Abstract
This study aimed to develop, characterize, and evaluate antibacterial and cytotoxic properties of novel fullerene derivative composed of C60 fullerenol and standard aminoglycoside antibiotic–gentamicin (C60 fullerenol-gentamicin conjugate). The successful introduction of gentamicin to fullerenol was confirmed by X-ray photoelectron spectroscopy which together with thermogravimetric and spectroscopic analysis revealing the formula of the composition as C60(OH)12(GLYMO)11(Gentamicin)0.8. The dynamic light scattering (DLS) revealed that conjugate possessed ability to form agglomerates in water (size around 115 nm), while Zeta potential measurements demonstrated that such agglomerates possessed neutral character. In vitro biological assays indicated that obtained C60 fullerenol-gentamicin conjugate possessed the same antibacterial activity as standard gentamicin against Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Escherichia coli, which proves that combination of fullerenol with gentamicin does not cause the loss of antibacterial activity of antibiotic. Moreover, cytotoxicity assessment demonstrated that obtained fullerenol-gentamicin derivative did not decrease viability of normal human fibroblasts (model eukaryotic cells) compared to control fibroblasts. Thus, taking into account all of the results, it can be stated that this research presents effective method to fabricate C60 fullerenol-gentamicin conjugate and proves that such derivative possesses desired antibacterial properties without unfavorable cytotoxic effects towards eukaryotic cells in vitro. These promising preliminary results indicate that obtained C60 fullerenol-gentamicin conjugate could have biomedical potential. It may be presumed that obtained fullerenol may be used as an effective carrier for antibiotic, and developed fullerenol-gentamicin conjugate may be apply locally (i.e., at the wound site). Moreover, in future we will evaluate possibility of its applications in inter alia tissue engineering, namely as a component of wound dressings and implantable biomaterials.
1. Introduction
The fullerenes, a family of carbon allotropes, represent very promising group of chemical molecules in the context of biomedical applications [1,2,3,4,5]. In this case, their hydrophilic derivatives have been gained the special scientific attention, thanks to water solubility [2] and biological activities, such as anti-oxidative and radical-quenching [6,7], antibacterial [8,9], antiviral [10,11], DNA photocleavage [12,13] as well as enzyme-inhibiting [14,15,16] or neuroprotective [17,18] properties. Thus, thanks to aforementioned features, it was suggested that water-soluble fullerene derivatives could be potentially used as antioxidant agents, therapeutics for the treatment of HIV infection, and also as photosensitizers or drug carriers [1,3,19,20].
The applications of water-soluble fullerenes as drug delivery vehicles are especially focused on anticancer therapy [1,3,21,22]. For instance, Chaudhuri et al. [23] demonstrated that a very popular chemotherapeutic agent–doxorubicin (DOX) together with polyethylene glycol (PEG) may be conjugated with polyhydroxylated fullerene (PHF) C60, which allows to obtain nanocomplex with high therapeutics efficacy and lower cytotoxicity compared to free doxorubicin. PHFs were also linked with other anti-cancer drug, namely methotrexate (MTX). Bahuguna et al. [24] received FLU-MTX nanocomplex, which was characterized by increased availability of drug to the biological system and increased cytotoxicity toward cancer cells compared to free MTX. In turn, Prylutska et al. [25] developed anti-cancer nanocomplex consisted of C60 fullerene aqueous colloid solution (C60FAS) and cisplatin (Cis). The authors showed that C60 + Cis nanocomplex exhibited higher toxicity towards human leukemia cells compared to cisplatin alone. Moreover, in vivo study with Lewis lung carcinoma (LLC) C57BL/6J male mice demonstrated that C60 + Cis conjugate inhibited tumor growth more potently than free cisplatin.
Nevertheless, to the best of our knowledge, there are no research reports which present adducts composed of fullerenols and antimicrobials. Such composition seems to be promising thanks to biological activities of fullerenols and antimicrobial agents. In other words, we assumed that C60 fullerenol will be not only a nanocarrier for gentamicin, but also may exhibit beneficial biological properties (for instance antioxidant or free-radical scavenging). It is especially important as an application of antibiotics most often is associated with unfavorable side effects. It is known that aminoglycoside antibiotics (such as gentamicin) may cause nephrotoxicity (damage of kidneys) and ototoxicity (damage of hearing organ) [26,27]. Thus, our preliminary study aimed to develop novel fullerene derivative composed of C60 fullerenol and standard aminoglycoside antibiotic–gentamicin. Desired product was synthesized by binding (3-glycidyloxypropyl)trimethoxysilane linker to fullerenol surface using part of its hydroxyl groups and further reaction of introduced oxirane moieties with gentamicin –NH2 groups. Synthesized nanomaterial was evaluated using X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FT-IR), thermogravimetry (TGA), and dynamic light scattering (DLS). This nanoconjugate was also subjected to initial evaluation of biological properties. The antibacterial activity of fullerenol-gentamicin nanocarrier was assessed towards Gram-positive and Gram-negative bacterial strains (i.e., Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa, and Escherichia coli, respectively). Moreover, its cytotoxicity was estimated using model eukaryotic cells–normal human skin fibroblasts (BJ cell line). To our best knowledge, it is first research, which demonstrates effective method allowing to conjugate fullerenol with aminoglycoside antibiotic.
