Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes
Abstract
:1. Introduction
2. Results and Discussion
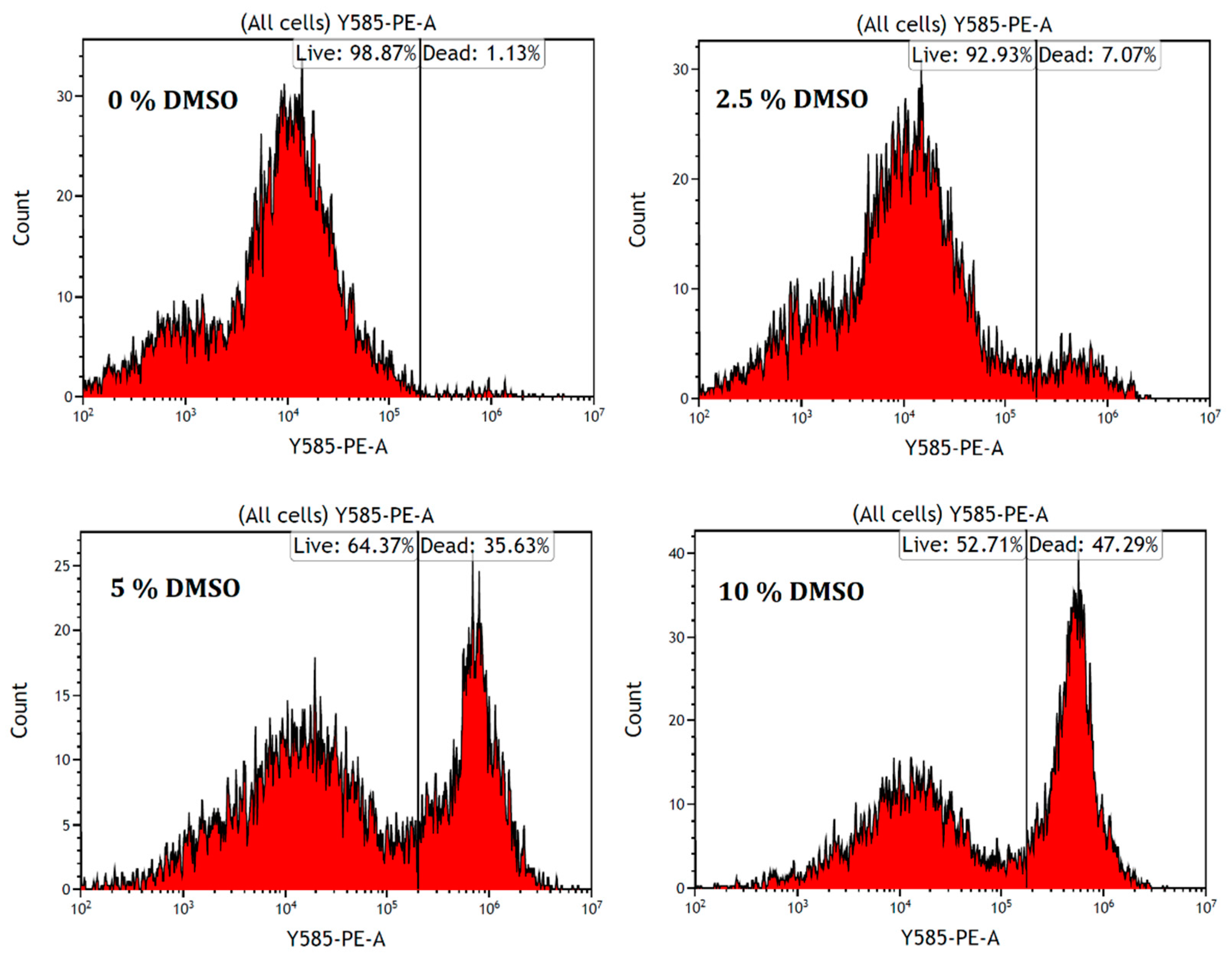
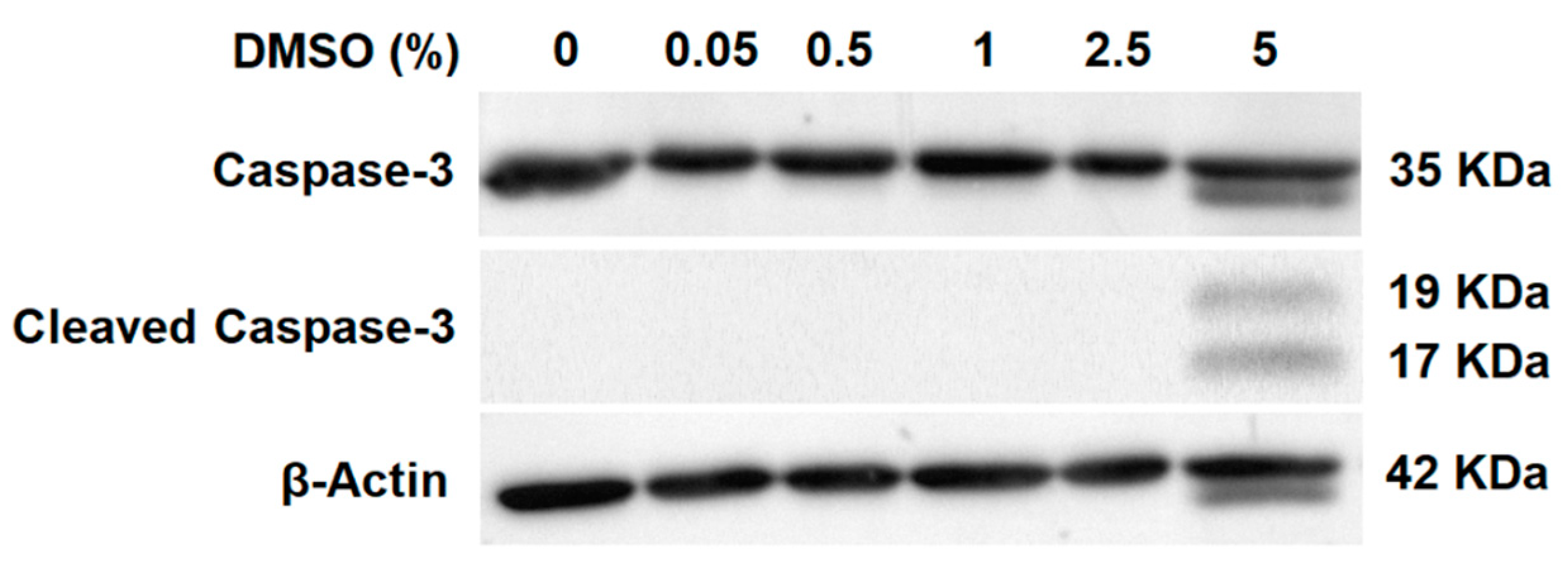
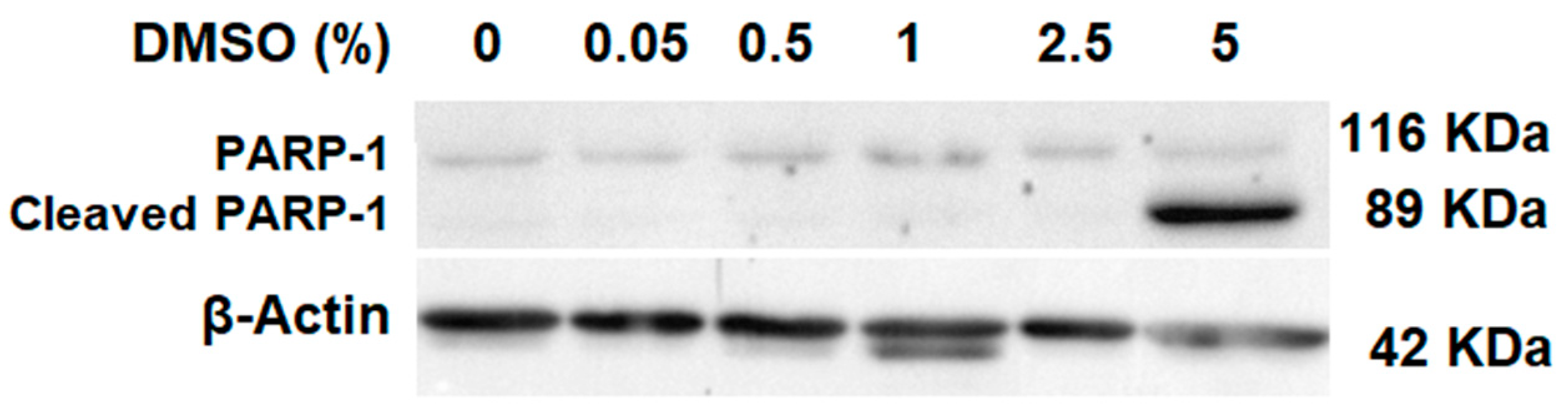
2.1. Cell Death Evaluation
2.2. Antiproliferative Assays
3. Conclusions
4. Materials and Methods
4.1. General
4.2. Preparation of Human RA Fibroblast-like Synoviocytes
4.3. Culture of Human RA FLSs and Treatment
4.4. Flow Cytometry
4.5. Protein Extraction and Western Blot Assays
4.6. Antiproliferative Assays
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hazen, K.C. Influence of DMSO on antifungal activity during susceptibility testing in vitro. Diagn. Microbiol. Infect. Dis. 2013, 75, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, A.A.; Gardini, D.; Baquié, M.; Juillerat-Jeanneret, L.; Serkova, T.P.; Shevtsova, E.P.; Dyson, P.J. Organometallic anticancer agents that interfere with cellular energy processes: A subtle approach to inducing cancer cell death. Dalton Trans. 2013, 42, 2347–2350. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.L.; Leger, D.Y.; Vergne-Salle, P.; Therrien, B.; Liagre, B. Ruthenium-based assemblies incorporating tetrapyridylporphyrin panels: A photosensitizer delivery strategy for the treatment of rheumatoid arthritis by photodynamic therapy. Dalton Trans. 2022, 51, 9673–9680. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Villagrán, M.; Paulus, L.; Charissoux, J.L.; Sutour, S.; Vergne-Salle, P.; Leger, D.Y.; Liagre, B.; Therrien, B. Evaluation of ruthenium-based assemblies as carriers of photosensitizers to treat rheumatoid arthritis by photodynamic therapy. Pharmaceutics 2021, 13, 2104. [Google Scholar] [CrossRef]
