Determination of Benzocaine in Pharmaceutical Formulations by Indirect SERRS Assay Combined with Azo Coupling
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Ag NPs
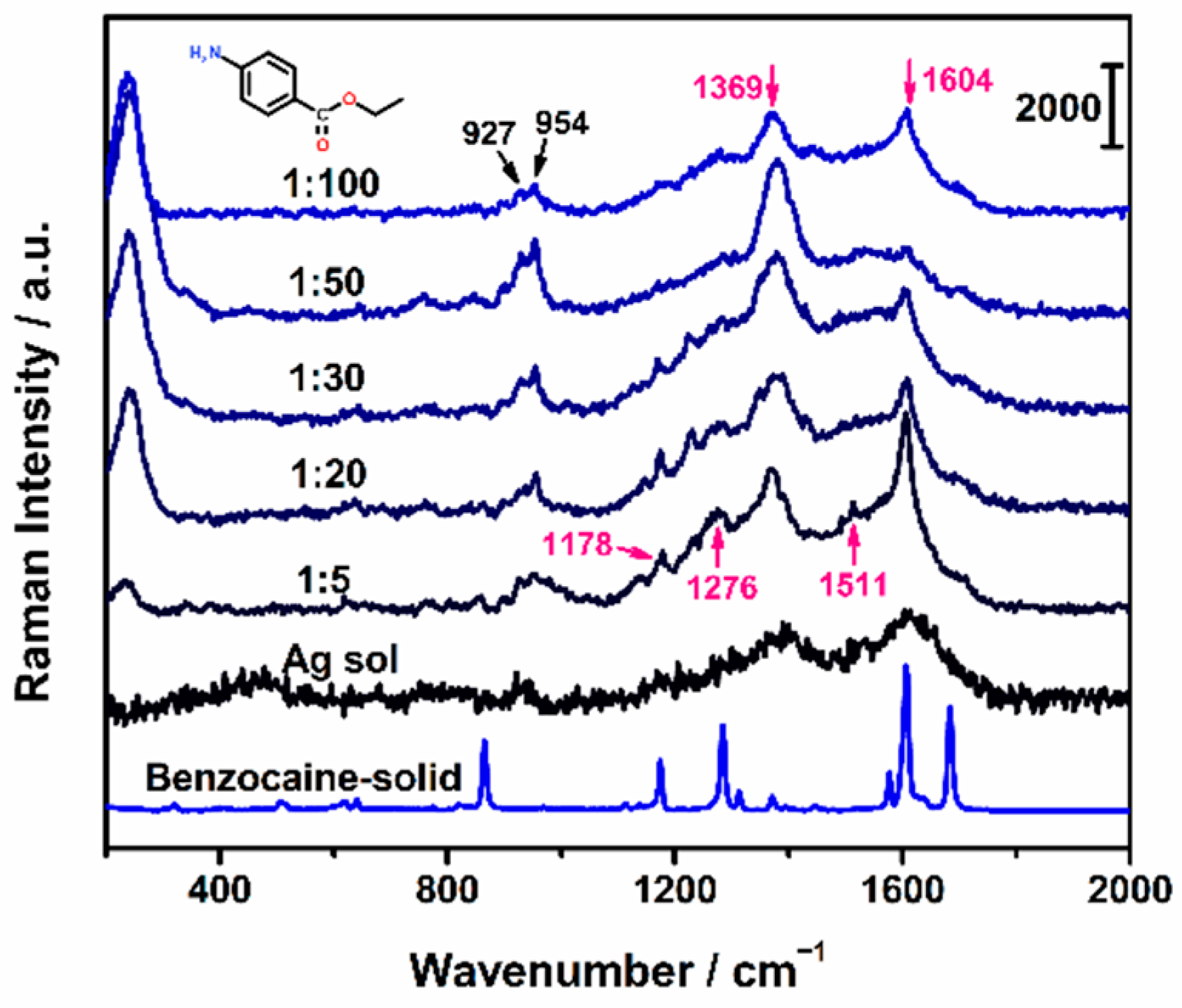
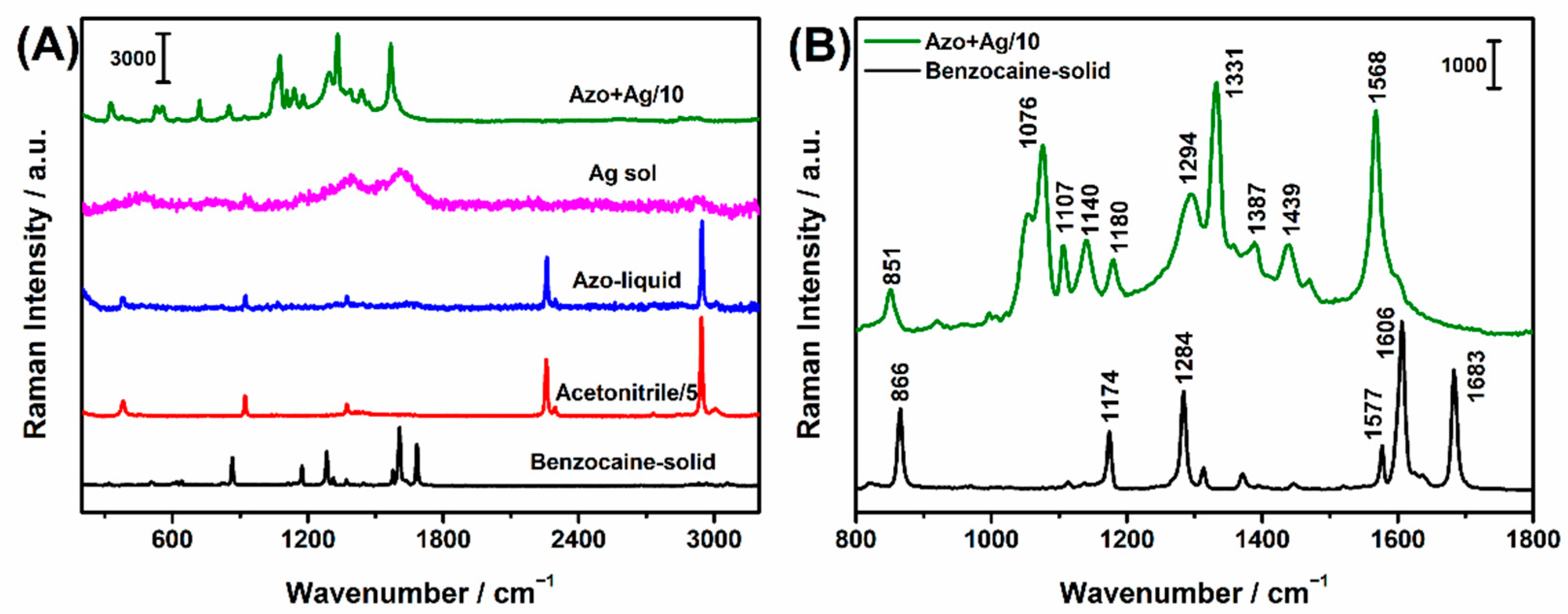
2.2. Intrinsic SERS Spectra of Benzocaine
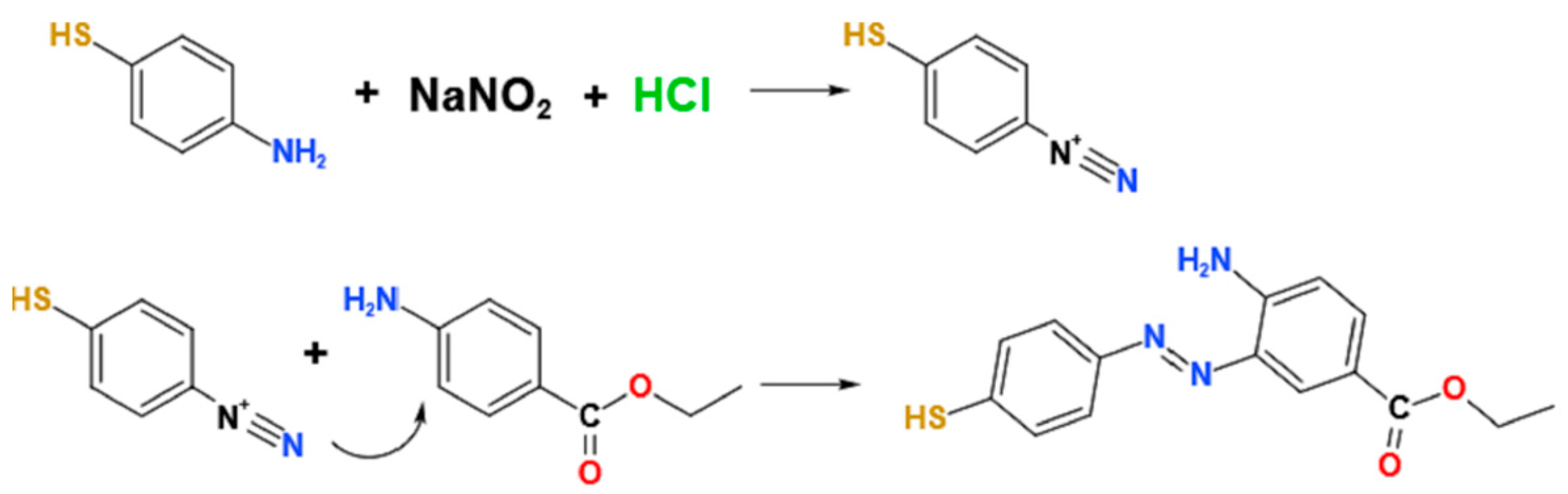
2.3. Azo Derivatization of Benzocaine
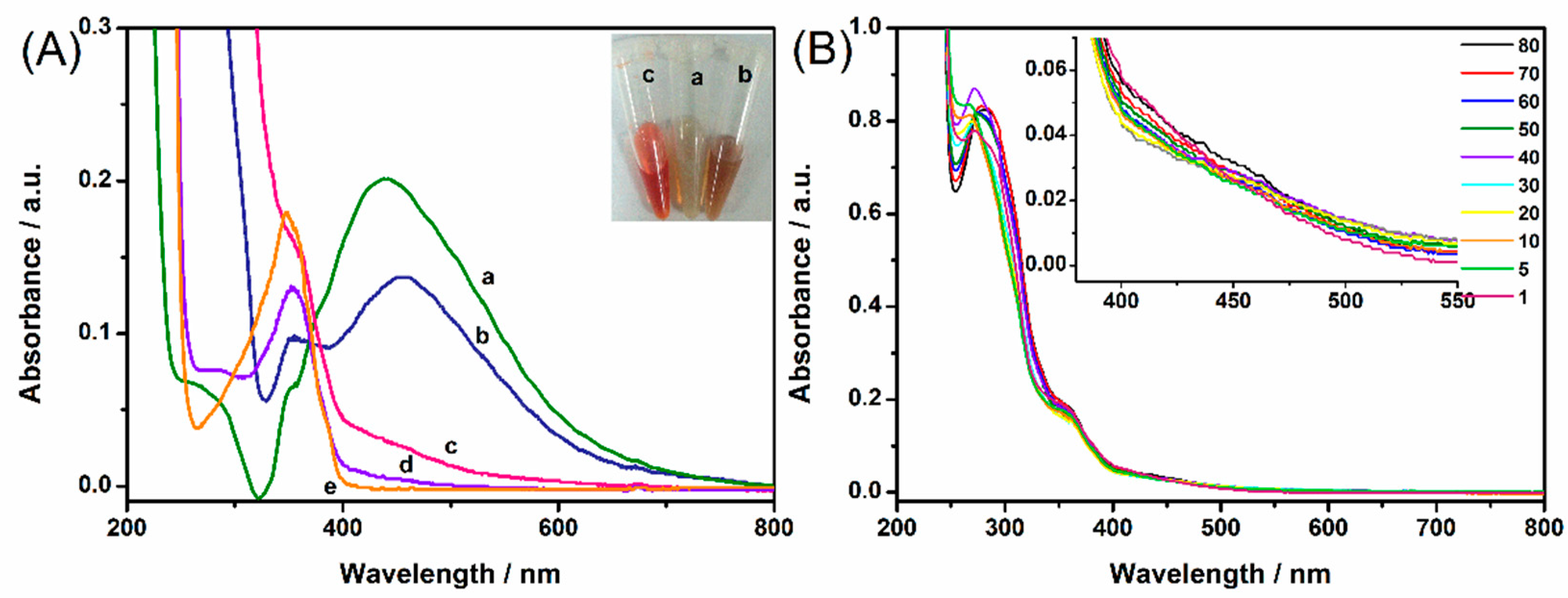
2.4. Optimization of Derivatization Time and Stability Investigation of Azo Product
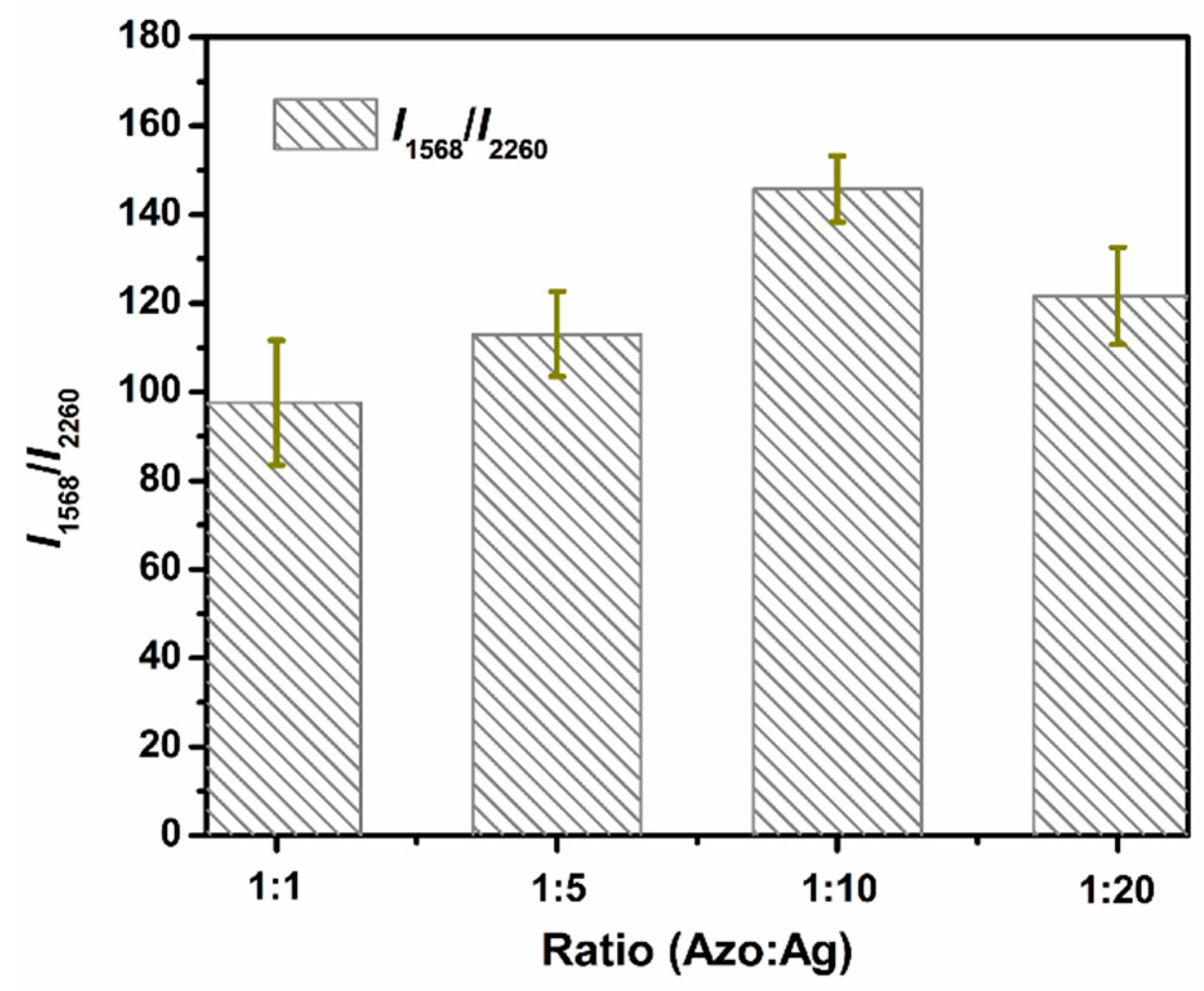
2.5. Mixing Ratio Optimization of Azo Solution and Ag NPs
2.6. SERRS Spectra of Azo Product
2.7. Concentration Dependent SERRS Characterization and Linear Investigation
2.8. Accuracy and Reproducibility
2.9. Determination in Real Samples
2.10. Comparison with Other Techniques for Benzocaine
3. Materials and Methods
3.1. Chemical Reagents
3.2. Apparatus and Measurement
3.3. Synthesis of Ag NPs
3.4. Derivatization Method of Benzocaine
3.5. Preparation of Benzocaine Standard Solution
3.6. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pysarevska, S.; Plotycya, S.; Dubenska, L. Voltammetry of local anesthetics: Theoretical and practical aspects. Crit. Rev. Anal. Chem. 2021, 51, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Ashraf, Z.; Valavoor, S.; Tinkel, J. Evaluation and management of acquired methemoglobinemia associated with topical benzocaine use. Am. J. Cardiovasc. Drugs 2013, 13, 325–330. [Google Scholar] [CrossRef]
- Weiss-Smith, S.; Deshpande, G.; Chung, S.; Gogolak, V. The FDA drug safety surveillance program: Adverse event reporting trends. Arch. Intern. Med. 2011, 171, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, S.; Ramirez, L.R.; Harmatz, A.J. S2071 Severe methemoglobinemia after benzocaine spray use during endoscopy. Am. J. Gastroenterol. 2020, 115, S1088. [Google Scholar] [CrossRef]