2. Results and Discussion
2.1. Characterization of C60 Fullerenol-Gentamicin Derivative
Composition of synthesized C60 fullerenol-gentamicin conjugate was investigated using X-ray photoelectron spectroscopy (XPS). Survey XPS spectrum obtained for the newly synthesized fullerene derivative revealed the presence of carbon, oxygen, nitrogen, and silicon atoms, namely elements expected for gentamicin functionalized fullerenol (see Supplementary Material, Figure S1).
C1s spectrum obtained for C60 fullerenol-gentamicin derivative (60FGG) (Figure 1a) was deconvoluted with very good correlation into four signals [28]. The lowest binding energy peak, centered at 284.6 eV is assigned to sp2 carbon from fullerene cage. Second signal was registered at 285.6 eV and can be attributed to the sp3 carbon. Next contribution (at 286.6eV) was ascribed to C-O carbon atoms from fullerenol, linker, and gentamicin moieties. The highest binding energy peak, arising from C = O carbon atoms, was registered at 288.0 eV and suggests that traces of hydroxyl groups present at the C60 surface were oxidized during the synthesis process.
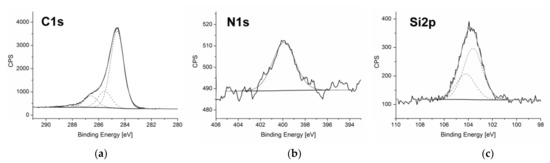
Figure 1.
The XPS spectra obtained for C60 fullerenol-gentamicin derivative (60FGG)–C1s spectrum (a), N1s core level region (b), and Si2p region (c).
Single peak observed in the N1s core level region (Figure 1b) with maximum located at 399.9 eV is assigned to the nitrogen atoms from amino groups of gentamicin bound to the fullerenol derivative surface. This result confirms successful introduction of aforementioned aminoglycoside antibiotic.
Another strong evidence of the modification of fullerenol surface was provided by Si2p region of XPS spectrum (Figure 1c), where double peak is observed with signals centered at 103.6 and 104.2 eV assigned to 2p3/2 and 2p1/2 silicon atoms from the GLYMO linker [29]. Those signals show expected area ratio of 2:1 and splitting of 1.2 eV.
Additionally, lack of signals associated with sulfur atoms implies that introduced gentamicin molecules were neutralized during the final step of synthesis. Released sulfate ions were removed during the dialysis process.
The XPS results along with thermogravimetric measurements [30] (Figure 2) allowed to calculate the approximate composition of fullerenol, its GLYMO derivative, and synthesized fullerenol-gentamicin conjugate. Single step thermal decomposition of 60F resulted in weight loss of 35%, which corresponds to 23 hydroxyl groups. GLYMO functionalized fullerenol and its gentamicin adduct revealed more complex TGA curves, both with shape close to two step decomposition. Total weight loss was determined to be 77% in case of 60FG and 80% for 60FGG. Thus, final composition of synthesized nanomaterial was estimated to be C60(OH)12(GLYMO)11(Gentamicin)0.8.
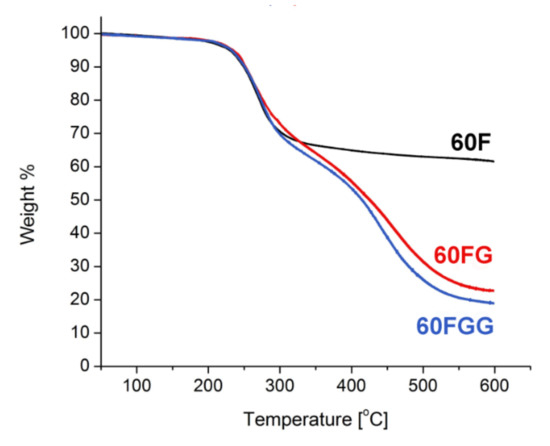
Figure 2.
Thermogravimetric analysis of fullerenol (60F), GLYMO modified fullerenol (60FG), and gentamicin functionalized C60 fullerene (60FGG) at 5 K/min under N2 atmosphere.
Functionalization of C60 fullerenol (60F) with GLYMO allowed to form 60FG and then to introduce gentamicin molecules in order to obtain desired conjugate, i.e., 60FGG that was also confirmed by means of FT-IR spectroscopy (Figure 3). First of all, 60FGG spectrum shows broad band from 3700 to 2400 cm−1, characteristic to gentamicin substrate. Despite relatively strong intensity of this signal, small peak at 2842 cm−1, also observed in 60FG spectrum is still present, indicating that final product has 60FG contributions associated with its alkane C-H stretching modes. Additionally, amine N-H bending mode is present at around 1635 cm−1 in both gentamicin functionalized fullerene and unmodified gentamicin samples. This band overlaps with signals attributed to C=C stretching vibrations, which are present in obtained fullerene derivatives at around 1600 cm−1. Presence of signal centered around 1420 cm−1 in all fullerene related samples: 60F, 60FG and 60FGG confirms presence of C60 core in those products [31]. Broad band at around 1130 cm−1 present in both 60FGG and G samples can be associated with C-O stretching vibrations coming from secondary and tertiary alcohol groups present in the gentamicin structure. Spectrum of 60FG shows band at 826 cm−1, which can be assigned to asymmetric ring deformation of epoxide groups [32,33,34], coming from introduced GLYMO linkers. Intensity of this signal is significantly lowered in the sample after reaction with gentamicin, which is in good agreement with expected reaction pathway, leading to epoxide ring opening due to reaction with amino groups from antibiotic molecules. Another very important signal, which is associated with gentamicin moiety can be observed in 60FGG spectrum at 614 cm−1, additionally confirming presence of the aminoglycoside antibiotic in final product.