- Gallardo-Villagrán, M.; Paulus, L.; Champavier, Y.; Leger, D.Y.; Therrien, B.; Liagre, B. Combination of tetrapyridylporphyrins and arene ruthenium (II) complexes to treat synovial sarcoma by photodynamic therapy. J. Porphyr. Phthalocyanines 2021, A–I. [Google Scholar] [CrossRef]
- Roldán-Fidalgo, A.; Trinidad, A.; Rodríguez-Valiente, A.; García-Berrocal, J.R.; Millán, I.; Coronado, M.J.; Ramírez-Camacho, R. Effect of intratympanic dimethyl sulphoxide (DMSO) in an in vivo model of cisplatin-related ototoxicity. Eur. Arch. Oto-Rhino Laryngol. 2014, 271, 3121–3126. [Google Scholar] [CrossRef]
- Santos, N.C.; Figueira-Coelho, J.; Martins-Silva, J.; Saldanha, C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003, 65, 1035–1041. [Google Scholar] [CrossRef]
- Wohnhaas, C.T.; Leparc, G.G.; Fernandez-Albert, F.; Kind, D.; Gantner, F.; Viollet, C.; Hildebrandt, T.; Baum, P. DMSO cryopreservation is the method of choice to preserve cells for droplet-based single-cell RNA sequencing. Sci. Rep. 2019, 9, 10699. [Google Scholar] [CrossRef] [Green Version]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Eroglu, A.; Bailey, S.E.; Toner, M.; Toth, T.L. Successful cryopreservation of mouse oocytes by using low concentrations of trehalose and dimethylsulfoxide. Biol. Reprod. 2009, 80, 70–78. [Google Scholar] [CrossRef] [Green Version]