- Song, T.Y.; Farrington, E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J. Pediatr. Health Care 2008, 22, 335–339. [Google Scholar]
- Taylor, D.; Miles, W.S.; Schiffern, L.; Thomason, M.; Larrazabal, C. Topical benzocaine-induced methemoglobinemia during transesophageal echocardiography in the intensive care unit. Crit. Care Med. 2006, 34, A168. [Google Scholar] [CrossRef]
- FDA. Fish and Fishery Products Hazards and Controls Guidance Fourth Edition. 2021. Available online: https://www.fda.gov/media/80637/download (accessed on 9 March 2022).
- Pérez-Lozano, P.; García-Montoya, E.; Orriols, A.; Miñarro, M.; Ticó, J.R.; Suñé-Negre, J.M. A new validated method for the simultaneous determination of benzocaine, propylparaben and benzyl alcohol in a bioadhesive gel by HPLC. J. Pharm. Biomed. Anal. 2005, 39, 920–927. [Google Scholar] [CrossRef]
- De Orsi, D.; Pellegrini, M.; Marchei, E.; Nebuloni, P.; Gallinella, B.; Scaravelli, G.; Martufi, A.; Gagliardi, L.; Pichini, S. High performance liquid chromatography-diode array and electrospray-mass spectrometry analysis of vardenafil, sildenafil, tadalafil, testosterone and local anesthetics in cosmetic creams sold on the Internet web sites. J. Pharm. Biomed. Anal. 2009, 50, 362–369. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Stehly, G.R.; Hubert, T.D.; Bernardy, J.A. Liquid chromatographic determination of benzocaine and N-acetylbenzocaine in the edible fillet tissue from rainbow trout. J. Chromatogr. A 1999, 855, 255–260. [Google Scholar] [CrossRef]
