - | - | - | - | - | - | - | - | - | 1.74 | - | - | - | - | - |
| 79 | Nonanal | 1105 | - | - | - | - | - | - | - | - | - | 9.75 | - | - | - | - | - |
| 80 | Decanal | 1206 | - | - | - | - | - | - | - | - | - | 1.33 | - | - | - | - | - |
| Aliphatic acids | |||||||||||||||||
| 81 | Acetic acid | <900 | - | 6.46 | 0.10 | 0.19 | - | - | - | 4.02 | - | 0.33 | 4.83 | 0.10 | - | - | - |
| 82 | Butanoic acid | <900 | 0.40 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 83 | Pentanoic acid (Valeric acid) | <900 | 0.70 | 0.20 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 84 | 3-Methylpentanoic acid (3-Methylvaleric acid) | 953 | - | - | - | 0.72 | - | - | - | - | - | - | - | - | - | - | - |
| 85 | Hexanoic acid | 987 | 1.30 | 1.00 | 1.88 | 0.36 | 1.86 | 1.90 | 0.15 | 0.65 | 0.96 | 0.64 | 0.58 | 0.69 | - | - | - |
| 86 | 2-Ethylhexanoic acid | 1132 | 0.85 | - | - | 0.76 | - | - | - | 1.46 | - | - | - | - | - | ||
| 87 | Octanoic acid | 1185 | 2.66 | 2.03 | 29.14 | 1.17 | 9.61 | 28.01 | 0.05 | 4.49 | 20.15 | 6.40 | 8.74 | 20.47 | - | 0.90 | 1.16 |
| 88 | Nonanoic acid | 1283 | 2.77 | - | - | 0.68 | - | - | - | 6.73 | - | - | - | - | - | ||
| 89 | Decanoic acid | 1379 | 0.21 | 0.10 | 12.96 | 0.10 | 0.31 | 12.55 | - | 0.92 | 13.21 | 0.71 | 2.51 | 9.09 | - | 0.92 | 1.17 |
| 90 | Dodecanoic acid | 1573 | - | - | - | - | - | - | - | - | 0.27 | - | - | - | - | - | 0.00 |
| 91 | Hexadecanoic acid | 1967 | - | - | 0.30 | - | - | - | - | - | - | - | - | - | - | - | - |
| Aliphatic acid esters | |||||||||||||||||
| 92 | Ethyl acetate | <900 | - | 5.08 | 1.91 | - | - | - | - | 2.45 | - | 1.34 | 5.03 | 1.77 | - | - | - |
| 93 | Ethyl butanoate | <900 | - | - | - | - | 0.11 | 0.10 | - | - | - | - | 0.09 | 0.08 | - | - | - |
| 94 | Isoamyl acetate | <900 | - | 3.10 | 2.57 | - | 0.66 | 1.30 | - | 0.58 | 0.61 | - | - | - | - | - | - |
| 95 | Ethyl hexanoate | 999 | - | 0.50 | 0.92 | 0.17 | 1.53 | 1.10 | 0.08 | 0.47 | 0.93 | 0.54 | 0.78 | 1.19 | - | 0.55 | 0.72 |
| 96 | Ethyl octanoate (Ethyl caprylate) | 1198 | - | 4.86 | 1.99 | 0.14 | 2.83 | 1.04 | - | 4.36 | 0.77 | - | 4.97 | 1.42 | - | 4.75 | 0.64 |
| 97 | Ethyl decanoate (Ethyl caprate) | 1396 | - | 23.91 | 4.26 | - | 17.34 | 3.41 | - | 10.22 | 3.40 | - | 5.70 | 1.55 | - | 3.06 | 0.82 |
| 98 | Ethyl dodecanoate | 1595 | - | 13.43 | 2.74 | - | 10.08 | 4.24 | - | 7.59 | 4.84 | - | 1.63 | 0.25 | - | 1.64 | 0.85 |
| 99 | 2-Methyl-1-(1,1-dimethylethyl)-2-methylpropanoic acid, 1,3-propanediyl ester | 1596 | - | - | - | 2.07 | - | - | - | - | - | 3.04 | - | 0.36 | - | - | - |
| 100 | Isopentyl decanoate | 1646 | - | - | - | - | - | 0.28 | - | 0.08 | 0.32 | - | - | - | - | - | - |
| 101 | Diethyl decanedioate (Diethyl sebacate) | 1789 | - | 0.30 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 102 | Ethyl tetradecanoate (Ethyl myristate) | 1794 | - | 1.25 | 1.40 | - | 0.05 | 0.82 | - | - | - | - | 0.28 | 0.11 | - | 0.21 | 0.35 |
| 103 | Ethyl hexadec-9-enoate | 1973 | - | - | 0.37 | - | - | - | - | - | - | - | - | - | - | - | - |
