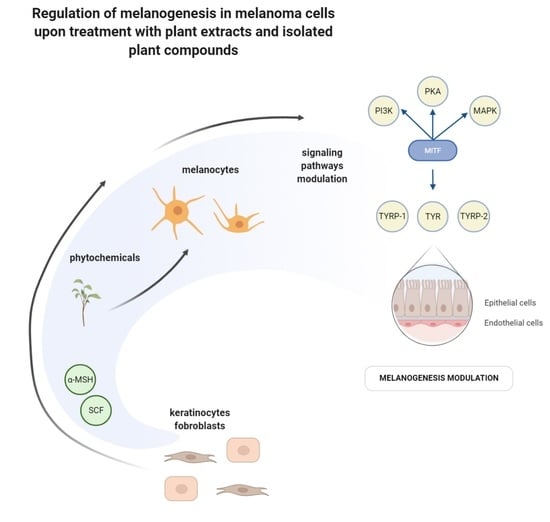
The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds
Abstract
1. Introduction
2. Study Design
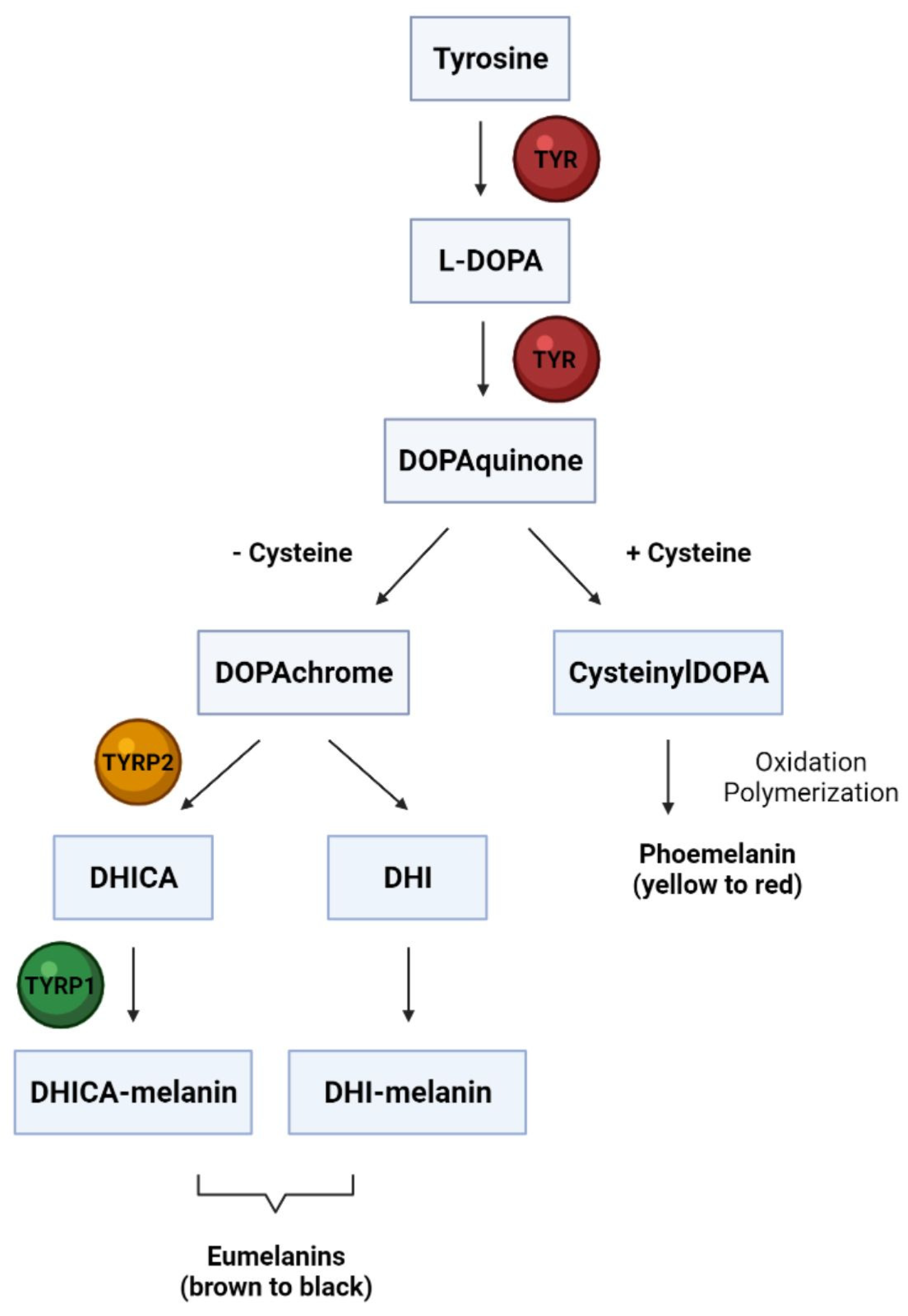
3. Melanocyte Biology
4. Natural Skin Agents against Hyper and Hypo-Pigmentation Disease
5. Mechanisms of Melanogenesis-Related Signaling Pathway Modulation by Plant Extracts and Isolated Compounds in B16 Cells
5.1. cAMP/PKA Signaling Pathway
5.2. MAPKs Signaling Pathway
5.3. PI3K/AKT Signaling Pathway
5.4. In Vivo Studies
6. Mechanisms of Melanogenesis-Related Signaling Pathway Modulation by Plant Extracts and Single-Derived Compounds in B16 Cells Stimulated by UV Radiation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postep. Dermatol. Alergol. 2013, 30, 30–41. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Plant secondary metabolism: Diversity, function and its evolution. Nat. Prod. Commun. 2008, 3, 1205–1216. [Google Scholar] [CrossRef]
- Videira, I.F.D.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, L.; Wu, L.; Wang, H.; Chen, K.; Wu, H.; Li, Y. Kaempferol, the melanogenic component of Sanguisorba officinalis, enhances dendricity and melanosome maturation/transport in melanocytes. J. Pharmacol. Sci. 2021, 147, 348–357. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Bennett, D.C.; Lamoreux, M.L. The color loci of mice-A genetic century. Pigment Cell Res. 2003, 16, 333–344. [Google Scholar] [CrossRef]
- Bennett, D.C.; Medrano, E.E. Molecular regulation of melanocyte senescence. Pigment Cell Res. 2002, 15, 242–250. [Google Scholar] [CrossRef]
- Becker, T.; Haferkamp, S. Molecular Mechanisms of Cellular Senescence. In Senescence and Senescence-Related Disorders; Wang, Z., Inuzuka, H., Eds.; IntechOpen Limited: London, UK, 2013; pp. 25–50. [Google Scholar]
- Nanni, V.; Canuti, L.; Gismondi, A.; Canini, A. Hydroalcoholic extract of Spartium junceum L. flowers inhibits growth and melanogenesis in B16-F10 cells by inducing senescence. Phytomedicine 2018, 46, 1–10. [Google Scholar] [CrossRef]
- Kim, E.S.; Shin, J.H.; Seok, S.H.; Kim, J.B.; Chang, H.; Park, S.J.; Jo, Y.K.; Choi, E.S.; Park, J.S.; Yeom, M.H.; et al. Autophagy mediates anti-melanogenic activity of 3′-ODI in B16F1 melanoma cells. Biochem. Biophys. Res. Commun. 2013, 442, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Low, E.; Alimohammadiha, G.; Smith, L.A.; Costello, L.F.; Przyborski, S.A.; von Zglinicki, T.; Miwa, S. How good is the evidence that cellular senescence causes skin ageing? Ageing Res. Rev. 2021, 71, 101456. [Google Scholar] [CrossRef] [PubMed]
- Martic, I.; Wedel, S.; Jansen-Dürr, P.; Cavinato, M. A new model to investigate UVB-induced cellular senescence and pigmentation in melanocytes. Mech. Ageing Dev. 2020, 190, 111322. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, A.; Sargsyan, E.; Vardanyan, A. Expression of secondary metabolites in plants and their useful perspective in animal health. Int. J. Bioflux Soc. 2011, 3, 129–134. [Google Scholar]
- Hussein, A.R.; El-Anssary, A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; Builders, P.F., Ed.; IntechOpen Limited: London, UK, 2019; pp. 11–30. [Google Scholar]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Szczurko, O.; Boon, H.S. A systematic review of natural health product treatment for vitiligo. BMC Dermatol. 2008, 8, 2. [Google Scholar] [CrossRef]
- Fisk, W.A.; Agbai, O.; Lev-Tov, H.A.; Sivamani, R.K. The use of botanically derived agents for hyperpigmentation: A systematic review. J. Am. Acad. Dermatol. 2014, 70, 352–365. [Google Scholar] [CrossRef]
- Smit, N.; Vicanova, J.; Pavel, S. The hunt for natural skin whitening agents. Int. J. Mol. Sci. 2009, 10, 5326–5349. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, J. The use of botanical extracts as topical skin-lightening agents for the improvement of skin pigmentation disorders. J. Investig. Dermatol. Symp. Proc. 2008, 13, 20–24. [Google Scholar] [CrossRef]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.Q.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. XA polymorphism in IRF4 affects human pigmentation through a tyrosinase-dependent MITF/TFAP2A pathway. Cell 2013, 155, 1022. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Kume, S.; Neffati, M.; Isoda, H. Upregulation of Mitf by Phenolic Compounds-Rich Cymbopogon schoenanthus Treatment Promotes Melanogenesis in B16 Melanoma Cells and Human Epidermal Melanocytes. Biomed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, J.; Jeong, S.G.; Hong, Y.H.; Yoo, S.; Han, S.Y.; Kim, J.H.; Kim, S.; Kim, J.S.; Chung, Y.S.; et al. Artemisia asiatica ethanol extract exhibits anti-photoaging activity. J. Ethnopharmacol. 2018, 220, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Dissanayake, C.Y.; Moon, H.H.; Yang, K.M.; Lee, Y.; Han, C.H. The effects of green tea (Camellia sinensis) flower extract on melanin synthesis in B16-F10 melanoma cells. Korean J. Vet. Res. 2018, 58, 66–72. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, G.N.; Kwak, J.H.; Jeong, C.H.; Jeong, H.R.; Lee, U.; Kim, M.J.; Heo, H.J. Inhibitory effects of chestnut inner skin extracts on melanogenesis. Food Sci. Biotechnol. 2012, 21, 1571–1576. [Google Scholar] [CrossRef]
- Lee, S.C.; Chen, C.H.; Yu, C.W.; Chen, H.L.; Huang, W.T.; Chang, Y.S.; Hung, S.H.; Lee, T.L. Inhibitory effect of Cinnamomum osmophloeum Kanehira ethanol extracts on melanin synthesis via repression of tyrosinase expression. J. Biosci. Bioeng. 2016, 122, 263–269. [Google Scholar] [CrossRef]
- Huang, H.C.; Hsieh, W.Y.; Niu, Y.L.; Chang, T.M. Inhibitory effects of adlay extract on melanin production and cellular oxygen stress in B16F10 melanoma cells. Int. J. Mol. Sci. 2014, 15, 16665–16679. [Google Scholar] [CrossRef]
- Chatatikun, M.; Yamauchi, T.; Yamasaki, K.; Aiba, S.; Chiabchalard, A. Anti melanogenic effect of Croton roxburghii and Croton sublyratus leaves in α-MSH stimulated B16F10 cells. J. Tradit. Complement. Med. 2019, 9, 66–72. [Google Scholar] [CrossRef]
- Ko, Y.J.; Yang, S.K.; Song, S.M.; Yoon, W.J.; Bae, K.H. Effect of Dendrobium moniliforme on Melanogenic Protein Expression in B16F10 Melanoma Cells. J. Biol. Act. Prod. Nat. 2015, 5, 12–17. [Google Scholar] [CrossRef]
- Park, S.A.; Lee, H.M.; Ha, J.H.; Jeon, S.H.; Park, S.N. Inhibitoiy effects of Dendropanax morbifera leaf extracts on melanogenesis through down-regulation of tyrosinase and TRP-2. Appl. Chem. Eng. 2014, 25, 468–473. [Google Scholar] [CrossRef]