- Joseph-Charles, J.; Montagut, M.; Langlois, M.H.; Boyer, C.; Dubost, J.P. Simultaneous determination of rutin and benzocaine in suppositories by reversed-phase high-performance liquid chromatography. Anal. Lett. 2001, 34, 2685–2692. [Google Scholar] [CrossRef]
- Elwalily, A.M.; Korany, M.A.; Bedair, M.M.; Elgindy, A. High-performance liquid-chromatography determination of benzocaine and phenindamine tartrate in cream. Anal. Lett. 1991, 24, 781–795. [Google Scholar] [CrossRef]
- Dejmkova, H.; Vokalova, V.; Zima, J.; Barek, J. Determination of benzocaine using HPLC and FIA with amperometric detection on a carbon paste electrode. Electroanalysis 2011, 23, 662–666. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Atty, S.A.; Merey, H.A.; Fattah, T.A.; Foster, C.W.; Banks, C.E. Titanium nanoparticles (TiO2)/graphene oxide nanosheets (GO): An electrochemical sensing platform for the sensitive and simultaneous determination of benzocaine in the presence of antipyrine. Analyst 2017, 142, 3674–3679. [Google Scholar] [CrossRef] [PubMed]
- de Lima, R.M.F.; Silva, M.D.D.O.; Felix, F.S.; Angnes, L.; dos Santos, W.T.P.; Saczk, A.A. Determination of benzocaine and tricaine in fish fillets using BIA with amperometric detection. Electroanalysis 2018, 30, 283–287. [Google Scholar] [CrossRef]
- Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using capsaicin modified multiwalled carbon nanotube based electrodes and p-chloranil modified carbon paste electrodes for the determination of amines: Application to benzocaine and lidocaine. Electroanalysis 2008, 20, 2495–2500. [Google Scholar] [CrossRef]