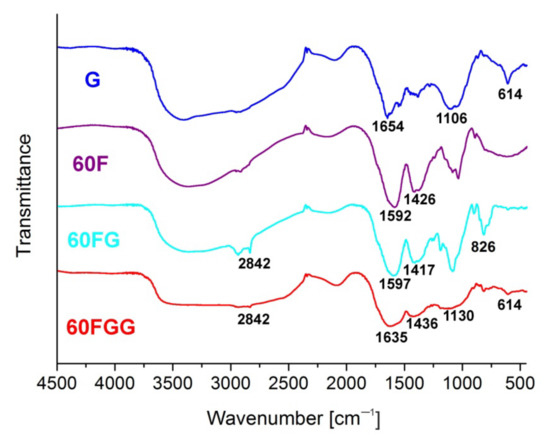
Figure 3.
FT-IR spectra recorded in KBr disk for: gentamicin (G), fullerenol (60F), GLYMO modified fullerenol (60FG), and gentamicin functionalized C60 fullerene (60FGG).
The dynamic light scattering (DLS) measurements allowed to determine the hydrodynamic diameter of the synthesized fullerene derivatives in water (Figure 4). Size distribution for 60F revealed single peak at approximately 92 nm, which is value typical for fullerenol associations [35,36,37]. Introduction of gentamicin onto fullerenol surface results in slight increase of the size of agglomerates, which was found to be around 115 nm.
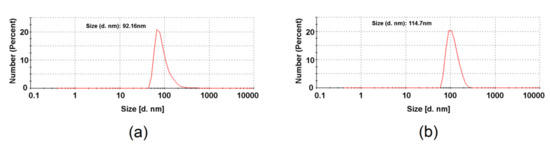
Figure 4.
Size distributions obtained from DLS measurements for: fullerenol 60F (a) and C60 fullerene gentamicin conjugate 60FGG (b).
DLS analysis was accompanied by Zeta potential measurements. Both fullerenol (60F) and gentamicin functionalized C60 fullerene (60FGG) revealed to form neutral agglomerates [38]. Corresponding Zeta potential values were determined to be −0.1 mV and 0.1 V for 60F and 60FGG, respectively (see Supplementary Material, Figures S2 and S3). Results obtained for C60 fullerenol-gentamicin conjugate are in good agreement with XPS data, which suggested that gentamicin was neutralized during 60FGG synthesis and purification process.
2.2. Antibacterial Properties of Gentamicin Functionalized C60 Fullerene Derivative
Firstly, antibacterial tests showed that hydroxylated C60 fullerene (60F) did not exhibit antibacterial activity towards all tested bacterial strain. Interestingly, some authors indicated that water-soluble C60 fullerene derivatives possessed antibacterial activities [8,9]. For instance, Deryabin et al. [8] developed water-soluble C60 fullerene derivatives bearing amine (AF) and carboxylic (CF) groups. The authors indicated that both derivatives had ability to form nanoclusters in aqueous solutions–diameters ranged from 2 to 200 nm (AF) and from 70 to 100 nm (CF) (based on DLS measurements). Nevertheless, only AF derivatives possessed antibacterial properties. The authors suggested that this phenomenon was associated with electrostatic interactions between fullerene derivatives and cells. Fullerene derivative (AF) with positive charge adhered to negatively charged bacterial cells, while negatively charged CF derivatives did not possess ability to interact with bacterial cells. Thus, it seems that charge of fullerene derivative has greater influence on antibacterial activity than particle size. In our study, we showed (DLS measurements, Section 2.1) that size distribution of 60F in water was 92 nm, while introduction of gentamicin onto fullerenol surface resulted in slight increase of the size of agglomerates, which was found to be around 115 nm. Thus, the particle size of both products (60F and 60FGG) was comparable. In turn, Zeta potential measurements showed that both possessed neutral charges. Thus, these results may explain the lack of bacterial properties of 60F–neutrally charged derivative rather should not have ability to adhere to negatively charged bacterial cells. Our study also demonstrated that both gentamicin sulfate salt (G) and conjugate composed of C60 fullerenol and gentamicin (60FGG) possessed the same activity–values of MIC and MBC for both compounds were identical and ranged from 0.125 to 1 μg/mL, depending on bacterial strain (Table 1 and Table 2). Thus, these results indicated, that conjugate composed of G and 60F did not exhibit greater antibacterial activity than G alone, but allowed to maintain the antibacterial properties of G. In other words, these antibacterial assays confirmed that newly developed method for fabrication of 60FGG allows to obtain effective, antibacterial agent. It is worth noting that the aim of this study is not to develop C60 fullerenol with antibacterial properties or C60 fullerenol-gentamicin conjugate with better activity than gentamicin alone. We aimed to develop nanoconjugate which will maintain antibacterial activity of gentamicin and will not exhibit cytotoxic properties towards eukaryotic cells.

