Facile Synthesis of Porous Ag Crystals as SERS Sensor for Detection of Five Methamphetamine Analogs
Abstract
:1. Introduction
2. Results and Discussion
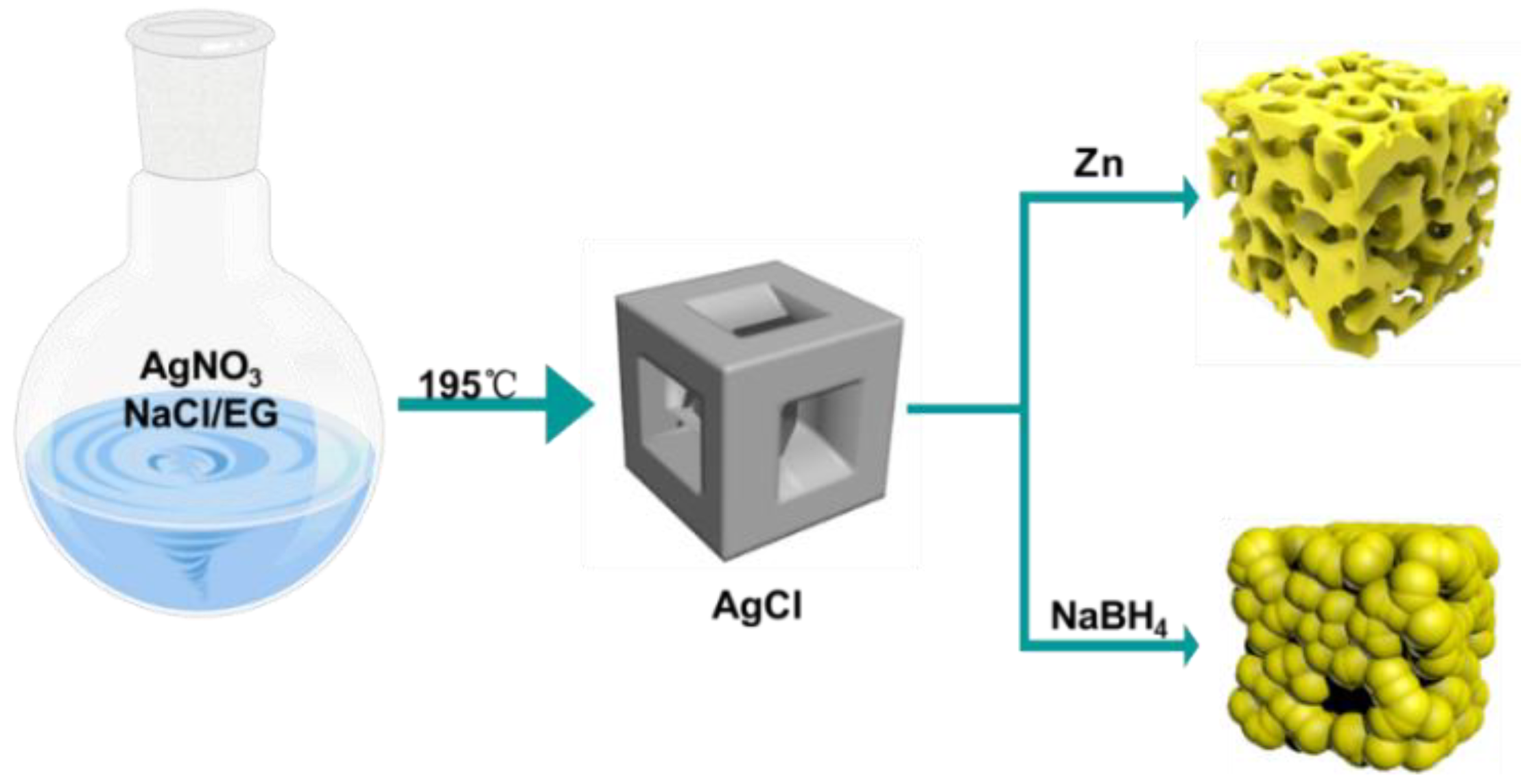
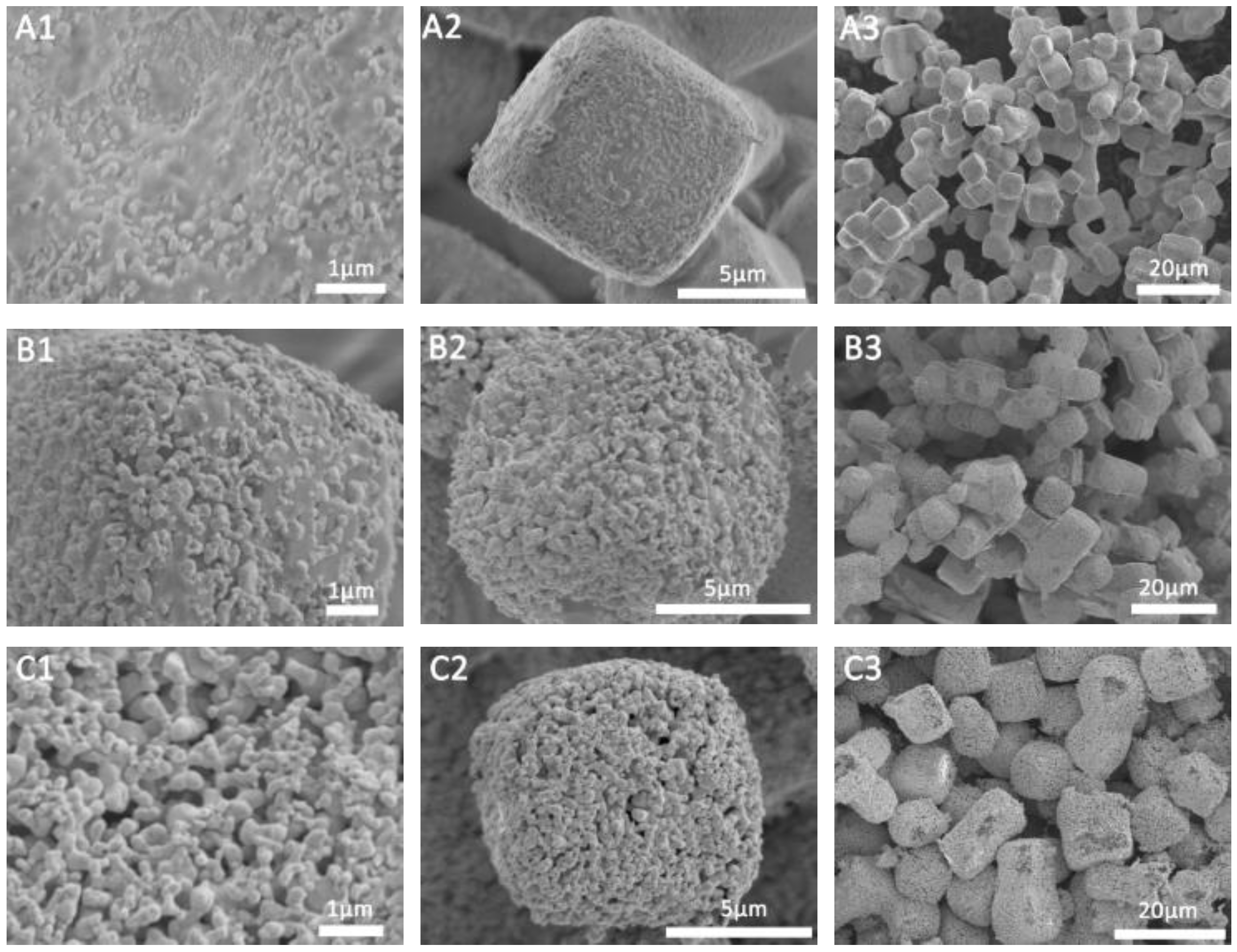
