Interfacial Adhesion between Fatty Acid Collectors and Hydrophilic Surfaces: Implications for Low-Rank Coal Flotation
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Density Functional Theory (DFT) Calculation
2.2. Molecular Dynamics (MD) Simulation
2.3. Atomic Force Microscopy (AFM) Test
2.4. Coal Surface Characteristics Tests
2.5. Flotation Tests
3. Results and Discussion
3.1. Interaction between the Low-Rank Coal Surface and Collectors Using DFT Calculations
3.2. Molecular Adsorption Structure of the Collectors
3.3. Analysis of AFM Force Measurement Results
3.4. Changes in the Coal Surface Properties after Adsorption of Collectors
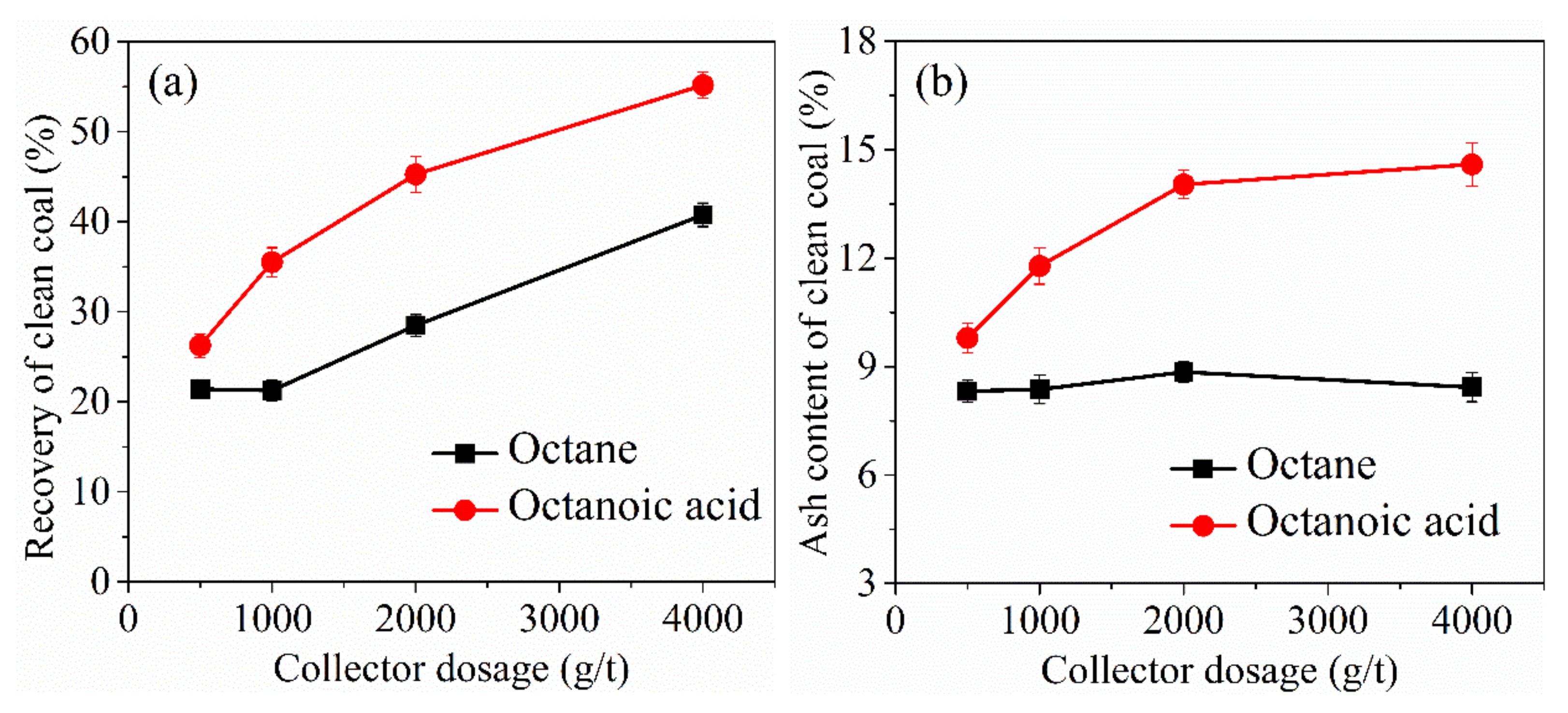
3.5. Implications for Low-Rank Coal Flotation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Osman, H.; Jangam, S.V.; Lease, J.D.; Mujumdar, A.S. Drying of low-rank coal (LRC)-a review of recent patents and innovations. Dry. Technol. 2011, 29, 1763–1783. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Yu, J.; Han, Y.; Li, X. A study of chemical structure changes of Chinese lignite during fluidized-bed drying in nitrogen and air. Fuel Processing Technol. 2012, 101, 85–93. [Google Scholar] [CrossRef]
- Gui, X.; Liu, J.; Cao, Y.; Miao, Z.; Li, S.; Xing, Y.; Wang, D. Coal preparation technology: Status and development in China. Energy Environ. 2015, 26, 26997–27013. [Google Scholar] [CrossRef]
