Cecropin-Loaded Zeolitic Imidazolate Framework Nanoparticles with High Biocompatibility and Cervical Cancer Cell Toxicity
Abstract
1. Introduction
2. Results and Discussion
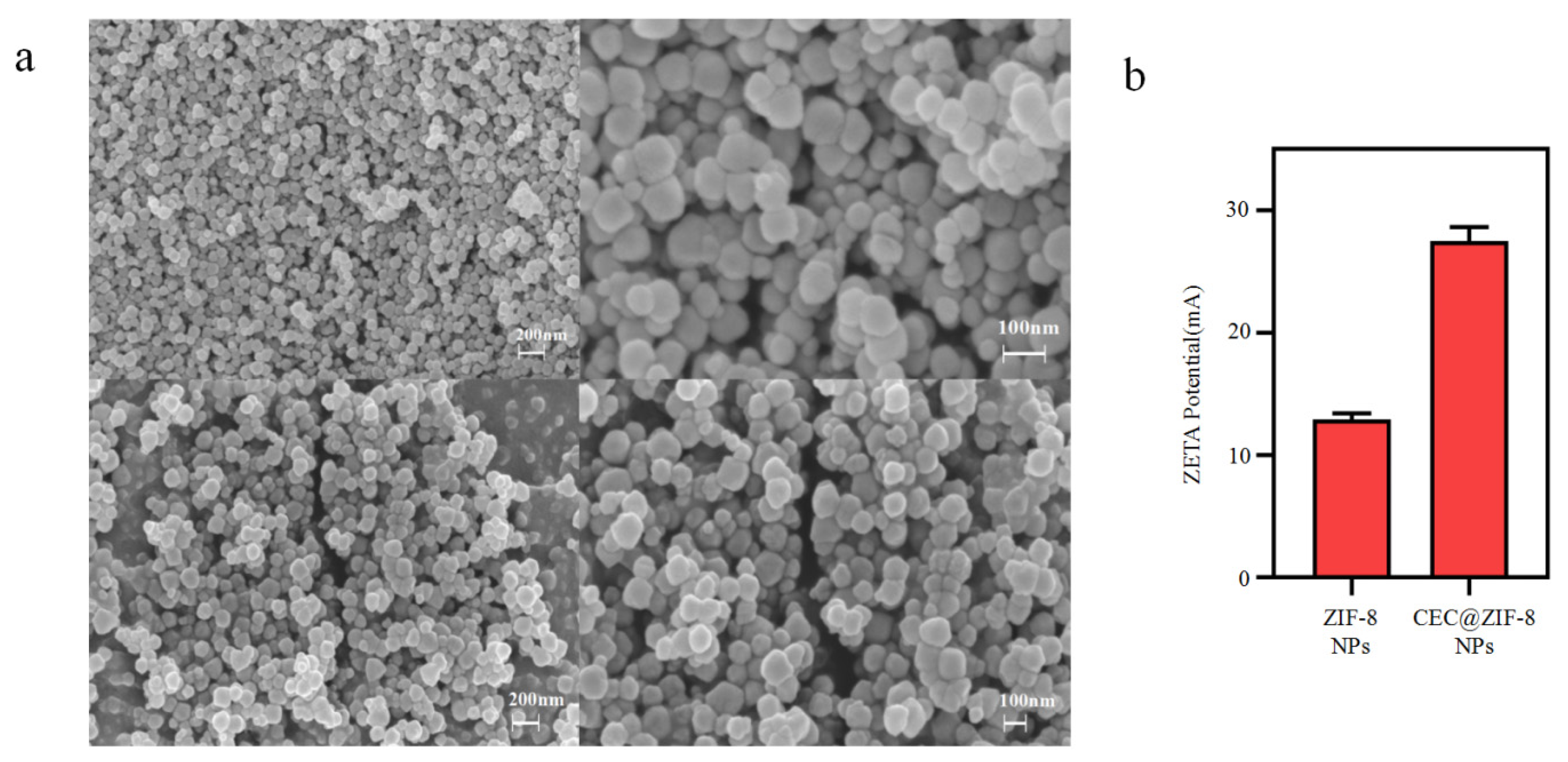
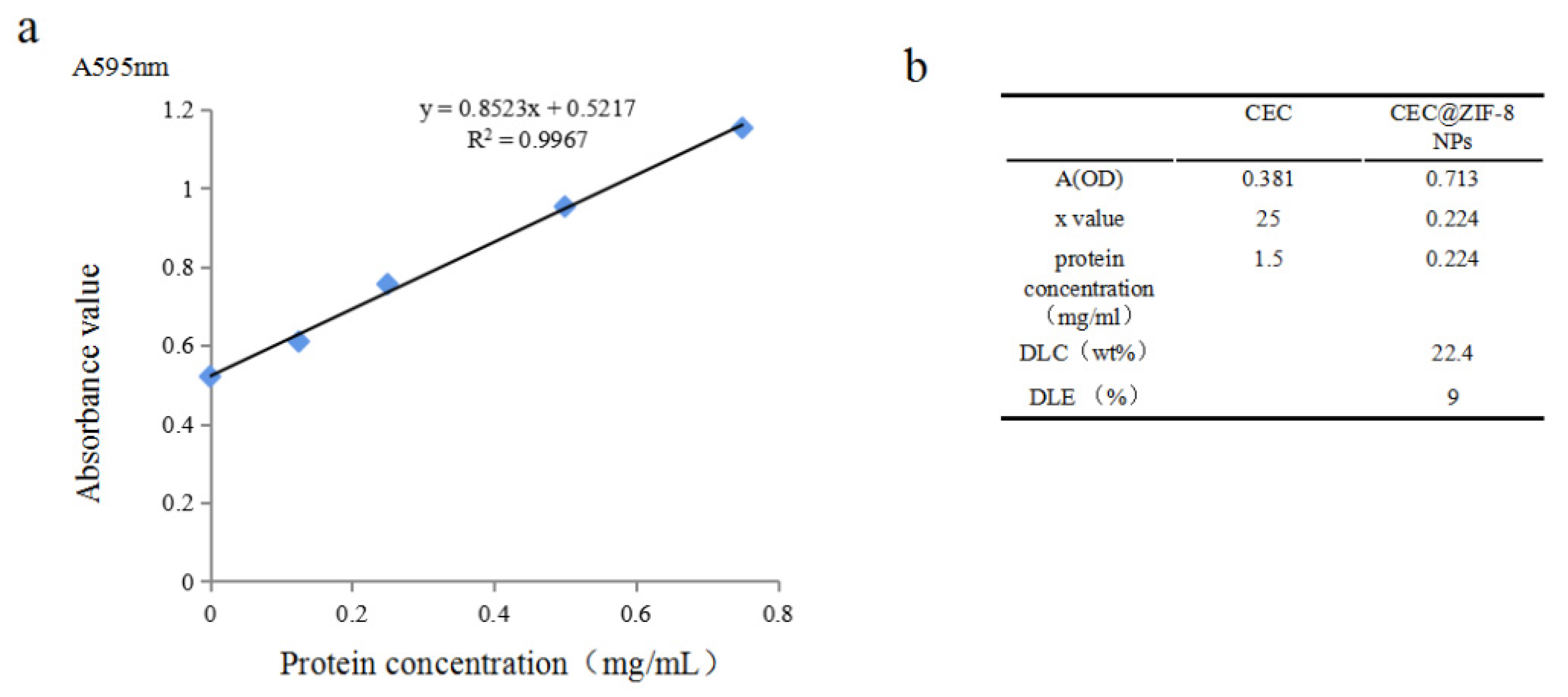
2.1. Evaluation of Drug Incorporation and Characterization of CEC@ZIF-8 NP Morphology
2.2. Uptake of Nanoparticles into Tumor Cells
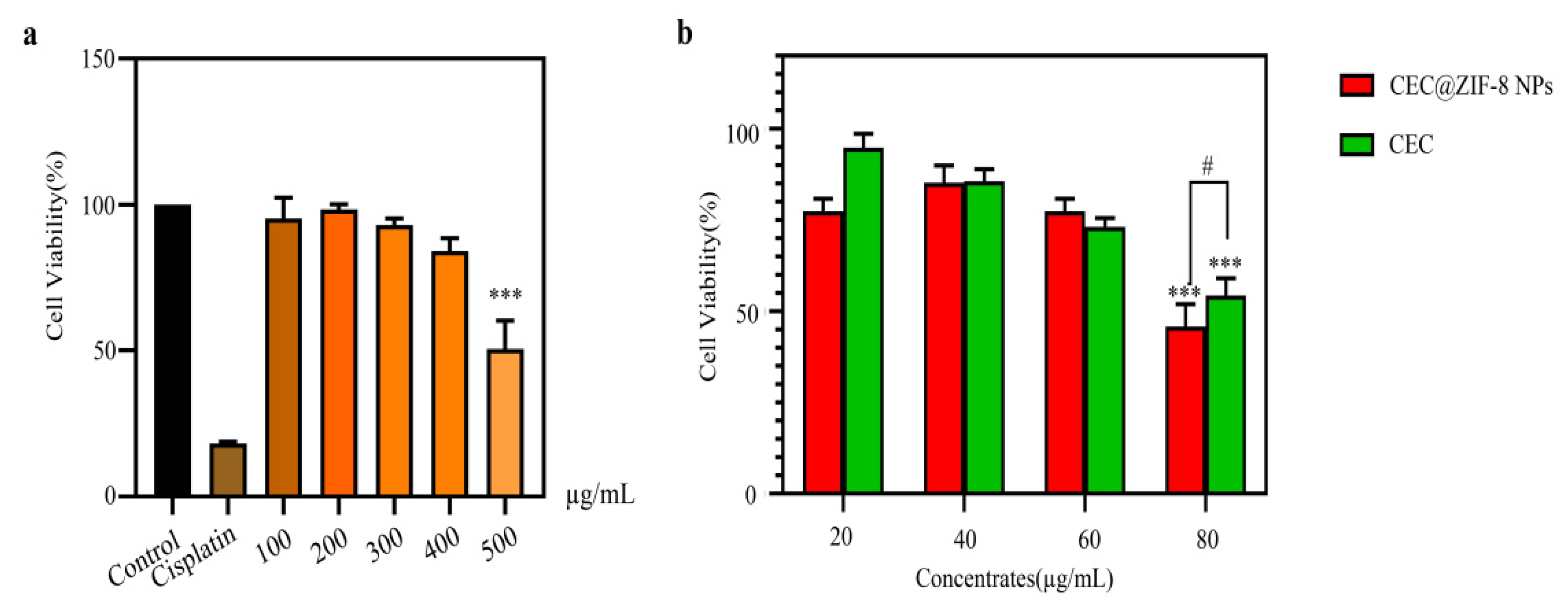
2.3. Effect of CEC@ZIF-8 Nanoparticles on Tumor Cell Viability
3. Materials and Methods
3.1. Materials
3.2. Synthesis of ZIF-8 Nanoparticles
3.3. Synthesis of CEC@ZIF-8 Nanoparticles
3.4. Characterization of Unloaded and CEC-Loaded NPs
3.5. Determination of CEC@ZIF-8 NPs Loading and Encapsulation Rates
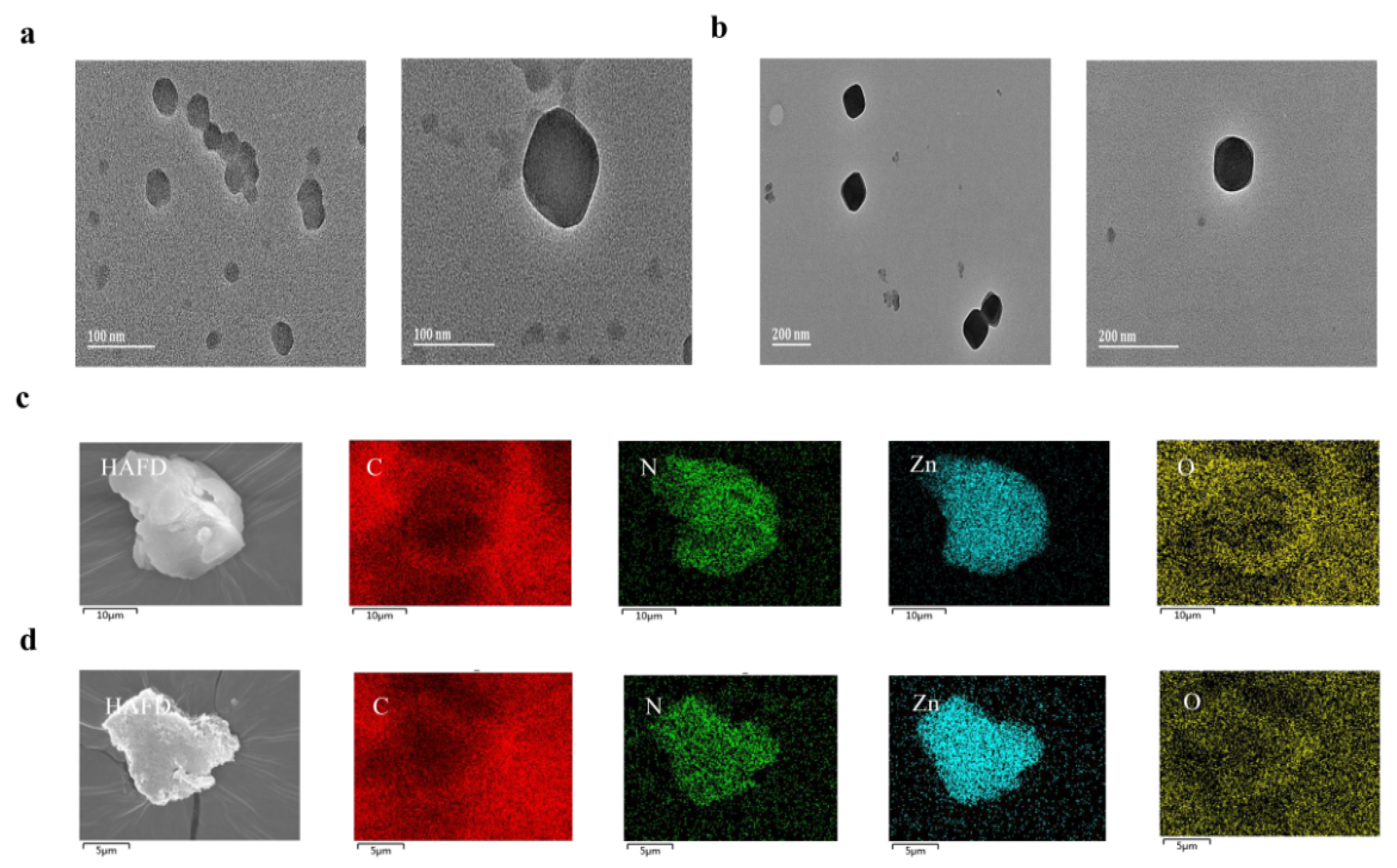
3.6. Confocal Laser Scanning Microscopy
3.7. Cell Lines and Cell Culture
3.8. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sarah, Z.H.; Rebecca, L.S.; Jeffrey, Y.L.; Akila, N.V. Adjuvant therapy after radical trachelectomy for stage I cervical cancer. Gynecol. Oncol. Rep. 2018, 25, 15–18. [Google Scholar]
- Wolford, J.E.; Tewari, K.S. Rational design for cervical cancer therapeutics: Cellular and non-cellular based strategies on the horizon for recurrent, metastatic or refractory cervical cancer. Expert Opin. Drug Dis. 2018, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lisa, B.; James, O.B. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliver. Rev. 2004, 56, 1649–1659. [Google Scholar]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/Metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ayyoubzadeh, S.M.; Ghorbani-Bidkorbeh, F.; Shahhosseini, S.; Dadashzadeh, S.; Asadian, E.; Mosayebnia, M.; Siavashy, S. An investigation of affecting factors on MOF characteristics for biomedical applications: A systematic review. Heliyon 2021, 7, e6914. [Google Scholar] [CrossRef]