Table 1.
MIC values for S. aureus, S. epidermidis, P. aeruginosa, and E. coli after 24 h incubation with aqueous solutions of gentamicin sulfate salt (G), C60 fullerenol (60F), and gentamicin functionalized C60 fullerene (60FGG).

Table 2.
MBC values for S. aureus, S. epidermidis, P. aeruginosa, and E. coli after treatment with aqueous solutions of gentamicin sulfate salt (G), C60 fullerenol (60F), and gentamicin functionalized C60 fullerene (60FGG).
2.3. Cytotoxic Properties of Gentamicin Functionalized C60 Fullerene
The MTT assay indicated that all tested solutions prepared from gentamicin sulfate salt (G), C60 fullerenol (60F), and gentamicin functionalized C60 fullerene (60FGG) were non-cytotoxic towards normal human skin fibroblasts (Figure 5). The cell viability after treatment with tested solutions of G, 60F, and 60FGG was comparable with viability of control cells (cultured without investigated compounds) and close to 100%. These results indicated that introduction of gentamicin to C60 fullerenol did not have unfavorable influence on cell behavior. Thus, obtained 60FGG conjugate possessed desired antibacterial activity (please see Section 2.2) and at the same time was safe for model eukaryotic cells (human fibroblasts) in vitro.
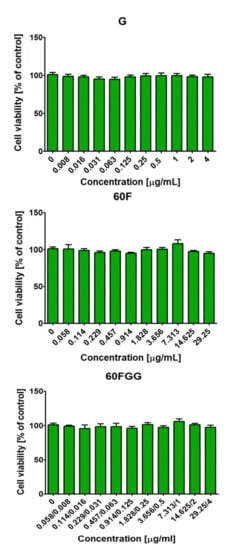
Figure 5.
Viability of normal human skin fibroblasts (BJ cell line, CRL-2522TM) after 24 h treatment with aqueous solutions of gentamicin sulfate salt (G), C60 fullerenol (60F), and gentamicin functionalized C60 fullerene (60FGG). Gentamicin (G) was evaluated at concentration of 4–0.008 μg/mL, C60 fullerenol (60F) at concentration of 29.25–0.058 μg/mL, while concentrations of gentamicin and C60 fullerenol in obtained conjugate (60FGG) ranged from 4–0.008 μg/mL and 29.25–0.058 μg/mL, respectively. The cell viability was assessed by MTT assay. The results were not statistically significant (p > 0.05) compared to control, namely cell incubated with culture medium without tested compounds; one-Way ANOVA test, followed by a Tukey’s multiple comparison test.
In general, water-soluble fullerene derivatives exhibit no or minimal cytotoxicity [19]. Nevertheless, it is also known that cytotoxicity of these derivatives may be controlled by inter alia derivatization of their surfaces. Thus, the increase in cytotoxicity of fullerene derivatives is suggested as a promising approach in antibacterial or cancer therapies [39]. Aforementioned positive-charged amine derivatives of fullerene (AF) (please see Section 2.2) possessed antibacterial activity thanks to their ability to adhere to negatively charged bacterial cells. Nevertheless, cell membrane of eukaryotic cells are also negative-charged, which suggests that such derivative will also adhere to these cells. Indeed, the authors of cited manuscript proved that AF derivatives adhered also to human erythrocytes, which suggests that they may have cytotoxic activity towards eukaryotic cells [8]. On the other hand, some authors demonstrated that water-soluble fullerene derivatives linked with anticancer agents exhibited increased cytotoxicity towards cancer cells compared to free anticancer agents [24,25]. However, they evaluated the influence of obtained derivatives only towards cancer cells. Thus, it is possible that such amine derivatives of fullerene may also have unfavorable influence on normal cells.
It is known that cytocompatibility is a mandatory feature of potential medicinal products [40,41]. Thus, non-cytotoxic 60FGG derivative seems to be promising candidate for further analysis.
3. Materials and Methods
The general plan of performed experiments was introduced in Figure 6.
Figure 6.
Schematic diagram presenting the experiments performed within this study.
3.1. Materials
Dimethylformamide (DMF), gentamicin sulfate salt, (3-glycidyloxypropyl)trimethoxysilane (GLYMO), methanol, penicillin-streptomycin solution, phosphate buffered saline (PBS), sodium dodecyl sulfate (SDS), sodium hydroxide, tetrabutylammonium hydroxide (TBAH), thiazolyl blue tetrazolium bromide (MTT), toluene, and trypsin-EDTA solution (0.25%) were obtained from Merck, Warsaw, Poland. Fetal bovine serum (FBS) was purchased from Pan-Biotech, Aidenbach, Germany. Eagle’s Minimum Essential Medium (EMEM), normal human skin fibroblasts (BJ cell line, CRL-2522TM), Staphylococcus aureus (ATCC 25923), Staphylococcus epidermidis (ATCC 12228), Pseudomonas aeruginosa (ATCC 27859), and Escherichia coli (ATCC 25922) were supplied by ATCC, Manassas, USA, while Mueller-Hinton agar (MHA) and Mueller-Hinton broth (MHB) by Oxoid, Hampshire, UK.