- Salim, A.S. Role of oxygen-derived free radical scavengers in the management of recurrent attacks of ulcerative colitis: A new approach. J. Lab. Clin. Med. 1992, 119, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Long, D.M. Comparative effects of direct and indirect hydroxyl radical scavengers on traumatic brain oedema. In Brain Edema VIII; Springer: Vienna, Austria, 1990; pp. 74–76. [Google Scholar] [CrossRef]
- Smith, R.S. A comprehensive macrophage-T-lymphocyte theory of schizophrenia. Med. Hypotheses 1992, 39, 248–257. [Google Scholar] [CrossRef]
- Patra, M.; Joshi, T.; Pierroz, V.; Ingram, K.; Kaiser, M.; Ferrari, P.-D.D.S.; Spingler, P.-D.D.B.; Keiser, J.; Gasser, G. DMSO-Mediated Ligand Dissociation: Renaissance for Biological Activity of N-Heterocyclic-[Ru(η6-arene)Cl2] Drug Candidates. Eur. J. Chem. 2013, 19, 14768–14772. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.D.; Telma, K.A.; Chang, K.-E.; Lee, T.D.; Madigan, J.P.; Lloyd, J.R.; Goldlust, I.S.; Hoeschele, J.D.; Gottesman, M.M. Say No to DMSO: Dimethylsulfoxide Inactivates Cisplatin, Carboplatin, and Other Platinum Complexes. Cancer Res. 2014, 74, 3913–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammler, D.H. The effect of DMSO on several enzyme systems. Ann. N. Y. Acad. Sci. 1967, 141, 291–299. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Nguyen, H.T.L.; Truong, K.D. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.B. Evaluation of the effects of dimethylsulphoxide on morphology, cellular viability, mRNA, and protein expression of stem cells culture in growth media. Biomed. Rep. 2017, 7, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Elisia, I.; Nakamura, H.; Lam, V.; Hofs, E.; Cederberg, R.; Cait, J.; Hughes, M.R.; Lee, L.; Jia, W.; Adomat, H.H.; et al. DMSO represses inflammatory cytokine production from human blood cells and reduces autoimmune arthritis. PLoS ONE 2019, 11, e0152538. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Martin, D.; Weise, A.; Niclas, H.J. The solvent dimethyl sulfoxide. Angew. Chem. Int. Ed. 1967, 6, 318–334. [Google Scholar] [CrossRef]
- Schläfer, H.L.; Schaffernicht, W. Dimethylsulfoxyd als Lösungsmittel für anorganische Verbindungen. Angew. Chem. 1960, 72, 618–626. [Google Scholar] [CrossRef]
- Huang, H.-L.; Hsing, H.-W.; Lai, T.-C.; Chen, Y.-W.; Lee, T.-R.; Chan, H.-T.; Lyu, P.-C.; Wu, C.-L.; Lu, Y.-C.; Lin, S.-T.; et al. Trypsin-induced proteome alteration during cell subculture in mammalian cells. J. Biomed. Sci. 2010, 17, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soler, A.P.; Knudsen, K.A.; Tecson-Miguel, A.; McBrearty, F.X.; Han, A.C.; Salazar, H. Expression of E-cadherin and N-cadherin in surface epithelial-stromal tumors of the ovary distinguishes mucinous from serous and endometrioid tumors. Hum. Pathol. 1997, 28, 734–739. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, J.; Pop, C.; Scott, F.L.; Drag, M.; Swartz, P.; Mattos, C.; Salvesen, G.S.; Clark, A.C. A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem. J. 2009, 424, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Parrish, A.B.; Freel, C.D.; Kornbluth, S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb. Perspect. Biol. 2013, 5, a008672. [Google Scholar] [CrossRef]
- Amé, J.C.; Spenlehauer, C.; de Murcia, G. The PARP superfamily. BioEssays 2004, 26, 882–893. [Google Scholar] [CrossRef]