- Li, X.; Song, H.; Wang, Q.; Meesri, C.; Wall, T.; Yu, J. Experimental study on drying and moisture re-adsorption kinetics of an Indonesian low rank coal. J. Environ. Sci. 2009, 21, S127–S130. [Google Scholar] [CrossRef]
- Sivrikaya, O. Cleaning study of a low-rank lignite with DMS, Reichert spiral and flotation. Fuel 2014, 119, 252–258. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, D.; Zhang, H. Clean low-rank-coal purification technique combining cyclonic-static microbubble flotation column with collector emulsification. J. Clean. Prod. 2017, 153, 657–672. [Google Scholar] [CrossRef]
- Fang, S.; Xu, L.; Wu, H.; Tian, J.; Lu, Z.; Sun, W.; Hu, Y. Adsorption of Pb(II)/benzohydroxamic acid collector complexes for ilmenite flotation. Miner. Eng. 2018, 126, 16–23. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Zhang, R.; Yang, Z.; Xing, Y.; Gui, X.; Cao, Y.; Sun, W. Enhancement of flotation response of fine low-rank coal using positively charged microbubbles. Fuel 2019, 245, 505–513. [Google Scholar] [CrossRef]
- Luo, L.; Wu, H.; Xu, L.; Meng, J.; Lu, J.; Zhou, H.; Huo, X.; Huang, L. An in situ ATR-FTIR study of mixed collectors BHA/DDA adsorption in ilmenite-titanaugite flotation system. Int. J. Min. Sci. Technol. 2021, 31, 689–697. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Yang, S. Effects of barite size on the fluorite flotation using the reagent scheme of GS/NaOI. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 626, 127101. [Google Scholar] [CrossRef]