| 104 | Ethyl hexadecanoate (Ethyl palmitate) | 1993 | - | 4.68 | 9.11 | - | 1.00 | 3.80 | - | 5.81 | 7.65 | - | 2.43 | 0.65 | - | 0.63 | 1.07 |
| 105 | Ethyl octadecanoate | 2194 | - | - | - | - | 0.15 | - | - | 0.37 | 0.51 | - | - | - | - | - | - |
| Furan derivatives | |||||||||||||||||
| 106 | 2-Methylfuran | <900 | - | - | - | - | - | - | - | - | - | - | - | - | 48.57 | - | - |
| 107 | 2,5-Dimethylfuran | <900 | - | - | - | - | - | - | - | - | - | 0.31 | - | - | - | - | - |
| 108 | 2-Furancarboxaldehyde | <900 | 2.07 | - | - | 2.06 | - | - | 0.31 | - | - | 1.30 | 1.27 | 1.97 | - | - | 0.05 |
| 109 | 2-Acetylfuran | 916 | 2.81 | - | - | - | - | - | - | - | - | 0.62 | - | - | - | - | - |
| 110 | 5-Hydroxymethylfurfural | 1247 | - | - | - | - | - | - | - | - | 0.32 | - | - | - | - | - | - |
| Pyran derivatives | |||||||||||||||||
| 111 | δ-Valerolactone | 961 | 2.72 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 112 | cis-Rose oxide | 1114 | - | - | - | - | - | - | 0.72 | - | - | - | - | - | - | - | - |
| 113 | trans-Rose oxide | 1131 | - | - | - | - | - | - | 0.31 | - | - | - | - | - | - | - | - |
| Other | |||||||||||||||||
| 114 | Dimethyl disulfide | <900 | 0.81 | - | - | 0.43 | - | - | - | - | - | - | - | - | - | - | - |
| 115 | Nonan-2-one | 1094 | - | - | - | - | - | - | - | - | - | 1.01 | - | - | - | - | - |
| Compound | RI 1 | Buckwheat | Canola | Linden | Honeydew | Control | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | A | B | C | A | B | C | |||
| Area [%] | |||||||||||||||||
| Benzene derivatives | |||||||||||||||||
| 1 | 2-Phenylethanol | 1116 | - | 1.95 | 4.06 | - | 1.56 | 3.70 | - | 2.37 | 1.79 | - | 8.61 | 17.11 | - | 25.32 | 8.07 |
| 2 | Benzoic acid | 1162 | - | - | - | - | 0.07 | - | - | - | - | 0.40 | - | - | - | - | - |
| 3 | 2,3-Dihydrobenzofuran (Coumaran) | 1249 | 7.64 | 0.94 | 4.33 | 0.63 | 0.03 | 0.77 | 0.50 | 0.10 | 0.09 | 0.23 | 0.53 | - | - | - | |
| 4 | Phenylacetic acid | 1269 | - | - | - | - | - | 0.19 | - | - | - | - | - | - | - | - | - |
| 5 | 4-Vinyl-2-methoxyphenol | 1314 | 0.32 | - | 0.32 | 1.18 | 0.05 | 0.79 | 0.64 | 0.10 | 0.39 | 0.53 | 0.16 | 0.95 | - | - | - |
| 6 | 4-Hydroxyphenethyl alcohol | 1445 | 0.35 | 1.23 | 0.09 | 0.35 | 0.91 | 0.73 | 3.04 | 4.42 | 9.11 | - | - | - | |||
| 7 | 2,4-Bis(1,1-dimethylethyl)phenol (BHT) | 1517 | 0.91 | 1.04 | 2.77 | 1.65 | 1.72 | 2.92 | 0.58 | 1.12 | 0.53 | 1.34 | 0.95 | 4.79 | 0.26 | 6.33 | 4.52 |
| 8 | p-Hydroxybenzoic acid | 1575 | 5.65 | 3.29 | 16.11 | - | - | - | - | - | - | - | - | - | - | - | - |
| 9 | 4-Hydroxybenzeneacetic acid * | 1608 | - | - | - | - | - | - | - | - | - | 6.40 | 0.84 | 6.94 | - | - | - |
| 10 | Homovanillic acid | 1657 | - | - | - | - | - | - | 0.36 | 0.35 | 4.89 | - | - | - | |||
| 11 | trans-Coniferyl alcohol | 1744 | - | - | - | - | - | - | - | - | - | 0.47 | 1.68 | 3.46 | - | - | - |
| 12 | Methyl syringate (Methyl 4-hydroxy-3,5-dimethoxybenzoate) | 1774 | 0.48 | 0.01 | 0.77 | 10.55 | 4.45 | 9.73 | 0.87 | 0.35 | 0.68 | - | - | - | - | - | - |