- Tamames-Tabar, C.; Cunha, D.; Imbuluzqueta, E.; Ragon, F.; Serre, C.; Blanco-Prieto, M.J.; Horcajada, P. Cytotoxicity of nanoscaled metal-organic frameworks. J. Mater. Chem. B 2014, 2, 262–271. [Google Scholar] [CrossRef]
- Abanades, L.I.; Wells, C.; Forgan, R.S. Multivariate modulation of the Zr MOF UiO-66 for Defect-Controlled combination anticancer drug delivery. Angew. Chem. Int. Ed. 2020, 59, 5211–5217. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Highly permeable zeolite imidazolate framework-8 membranes for CO2/CH4 separation. J. Am. Chem. Soc. 2010, 132, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, X.; Wang, W.; Gai, C.; Zhang, W.; Li, W.; Ding, D. Fe(II) and tannic Acid-Cloaked MOF as carrier of artemisinin for supply of ferrous ions to enhance treatment of Triple-Negative breast cancer. Nanoscale Res. Lett. 2021, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Keywanlu, M.; Tayebee, R. Experimental and molecular dynamics simulation study on the delivery of some common drugs by ZIF, ZIF, and ZIF zeolitic imidazolate frameworks. Appl. Organomet. Chem. 2021, 8, 2–3. [Google Scholar] [CrossRef]
- de Moura, F.L.R.; Tabosa, A.É.G.A.; Da, S.N.D.D.; Ferreira, A.S.; Silva, J.Y.R.; Junior, S.A.; Rolim, L.A.; Rolimneto, P.J. Benznidazole in vitro dissolution release from a pH-sensitive drug delivery system using ZIF-8 as a carrier. J. Mater. Sci. Mater. Med. 2021, 32, 6. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Feng, J.; Chu, C.; Ma, Z.; Han, H. Cascade controlled release system based on pH-responsive ZIF-8 capsule and enzyme-responsive hyaluronic acid hydrogel for tumor marker detection using electro-readout-mode. Sens. Actuators B Chem. 2021, 348, 130701–130706. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Mi. 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered polymer nanoparticles with unprecedented antimicrobial efficacy and therapeutic indices against Multidrug-Resistant bacteria and biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Amirkhanov, N.V.; Bardasheva, A.V.; Tikunova, N.V.; Pyshnyi, D.V. Synthetic antimicrobial peptides: III—Effect of cationic groups of lysine, arginine, and histidine on antimicrobial activity of peptides with a linear type of amphipathicity. Russ. J. Bioorg. Chem. 2021, 47, 681–690. [Google Scholar] [CrossRef]
- André, M.N.; Patrícia, A.; Hugo, C.P.; Larissa, F.; Fabricio, F.C.; Erika, S.K.; Ana, K.R.A.; Anamélia, L.B.; Maria, S.F. Antifungal drugs: New insights in research & development. Pharmacol. Ther. 2018, 195, 21–38. [Google Scholar]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. Clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Li, F.; Xia, Z.; Guo, X.; Gao, M.; Sun, F.; Cheng, Y.; Wu, Y.; Li, W.; Ali, S.A.; et al. The scorpion venom peptide smp76 inhibits viral infection by regulating Type-I interferon response. Virol. Sin. 2018, 33, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, A.; Skrygan, M.; Gambichler, T.; Brockmeyer, N.H.; Stücker, M.; Herzler, C.; Potthoff, A.; Altmeyer, P.; Pfister, H.; Wieland, U. Human papillomavirus-associated induction of human beta-defensins in anal intraepithelial neoplasia. Br. J. Dermatol. 2009, 160, 1197–1205. [Google Scholar] [CrossRef]