- Çınar, M. Floatability and desulfurization of a low-rank (Turkish) coal by low-temperature heat treatment. Fuel Processing Technol. 2009, 90, 1300–1304. [Google Scholar] [CrossRef]
- Harris, G.H.; Diao, J.; Fuerstenau, D.W. Coal flotation with nonionic surfactants. Coal Prep. 1995, 16, 135–147. [Google Scholar] [CrossRef]
- Polat, M.; Polat, H.; Chander, S. Physical and chemical interactions in coal flotation. Int. J. Mineral. Processing 2003, 72, 199–213. [Google Scholar] [CrossRef] [Green Version]
- Ateşok, G.; Çelik, M.S. A new flotation scheme for a difficult-to-float coal using pitch additive in dry grinding. Fuel 2000, 79, 1509–1513. [Google Scholar] [CrossRef]
- Ren, H.; Liao, Y.; Yang, Z.; An, M.; Hao, X.; Song, X.; Liu, Z. Effect of Fe2+ on low rank coal flotation using oleic acid as collector. Powder Technol. 2021, 393, 250–256. [Google Scholar] [CrossRef]
- Yu, J.; Tahmasebi, A.; Han, Y.; Yin, F.; Li, X. A review on water in low rank coals: The existence, interaction with coal structure and effects on coal utilization. Fuel Processing Technol. 2013, 106, 9–20. [Google Scholar] [CrossRef]