- Li, P.H.; Chiu, Y.P.; Shih, C.C.; Wen, Z.H.; Ibeto, L.K.; Huang, S.H.; Chiu, C.C.; Ma, D.L.; Leung, C.H.; Chang, Y.N.; et al. Biofunctional Activities of Equisetum ramosissimum Extract: Protective Effects against Oxidation, Melanoma, and Melanogenesis. Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Baek, S.H.; Nam, I.J.; Kwak, H.S.; Kim, K.C.; Lee, S.H. Cellular anti-melanogenic effects of a Euryale ferox seed extract ethyl acetate fraction via the lysosomal degradation machinery. Int. J. Mol. Sci. 2015, 16, 9217–9235. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Shin, S.; Lee, J.A.; Park, D.; Lee, J.; Jung, E. Inhibition of melanogenesis by Gaillardia aristata flower extract. BMC Complement. Altern. Med. 2015, 15, 449. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M.A.; Sarmidi, M.R.; Park, C.S. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int. J. Mol. Med. 2012, 29, 209–217. [Google Scholar] [CrossRef]
- Shim, E.; Song, E.; Choi, K.S.; Choi, H.J.; Hwang, J. Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma. Nutr. Res. Pract. 2017, 11, 173–179. [Google Scholar] [CrossRef]
- Qiao, Z.; Koizumi, Y.; Zhang, M.; Natsui, M.; Flores, M.J.; Gao, L.; Yusa, K.; Koyota, S.; Sugiyama, T. Anti-melanogenesis effect of Glechoma hederacea L. extract on B16 murine melanoma cells. Biosci. Biotechnol. Biochem. 2012, 76, 1877–1883. [Google Scholar] [CrossRef]
- Bodurlar, Y. Inhibitory Activity of Soy Cell Culture Extract on Tyrosinase Activity and Melanin Formation in ⍺-MSH Induced B16-F10 Melanoma Cells 2022, 2022, 1–14. Res. Sq. 2022, 1–14. [Google Scholar] [CrossRef]
- Chao, H.C.; Najjaa, H.; Villareal, M.O.; Ksouri, R.; Han, J.; Neffati, M.; Isoda, H. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013, 22, 131–136. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cho, Y.R.; Park, J.H.; Ahn, E.K.; Jeong, W.; Shin, H.S.; Kim, M.S.; Yang, S.H.; Oh, J.S. Anti-melanogenic and anti-oxidant activities of ethanol extract of Kummerowia striata: Kummerowia striata regulate anti-melanogenic activity through down-regulation of TRP-1, TRP-2 and MITF expression. Toxicol. Rep. 2019, 6, 10–17. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, J.Y.; Cho, Y.-R.; Ahn, E.-K.; Jeong, W.; Shin, H.S.; Kim, M.-S.; Oh, J.S. Anti-Melanogenic and Anti-Oxidant Activities of an Ethanolic Extract of Kummerowia Striata and Its Active Compounds, P-Coumaric Acid and Quercetin. Preprints 2018, 2018060341. [Google Scholar] [CrossRef]
- Yao, C.; Jin, C.L.; Oh, I.G.; Park, C.H.; Chung, J.H. Melia azedarach extract stimulates melanogenesis through increase of tyrosinase-related protein 1 expression in B16F10 mouse melanoma cells. Int. J. Mol. Med. 2015, 35, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Akaberi, M.; Vatani, M.; Emami, S.A. Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of nepeta binaludensis jamzad in murine melanoma B16F10 cells. Iran. J. Basic Med. Sci. 2016, 19, 662–669. [Google Scholar]
- Akaberi, M.; Emami, S.A.; Vatani, M.; Tayarani-Najaran, Z. Evaluation of antioxidant and anti-melanogenic activity of different extracts of aerial parts of N. Sintenisii in murine melanoma B16F10 cells. Iran. J. Pharm. Res. 2018, 17, 225–235. [Google Scholar] [PubMed]
- Park, H.J.; Lee, E.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Effects of phenolics from oplismenus undulatifolius in α-MSH-stimulated B16F10 melanoma cells. J. Appl. Biol. Chem. 2020, 63, 89–93. [Google Scholar] [CrossRef]
- Han, J.H.; Byeon, S.H.; Hyun, C.G.; Lee, N.H. Melanogenesis inhibitory activity in the extracts of Oreocnide fruticosa (Gaudich.) Hand.-Mazz. branches. J. Appl. Pharm. Sci. 2014, 4, 166–169. [Google Scholar] [CrossRef]
- Wang, Y.C.; Haung, X.Y.; Chiu, C.C.; Lin, M.Y.; Lin, W.H.; Chang, W.T.; Tseng, C.C.; Wang, H.M.D. Inhibitions of melanogenesis via Phyllanthus emblica fruit extract powder in B16F10 cells. Food Biosci. 2019, 28, 177–182. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jang, T.-W.; Choi, J.-S.; Mun, J.-Y.; Park, J.-H. Inhibitory Effects of Pine Cone (Pinus densiflora) on Melanogenesis in B16F10 Melanoma Cells. Korean J. Plant Resour. 2019, 32, 275–281. [Google Scholar]
- Ghali, S.K.; Chakraborty, A.; Pallapolu, P.; Rafeeqi, T.A.; Husain, G.M.; Javed, G.; Waheed, M.A.; Kazmi, M.H. Expression of MITF Gene in Melanogenesis with Psoralea corylifolia, Zingiber officinale, Terminalia chebula, Punica granatum and Eclipta alba Based on Poly-Herbal Formulation. J. Biol. Act. Prod. Nat. 2021, 11, 82–96. [Google Scholar] [CrossRef]
- Junlatat, J.; Fangkrathok, N.; Sripanidkulchai, B. Antioxidative and melanin production inhibitory effects of syzygium cumini extracts. Songklanakarin J. Sci. Technol. 2018, 40, 1136–1143. [Google Scholar] [CrossRef]
- Dong, Y.; Woo, Y.M.; Cha, J.H.; Cha, J.Y.; Lee, N.W.; Back, M.W.; Park, J.; Lee, S.; Ha, J.; Kim, A. The Effect of Inhibition of Uncaria rhynchophylla as an Inhibitor of Melanogenesis and an Antioxidant in B16F10 Melanoma Cells. J. Life Sci. 2020, 30, 1033–1041. [Google Scholar] [CrossRef]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape extract promoted α-msh-induced melanogenesis in b16f10 melanoma cells, which was inverse to resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, J.; DaSilva, N.A.; Wang, L.; Wei, Z.; Guo, L.; Johnson, S.L.; Lu, W.; Xu, J.; Gu, Q.; et al. Cosmetic applications of glucitol-core containing gallotannins from a proprietary phenolic-enriched red maple (Acer rubrum) leaves extract: Inhibition of melanogenesis via down-regulation of tyrosinase and melanogenic gene expression in B16F10 melanoma ce. Arch. Dermatol. Res. 2017, 309, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Won, K.J.; Kim, D.Y.; Kim, M.J.; Won, Y.R.; Kim, N.Y.; Lee, H.M. Wound healing-promoting and melanogenesis-inhibiting activities of Angelica polymorpha maxim. Flower absolute in vitro and its chemical composition. Molecules 2021, 26, 6172. [Google Scholar] [CrossRef] [PubMed]
- Bourhim, T.; Villareal, M.O.; Couderc, F.; Hafidi, A.; Isoda, H.; Gadhi, C. Melanogenesis promoting effect, antioxidant activity, and uplc-esi-hrms characterization of phenolic compounds of argan leaves extract. Molecules 2021, 26, 371. [Google Scholar] [CrossRef]
- Woo, S.M.; Choi, W.R.; Lee, D.R.; Kim, H.S.; Yi, C.; Kim, K.H.; Kim, H.L.; Cheng, J.; Le, B.; Yang, S.H.; et al. Leukodin isolated from Artemisia capillaris inhibits alpha-melanocyte stimulating hormone induced melanogenesis in B16F10 melanoma cells. Eur. J. Integr. Med. 2019, 25, 85–91. [Google Scholar] [CrossRef]
- Panichakul, T.; Rodboon, T.; Suwannalert, P.; Tripetch, C.; Rungruang, R.; Boohuad, N.; Youdee, P. Additive effect of a combination of Artocarpus Lakoocha and Glycyrrhiza Glabra extracts on tyrosinase inhibition in melanoma B16 cells. Pharmaceuticals 2020, 13, 1–17. [Google Scholar] [CrossRef]