- Hassa, P.O.; Covic, M.; Hasan, S.; Imhof, R.; Hottiger, M.O. The enzymatic and DNA binding activity of PARP-1 are not required for NF-κB coactivator function. J. Biol. Chem. 2001, 276, 45588–45597. [Google Scholar] [CrossRef] [Green Version]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly (ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [Green Version]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; McShane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S.; et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988, 31, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Liagre, B.; Vergne-Salle, P.; Corbiere, C.; Charissoux, J.L.; Beneytout, J.L. Diosgenin, a plant steroid, induces apoptosis in human rheumatoid arthritis synoviocytes with cyclooxygenase-2 overexpression. Arthritis Res. Ther. 2004, 6, R373–R383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glant, T.T.; Jacobs, J.J.; Molnár, G.; Shanbhag, A.S.; Valyon, M.; Galante, J.O. Bone resorption activity of particulate-stimulated macrophages. J. Bone Miner. Res. 1993, 8, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
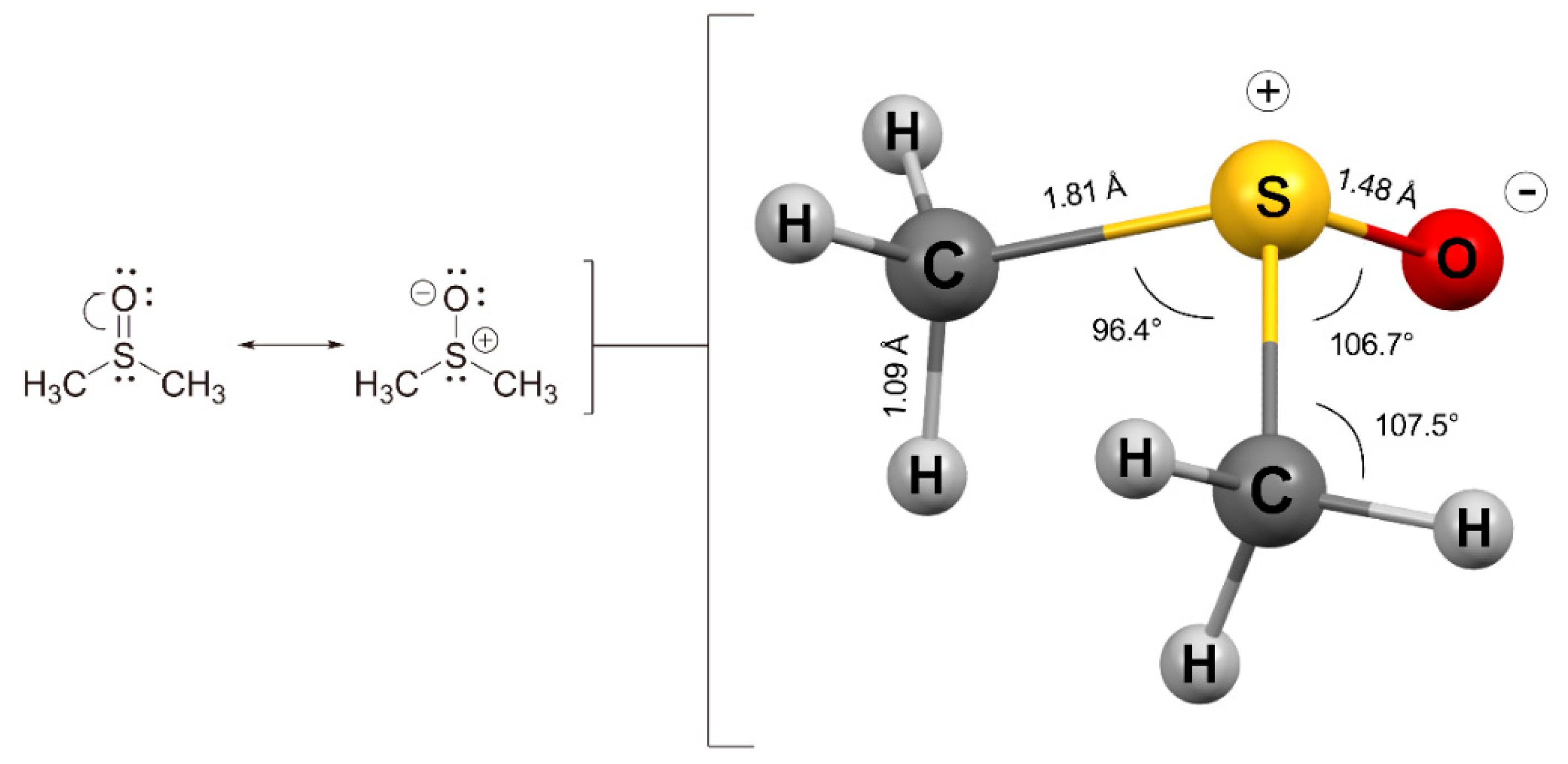


| Water | DMSO | |
|---|---|---|
| Chemical formula | H2O | C2H6OS |
| Appearance | Colorless liquid | Colorless liquid |
| Molecular weight | 18.02 | 78.13 |
| Polarity | 1.000 | 0.444 |
| Boiling point | 100.0 | 189.0 |
| Melting point | 0.0 | 17.9 |
| Density (g/mL) | 0.9982 | 1.1010 |
| Viscosity (cP) at 20 °C | 1.002 | 1.996 |
| Dipole moment (Debye) | 1.850 | 3.960 |
| Ebullioscopy constant (K·kg/mol) | 0.513 | 3.220 |
| Cryoscopic constant (K·kg/mol) | 1.860 | 3.850 |
| Specific heat at 25 °C (cal/g) | 1.000 | 0.470 |
| pKa (25 °C) | 14.0 | 35.1 |