- Zhang, X.R.; Baeyens, W.R.G.; Vanderweken, G.; Calokerinos, A.C.; Imai, K. Chemiluminescence determination of some local anaesthetics. Anal. Chim. Acta 1995, 303, 137–142. [Google Scholar] [CrossRef]
- Pasekova, H.; Polasek, M. Determination of procaine, benzocaine and tetracaine by sequential injection analysis with pel-manganate-induced chemiluminescence detection. Talanta 2000, 52, 67–75. [Google Scholar] [CrossRef]
- Amin, A.S.; EL-Didamony, A.M. Colorimetric determination of benzocaine, lignocaine and procaine hydrochlorides in pure form and in pharmaceutical formulations using p-benzoquinone. Anal. Sci. 2003, 19, 1457–1459. [Google Scholar] [CrossRef][Green Version]
- Carmona, M.; Silva, M.; Perezbendito, D. A selective and sensitive kinetic method for the determination of procaine and benzocaine in pharmaceuticals. J. Pharm. Biomed. Anal. 1992, 10, 145–152. [Google Scholar] [CrossRef]
- Paschoal, L.R.; Ferreira, W.A. Simultaneous determination of benzocaine and cetylpiridinium chloride in tablets by first-derivative spectrophotometric method. Farmaco 2000, 55, 687–693. [Google Scholar] [CrossRef]
- Khalil, R.A.; Hussain, S.A. Surfactant enhanced reaction between benzocaine and p-dimethylaminobenzaldehyde: Kinetic study and its analytical application. Arab. J. Sci. Eng. 2010, 35, 55–66. [Google Scholar]
- Li, D.; Yao, D.; Li, C.; Luo, Y.; Liang, A.; Wen, G.; Jiang, Z. Nanosol SERS quantitative analytical method: A review. TrAC Trends Anal. Chem. 2020, 127, 115885. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, Y.J.; Ding, S.Y.; Panneerselvam, R.; Tian, Z.Q. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.C.; Hu, S.; Yan, S.; Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2020, 2, 253–271. [Google Scholar] [CrossRef]