- Yamahara, M.; Sugimura, K.; Kumagai, A.; Fuchino, H.; Kuroi, A.; Kagawa, M.; Itoh, Y.; Kawahara, H.; Nagaoka, Y.; Iida, O.; et al. Callicarpa longissima extract, carnosol-rich, potently inhibits melanogenesis in B16F10 melanoma cells. J. Nat. Med. 2016, 70, 28–35. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Momtaz, S.; Hussein, A.; Naidoo, S.; Nqephe, M.; Crampton, B. Extract from Ceratonia siliqua exhibits depigmentation properties. Phyther. Res. 2015, 29, 1729–1736. [Google Scholar] [CrossRef]
- Kang, M.H.; Jang, G.Y.; Ji, Y.J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and anti-melanogenic activities of heat-treated licorice (Wongam, glycyrrhiza glabra × g. uralensis) extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef]
- Choi, J.H.; Jung, J.G.; Kim, J.E.; Bang, M.A. Anti-melanogenic effects of Hordeum vulgare L. Barely sprout extract in murine B16F10 melanoma cells. J. Nutr. Health 2019, 52, 168–175. [Google Scholar] [CrossRef]
- Jegal, J.; Chung, K.W.; Chung, H.Y.; Jeong, E.J.; Yang, M.H. The standardißed extract of juniperus communis alleviates hyperpigmentation in vivo HRM-2 hairless mice and in vitro murine B16 melanoma cells. Biol. Pharm. Bull. 2017, 40, 1381–1388. [Google Scholar] [CrossRef]
- Pedrosa, T.D.N.; Barros, A.O.; Nogueira, J.R.; Fruet, A.C.; Rodrigues, I.C.; Calcagno, D.Q.; Smith, M.D.A.C.; de Souza, T.P.; Barros, S.B.D.M.; de Vasconcellos, M.C.; et al. Anti-wrinkle and anti-whitening effects of jucá (Libidibia ferrea Mart.) extracts. Arch. Dermatol. Res. 2016, 308, 643–654. [Google Scholar] [CrossRef]
- Lee, S.G.; Karadeniz, F.; Seo, Y.; Kong, C.S. Anti-melanogenic effects of flavonoid glycosides from limonium tetragonum (thunb.) bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017, 22, 1480. [Google Scholar] [CrossRef]
- Juang, L.J.; Gao, X.Y.; Mai, S.T.; Lee, C.H.; Lee, M.C.; Yao, C.L. Safety assessment, biological effects, and mechanisms of Myrica rubra fruit extract for anti-melanogenesis, anti-oxidation, and free radical scavenging abilities on melanoma cells. J. Cosmet. Dermatol. 2019, 18, 322–332. [Google Scholar] [CrossRef]
- Li, H.; Dasilva, N.A.; Liu, W.; Xu, J.; Dombi, G.W.; Dain, J.A.; Li, D.; Chamcheu, J.C.; Seeram, N.P.; Ma, H. Thymocid®, a standardized black cumin (Nigella sativa) seed extract, modulates collagen cross-linking, collagenase and elastase activities, and melanogenesis in murine B16F10 melanoma cells. Nutrients 2020, 12, 2146. [Google Scholar] [CrossRef]
- Pliego-Arreaga, R.; Regalado, C.; Amaro-Reyes, A.; García-Almendárez, B.E. Changes in the phenolic compounds profile, antioxidant and anti-melanogenic activity from organs of Petasites japonicas under di erent extraction methods. Rev. Mex. Ing. Química 2013, 12, 505–511. [Google Scholar]
- Sripanidkulchai, B.; Junlatat, J. Bioactivities of alcohol based extracts of Phyllanthus emblica branches: Antioxidation, antimelanogenesis and anti-inflammation. J. Nat. Med. 2014, 68, 615–622. [Google Scholar] [CrossRef]
- Han, E.B.; Chang, B.Y.; Kim, D.S.; Cho, H.K.; Kim, S.Y. Melanogenesis inhibitory effect of aerial part of Pueraria thunbergiana in vitro and in vivo. Arch. Dermatol. Res. 2014, 307, 57–72. [Google Scholar] [CrossRef]
- Gao, D.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Kim, H.M.; Sim, J.; Kang, J.S. Evaluation of anti-melanogenesis activity of enriched pueraria lobata stem extracts and characterization of its phytochemical components using hplc–pda–esi–ms/ms. Int. J. Mol. Sci. 2021, 22, 8105. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, S.S.; Yun, S.H.; Kim, S.Y.; Hyun, K.H.; Lee, J.; Lee, N.H.; Hyun, C.G. Melanogenesis inhibitory activity of Rhododendron weyrichii in mouse B16 melanoma cells. Orient. J. Chem. 2016, 32, 1899–1907. [Google Scholar] [CrossRef][Green Version]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. 2020 nutrients Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 melanoma cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef]
- Tuerxuntayi, A.; Liu, Y.Q.; Tulake, A.; Kabas, M.; Eblimit, A.; Aisa, H.A. Kaliziri extract upregulates tyrosinase, TRP-1, TRP-2 and MITF expression in murine B16 melanoma cells. BMC Complement. Altern. Med. 2014, 14, 166. [Google Scholar] [CrossRef]
- Yoon, W.J.; Ham, Y.M.; Yoon, H.S.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Acanthoic acid inhibits melanogenesis through tyrosinase down-regulation and melanogenic gene expression in B16 melanoma cells. Nat. Prod. Commun. 2013, 8, 1359–1362. [Google Scholar] [CrossRef]
- Yim, S.H.; Tabassum, N.; Kim, W.H.; Cho, H.; Lee, J.H.; Batkhuu, G.J.; Kim, H.J.; Oh, W.K.; Jung, D.W.; Williams, D.R. Isolation and Characterization of Isofraxidin 7-O-(6′-O-p-Coumaroyl)-β-glucopyranoside from Artemisia capillaris Thunberg: A Novel, Nontoxic Hyperpigmentation Agent That Is Effective In Vivo. Evid.-Based Complement. Altern. Med. 2017, 2017, 867494. [Google Scholar] [CrossRef]
- Tabassum, N.; Lee, J.H.; Yim, S.H.; Batkhuu, G.J.; Jung, D.W.; Williams, D.R. Isolation of 4,5-O-Dicaffeoylquinic Acid as a Pigmentation Inhibitor Occurring in Artemisia capillaris Thunberg and Its Validation In Vivo. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Chang, T.S.; Chao, S.Y.; Ding, H.Y. Melanogenesis inhibition by homoisoflavavone sappanone a from Caesalpinia sappan. Int. J. Mol. Sci. 2012, 13, 10359–10367. [Google Scholar] [CrossRef]
- Hashemi-Shahri, S.H.; Golshan, A.; Mohajeri, S.A.; Baharara, J.; Amini, E.; Salek, F.; Sahebkar, A.; Tayarani-Najaran, Z. ROS-scavenging and Anti-tyrosinase Properties of Crocetin on B16F10 Murine Melanoma Cells. Anticancer. Agents Med. Chem. 2017, 18, 1064–1069. [Google Scholar] [CrossRef]
- Liu, Z.J.; Wang, Y.L.; Li, Q.L.; Yang, L. Improved antimelanogenesis and antioxidant effects of polysaccharide from cuscuta Chinensis lam seeds after enzymatic hydrolysis. Brazilian J. Med. Biol. Res. 2018, 51, e7256. [Google Scholar] [CrossRef]
- Kim, I.S.; Yoon, S.J.; Park, Y.J.; Lee, H.B. Inhibitory effect of ephedrannins A and B from roots of Ephedra sinica STAPF on melanogenesis. Biochim. Biophys. Acta-Gen. Subj. 2015, 1850, 1389–1396. [Google Scholar] [CrossRef]
- Han, S.K.; Kim, Y.G.; Kang, H.C.; Huh, J.R.; Kim, J.Y.; Baek, N.I.; Lee, D.K.; Lee, D.G. Oleanolic acid from Fragaria ananassa calyx leads to inhibition of α-MSH-induced melanogenesis in B16-F10 melanoma cells. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 735–742. [Google Scholar] [CrossRef]
- Manse, Y.; Ninomiya, K.; Okazaki, A.; Okada-Nishida, E.; Imagawa, T.; Imamura-Mizushima, M.; Yamano, Y.; Kaname, K.; Nakamura, S.; Morikawa, T. Melanogenesis inhibitory activity of diterpenoid and triterpenoid constituents from the aerial part of Isodon trichocarpus. Natural Product. Commun. 2017, 12, 1185–1188. [Google Scholar] [CrossRef]