- Liu, X.; Liu, S.; Fan, M.; Zhang, L. Decrease of hydrophilicity of lignite using CTAB: Effects of adsorption differences of surfactant onto mineral composition and functional groups. Fuel 2017, 197, 474–481. [Google Scholar] [CrossRef]
- Lyu, X.; You, X.; He, M.; Zhang, W.; Wei, H.; Li, L.; He, Q. Adsorption and molecular dynamics simulations of nonionic surfactant on the low rank coal surface. Fuel 2018, 211, 529–534. [Google Scholar] [CrossRef]
- Xia, Y.; Rong, G.; Xing, Y.; Gui, X. Synergistic adsorption of polar and nonpolar reagents on oxygen-containing graphite surfaces: Implications for low-rank coal flotation. J. Colloid Interface Sci. 2019, 557, 276–281. [Google Scholar] [CrossRef]
- Dey, S. Enhancement in hydrophobicity of low rank coal by surfactants-A critical overview. Fuel Processing Technol. 2012, 94, 151–158. [Google Scholar] [CrossRef]
- Singh, B.P.; Besra, L.; Reddy, P.S.R.; Sengupta, D.K. Use of surfactants to aid the dewatering of fine clean coal. Fuel 1998, 77, 1349–1356. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Xing, Y.; Gui, X. Improving the adsorption of oily collector on the surface of low-rank coal during flotation using a cationic surfactant: An experimental and molecular dynamics simulation study. Fuel 2019, 235, 687–695. [Google Scholar] [CrossRef]
- Chander, S.; Polat, H.; Polat, M. Mechanism of coal flotation by insoluble collectors in the presence of PEO/PPO/PEO block co-polymers. In Proceedings of the 6th International Mineral Processing Symposium, Kusadasi, Turkey, 24–26 September 1996; pp. 461–467. [Google Scholar]