- Si, K.J.; Guo, P.Z.; Shi, Q.Q.; Cheng, W.L. Self-assembled nanocube-based plasmene nanosheets as soft Surface-enhanced Raman scattering substrates toward direct quantitative drug identification on surfaces. Anal. Chem. 2015, 87, 5263–5269. [Google Scholar] [CrossRef]
- Štolcová, L.; Peksa, V.; Proška, J.; Procházka, M. Gold film over very small (107 nm) spheres as efficient substrate for sensitive and reproducible surface-enhanced Raman scattering (SERS) detection of biologically important molecules. J. Raman Spectrosc. 2017, 49, 499–505. [Google Scholar] [CrossRef]
- Stacy, A.M.; Van Duyne, R.P. Surface enhanced Raman and resonance Raman-spectroscopy in a non-aqueous electrochemical environment-tri (2,2′-bipyridine) Ruthenium (II) adsorbed on silver from acetonitrile. Chem. Phys. Lett. 1983, 102, 365–370. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Iancu, S.D.; Stefancu, A.; Moisoiu, V.; Leopold, L.F.; Leopold, N. The role of Ag+, Ca2+, Pb2+ and Al3+ adions in the SERS turn-on effect of anionic analytes. Beilstein J. Nanotech. 2019, 10, 2338–2345. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, B.; Xu, W.; Li, B.; Jung, Y.M.; Ozaki, Y. Near-infrared surface-enhanced Raman scattering study of ultrathin films of azobenzene-containing long-chain fatty acids on a silver surface prepared by silver mirror and nitric acid etched silver foil methods. Langmuir 1999, 15, 4625–4629. [Google Scholar] [CrossRef]
- Szele, I.; Zollinger, H. Azo coupling reactions structures and mechanisms. In Preparative Organic Chemistry; Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 1983; Volume 112, pp. 1–66. [Google Scholar]