- Woo, S.Y.; Wong, C.P.; Win, N.N.; Lae, K.Z.W.; Woo, B.; Elsabbagh, S.A.; Liu, Q.Q.; Ngwe, H.; Morita, H. Anti-melanin deposition activity and active constituents of Jatropha multifida stems. J. Nat. Med. 2019, 73, 805–813. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsumoto, T.; Chaipech, S.; Miyake, S.; Katsuyama, Y.; Tsuboyama, A.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016, 70, 179–189. [Google Scholar] [CrossRef]
- Lee, Y.K.; Hwang, B.S.; Hwang, Y.; Lee, S.Y.; Oh, Y.T.; Kim, C.H.; Nam, H.J.; Jeong, Y.T. Melanogenesis inhibitory activity of epicatechin-3-O-gallate isolated from Polygonum amphibium L. Microbiol. Biotechnol. Lett. 2021, 49, 24–31. [Google Scholar] [CrossRef]
- Kuroi, A.; Sugimura, K.; Kumagai, A.; Kohara, A.; Nagaoka, Y.; Kawahara, H.; Yamahara, M.; Kawahara, N.; Takemori, H.; Fuchino, H. The Importance of 11α-OH, 15-oxo, and 16-en Moieties of 11α-Hydroxy-15-oxo-kaur-16-en-19-oic Acid in Its Inhibitory Activity on Melanogenesis. Skin Pharmacol. Physiol. 2017, 30, 205–215. [Google Scholar] [CrossRef]
- Shim, S.Y.; Lee, Y.E.; Song, H.Y.; Lee, M. P-hydroxybenzoic acid β-d-glucosyl ester and cimidahurinine with antimelanogenesis and antioxidant effects from Pyracantha angustifolia via bioactivity-guided fractionation. Antioxidants 2020, 9, 258. [Google Scholar] [CrossRef]
- Seong, Z.K.; Kim, H.S.; Won, Y.M.; Kim, J.L.; Song, H.H.; Kim, D.Y.; Oh, S.R.; Cho, H.W.; Cho, J.H.; Lee, H.K. Phenylacylphenol derivatives with anti-melanogenic activity from Stewartia pseudocamellia. Arch. Pharm. Res. 2016, 39, 636–645. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Zhang, J.; Kurita, M.; Ebina, K.; Ukiya, M.; Tokuda, H.; Yasukawa, K.; Masters, E.T.; Shimizu, N.; Akihisa, M.; Feng, F.; et al. Melanogenesis-inhibitory activity and cancer chemopreventive effect of glucosylcucurbic acid from shea (vitellaria paradoxa) kernels. Chem. Biodivers. 2015, 12, 547–558. [Google Scholar] [CrossRef]
- Won, Y.M.; Seong, Z.K.; Kim, J.L.; Kim, H.S.; Song, H.H.; Kim, D.Y.; Kim, J.H.; Oh, S.R.; Cho, H.W.; Cho, J.H.; et al. Triterpene glycosides with stimulatory activity on melanogenesis from the aerial parts of Weigela subsessilis. Arch. Pharm. Res. 2015, 38, 1541–1551. [Google Scholar] [CrossRef]
- Mamat, N.; Lu, X.Y.; Kabas, M.; Aisa, H.A. Potential anti-vitiligo properties of cynarine extracted from Vernonia anthelmintica (L.) Willd. Int. J. Mol. Med. 2018, 42, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Jo, H.G.; Yang, J.H.; Ki, S.H.; Shin, H.J. Antioxidative and anti-melanogenic activities of bamboo stems (Phyllostachys nigra variety henosis) via PKA/CREB-mediated MITF downregulation in B16F10 melanoma cells. Int. J. Mol. Sci. 2018, 19, 409. [Google Scholar] [CrossRef] [PubMed]
- Park, J.U.; Yang, S.Y.; Guo, R.H.; Li, H.X.; Kim, Y.H.; Kim, Y.R. Anti-Melanogenic Effect of Dendropanax morbiferus and Its Active Components via Protein Kinase A/Cyclic Adenosine Monophosphate-Responsive Binding Protein- and p38 Mitogen-Activated Protein Kinase-Mediated Microphthalmia−Associated Transcription Factor Do. Front. Pharmacol. 2020, 11, 507. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.Y.; Lin, H.H.; Li, T.S.; Tseng, C.Y.; Wong, Y.; Chen, J.H. Anti-melanogenesis effects of Guinea Pigs in vitro and in vivo. Nutrients 2020, 12, 3535. [Google Scholar] [CrossRef]
- Roh, E.; Yun, C.Y.; Yun, J.Y.; Park, D.; Doo Kim, N.; Yeon Hwang, B.; Jung, S.H.; Park, S.K.; Kim, Y.B.; Han, S.B.; et al. CAMP-binding site of PKA as a molecular target of bisabolangelone against melanocyte-specific hyperpigmented disorder. J. Invest. Dermatol. 2013, 133, 1072–1079. [Google Scholar] [CrossRef]
- Park, H.; Song, K.H.; Jung, P.M.; Kim, J.E.; Ro, H.; Kim, M.Y.; Ma, J.Y. Inhibitory effect of arctigenin from fructus arctii extract on melanin synthesis via repression of tyrosinase expression. Evid.-Based Complement. Altern. Med. 2013, 2013, 965312. [Google Scholar] [CrossRef]
- Kim, M.O.; Park, S.J.; Park, S.H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Kwon, K.; Kim, J.; Kim, M.H.; Cho, J.Y.; et al. Ethanolic extract of Melia azedarach L. induces melanogenesis through the cAMP-PKA-CREB signaling pathway. Mol. Cell. Toxicol. 2019, 15, 75–83. [Google Scholar] [CrossRef]
- Oh, S.W.; Park, S.H.; Lee, H.S.; Kang, M.; Lee, S.E.; Yoo, J.A.; Cho, J.Y.; Lee, J. Melanogenic mechanism of ethanolic extract of Dalbergia odorifera. Mol. Cell. Toxicol. 2017, 13, 453–459. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, I.S.; Dong, Y.; Lee, I.S.; Kim, J.S.; Kim, J.S.; Woo, J.T.; Cha, B.Y. Melanogenesis-inducing effect of cirsimaritin through increases in microphthalmia-associated transcription factor and tyrosinase expression. Int. J. Mol. Sci. 2015, 16, 8772–8788. [Google Scholar] [CrossRef]
- Huang, Y.C.; Liu, K.C.; Chiou, Y.L. Melanogenesis of murine melanoma cells induced by hesperetin, a Citrus hydrolysate-derived flavonoid. Food Chem. Toxicol. 2012, 50, 653–659. [Google Scholar] [CrossRef]
- Ahn, M.J.; Hur, S.J.; Kim, E.H.; Lee, S.H.; Shin, J.S.; Kim, M.K.; Uchizono, J.A.; Whang, W.K.; Kim, D.S. Scopoletin from Cirsium setidens increases melanin synthesis via CREB phosphorylation in B16F10 cells. Korean J. Physiol. Pharmacol. 2014, 18, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-J.; Kao, E.-S.; Chen, S.-R.; Huang, Y.-T.; Wang, C.-J.; Huang, H.-P. Nelumbo nucifera Leaf Extracts Inhibit Melanogenesis in B16 Melanoma Cells and Guinea Pigs through Downregulation of CREB/MITF Activation. J. Food Nutr. Res. 2020, 8, 459–465. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chien, Y.C.; Wu, C.H.; Kuo, Y.H.; Wu, W.C.; Pan, Y.Y.; Su, Y.H.; Wen, K.C. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem. Toxicol. 2014, 65, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, B.; Jeon, Y.D.; Song, H.W.; Lee, Y.M.; Song, B.J.; Kim, D.K. Inhibitory effect of elaeagnus umbellata fractions on melanogenesis in α-MSH-stimulated B16-F10 melanoma cells. Molecules 2021, 26, 1308. [Google Scholar] [CrossRef]
- Ko, H.H.; Chang, Y.T.; Kuo, Y.H.; Lin, C.H.; Chen, Y.F. Oenothera laciniata Hill Extracts Exhibits Antioxidant Effects and Attenuates Melanogenesis in B16-F10 Cells via Downregulating CREB/MITF/Tyrosinase and Upregulating p-ERK and p-JNK. Plants 2021, 10, 727. [Google Scholar] [CrossRef]
- Jeon, J.S.; Kang, H.M.; Park, J.H.; Kang, J.S.; Lee, Y.J.; Park, Y.H.; Je, B.I.; Park, S.Y.; Choi, Y.W. A comparative study on photo-protective and anti-melanogenic properties of different kadsura coccinea extracts. Plants 2021, 10, 1633. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Wong, Z.C.F.; Wang, C.; Fung, A.H.Y.; Wong, E.O.Y.; Chan, G.K.L.; Dong, T.T.X.; Chen, Y.; Tsim, K.W.K. Isoorientin derived from Gentiana veitchiorum Hemsl. flowers inhibits melanogenesis by down-regulating MITF-induced tyrosinase expression. Phytomedicine 2019, 57, 129–136. [Google Scholar] [CrossRef]