| Flash point (open dish, °C) | n/a | 95 |
| Dielectric constant at 20 °C | 80.4 | 48.9 |
| Refractive index | 1.4793 | 1.333 |
| Point group | C2v | Cs |
| Entry | DMSO (%) | 60F | 65H | 69P | 71F | 72F | Average (%) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 100.0 ± 9.7 | 100.0 ± 8.8 | 100.0 ± 7.8 | 100.0 ± 4.1 | 100.0 ± 7.1 | 100.0 ± 7.6 |
| 2 | 0.01 | 99.6 ± 5.7 | 98.7 ± 7.1 | 99.8 ± 4.5 | 100.9 ± 3.9 | 98.6 ± 8.4 | 99.7 ± 5.9 |
| 3 | 0.05 | 97.1 ± 2.7 | 96.4 ± 7.8 | 91.8 ± 6.1 | 95.6 ± 8.7 | 95.4 ± 6.7 | 95.5 ± 6.4 |
| 4 | 0.1 | 89.2 ± 8.0 | 86.7 ± 7.1 | 85.6 ± 4.4 | 87.7 ± 9.6 | 85.4 ± 4.0 | 86.6 ± 6.6 |
| 5 | 0.5 | 74.1 ± 5.1 | 70.9 ± 9.4 | 69.6 ± 8.0 | 72.9 ± 5.6 | 70.5 ± 2.5 | 71.7 ± 6.1 |
| 6 | 1 | 61.4 ± 7.5 | 60.2 ± 7.7 | 58.9 ± 7.3 | 64.1 ± 5.2 | 60.4 ± 5.1 | 62.2 ± 6.6 |
| 7 | 5 | 9.8 ± 1.6 | 11.5 ± 7.3 | 2.5 ± 3.5 | 6.4 ± 7.0 | 1.4 ± 2.5 | 3.9 ± 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. https://doi.org/10.3390/molecules27144472
Gallardo-Villagrán M, Paulus L, Leger DY, Therrien B, Liagre B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules. 2022; 27(14):4472. https://doi.org/10.3390/molecules27144472
Chicago/Turabian StyleGallardo-Villagrán, Manuel, Lucie Paulus, David Yannick Leger, Bruno Therrien, and Bertrand Liagre. 2022. "Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes" Molecules 27, no. 14: 4472. https://doi.org/10.3390/molecules27144472
APA StyleGallardo-Villagrán, M., Paulus, L., Leger, D. Y., Therrien, B., & Liagre, B. (2022). Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules, 27(14), 4472. https://doi.org/10.3390/molecules27144472