- Polat, H.; Chander, S. Interaction between physical and chemical variables in the flotation of low rank coals. Miner. Metall. Processing 1998, 15, 41–47. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.H.; Fuerstenau, D.W. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Processing 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Cebeci, Y. The investigation of the floatability improvement of Yozgat Ayrdam lignite using various collectors. Fuel 2002, 81, 281–289. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, X.; Peng, Y. The interaction between diesel and surfactant Triton X-100 and their adsorption on coal surfaces with different degrees of oxidation. Powder Technol. 2019, 342, 840–847. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, R.; Cao, Y.; Xing, Y.; Gui, X. Role of molecular simulation in understanding the mechanism of low-rank coal flotation: A review. Fuel 2020, 262, 116535. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef]
- Liu, S.; Xie, L.; Liu, J.; Liu, G.; Zhong, H.; Wang, Y.; Zeng, H. Probing the interactions of hydroxamic acid and mineral surfaces: Molecular mechanism underlying the selective separation. Chem. Eng. J. 2019, 374, 123–132. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; Mantilla, C.A.; van den Berg, F.G.A.; Zeng, H. Interaction mechanism of oil-in-water emulsions with asphaltenes determined using droplet probe AFM. Langmuir 2016, 32, 2302–2310. [Google Scholar] [CrossRef]