- Lombardi, J.R.; Birke, R.L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef]
- Sui, H.; Wang, Y.; Yu, Z.; Cong, Q.; Han, X.X.; Zhao, B. A rapid and ultrasensitive SERRS assay for histidine and tyrosine based on azo coupling. Talanta 2016, 159, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Wang, Y.; Zhang, X.; Wang, X.; Cheng, W.; Su, H.; Wang, X.; Sun, X.; Han, X.X.; Zhao, B.; et al. Ultrasensitive detection of thyrotropin-releasing hormone based on azo coupling and surface-enhanced resonance Raman spectroscopy. Analyst 2016, 141, 5181–5188. [Google Scholar] [CrossRef] [PubMed]
- Balci, K.; Akyuz, S. A vibrational spectroscopic investigation on benzocaine molecule. Vib. Spectrosc. 2008, 48, 215–228. [Google Scholar] [CrossRef]
- Biswas, N.; Umapathy, S. Density functional calculations of structures, vibrational frequencies, and normal modes of trans- and cis-azobenzene. J. Phys. Chem. A 1997, 101, 5555–5566. [Google Scholar] [CrossRef]
- Moskovits, M.; Suh, J.S. Surface selection-rules for surface-enhanced Raman spectroscopy: Calculations and application to the surface-enhanced Raman spectrum of phthalazine on silver. J. Phys. Chem. 1984, 88, 5526–5530. [Google Scholar] [CrossRef]

| Pharmaceutical | Benzocaine Content by Our Method (g/g or mg/mL) | Declared Benzocaine Content (g/g or mg/mL) | RSD (%) |
|---|---|---|---|
| Lidinuo compound benzocaine gel | 0.210 ± 0.013 | 0.2 | 6.2 |
| Enemeez | 3.98 ± 0.21 | 4 | 5.3 |
| Americaine benzocaine topical anesthetic spray | 0.201 ± 0.018 | 0.2 | 9.0 |
| Methods | Samples | Sample Treatment | Instrument Test Time | LOD or Linear Range | Reference |
|---|---|---|---|---|---|
| HPLC-DAD | Bioadhesive gel | Stirred 90 min, ultrasonicated 10 min | 30 min | / | [8] |