| 13 | p-Coumaric acid | 1849 | 0.02 | - | 0.81 | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | Diisobutyl phthalate | 1869 | - | 0.09 | 0.87 | - | 0.32 | 0.57 | - | 0.05 | 0.12 | - | 0.12 | 0.50 | 6.90 | 5.11 | 2.61 |
| 15 | Dibutyl phthalate | 1962 | - | 0.28 | 1.13 | 0.67 | 0.66 | 1.31 | 0.24 | 0.24 | 0.18 | 0.71 | 0.29 | 2.21 | 18.91 | 11.87 | 5.99 |
| Monoterpenes | |||||||||||||||||
| 16 | p-Mentha-1,5,8-triene | 1030 | - | - | - | - | - | - | 0.08 | - | - | - | - | - | - | - | - |
| 17 | p-Cymenene | 1092 | - | - | - | - | - | - | 0.37 | 0.11 | - | - | - | - | - | - | |
| 18 | Limonen-1,2-diol | 1348 | - | - | - | - | - | - | 0.37 | 0.10 | 0.24 | - | - | - | - | - | - |
| 19 | 4-(1-Methylethyl)benzoic acid (Cumic acid) | 1437 | - | - | - | - | - | - | 2.62 | 1.27 | 3.83 | - | - | - | - | - | - |
| 20 | 4-Isopropenylcyclohexa-1,3-diene-1-carboxylic acid | 1531 | - | - | - | - | - | - | 22.83 | 15.05 | 23.64 | - | - | - | - | - | - |
| 21 | 4-(1-Hydroxy-2-propanyl)cyclohexa-1,3-diene-1-carboxylic acid | 1611 | - | - | - | - | - | - | 29.27 | 18.35 | 22.37 | - | - | - | - | - | - |
| 22 | 4-Hydroxy-3,5,6-trimethyl-4-(3-oxobut-1-enyl)cyclohex-2-en-1-one | 1790 | 1.54 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Aliphatic hydrocarbons | |||||||||||||||||
| 23 | Heneicosane | 2100 | 0.73 | - | - | 0.92 | - | 0.31 | 0.86 | 0.28 | 0.18 | 0.94 | 0.13 | - | 0.01 | 0.02 | 0.19 |
| 24 | (Z)-Tricos-9-ene | 2265 | 6.74 | 0.53 | 0.01 | 2.88 | 0.01 | 0.40 | 2.31 | 0.03 | 0.37 | 5.11 | 1.25 | 3.07 | - | - | - |
| 25 | Tricosane | 2300 | 25.35 | 5.20 | 0.89 | 14.23 | 3.87 | 3.82 | 11.33 | 3.55 | 2.16 | 23.93 | 4.52 | 1.34 | - | - | - |
| 26 | Tetracosane | 2400 | 2.72 | 0.20 | 0.10 | 0.98 | 3.98 | 0.10 | 0.31 | 0.10 | 0.02 | 3.59 | 0.20 | 0.67 | 1.00 | 1.23 | 0.91 |
| Aliphatic alcohols | |||||||||||||||||
| 27 | Hexadecan-1-ol | 1882 | 0.08 | - | - | - | - | 0.39 | - | - | 0.15 | - | 0.02 | 1.31 | 3.40 | 0.28 | 3.33 |
| 28 | (Z)-Octadec-9-en-1-ol | 2060 | 2.18 | - | 3.67 | 0.49 | 1.89 | 2.41 | 0.62 | 0.36 | 0.61 | 1.62 | 0.64 | 3.81 | 30.46 | 18.90 | 30.42 |
| 29 | Octadecan-1-ol | 2084 | 1.23 | - | 2.07 | 0.02 | 2.99 | 1.77 | 0.20 | 0.35 | 0.43 | 4.82 | 0.35 | 4.54 | 13.97 | 12.74 | 13.09 |
| Aliphatic aldehydes | |||||||||||||||||
| 30 | 3-Methylpentanal | 1028 | 0.56 | 0.01 | 0.04 | - | - | - | - | - | 0.06 | - | 0.05 | 0.08 | - | - | - |
| Aliphatic acids | |||||||||||||||||
| 31 | Octanoic acid | 1185 | - | 0.16 | 1.64 | - | - | 1.62 | - | 0.02 | 0.27 | - | 0.14 | 1.14 | - | - | - |
| 32 | Decanoic acid | 1380 | - | - | 0.90 | - | - | 0.62 | - | 0.01 | 0.17 | - | 0.02 | 0.63 | - | - | - |
| 33 | Dodecanoic acid | 1573 | - | - | - | - | - | - | - | - | 0.09 | - | - | - | - | - | - |
| 34 | Hexadecanoic acid | 1963 | 0.58 | 1.04 | 3.48 | 0.74 | 1.28 | 4.92 | - | 0.56 | 1.37 | - | 0.88 | 3.87 | 0.02 | 4.92 | 1.82 |
| 35 | Oleic acid | 2141 | 2.23 | - | - | 5.81 | - | - | - | - | - | 2.87 | - | - | - | - | - |