- Cai, Z.N.; Li, W.; Mehmood, S.; Pan, W.J.; Wu, Q.X.; Chen, Y.; Lu, Y.M. Effect of polysaccharide FMP-1 from Morchella esculenta on melanogenesis in B16F10 cells and zebrafish. Food Funct. 2018, 9, 5007–5015. [Google Scholar] [CrossRef]
- Jung, H.J.; Bang, E.; Kim, B.M.; Jeong, S.H.; Lee, G.H. Loganin Inhibits α-MSH and IBMX-induced Melanogenesis by Suppressing the Expression of Tyrosinase in B16F10 Melanoma Cells. J. Life Sci. 2019, 29, 1200–1207. [Google Scholar] [CrossRef]
- Seong, Z.K.; Lee, S.Y.; Poudel, A.; Oh, S.R.; Lee, H.K. Constituents of Cryptotaenia japonica inhibit melanogenesis via CREB- and MAPK-associated signaling pathways in murine B16 melanoma cells. Molecules 2016, 21, 1296. [Google Scholar] [CrossRef]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of anti-melanogenesis constituents from morus alba L. Leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.K.; Pham, C.H.; Lee, I.S.; Jung, S.H.; Jeong, J.H.; Shin, H.S.; Yoo, H.M. Anti-melanogenesis activity of 6-O-isobutyrylbritannilactone from inula britannica on B16F10 melanocytes and in vivo zebrafish models. Molecules 2020, 25, 3887. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Wang, S.S.; Tsai, T.C.; Ko, W.P.; Chang, T.M. Phoenix dactylifera L. Seed extract exhibits antioxidant effects and attenuates melanogenesis in B16F10 murine melanoma cells by downregulating PKA signaling. Antioxidants 2020, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and Function of the MAPKs and Their Substrates, the MAPK-Activated Protein Kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef]
- Buscà, R.; Abbe, P.; Mantoux, F.; Aberdam, E.; Peyssonnaux, C.; Eychène, A.; Ortonne, J.P.; Ballotti, R. Ras mediates the cAMP-dependent activation of extracellular signal-regulated kinases (ERKs) in melanocytes. EMBO J. 2000, 19, 2900–2910. [Google Scholar] [CrossRef]
- Gerits, N.; Kostenko, S.; Shiryaev, A.; Johannessen, M.; Moens, U. Relations between the mitogen-activated protein kinase and the cAMP-dependent protein kinase pathways: Comradeship and hostility. Cell. Signal. 2008, 20, 1592–1607. [Google Scholar] [CrossRef]
- Lee, A.Y. Recent progress in melasma pathogenesis. Pigment Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef]
- Kim, M.; Shibata, T.; Kwon, S.; Park, T.J.; Kang, H.Y. Ultraviolet-irradiated endothelial cells secrete stem cell factor and induce epidermal pigmentation. Sci. Rep. 2018, 8, 4235. [Google Scholar] [CrossRef]
- Li, P.H.; Liu, L.H.; Chang, C.C.; Gao, R.; Leung, C.H.; Ma, D.L.; David Wang, H.M. Silencing stem cell factor gene in fibroblasts to regulate paracrine factor productions and enhance c-Kit expression in melanocytes on melanogenesis. Int. J. Mol. Sci. 2018, 19, 19. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jin, S.H.; Kang, H.Y. LPS induces melanogenesis through p38 MAPK activation in human melanocytes. Arch. Dermatol. Res. 2008, 300, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, H.J.; Jun, H.S. Polygonum multiflorum Thunb. Extract Stimulates Melanogenesis by Induction of COX2 Expression through the Activation of p38 MAPK in B16F10 Mouse Melanoma Cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shang, J.; Ping, F.; Zhao, G. Alcohol extract from Vernonia anthelmintica (L.) willd seed enhances melanin synthesis through activation of the p38 MAPK signaling pathway in B16F10 cells and primary melanocytes. J. Ethnopharmacol. 2012, 143, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.A.; Kang, H.R.; Moon, J.Y.; Ediriweera, M.K.; Eum, S.; Bach, T.T.; Cho, S.K. Annona squamosa L. leaves inhibit alpha-melanocyte-stimulating hormone (α-MSH) stimulated melanogenesis via p38 signaling pathway in B16F10 melanoma cells. J. Cosmet. Dermatol. 2020, 19, 1785–1792. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kim, H.N.; Kim, Y.R.; Choi, Y.H.; Kim, B.W.; Shin, H.K.; Choi, B.T. Aqueous fraction from Cuscuta japonica seed suppresses melanin synthesis through inhibition of the p38 mitogen-activated protein kinase signaling pathway in B16F10 cells. J. Ethnopharmacol. 2012, 141, 338–344. [Google Scholar] [CrossRef]
- Masuda, M.; Itoh, K.; Murata, K.; Naruto, S.; Uwaya, A.; Isami, F.; Matsuda, H. Inhibitory Effects of Morinda citrifolia Extract and Its Constituents on Melanogenesis in Murine B16 Melanoma Cells. Biol. Pharm. Bull. 2012, 35, 78–83. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.S.; Woo, J.T.; Lee, I.S.; Cha, B.Y. Hyperpigmentation mechanism of methyl 3,5-di-caffeoylquinate through activation of p38 and MITF induction of tyrosinase. Acta Biochim. Biophys. Sin. 2015, 47, 548–556. [Google Scholar] [CrossRef]
- Yao, C.; Jin, C.L.; Oh, J.H.; Oh, I.G.; Park, C.H.; Chung, J.H. Ardisia crenata extract stimulates melanogenesis in B16F10 melanoma cells through inhibiting ERK1/2 and Akt activation. Mol. Med. Rep. 2015, 11, 653–657. [Google Scholar] [CrossRef]
- Villareal, M.O.; Han, J.; Matsuyama, K.; Sekii, Y.; Smaoui, A.; Shigemori, H.; Isoda, H. Lupenone from erica multiflora leaf extract stimulates melanogenesis in B16 murine melanoma cells through the inhibition of ERK1/2 activation. Planta Med. 2013, 79, 236–243. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Kuo, C.Y.; Kuan, Y.D.; Lin, H.C.; Wu, L.H.; Lee, C.H. The extracts of astragalus membranaceus inhibit melanogenesis through the ERK signaling pathway. Int. J. Med. Sci. 2017, 14, 1049–1053. [Google Scholar] [CrossRef]
- Lee, J.I.; Seo, J.H.; Ko, E.S.; Cho, S.M.; Kang, J.R.; Jeong, J.H.; Jeong, Y.J.; Kim, C.Y.; Cha, J.D.; Kim, W.S.; et al. Inhibition of melanogenesis by aster yomena callus pellet extract in melanoma cells and patients with skin pigmentation. Int. J. Med. Sci. 2021, 18, 3299–3308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.H.; Tian, Y.D.; Oh, J.H.; Bach, T.T.; Chung, J.H.; Jin, Z.H. Melochia corchorifolia extract inhibits melanogenesis in B16F10 mouse melanoma cells via activation of the ERK signaling. J. Cosmet. Dermatol. 2020, 19, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Saba, E.; Oh, M.J.; Lee, Y.Y.; Kwak, D.; Kim, S.; Rhee, M.H. Artemisia capillaris Thunb. inhibits melanin synthesis activity via ERK-dependent MITF pathway in B16/F10 melanoma cells. Korean J. Vet. Res. 2018, 58, 1–7. [Google Scholar] [CrossRef]
- Im, D.S.; Lee, J.M.; Lee, J.; Shin, H.J.; No, K.T.; Park, S.H.; Kim, K. Inhibition of collagenase and melanogenesis by ethanol extracts of Orostachys japonicus A. Berger: Possible involvement of Erk and Akt signaling pathways in melanoma cells. Acta Biochim. Biophys. Sin. 2017, 49, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.Y.; Choung, S.Y. Anti-melanogenic effects of Aster spathulifolius extract in UVB-exposed C57BL/6J mice and B16F10 melanoma cells through the regulation of MAPK/ERK and AKT/GSK3β signalling. J. Pharm. Pharmacol. 2016, 68, 503–513. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.J.; Ahn, Y.; Park, S.J.; Kim, S.H.; Oh, S.H. A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine 2019, 57, 57–64. [Google Scholar] [CrossRef]