3.2. Synthesis and Characterization of C60 Fullerenol-Gentamicin Derivative
Gentamicin functionalized C60 fullerene (60FGG) was obtained in three-step synthesis. In the first step we obtained hydroxylated C60 using modified method reported by Li and Takeuchi [42]. Briefly, C60 (200 mg) was dissolved in 150 mL of toluene, then 10 mL of saturated NaOH solution was added, followed by addition of catalytic amount of TBAH. The solution was then stirred for an hour, until the organic layer became colorless and brown precipitate appeared. Toluene was decanted from the solid and 25 mL of deionized water was added. After the solution was stirred for additional 10 h, another 50 mL of water were added and the resultant mixture was filtered through a fluted filter. Obtained orange solution was concentrated on rotary evaporator to c.a. 20 mL and 125 mL of methanol was added to precipitate product. Afterwards, crude product was re-dissolved in deionized water and dialyzed (1 kDa cut off). Resultant solution was precipitated using methanol, centrifuged and dried in a vacuum oven. TGA measurements allowed to estimate the composition of synthesized fullerenol to be C60(OH)24.
The second step was concentrated on the addition of (3-glycidyloxypropyl)trimethoxysilane (GLYMO) to the hydroxyl groups previously introduced onto fullerene surface (Figure 7). 150 mg of synthesized fullerenol was dispersed in 12 mL of anhydrous toluene using ultrasound. Then 1.2 mL of GLYMO was added and resultant mixture was refluxed in argon atmosphere for 24 h. Then, methanol was added and mixture was centrifuged. The liquids were decanted from the solid, which was washed with methanol and then dried in vacuo.
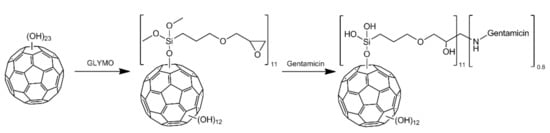
Figure 7.
Scheme for synthesis of gentamicin functionalized C60 fullerene (60FGG) using fullerenol as a substrate.
The final step was performed using the modified method reported by Lizza et al. [43], where epoxide ring was opened using primary amino groups from gentamicin molecules. Crude GLYMO functionalized fullerene 60FG (65 mg, 0.02 mmoL) was sonicated in 8 mL of DMF, followed by addition of gentamicin (94 mg, 0.2 mmol) dissolved in small amount of DMF. The reaction was carried out at 60 °C for 24 h. The water (18 mL, 1 mmoL) was added and the mixture was centrifuged to separate insoluble residue. Obtained brown liquid was decanted and dialyzed (1 kDa cut off). Then, it was concentrated on rotary evaporator and methanol was added in order to form a precipitate of the final product, which composition was estimated to be C60(OH)12(GLYMO)11(Gentamicin)0.8.
Obtained products were characterized using X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FT-IR), and thermogravimetry (TGA). XPS measurements were carried out using a VG ESCALAB 210 electron spectrometer (Thermo Fisher Sceintific, Bothell, WA, USA) equipped with an Al-Kα source (1486.6 eV). XPS data were calibrated using the binding energy of C1s = 284.6 eV as the internal standard. The infrared experiments were performed on the Shimadzu FTIR-8400S (Shimadzu, Kioto, Japan). The samples were prepared as KBr disks. Thermogravimetric measurements were performed using Q50 TGA (TA Instruments, New Castle, DE, USA). The analyzed sample was previously dried under vacuum at 60 °C and the measurement was carried out under flow of nitrogen with heating rate of 5 K/min. Size of synthesized fullerene derivatives their Zeta potential were measured in water using Zetasizer Nano (Malvern, UK).
3.3. Biological Properties of PEG Functionalized C60 Fullerenol-Gentamicin Conjugate
For biological assays, the following samples were used: C60 fullerenol (60F), gentamicin sulfate salt (G), and C60 fullerenol-gentamicin conjugate (60FGG). The samples were put into 1.5 mL eppendorf tubes and sterilized by ethylene oxide. During preparation of aqueous solutions of samples (in sterile deionized water), estimated structure of synthesized fullerene derivative (C60(OH)12(GLYMO)11(Gentamicin)0.8) was taken into account. It was assumed that 60FGG contains 88 wt.% of modified C60 fullerenol and 12 wt.% of gentamicin. Thus, tested concentrations of 60F and G in solutions of 60FGG ranged 29.25–0.057 μg/mL and 4–0.007 μg/mL, respectively. In parallel, 60F was tested at concentrations ranged 29.25–0.057 μg/mL, while G at concentrations ranged 4–0.007 μg/mL.
3.3.1. Antibacterial Properties In Vitro
In order to evaluate antibacterial activity of tested samples, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined. The MIC was estimated using microdilution method according to the CLSI M7A7 standard [44]. Briefly, the two-fold dilutions of tested samples were prepared in MHB broth. Then, 100 μL of solutions were added to 96-well plate. 100 μL of MHB was used as a control solution. The bacteria inoculum at 0.5 McFarland standard density (1 × 108 CFU/mL) was prepared in sterile 0.85% saline using nephelometer (BD PhoenixSpecTM Nephelometer, Thermo Fisher Scientific, Waltham, WA, USA). Then, inoculum was 200-times diluted in order to obtain 5 × 105 CFU/mL. 100 μL of diluted inoculum was added to 100 μL of tested sample solutions or MHB (control), which were placed in 96-well plate. The plates were incubated in air at 37 °C without agitation for 24 h. Then, the absorbance was read at 600 nm (Synergy H4 automatic plate reader, BioTek, Winooski, VT, USA). The experiment was performed in three separate measurements in triplicate. The MIC was defined as the lowest compound concentration that inhibits more than 90% of bacterial growth. Then, in order to determine MBC, 100 μL of each well without bacterial growth was seeded on Petri dish containing MHA. After 24 h incubation at 37 °C without agitation, the colonies were count. MBC was defined as the lowest compound concentration that decreased the number of colonies by ≥99.99% compared to the control growth. The experiment was performed in three separate replicates.