- Chen, Y.; Shih, P.; Feng, C.; Wu, C.; Tsui, K.; Lin, Y.; Kuo, H.; Wen, Z. Pardaxin activates excessive mitophagy and Mitochondria-Mediated apoptosis in human ovarian cancer by inducing reactive oxygen species. Antioxidants 2021, 10, 1883. [Google Scholar] [CrossRef] [PubMed]
- Kachel, H.S.; Buckingham, S.D.; Sattelle, D.B. Insect toxins-selective pharmacological tools and drug/chemical leads. Curr. Opin. Insect. Sci. 2018, 30, 93–98. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Chai, J.; Yang, W.; Gao, Y.; Guo, R.; Peng, Q.; Abdelrahman, M.A.; Xu, X. Antitumor effects of scorpion peptide smp43 through mitochondrial dysfunction and membrane disruption on hepatocellular carcinoma. J. Nat. Prod. 2021, 84, 3147–3160. [Google Scholar] [CrossRef]
- Kim, S.; Lee, D.G. Role of calcium in reactive oxygen species-induced apoptosis in Candida albicans: An antifungal mechanism of antimicrobial peptide, PMAP-23. Free Radical Res. 2019, 53, 1–10. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Fareed, M.S.; He, Y.; Lu, Y.; Yang, C.; Wang, Z.; Su, J.; Wang, P.; Yan, W.; et al. An injectable peptide hydrogel constructed of natural antimicrobial peptide j-1 and ADP shows Anti-Infection, hemostasis, and antiadhesion efficacy. ACS Nano 2022, 16, 7636–7650. [Google Scholar] [CrossRef]
- Wang, J.; Ma, K.; Ruan, M.; Wang, Y.; Li, Y.; Fu, Y.V.; Song, Y.; Sun, H.; Wang, J. A novel cecropin B-derived peptide with antibacterial and potential anti-inflammatory properties. PeerJ 2018, 6, 5634–5646. [Google Scholar] [CrossRef] [PubMed]
- Erdem, B.M.; Kesmen, Z. Antimicrobial peptides (AMPs): A promising class of antimicrobial compounds. J. Appl. Microbiol. 2021, 132, 1573–1596. [Google Scholar] [CrossRef] [PubMed]