- Choo, S.J.; Ryoo, I.J.; Kim, K.C.; Na, M.; Jang, J.H.; Ahn, J.S.; Yoo, I.D. Hypo-pigmenting effect of sesquiterpenes from Inula britannica in B16 melanoma cells. Arch. Pharm. Res. 2014, 37, 567–574. [Google Scholar] [CrossRef]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale Roscoe, attenuates α-MSH-induced melanogenesis in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef]
- Kim, K.N.; Yang, H.M.; Kang, S.M.; Kim, D.; Ahn, G.; Jeon, Y.J. Octaphlorethol A isolated from Ishige foliacea inhibits α-MSH-stimulated induced melanogenesis via ERK pathway in B16F10 melanoma cells. Food Chem. Toxicol. 2013, 59, 521–526. [Google Scholar] [CrossRef]
- Kim, D.E.; Chang, B.Y.; Ham, S.O.; Kim, Y.C.; Kim, S.Y. Neobavaisoflavone inhibits melanogenesis through the regulation of Akt/GSK-3β and MEK/ERK pathways in B16F10 cells and a reconstructed human 3D skin model. Molecules 2020, 25, 2683. [Google Scholar] [CrossRef]
- Hong, C.O.; Lee, H.A.; Rhee, C.H.; Choung, S.Y.; Lee, K.W. Separation of the antioxidant compound quercitrin from Lindera obtusiloba blume and its antimelanogenic effect on B16F10 melanoma cells. Biosci. Biotechnol. Biochem. 2013, 77, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Kim, D.O.; Heo, H.J. Melanogenesis regulatory activity of the ethyl acetate fraction from Arctium lappa L. leaf on α-MSH–induced B16/F10 melanoma cells. Ind. Crops Prod. 2019, 138, 111581. [Google Scholar] [CrossRef]
- Fu, Y.T.; Lee, C.W.; Ko, H.H.; Yen, F.L. Extracts of artocarpus communis decrease-melanocyte stimulating hormone-induced melanogenesis through activation of ERK and JNK signaling pathways. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Yen, F.L.; Wang, M.C.; Liang, C.J.; Ko, H.H.; Lee, C.W. Melanogenesis inhibitor(s) from Phyla nodiflora extract. Evidence-Based Complement. Altern. Med. 2012, 2012, 867494. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.; Lee, J.; Park, S.H.; Kim, Y.A.; Park, B.J.; Oh, J.; Sung, G.H.; Aravinthan, A.; Kim, J.H.; Kang, H.; et al. Antiphotoaging and antimelanogenic effects of penthorum chinense pursh ethanol extract due to antioxidant- and autophagy-inducing properties. Oxid. Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- In, M.-H.; Jeon, B.K.; Mun, Y.-J.; Woo, W.-H. Hexane Extract of Kaempferia galanga L. Suppresses Melanogenesis via p38, JNK and Akt. Korean J. Orient. Physiol. Pathol. 2016, 30, 47. [Google Scholar] [CrossRef]
- Sim, M.O.; Ham, J.R.; Lee, M.K. Young leaves of reed (Phragmites communis) suppress melanogenesis and oxidative stress in B16F10 melanoma cells. Biomed. Pharmacother. 2017, 93, 165–171. [Google Scholar] [CrossRef]
- Ko, H.H.; Tsai, Y.T.; Yen, M.H.; Lin, C.C.; Liang, C.J.; Yang, T.H.; Lee, C.W.; Yen, F.L. Norartocarpetin from a folk medicine Artocarpus communis plays a melanogenesis inhibitor without cytotoxicity in B16F10 cell and skin irritation in mice. BMC Complement. Altern. Med. 2013, 13, 348. [Google Scholar] [CrossRef]
- Makbal, R.; Villareal, M.O.; Gadhi, C.; Hafidi, A.; Isoda, H. Argania spinosa fruit shell extract-induced melanogenesis via cAMP signaling pathway activation. Int. J. Mol. Sci. 2020, 21, 2539. [Google Scholar] [CrossRef]
- Ko, H.H.; Chiang, Y.C.; Tsai, M.H.; Liang, C.J.; Hsu, L.F.; Li, S.Y.; Wang, M.C.; Yen, F.L.; Lee, C.W. Eupafolin, a skin whitening flavonoid isolated from Phyla nodiflora, downregulated melanogenesis: Role of MAPK and Akt pathways. J. Ethnopharmacol. 2014, 151, 386–393. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lee, J.N.; Kim, B.S.; Hyun, C.G. Anti-melanogenic effects of Paederia foetida L. Extract via mapk signaling-mediated mitf downregulation. Cosmetics 2021, 8, 22. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, W.C.; Han, A.; Lee, M.H.; Shin, E.J.; Lee, K.M.; Nam, T.G.; Lim, T.G. Rose Petal Extract (Rosa gallica) Exerts Skin Whitening and Anti-Skin Wrinkle Effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Liao, C.C.; Peng, C.C.; Lim, J.M.; Siao, J.H.; Wei, C.M.; Chen, C.C.; Wu, C.S.; Chang, T.M. Dihydromyricetin from Ampelopsis grossedentata inhibits melanogenesis through down-regulation of MAPK, PKA and PKC signaling pathways. Chem. Biol. Interact. 2016, 258, 166–174. [Google Scholar] [CrossRef]
- Khaled, M.; Larribere, L.; Bille, K.; Aberdam, E.; Ortonne, J.P.; Ballotti, R.; Bertolotto, C. Glycogen synthase kinase 3β is activated by cAMP and plays an active role in the regulation of melanogenesis. J. Biol. Chem. 2002, 277, 33690–33697. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, L.; Su, Q.; Fan, X.; Wang, Y.; Gao, S.; Wang, H.; Chen, H.; Chan, C.B.; Liu, Z. Phosphorylation of MITF by AKT affects its downstream targets and causes TP53-dependent cell senescence. Int. J. Biochem. Cell Biol. 2016, 80, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Mosca, S.; Cardinali, G.; Flori, E.; Briganti, S.; Bottillo, I.; Mileo, A.M.; Maresca, V. The PI3K pathway induced by αMSH exerts a negative feedback on melanogenesis and contributes to the release of pigment. Pigment Cell Melanoma Res. 2021, 34, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; Ratti, S.; Candido, S.; Libra, M.; Montalto, G.; et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef]
- Phacharapiyangkul, N.; Thirapanmethee, K.; Sa-Ngiamsuntorn, K.; Panich, U.; Lee, C.H.; Chomnawang, M.T. The ethanol extract of musa sapientum linn. Peel inhibits melanogenesis through akt signaling pathway. Cosmetics 2021, 8, 70. [Google Scholar] [CrossRef]
- Ku, B.; Kim, D.; Choi, E.M. Anti-melanogenic effect of the aqueous ethanol extract of Ginkgo biloba leaf in B16F10 cells. Toxicol. Environ. Health Sci. 2020, 12, 287–295. [Google Scholar] [CrossRef]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef]
- Box, N.F.; Terzian, T. The role of p53 in pigmentation, tanning and melanoma. Pigment Cell Melanoma Res. 2008, 21, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Weon, K.Y.; Nam, D.Y.; Nam, J.H.; Kim, W.K. Skin protective effect of guava leaves against UV-induced melanogenesis via inhibition of ORAI1 channel and tyrosinase activity. Exp. Dermatol. 2016, 25, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Lee, D.U. Foeniculum vulgare extract and its constituent, trans-anethole, inhibit UV-induced melanogenesis via ORAI1 channel inhibition. J. Dermatol. Sci. 2016, 84, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Nam, D.Y.; Lee, D.U. Valencene from the Rhizomes of Cyperus rotundus Inhibits Skin Photoaging-Related Ion Channels and UV-Induced Melanogenesis in B16F10 Melanoma Cells. J. Nat. Prod. 2016, 79, 1091–1096. [Google Scholar] [CrossRef]