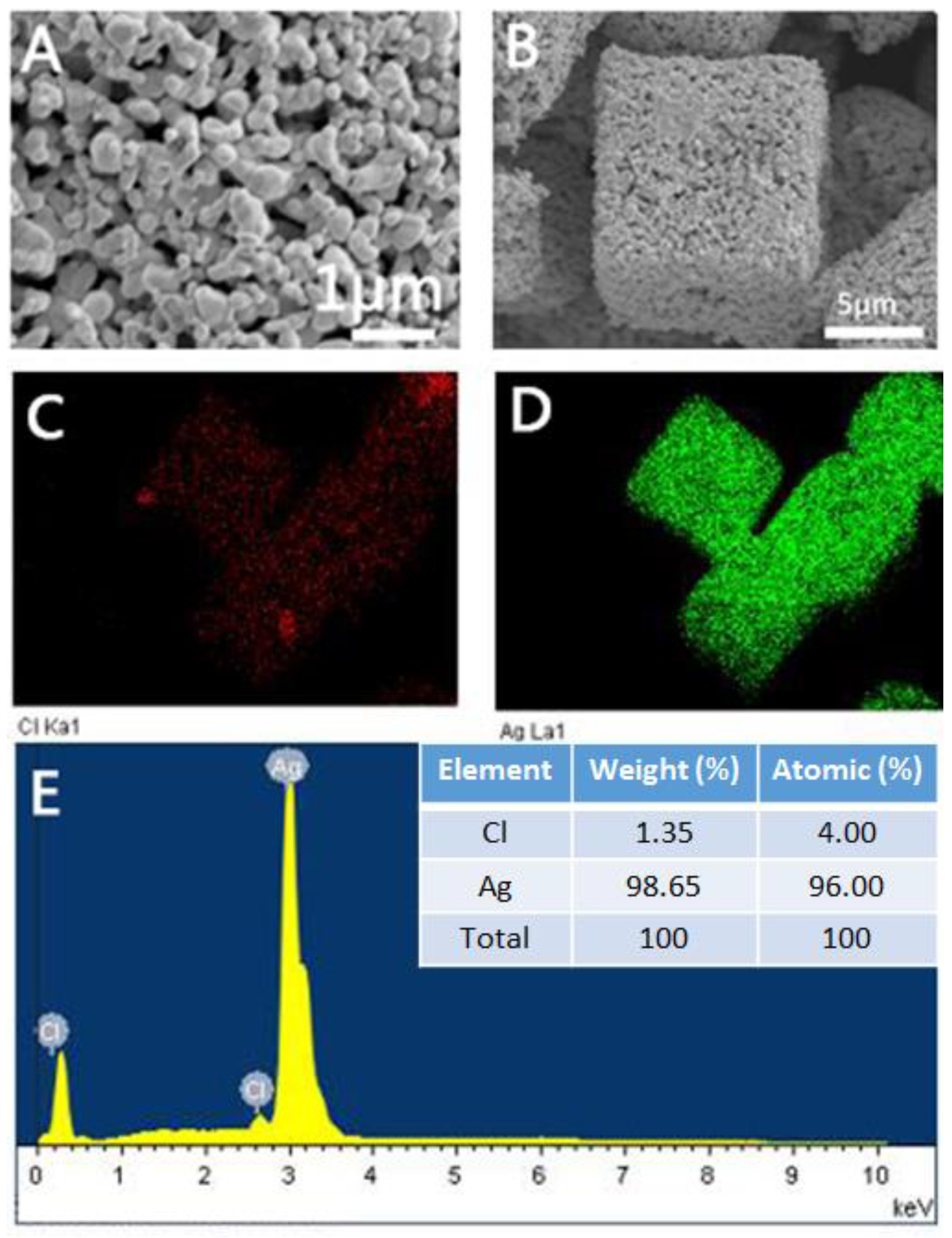
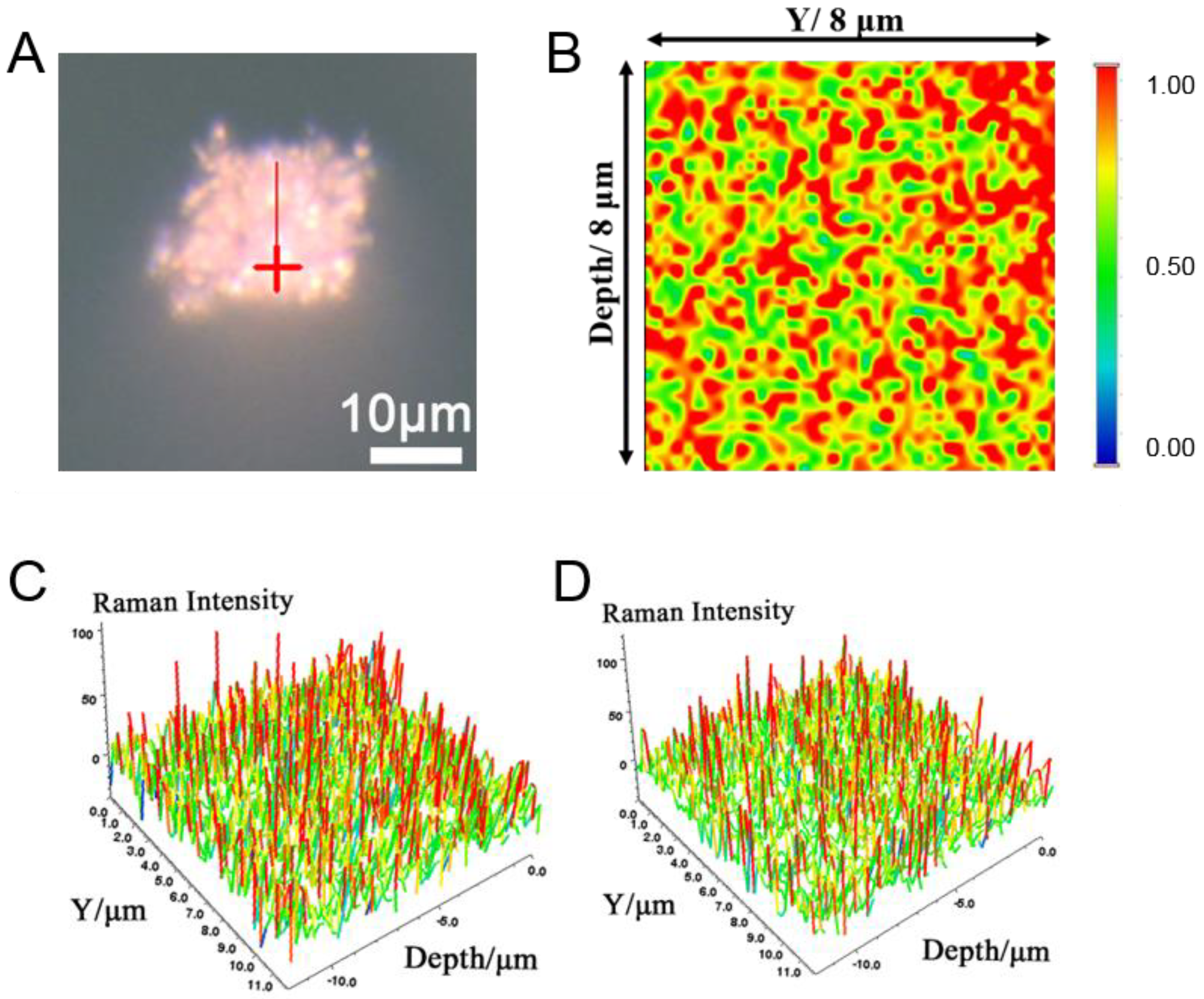