- Lijie, X.; Zhongyuan, L.; Ji, M.; Surong, S.; Jianhua, Y.; Fuchun, Z. Expression, purification and characterization of cecropin antibacterial peptide from Bombyx mori in Saccharomyces cerevisiae. Protein Expres. Purif. 2013, 90, 47–54. [Google Scholar]
- Wu, Y.; Xia, L.; Li, J.; Zhang, F. CecropinXJ inhibits the proliferation of human gastric cancer BGC823 cells and induces cell death in vitro and in vivo. Int. J. Oncol. 2015, 46, 2181–2193. [Google Scholar] [CrossRef][Green Version]
- Xia, L.; Wu, Y.; Ma, J.; Zhang, F. Therapeutic effects of antimicrobial peptide on malignant ascites in a mouse model. Mol. Med. Rep. 2018, 17, 6245–6252. [Google Scholar] [CrossRef]
- Dang, Y.; Li, H.; Wu, Y. Construction of a supramolecular Förster resonance energy transfer system and its application based on the interaction between Cy3-labeled melittin and phosphocholine encapsulated quantum dots. ACS Appl. Mater. Inter. 2012, 4, 6245–6252. [Google Scholar] [CrossRef]
- Moorcroft, S.C.T.; Roach, L.; Jayne, D.G.; Ong, Z.Y.; Evans, S.D. Nanoparticle-Loaded hydrogel for the Light-Activated release and photothermal enhancement of antimicrobial peptides. ACS Appl. Mater. Inter. 2020, 12, 24544. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Qi, G.; Ma, K.; Qu, X.; Xu, W.; Xu, S.; Jin, Y. Tumor Microenvironment-Activated degradable multifunctional nanoreactor for synergistic cancer therapy and glucose SERS feedback. iScience 2020, 23, 101274. [Google Scholar] [CrossRef]
- Chunli, H.; Dairan, Z.; Jianxiang, X.; Shi, H.; Wenmei, W.; Tingting, Z.; Huabin, H.; Weijun, F. One-pot synthesis of vancomycin-encapsulated ZIF-8 nanoparticles as multivalent and photocatalytic antibacterial agents for selective-killing of pathogenic gram-positive bacteria. J. Mater. Sci. 2021, 56, 9434–9444. [Google Scholar]