| HPLC-UV-DAD HPLC-ESI-MS | Cosmetic creams | Dispersed, ultrasonicated 10 min | 30 min | 1.8 μg/g | [9] |
| 1.7 ng/g | |||||
| HPLC-UV | Edible fillet tissue from rainbow trout | Inorganic salt treatment, a glass extraction column and a Baker SPE-24G column, rotary evaporation. Then solid-phase extraction | 7.5 min | 6 ng/g | [10] |
| RP-HPLC-UV | Suppository | Extracted three times, centrifuged 5 min | 5 min | 0.22 μg/mL | [11] |
| HPLC-UV | Cream | Ultrasonicated 10 min, centrifuged 20 min. Repeated three times. | 2.5 min | 6~15.6 μg/mL | [12] |
| HPLC-ED FIA-ED (electrochemical detection) | Pastilles and mouthwash | Dissolved then diluted | 10 min | 2.0 × 10−7 mol/L | [13] |
| 12 min | 1.9 × 10−7 mol/L | ||||
| TiO2-GO/CPE-based ED | Ear drops, tablets, and oral fluid | Dissolved and diluted. Oral fluid: centrifuged 5 min | / | 2.48 × 10−7 mol/L | [14] |
| BIA-amperometric method | Fish fillets | Freeze-drying, vortexed 5 min and centrifuged 5 min, frozen and thawed | / | 3.02 × 10−8 mol/L | [15] |
| Capsaicin-modified MWCNT and p-chloranil-modified CPE-based ED | Analytical grade chemicals | Dissolved and diluted | / | 2.45 ± 0.2 μmol/L | [16] |
| Chemiluminescence (MnO4−,H+) | Spray | Dissolved and diluted | / | 0.03 μg/mL | [17] |
| SIA-CL (MnO4−,H+) | Babydent STADA solution | Diluted | 120 h−1 | 0.3 μg/mL | [18] |
| Colorimetric method | Dentocalm ointment | Extract four times, evaporated to dryness, then dissolved. React for 10 min | 10~60 μg/mL | [19] | |
| Kinetic-spectrophotometry (Stopped-flow method) | Tablets and solutions | Dissolved and diluted | 100 h−1 | 30 ng/mL | [20] |
| First-derivative spectrophotometric method | Tablets | Dissolved then diluted | / | 10~25 μg/mL | [21] |