- Stanisz, H.; Vultur, A.; Herlyn, M.; Roesch, A.; Bogeski, I. The role of Orai-STIM calcium channels in melanocytes and melanoma. J. Physiol. 2016, 594, 2825–2835. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhang, A.; Gupte, A.A.; Hamilton, D.J. The Role of Calcium Signaling in Melanoma. Int. J. Mol. Sci. 2022, 23, 1010. [Google Scholar] [CrossRef]

| Name of Species/Family | Part of Plant | Type of Solvent | Concentration | Methods | Effects | Ref. |
|---|---|---|---|---|---|---|
| Artemisia asiatica Nakai ex Pamp./Asteraceae | whole plant | ethanol | 25–50 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [25] |
| Camellia sinensis (L.) Kuntze/Theaceae | flower | ethanol | 20–40 µg/mL | RT-PCR | Reduced expression: TYR | [26] |
| Castanea crenata Siebold & Zucc./Fagaceae | inner skin | ethyl acetate | 10–100 µg/mL | Western blotting | Reduced expression: TYR | [27] |
| Cinnamomum osmophloeum Kaneh./Lauraceae | leaves | ethanol | 21.25 µg/mL | RT-PCR | Reduced expression: MITF, TYR | [28] |
| Coix lacryma-jobi L./Poaceae | seeds | ethanol | 20–40 mg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [29] |
| Croton roxburghii N.P.Balakr. and Croton sublyratus Kurz/Euphorbiaceae | leaves | ethanol | 25–100 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [30] |
| Dendrobium moniliforme (L.) Sw./Orchidaceae | leaves | ethanol | 12.5–50 µg/mL | Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [31] |
| Dendropanax morbiferus H.Lév./Araliaceae | leaves | ethanol | 12.5–50 µg/mL | Western blotting | Reduced expression: TYR, TYRP-2 | [32] |
| Equisetum ramosissimum Desf./Equisetaceae | whole plant | ethyl acetate, dichloromethane | 10–100 µg/mL | Western blotting Western blotting | ethyl acetate: Reduced expression: MITF, TYR, TYRP-1, TYRP-2; dichloromethane: Increased expression: MITF, TYR, TYRP-1, TYRP-2 | [33] |
| Euryale ferox Salisb./Nymphaeaceae | seeds | ethyl acetate | 30 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [34] |
| Gaillardia aristata Pursh/Asteraceae | flowers | ethanol | 10–20 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [35] |
| Garcinia mangostana L./Clusiaceae | leaves | water | 4–32 µg/mL | Western blotting | Increased expression: TYR | [36] |
| Gastrodia elata Blume/Orchidaceae | whole plant | water | 0.5–5 mg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [37] |
| Glechoma hederacea L./Lamiaceae | whole plant | water | 0.1–1 mg/mL | RT-PCR, Western blotting | Reduced expression: TYR | [38] |
| Glycine max (L.) Merr./Fabaceae | seeds cell culture | ethanol | 0.5–1 mg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [39] |
| Haloxylon scoparium Pomel/Amaranthaceae | stems | ethanol | 0.017%(w/v) | RT-PCR, Western blotting | Reduced expression: MC1R, TYR, TYRP-1 | [40] |
| Kummerowia striata (Thunb.) Schindl./Fabaceae | aerial parts | ethanol | 100–400 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [41] |
| Kummerowia striata (Thunb.) Schindl./Fabaceae | aerial parts | ethanol | 100–400 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [42] |
| Melia azedarach L./Meliaceae | whole plant | ethanol | 20 µg/mL | RT-PCR, Western blotting | Increased expression: TYRP-1 | [43] |
| Nepeta binaludensis Jamzad/Lamiaceae | aerial parts | methanol | 50 µg/mL | Western blotting | Reduced expression: TYR | [44] |
| Nepeta sintenisii Bornm./Lamiaceae | aerial parts | n-hexane, methanol, water | 50 µg/mL | Western blotting | Reduced expression: MITF | [45] |
| Oplismenus undulatifolius (Ard.) P.Beauv./Poaceae | whole plant | ethanol | 5–15 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [46] |
| Oreocnide fruticosa (Gaudich.) Hand. Mazz./Urticaceae | branches | ethyl acetate | 25–100 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [47] |
| Phyllanthus emblica L./Phyllanthaceae | fruits | water | 0.05–1 mg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [48] |
| Pinus densiflora Siebold & Zucc./Pinaceae | pine cone | ethyl acetate | 12.5–50 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [49] |
| Psoralea corylifolia (Babchi)/Fabaceae and Zingiber officinale Roscoe/Zingiberaceae; Psoralea corylifolia (Babchi)/Fabaceae and Eclipta prostrata (L.) L./Asteraceae | whole plants | methanol | 10–100 µg/mL | RT-PCR, Western blotting | Increased expression: MITF | [50] |
| Syzygium cumini (L.) Skeels/Myrtaceae | leaves and branch | ethanol | 25–100 µg/mL | RT-PCR | Reduced expression: TYR, TYRP-1, TYRP-2 | [51] |
| Uncaria rhynchophylla (Miq.) Miq./Rubiaceae | stems and hooks | ethanol | 0.1–1 mg/mL | RT-PCR | Reduced expression: TYR | [52] |
| Vitis vinifera L./Vitaceae | pericarp, seed, flesh, and grape stem | ethanol | 100 µg/mL | Western blotting | Increased expression: MITF, TYR, TYRP-1, TYRP-2 | [53] |
| Name of Species/Family | Part of Plant | Type of Solvent | Identified Compounds | Concentration | Methods | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Acer rubrum L./Sapindaceae | leaves | ethanol | phenolic compounds | 10 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [54] |
| Angelica polymorpha Maxim./Apiaceae | flowers | hexane | aromadendrene, methoxsalen, bergapten, isopimpinellin, nonadencane | 0.1–100 µg/mL | Western blotting | Reduced expression: MITF, TYR | [55] |
| Argania spinosa L.) Skeels/Sapotaceae | leaves | ethanol | 14 compounds | 30 µg/mL | Western blotting | Increased expression: TYR, TYRP-1, | [56] |
| Artemisia capillaris Thunb./Asteraceae | whole plant | ethanol | leukodin | 12.5–50 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [57] |
| Artocarpus lacucha Buch.-Ham./Moraceae and Glycyrrhiza glabra L./Fabaceae | heartwood and root | ethanol | gallic acid, oxyresveratrol, resveratrol and glabridin | 0.1 mg/mL | Western blotting | Reduced expression: MITF, TYRP-2 | [58] |
| Callicarpa longissima (Hemsl.) Merr./Lamiaceae | whole plant | ethanol | carnosol and carnosic acid | 0.1–10 µg/mL | RT-PCR | Reduced expression: MITF | [59] |
| Ceratonia siliqua L./Fabaceae | leaves, bark and fruits | ethanol | epicatechin-3-O-gallate, 1,2,3,6-tetra-O-galloyl-ß-D-glucose and gallocatechin-3-O-gallate | 100 µg/mL | RT-PCR | Reduced expression: TYR | [60] |
| Glycyrrhiza glabra L. and Glycyrrhiza uralensis Fisch. ex DC./Fabaceae | whole plant/heat treated | ethanol | isoliquiritigenin | 100 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [61] |
| Hordeum vulgare L./Poaceae | barely sprout | water | p-coumaric, ferulic, and vanillic acids | 50–250 µg/mL | Western blotting | Reduced expression: MITF, TYR | [62] |