- Yunhui, S.; Xiaorong, L.; Guie, Y.; Xiaoli, M.; Laobao, G. Fabrication of a novel core–shell CQDs@ZIF-8 composite with enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 13049–13061. [Google Scholar]
- Li, N.; Zhou, L.; Jin, X.; Owens, G.; Chen, Z. Simultaneous removal of tetracycline and oxytetracycline antibiotics from wastewater using a ZIF-8 metal organic-framework. J. Hazard. Mater. 2019, 366, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Amritpal, K.; Divya, G.; Rajesh, K. Surfactant mediated interaction of vancomycin with silver nanoparticles. Appl. Surf. Sci. 2018, 449, 23–30. [Google Scholar]
- Li, Y.; Xu, N.; Zhu, W.; Wang, L.; Liu, B.; Zhang, J.; Xie, Z.; Liu, W. Nanoscale melittin@zeolitic imidazolate frameworks for enhanced anticancer activity and mechanism analysis. ACS Appl. Mater. Interfaces 2018, 10, 22974–22984. [Google Scholar] [CrossRef]
- Yun, H.S.; Ya, Y.L.; Yu, X.; Shao, K.S.; Xin, B.X.; Xie, R.Z.; Ji, Z. Fabrication of novel ZIF-8@BiVO4 composite with enhanced photocatalytic performance. Crystals 2018, 8, 432. [Google Scholar]
- Gagnon, M.C.; Strandberg, E.; Grau-Campistany, A.; Wadhwani, P.; Reichert, J.; Burck, J.; Rabanal, F.; Auger, M.; Paquin, J.F.; Ulrich, A.S. Influence of the length and charge on the activity of alpha-Helical amphipathic antimicrobial peptides. Biochemistry 2017, 56, 1680–1695. [Google Scholar] [CrossRef] [PubMed]
- Margitta, D.; Torsten, W. Structural features of helical antimicrobial peptides: Their potential to modulate activity on model membranes and biological cells. BBA Biomembr. 1999, 1462, 71–87. [Google Scholar]