3.3.2. Cytotoxic Properties In Vitro
Cytotoxicity of compounds was evaluated using normal skin fibroblasts (BJ cell lines) as described in details previously [45]. Briefly, BJ cells were seeded in 96-well plate at concentration of 2 × 104 cell/well and incubated at 37 °C for 24 h. On the next day, the two-fold dilutions of tested samples were prepared in EMEM medium. Then, cell medium from above the cells was gently removed and 100 μL of prepared solutions were added. 100 μL of fresh EMEM medium was used as a control solution. After 24 h incubation at 37 °C, fibroblast viability was assessed using MTT assay. The experiment was performed in three separate measurements in octuplicate.
4. Conclusions
In this study, (3-glycidyloxypropyl)trismethoxysilane functionalized fullerene (60FG) was used as a vehicle for a standard aminoglycoside antibiotic–gentamicin (G). It is worth underlining that this is the first study which shows effective method to conjugate fullerenol with aminoglycoside antibiotic. We believe that such conjugate may allow to link biological properties of fullerenol (for instance antioxidant or free-radical scavenging) and antibacterial properties of gentamicin. It seems to be very important since the application of antibiotics (such as gentamicin) is associated with unfavorable generation of free radicals, which may lead to nephrotoxicity. Our preliminary results demonstrated that applied three-step fabrication procedure allowed to obtain gentamicin functionalized C60 fullerene (60FGG), as proven by XPS analysis, termogravimetric evaluation, and FT-IR measurements. Obtained 60FGG derivative exhibited beneficial antibacterial activity against Gram-positive and Gram-negative bacterial strains, indicating that such conjugate enabled the maintaining of inhibition activity of gentamicin. Moreover, cell culture experiments proved that 60FGG derivative did not possess cytotoxic properties towards normal human fibroblasts (model eukaryotic cells). From biomedical point of view, these preliminary results are very promising and allow to consider further application of obtained 60FGG derivative. For instance, obtained fullerenol-gentamicin conjugate could be potentially used as injection solution with antibacterial properties for the treatment of chronic wounds or osteochondral defects. It may be also applied as a component of bioactive biomaterials, such as wound dressings or implantable biomaterials, which are prone to bacterial infections. Presumably, addition of fullerenol-gentamicin conjugate to biomaterial, instead of gentamicin alone, allow to obtain construct possessing ability to release antibiotic in controllable manner and hopefully with antioxidant properties, thanks to presence of C60 fullerenol. Nevertheless, in order to verify these hypotheses, additional experiments are needed. Thus, we plan to expand our research involving (e.g., evaluation of antioxidant properties of 60FGG derivative and possibility to use it as a component of bioactive biomaterials).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27144366/s1, Figure S1: Survey XPS spectrum of gentamicine modified C60 fullerene (60FGG), Figure S2: Zeta potential measurements of C60 fullerenol (60F), Figure S3: Zeta potential measurements of gentamicin functionalized C60 fullerene (60FGG).
Author Contributions
Conceptualization, A.N., A.K. and G.G.; methodology, P.P. and K.K.; software, A.N.; validation, A.N., P.P. and K.K.; formal analysis, K.K.; investigation, A.N., P.P., K.K. and J.K.; resources, A.K. and G.G.; data curation, K.K.; writing—original draft preparation, A.N. and P.P.; writing—review and editing, A.N., K.K., A.K. and G.G.; visualization, A.N.; supervision, A.K. and G.G.; project administration, G.G.; funding acquisition, A.K. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DS2 project of Medical University of Lublin, Poland. This paper was developed using the equipment purchased within agreement No. POPW.01.03.00-06-010/09-00 Operational Program Development of Eastern Poland 2007–2013, Priority Axis I, Modern Economy, Operations 1.3. Innovations Promotion.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of C60 fullerenol-gentamicin derivative are available from the authors.