- Zhang, Z.; Yun, T.; Zhang, H.; Yan, K. Density function study of the interaction of a surface modifier with the oxidized coal surface model. ACS Omega 2018, 3, 14585–14591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, Y.; Li, C.; Gui, X.; Cao, Y. Interaction forces between paraffin/stearic acid and fresh/oxidized coal particles measured by atomic force microscopy. Energy Fuels 2017, 31, 3305–3312. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Yang, H.; Xu, S.; Xie, Q.; Zhang, M.; Chen, T.; Hu, G.; Wang, J. Effect of specific functional groups on oil adhesion from mica substrate: Implications for low salinity effect. J. Ind. Eng. Chem. 2016, 17, 342–349. [Google Scholar] [CrossRef]
- Feng, L.; Manica, R.; Grundy, J.S.; Liu, Q. Unraveling Interaction Mechanisms between Molybdenite and a Dodecane Oil Droplet Using Atomic Force Microscopy. Langmuir 2019, 35, 6024–6031. [Google Scholar] [CrossRef]
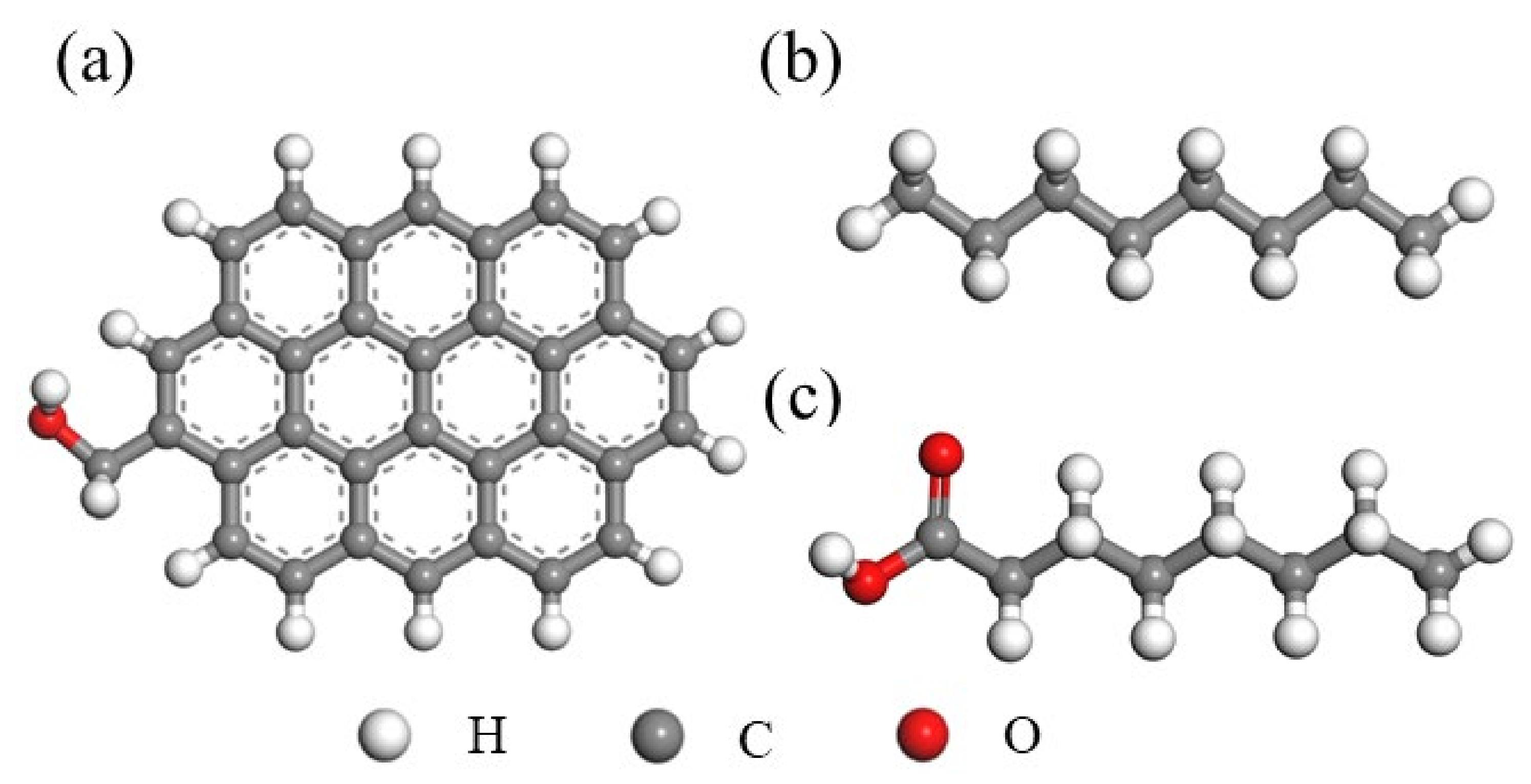



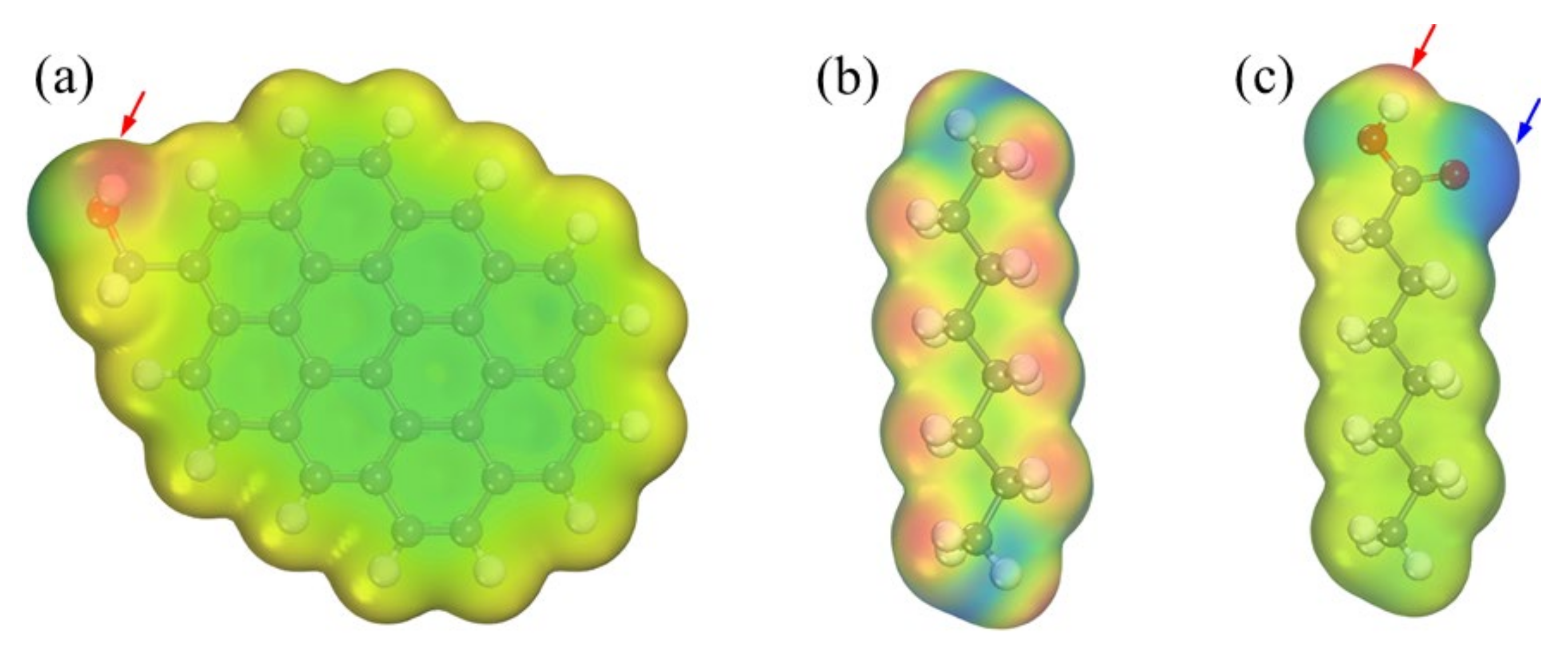


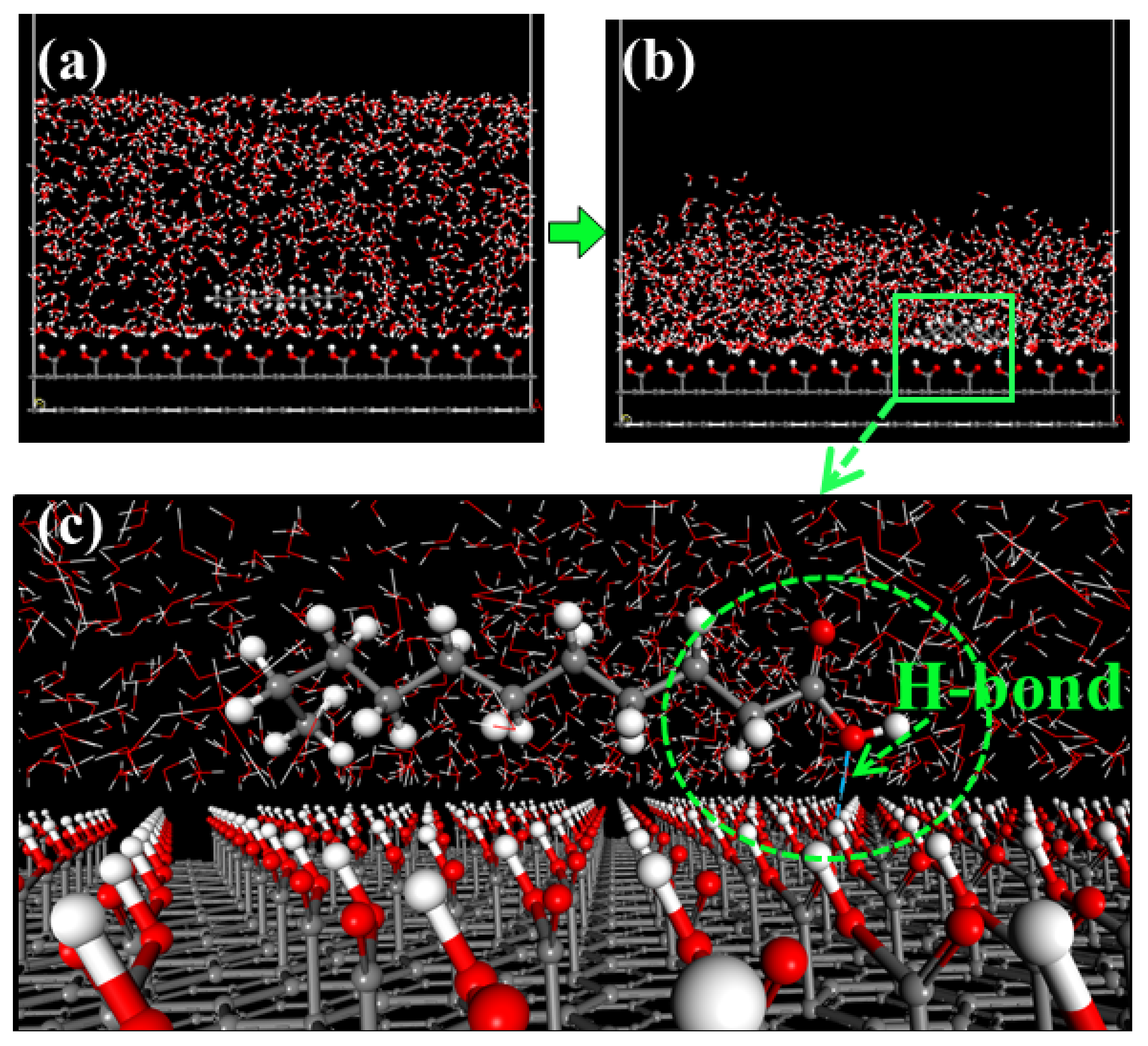

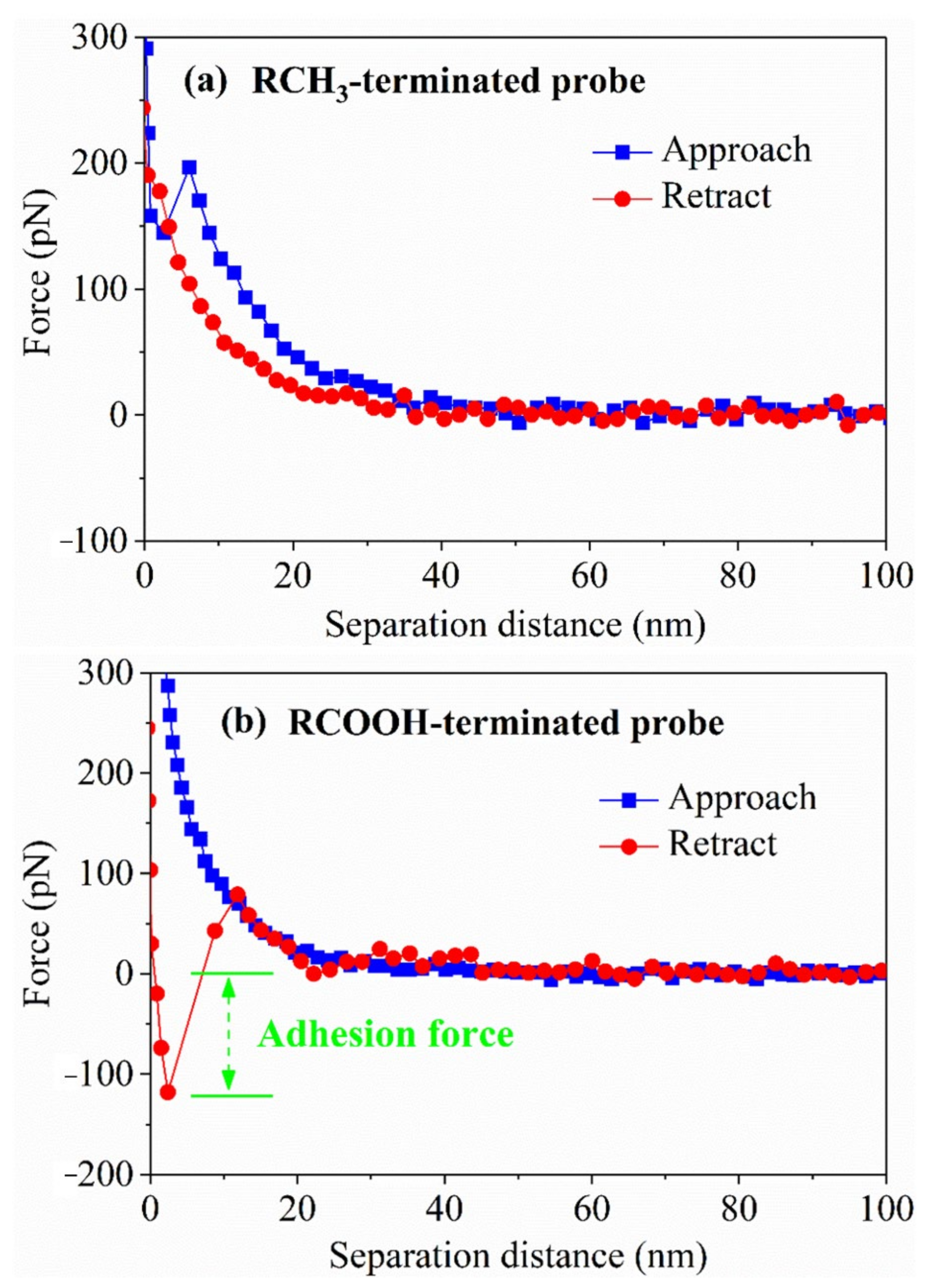

| Size (mm) | Yield (%) | Ash (%) |
|---|---|---|
| 0.5~0.25 | 39.93 | 14.16 |