| Juniperus communis L./Cupressaceae | fruits | ethanol | hypolaetin-7-O-β-D-xylopyranoside and isoscutellarein-7-O-β-D-xylopyranoside | 50 µg/mL | Western blotting | Reduced expression: TYR | [63] |
| Libidibia ferrea (Mart. ex Tul.) L.P.Queiroz/Fabaceae | bark and pods | ethanol | 18 compounds | 25 µg/mL | RT-PCR, Western blotting | Reduced expression: TYR | [64] |
| Limonium tetragonum (Thunb.) Bullock/Plumbaginaceae | whole plant | water, methanol, buthanol | myricetin 3-galactoside and quercetin 3-O- -galactopyronaside | 5–20 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [65] |
| Myrica rubra (Lour.) Siebold & Zucc./Myricaceae | fruits | water | myricetin-O-deoxyhexoside, quercetin-O-deoxyhexoside, and aempferol-O-hexoside | 0.5–2 mg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYRP-1, | [66] |
| Nigella sativa L./Ranunculaceae | seed | Thymocid® | thymoquinone | 20 µg/mL | RT-PCR, Western blotting | Reduced expression: MITF, TYRP-1, TYRP-2 | [67] |
| Petasites japonicus (Siebold & Zucc.) Maxim./Asteraceae | leaves, stems, and roots | water | leaf extract-isorhamnetin (main) root extract-p-coumaric acid (main) | 50–200 µg/mL | RT-PCR, Western blotting | Reduced expression: TYR | [68] |
| Phyllanthus emblica L./Phyllanthaceae | branch | ethanol | gallic acid and vanillic acid | 6.25–25 µg/mL | RT-PCR | Reduced expression: TYR, TYRP-1, TYRP-2 | [69] |
| Pueraria montana (Lour.) Merr./Fabaceae | aerial parts | ethanol | daidzein, daidzin, glycitein, glycitin, genistein, genistin | 10–100 µg/mL | RT-PCR, Western blotting | Reduced expression: TYR | [70] |
| Pueraria montana (Lour.) Merr./Fabaceae | stems | n-hexane | 12 compounds | 50 µg/mL | RT-PCR | Reduced expression: TYR | [71] |
| Rhododendron weyrichii Maxim./Ericaceae Durande | flowers | ethanol | p-coumaric acid | 25–200 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [72] |
| Sorghum bicolor (L.) Moench/Poaceae | whole plant | ethanol | 1-O-ca eoylglycerol, dica eoylglycerides, 1,3-O-dica eoylglycerol, p-coumaroyl-ca eoylglycerol, feruloyl-ca eoylglycerol, Tricin, 9-hydroxyoctadecadienoic acid | 2–10 µg/mL | Western blotting | Reduced expression: MITF, TYRP-1, | [73] |
| Vernonia anthelmintica (L.) Willd./Asteraceae | whole plant | ethanol | 15 compounds (mainly flavonoids) | 20 µg/mL | Western blotting | Increased expression: TYR | [74] |
| Name of Species/Family | Part of Plant | Compounds | Concentration | Methods | Effects | Ref. |
|---|---|---|---|---|---|---|
| Acanthopanax koreanum Nakai/Araliaceae | roots | acanthoic acid | 25–100 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [75] |
| Artemisia capillaris Thunb./Asteraceae | whole plant | leukodin | 37.5–150 µg/mL | Western blotting | Reduced expression: TYRP-1, TYRP-2 | [57] |
| Artemisia capillaris Thunb./Asteraceae | whole plant | isofraxidin 7-O-(6′-O-p-coumaroyl)-𝛽-glucopyranoside | 25 µg/mL | RT-PCR | Increased expression: MITF, TYR | [76] |
| Artemisia capillaris Thunb./Asteraceae | leaves and stems | 4,5-𝑂-dicaffeoylquinic acid | 25 µg/mL | RT-PCR | Reduced expression: TYRP-1 | [77] |
| Caesalpinia sappan L./Fabaceae | heartwood | sappanone A | 4.4 µg/mL | RT-PCR | Reduced expression: TYR | [78] |
| Crocus sativus L./Iridaceae | stigmas | crocetin | 0.5–32 µg/mL | Western blotting | Reduced expression: MITF | [79] |
| Cuscuta chinensis Lam./Convolvulaceae | whole plant | polysaccharide | 40–160 µg/mL | Western blotting | Reduced expression: MITF, TYR, TYRP-1 | [80] |
| Ephedra sinica Stapf/Ephedraceae | roots | ephedrannins A and B | A: 18–72 µg/mL; B: 1.85–7.4 µg/mL | RT-PCR | Reduced expression: TYR | [81] |
| Fragaria × ananassa (Duchesne ex Weston) Duchesne ex Rozier/Rosaceae | calyx | oleanolic acid | 12.5 µg/mL | Western blotting | Reduced expression: TYR, TYRP-1, TYRP-2 | [82] |
| Isodon trichocarpus (Maxim.) Kudô./Lamiaceae | aerial parts | enmein, isodocarpin, nodosin, oridonin | 1–3 µg/mL | RT-PCR | Reduced expression: TYR, TYRP-1, TYRP-2 | [83] |
| Jatropha multifida L./Euphorbiaceae | stems | Secoisolariciresinol | 6.25–200 µg/mL | RT-PCR | Reduced expression: TYR | [84] |
| Kaempferia parviflora Wall. ex Baker/Zingiberaceae | rhizomes | 5-hydroxy-7,3′,4′-trimethoxyflavone, 5,7,3′,4′-tetramethoxyflavone, 5,3′- dihydroxy-3,7,4′-trimethoxyflavone and 5-hydroxy-3,7,3′,4′-tetramethoxyflavone | 3–30 µg/mL | RT-PCR | Reduced expression: TYR, TYRP-1, TYRP-2 | [85] |
| Limonium tetragonum (Thunb.) Bullock/Plumbaginaceae | whole plant | myricetin 3-galactoside and quercetin 3-O-galactopyronaside | 10 µg/mL | Western blotting | Reduced expression: TYRP-1, TYRP-2 | [65] |
| Persicaria amphibia (L.) Delarbre/Polygonaceae | whole plant | epicatechin-3-O-gallate | 25–200 µg/mL | Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [86] |
| Pteris dispar Kunze/Pteridaceae | leaves | ent -11α-hydroxy-15-oxo-kaur-16-en-19-oic acid | 10 µg/mL | RT-PCR, Western blotting | Reduced expression: TYR | [87] |
| Pyracantha angustifolia (Franch.) C.K.Schneid./Rosaceae | leaves, twigs, and fruits | β-D-glucosylester and cimidahurinine | 10–100 µg/mL | Western blotting | Reduced expression: TYRP-1, TYRP-2 | [88] |
| Stewartia pseudocamellia Maxim. | twigs | stewartianol and stewartianol- 3-O-glucoside | 20–80 µg/mL | Western blotting | Reduced expression: MITF | [89] |
| Tetragonia tetragonoides (Pall.) Kuntze/Aizoaceae | whole plant | ferulic acid | 5–20 µg/mL | Western blotting | Reduced expression: MITF, TYR | [90] |
| Vitellaria paradoxa C.F.Gaertn./Sapotaceae | fruit | glucosylcucurbic acid and cucurbic acid | 30–100 µg/mL | Western blotting | Reduced expression: MITF, TYR, TYRP-1, TYRP-2 | [91] |
| Weigela subsessilis (Nakai) L.H.Bailey/Caprifoliaceae | aerial parts | loniceroside A, loniceroside L | 1–20 µg/mL | Western blotting | Increased expression: MITF, TYR | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules 2022, 27, 4360. https://doi.org/10.3390/molecules27144360
Merecz-Sadowska A, Sitarek P, Kowalczyk T, Zajdel K, Kucharska E, Zajdel R. The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules. 2022; 27(14):4360. https://doi.org/10.3390/molecules27144360
Chicago/Turabian StyleMerecz-Sadowska, Anna, Przemysław Sitarek, Tomasz Kowalczyk, Karolina Zajdel, Ewa Kucharska, and Radosław Zajdel. 2022. "The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds" Molecules 27, no. 14: 4360. https://doi.org/10.3390/molecules27144360
APA StyleMerecz-Sadowska, A., Sitarek, P., Kowalczyk, T., Zajdel, K., Kucharska, E., & Zajdel, R. (2022). The Modulation of Melanogenesis in B16 Cells Upon Treatment with Plant Extracts and Isolated Plant Compounds. Molecules, 27(14), 4360. https://doi.org/10.3390/molecules27144360