References
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Lidija, M.; Roumiana, T.; Jelena, M.; Mari, M.; Kyoko, B.; Marija, T.; Branislava, J. Fullerene based nanomaterials for biomedical applications: Engineering, functionalization and characterization. Adv. Mater. Res. 2013, 633, 224–238. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- Pochkaeva, E.I.; Podolsky, N.E.; Zakusilo, D.N.; Petrov, A.V.; Charykov, N.A.; Vlasov, T.D.; Penkova, A.V.; Vasina, L.V.; Murin, I.V.; Sharoyko, V.V.; et al. Fullerene derivatives with amino acids, peptides and proteins: From synthesis to biomedical application. Prog. Solid State Chem. 2020, 57, 100255. [Google Scholar] [CrossRef]
- Zhu, X.; Sollogoub, M.; Zhang, Y. Biological applications of hydrophilic C60 derivatives—A structural perspective. Eur. J. Med. Chem. 2016, 115, 438–452. [Google Scholar] [CrossRef] [Green Version]
- Gudkov, S.V.; Guryev, E.L.; Gapeyev, A.B.; Sharapov, M.G.; Bunkin, N.F.; Shkirin, A.V.; Zabelina, T.S.; Glinushkin, A.P.; Sevost’yanov, M.A.; Belosludtsev, K.N.; et al. Unmodified hydrated C60 fullerene molecules exhibit antioxidant properties, prevent damage to DNA and proteins induced by reactive oxygen species and protect mice against injuries caused by radiation-induced oxidative stress. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Gudkov, S.V.; Simakin, A.V.; Sarimov, R.M.; Kurilov, A.D.; Chausov, D.N. Novel biocompatible with animal cells composite material based on organosilicon polymers and fullerenes with Light-induced bacteriostatic properties. Nanomaterials 2021, 11, 2804. [Google Scholar] [CrossRef]
- Deryabin, D.G.; Davydova, O.K.; Yankina, Z.Z.; Vasilchenko, A.S.; Miroshnikov, S.A.; Kornev, A.B.; Ivanchikhina, A.V.; Troshin, P.A. The activity of [60]fullerene derivatives bearing amine and carboxylic solubilizing groups against Escherichia coli: A comparative study. J. Nanomater. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lyon, D.Y.; Alvarez, P.J.J. Fullerene water suspension (nC60) exerts antibacterial effects via ROS-independent protein oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef]
- Martinez, Z.S.; Castro, E.; Seong, C.S.; Cerón, M.R.; Echegoyen, L.; Llano, M. Fullerene derivatives strongly inhibit HIV-1 replication by affecting virus maturation without impairing protease activity. Antimicrob. Agents Chemother. 2016, 60, 5731–5741. [Google Scholar] [CrossRef] [Green Version]
- Marforio, T.D.; Mattioli, E.J.; Zerbetto, F.; Calvaresi, M. Fullerenes against COVID-19: Repurposing C60 and C70 to Clog the Active Site of SARS-CoV-2 Protease. Molecules 2022, 27, 1916. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Doi, Y.; Hashizume, M.; Kikuchi, J.I.; Konishi, T. An extremely effective DNA photocleavage utilizing functionalized liposomes with a fullerene-enriched lipid bilayer. J. Am. Chem. Soc. 2007, 129, 4140–4141. [Google Scholar] [CrossRef]
- Gao, Y.; Ou, Z.; Yang, G.; Liu, L.; Jin, M.; Wang, X.; Zhang, B.; Wang, L. Efficient photocleavage of DNA utilizing water soluble riboflavin/naphthaleneacetate substituted fullerene complex. J. Photochem. Photobiol. A Chem. 2009, 203, 105–111. [Google Scholar] [CrossRef]
- Soldatova, Y.V.; Areshidze, D.A.; Zhilenkov, A.V.; Kraevaya, O.A.; Peregudov, A.S.; Poletaeva, D.A.; Faingold, I.I.; Troshin, P.A.; Kotelnikova, R.A. Water-soluble fullerene derivatives: The inhibition effect on polyol pathway enzymes and antidiabetic potential on high-fat diet/low-dose streptozotocin-induced diabetes in rats. J. Nanoparticle Res. 2021, 23, 1–13. [Google Scholar] [CrossRef]
- Meng, X.; Li, B.; Chen, Z.; Yao, L.; Zhao, D.; Yang, X.; He, M.; Yu, Q. Inhibition of a thermophilic deoxyribonucleic acid polymerase by fullerene derivatives. J. Enzym. Inhib. Med. Chem. 2007, 22, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease, A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [Green Version]
- Vorobyov, V.; Kaptsov, V.; Gordon, R.; Makarova, E.; Podolski, I.; Sengpiel, F. Neuroprotective effects of hydrated fullerene C60: Cortical and hippocampal EEG interplay in an amyloid-infused rat model of alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 217–233. [Google Scholar] [CrossRef]
- Golomidov, I.; Bolshakova, O.; Komissarov, A.; Sharoyko, V.; Slepneva, E.; Slobodina, A.; Latypova, E.; Zherebyateva, O.; Tennikova, T.; Sarantseva, S. The neuroprotective effect of fullerenols on a model of Parkinson’s disease in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2020, 523, 446–451. [Google Scholar] [CrossRef]
- Rašović, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. (UK) 2017, 33, 777–794. [Google Scholar] [CrossRef]
- Nakamura, S.; Mashino, T. Water-Soluble Fullerene Derivatives for Drug Discovery. J. Nippn. Med. Sch. 2012, 79, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Rašović, I.; Porfyrakis, K. Functionalisation of fullerenes for biomedical applications. Compr. Nanosci. Nanotechnol. 2019, 1–5, 109–122. [Google Scholar] [CrossRef]