| 0.25~0.125 | 17.12 | 11.76 |
| 0.125~0.074 | 13.74 | 12.80 |
| 0.074~0.045 | 9.09 | 12.93 |
| <0.045 | 20.12 | 24.16 |
| Total | 100.00 | 15.46 |
| Coal | Carbon Form (Relative % of C 1s) | |||
|---|---|---|---|---|
| C-C/C-H | C-O | C=O | O=C-O | |
| Raw coal | 77.89 | 13.66 | 4.36 | 4.10 |
| Coal-octane | 80.20 | 14.30 | 2.58 | 2.92 |
| Coal-octanoic acid | 83.42 | 12.48 | 2.50 | 1.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Fang, D.; Qu, P.; Li, Y. Interfacial Adhesion between Fatty Acid Collectors and Hydrophilic Surfaces: Implications for Low-Rank Coal Flotation. Molecules 2022, 27, 4392. https://doi.org/10.3390/molecules27144392
Xia Y, Fang D, Qu P, Li Y. Interfacial Adhesion between Fatty Acid Collectors and Hydrophilic Surfaces: Implications for Low-Rank Coal Flotation. Molecules. 2022; 27(14):4392. https://doi.org/10.3390/molecules27144392
Chicago/Turabian StyleXia, Yangchao, Dan Fang, Pengcheng Qu, and Yonggai Li. 2022. "Interfacial Adhesion between Fatty Acid Collectors and Hydrophilic Surfaces: Implications for Low-Rank Coal Flotation" Molecules 27, no. 14: 4392. https://doi.org/10.3390/molecules27144392
APA StyleXia, Y., Fang, D., Qu, P., & Li, Y. (2022). Interfacial Adhesion between Fatty Acid Collectors and Hydrophilic Surfaces: Implications for Low-Rank Coal Flotation. Molecules, 27(14), 4392. https://doi.org/10.3390/molecules27144392