| Surfactant-enhanced spectrophotometric method | Throat lozenges and lozenges of benzocaine | Dissolved, diluted, react 20 min | / | 0.0825~4.9558 μg/mL | [22] |
| SAM nanocube-based plasmene nanosheets for SERS | Banknotes | Dissolved | / | 0.9 × 10−6 g/cm2 | [28] |
| Gold film over PS spheres for SERS | Benzocaine in analytical grade | Dissolved | / | 4.3 × 10−7 mol/L | [29] |
| Our method | Gel, enema, and spray | Dissolved, ultrasonicated 5 min (not for spray). React 5 min | 0.139 μg/mL (8.39 × 10−7 mol/L) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.-Y.; Sui, H.; Xue, E.; Li, L.; Zhang, J.; Xu, T.; Liang, X.; Yang, Y. Determination of Benzocaine in Pharmaceutical Formulations by Indirect SERRS Assay Combined with Azo Coupling. Molecules 2022, 27, 4492. https://doi.org/10.3390/molecules27144492
Zhao C-Y, Sui H, Xue E, Li L, Zhang J, Xu T, Liang X, Yang Y. Determination of Benzocaine in Pharmaceutical Formulations by Indirect SERRS Assay Combined with Azo Coupling. Molecules. 2022; 27(14):4492. https://doi.org/10.3390/molecules27144492
Chicago/Turabian StyleZhao, Chao-Yang, Huimin Sui, Endi Xue, Li Li, Jie Zhang, Tao Xu, Xin Liang, and Ying Yang. 2022. "Determination of Benzocaine in Pharmaceutical Formulations by Indirect SERRS Assay Combined with Azo Coupling" Molecules 27, no. 14: 4492. https://doi.org/10.3390/molecules27144492
APA StyleZhao, C.-Y., Sui, H., Xue, E., Li, L., Zhang, J., Xu, T., Liang, X., & Yang, Y. (2022). Determination of Benzocaine in Pharmaceutical Formulations by Indirect SERRS Assay Combined with Azo Coupling. Molecules, 27(14), 4492. https://doi.org/10.3390/molecules27144492