- Zaręba, N.; Więcławik, K.; Kizek, R.; Hosnedlova, B.; Kepinska, M. The Impact of Fullerenes as Doxorubicin Nano-Transporters on Metallothionein and Superoxide Dismutase Status in MCF-10A Cells. Pharmaceutics 2022, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, P.; Paraskar, A.; Soni, S.; Mashelkar, R.A.; Sengupta, S. Fullerenol-cytotoxic conjugates for cancer chemotherapy. ACS Nano 2009, 3, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, S.; Kumar, M.; Sharma, G.; Kumar, R.; Singh, B.; Raza, K. Fullerenol-Based Intracellular Delivery of Methotrexate: A Water-Soluble Nanoconjugate for Enhanced Cytotoxicity and Improved Pharmacokinetics. AAPS PharmSciTech 2018, 19, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 fullerene enhances cisplatin anticancer activity and overcomes tumor cell drug resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.H.; Park, S.K.; Cho, Y.S.; Lee, H.S.; Kim, K.R.; Kim, M.G.; Chung, W.H. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear. Res. 2006, 211, 46–53. [Google Scholar] [CrossRef]
- Lopez-Novoa, J.M.; Quiros, Y.; Vicente, L.; Morales, A.I.; Lopez-Hernandez, F.J. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011, 79, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-Spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Hafeez, H.; Choi, D.K.; Lee, C.M.; Jesuraj, P.J.; Kim, D.H.; Song, A.; Chung, K.B.; Song, M.; Ma, J.F.; Kim, C.S.; et al. Replacement of n-type layers with a non-toxic APTES interfacial layer to improve the performance of amorphous Si thin-film solar cells. RSC Adv. 2019, 9, 7536–7542. [Google Scholar] [CrossRef] [Green Version]
- Goswami, T.H.; Singh, R.; Alam, S.; Mathur, G.N. Thermal analysis: A unique method to estimate the number of substituents in fullerene derivatives. Thermochim. Acta 2004, 419, 97–104. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Kadish, K.M.; Boulas, P.; Chen, E.C.M. Relationship between the electron affinities and half-wave reduction potentials of fullerenes, aromatic hydrocarbons, and metal complexes. J. Phys. Chem. 1995, 99, 8843–8850. [Google Scholar] [CrossRef]
- Karadeniz, K.; Aki, H.; Sen, M.Y.; Çalikoʇlu, Y. Ring opening of epoxidized soybean oil with compounds containing two different functional groups. JAOCS J. Am. Oil Chem. Soc. 2015, 92, 725–731. [Google Scholar] [CrossRef]
- Mijović, J.; Andjelić, S. A Study of Reaction Kinetics by Near-Infrared Spectroscopy. 1. Comprehensive Analysis of a Model Epoxy/Amine System. Macromolecules 1995, 28, 2787–2796. [Google Scholar] [CrossRef]
- Komartin, R.S.; Balanuca, B.; Necolau, M.I.; Cojocaru, A.; Stan, R. Composite materials from renewable resources as sustainable corrosion protection coatings. Polymers 2021, 13, 3792. [Google Scholar] [CrossRef]
- Brant, J.A.; Labille, J.; Robichaud, C.O.; Wiesner, M. Fullerol cluster formation in aqueous solutions: Implications for environmental release. J. Colloid Interface Sci. 2007, 314, 281–288. [Google Scholar] [CrossRef]
- Mbizvo, G.K.; Bennett, K.; Simpson, C.R.; Susan, E.; Chin, R.F.M. Epilepsy-related and other nca l Puses of mortality in people with epilepsy: A systematic review of systematic reviews. Epilepsy Res. 2019, 157, 106192. [Google Scholar] [CrossRef]
- Afreen, S.; Kokubo, K.; Muthoosamy, K.; Manickam, S. Hydration or hydroxylation: Direct synthesis of fullerenol from pristine fullerene [C60] via acoustic cavitation in the presence of hydrogen peroxide. RSC Adv. 2017, 7, 31930–31939. [Google Scholar] [CrossRef] [Green Version]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta potential: A case study of cationic, anionic, and neutral liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Klimek, K.; Przekora, A.; Benko, A.; Niemiec, W.; Blazewicz, M.; Ginalska, G. The use of calcium ions instead of heat treatment for β-1,3-glucan gelation improves biocompatibility of the β-1,3-glucan/HA bone scaffold. Carbohydr. Polym. 2017, 164, 170–178. [Google Scholar] [CrossRef]
- Nurzynska, A.; Klimek, K.; Palka, K.; Szajnecki, Ł.; Ginalska, G. Curdlan-based hydrogels for potential application as dressings for promotion of skin wound healing-preliminary in vitro studies. Materials 2021, 14, 2344. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Takeuchi, A.; Ozawa, M.; Li, X.; Saigo, K.; Kitazawa, K. C60 Fullerol formation catalysed by quaternary ammonium hydroxides. J. Chem. Soc. Chem. Commun. 1993, 1784–1785. [Google Scholar] [CrossRef]
- Lizza, J.R.; Moura-Letts, G. Solvent-Directed Epoxide Opening with Primary Amines for the Synthesis of β-Amino Alcohols. Synthesis. 2017, 49, 1231–1242. [Google Scholar]
- CLSI M07. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Pitucha, M.; Woś, M.; Miazga-Karska, M.; Klimek, K.; Mirosław, B.; Pachuta-Stec, A.; Gładysz, A.; Ginalska, G. Synthesis, antibacterial and antiproliferative potential of some new 1-pyridinecarbonyl-4-substituted thiosemicarbazide derivatives. Med. Chem. Res. 2016, 25, 1666–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).