- Sok, M.; Sentjurc, M.; Schara, M. Membrane fluidity characteristics of human lung cancer. Cancer Lett. 1999, 139, 215–220. [Google Scholar] [CrossRef]
- Chen, X.; Huang, Y.; Chen, H.; Chen, Z.; Chen, J.; Wang, H.; Li, D.; Su, Z. Correction to: Augmented EPR effect post IRFA to enhance the therapeutic efficacy of arsenic loaded ZIF-8 nanoparticles on residual HCC progression. J. Nanobiotechnol. 2022, 20, 175. [Google Scholar] [CrossRef]
- Yan, J.; Liu, C.; Wu, Q.; Zhou, J.; Xu, X.; Zhang, L.; Wang, D.; Yang, F.; Zhang, H. Mineralization of pH-Sensitive doxorubicin prodrug in ZIF-8 to enable targeted delivery to solid tumors. Anal. Chem. 2020, 92, 11453–11461. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Pan, Y.; Li, J.; Xia, L. Cecropin-Loaded Zeolitic Imidazolate Framework Nanoparticles with High Biocompatibility and Cervical Cancer Cell Toxicity. Molecules 2022, 27, 4364. https://doi.org/10.3390/molecules27144364
Jiang J, Pan Y, Li J, Xia L. Cecropin-Loaded Zeolitic Imidazolate Framework Nanoparticles with High Biocompatibility and Cervical Cancer Cell Toxicity. Molecules. 2022; 27(14):4364. https://doi.org/10.3390/molecules27144364
Chicago/Turabian StyleJiang, Jingwen, Yanzhu Pan, Jinyao Li, and Lijie Xia. 2022. "Cecropin-Loaded Zeolitic Imidazolate Framework Nanoparticles with High Biocompatibility and Cervical Cancer Cell Toxicity" Molecules 27, no. 14: 4364. https://doi.org/10.3390/molecules27144364
APA StyleJiang, J., Pan, Y., Li, J., & Xia, L. (2022). Cecropin-Loaded Zeolitic Imidazolate Framework Nanoparticles with High Biocompatibility and Cervical Cancer Cell Toxicity. Molecules, 27(14), 4364. https://doi.org/10.3390/molecules27144364