| 36 | Octadecanoic acid | 2181 | 19.07 | 6.68 | 1.47 | 17.84 | 8.47 | 0.30 | 10.53 | 0.58 | 1.85 | 32.37 | 5.12 | 0.10 | 0.01 | 0.22 | 4.15 |
| Aliphatic acid esters | |||||||||||||||||
| 37 | Ethyl decanoate | 1396 | - | 0.62 | 0.29 | - | 0.18 | 0.39 | - | 0.24 | 0.19 | - | 0.61 | 0.15 | - | - | - |
| 38 | Ethyl dodecanoate | 1595 | - | 1.42 | - | - | 0.49 | 1.17 | - | 0.56 | 0.36 | - | 1.06 | 0.05 | - | - | - |
| 39 | Ethyl tetradecanoate | 1794 | - | 0.75 | 0.89 | - | 0.51 | 0.65 | - | 0.73 | 0.37 | - | 0.54 | - | - | - | - |
| 40 | Ethyl hexadec-9-enoate | 1972 | - | 0.77 | 1.25 | 0.78 | 1.53 | - | 0.20 | 0.23 | - | 0.40 | 0.83 | - | - | - | |
| 41 | Ethyl hexadecanoate | 1993 | 0.22 | 24.98 | 14.47 | 0.70 | 14.88 | 22.65 | - | 18.90 | 12.05 | - | 21.63 | 4.24 | - | - | - |
| 42 | Ethyl linoleate | 2157 | - | - | - | 1.05 | - | - | - | - | - | - | - | - | - | - | - |
| 43 | Ethyl oleate | 2164 | 7.71 | 7.33 | 2.77 | 28.22 | 11.72 | 9.03 | 4.00 | 2.31 | 1.99 | 9.10 | 4.95 | 1.67 | - | - | - |
| 44 | Ethyl octadecanoate | 2194 | - | 37.17 | 14.46 | 3.56 | 34.34 | 16.67 | - | 22.34 | 9.23 | 0.81 | 37.01 | 11.02 | - | - | - |
| 45 | Tributyl acetylcitrate | 2260 | - | - | - | - | - | - | - | - | - | - | - | - | 18.08 | 1.18 | 14.50 |
| Nitrogen compounds | |||||||||||||||||
| 46 | 1-Isoquinolinecarbonitrile * | 1446 | 1.37 | 0.49 | 0.93 | - | - | - | - | - | - | - | - | - | - | - | - |
| 47 | Tryptophol | 1769 | - | 0.92 | 1.75 | - | 0.98 | 1.91 | - | 0.10 | 1.04 | - | 1.68 | 4.81 | - | 2.79 | 1.38 |
| 48 | 1H-Indole-3-acetonitrile | 1816 | 3.58 | 0.02 | 1.38 | - | - | - | - | - | - | - | - | - | - | - | - |
| 49 | 1H-Indole-3-carboxaldehyde | 1824 | 3.30 | 0.78 | 5.00 | - | - | - | - | - | - | - | - | - | - | - | - |
| Furan derivatives | |||||||||||||||||
| 50 | Dihydro-5-methyl-2(3H)-furanone (γ-Valerolactone) | 964 | 0.14 | 0.04 | 0.29 | - | - | - | - | - | - | - | - | - | - | - | - |
| 51 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | 982 | 0.38 | 0.10 | 0.38 | 0.08 | 0.04 | 0.46 | 0.97 | 0.16 | 0.42 | 0.44 | 0.10 | - | - | - | - |
| 52 | Succinic anhydride | 1024 | - | - | - | - | - | - | - | - | 0.22 | - | 0.20 | - | - | - | - |
| 53 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 1151 | - | - | - | - | - | - | 0.10 | 0.10 | 0.06 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuś, P.M.; Czabaj, S.; Jerković, I. Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers. Molecules 2022, 27, 4558. https://doi.org/10.3390/molecules27144558
Kuś PM, Czabaj S, Jerković I. Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers. Molecules. 2022; 27(14):4558. https://doi.org/10.3390/molecules27144558
Chicago/Turabian StyleKuś, Piotr M., Sławomir Czabaj, and Igor Jerković. 2022. "Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers" Molecules 27, no. 14: 4558. https://doi.org/10.3390/molecules27144558
APA StyleKuś, P. M., Czabaj, S., & Jerković, I. (2022). Comparison of Volatile Profiles of Meads and Related Unifloral Honeys: Traceability Markers. Molecules, 27(14), 4558. https://doi.org/10.3390/molecules27144558