2.1. Characterization of the Prepared Porous Ag
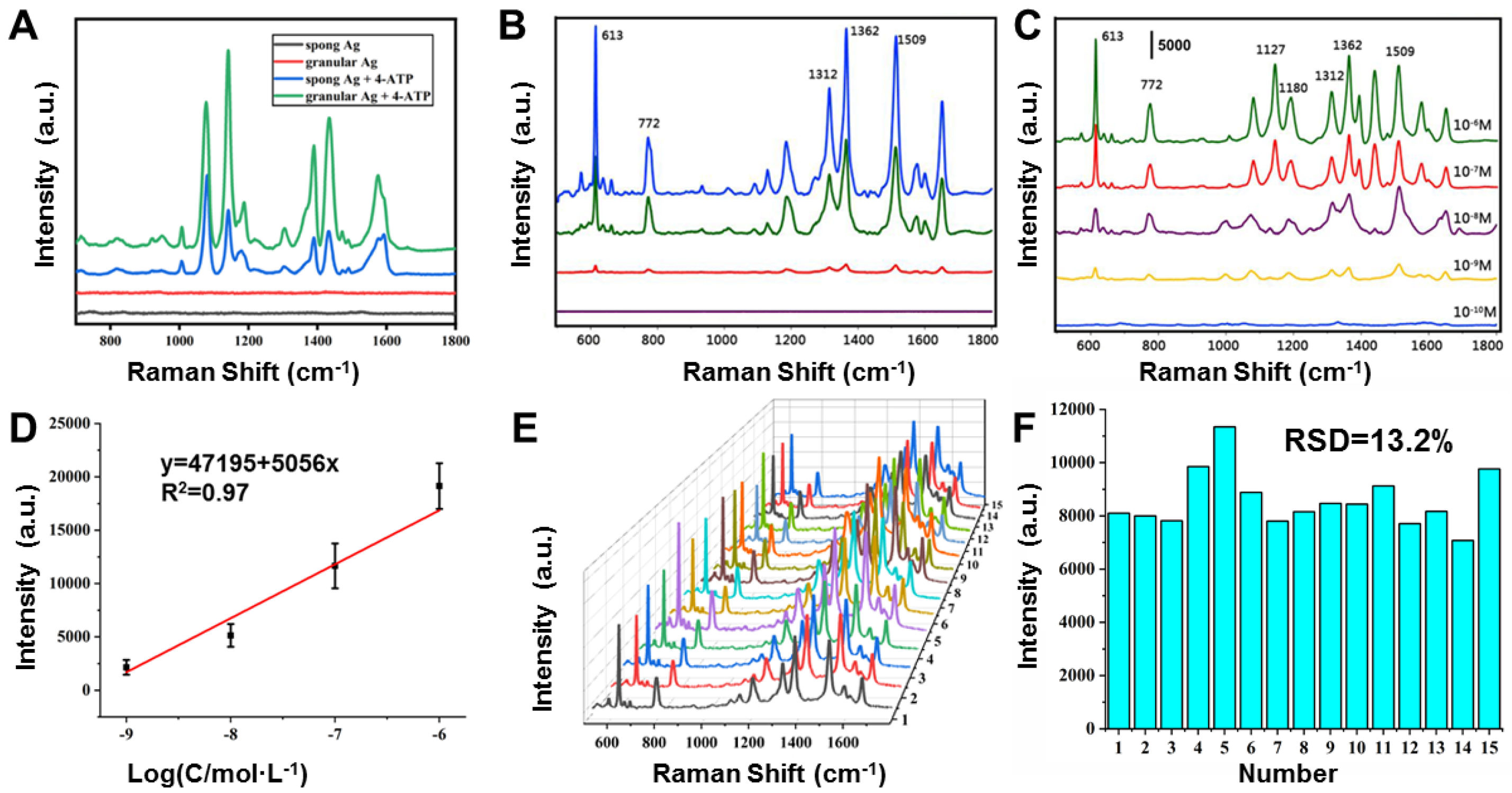
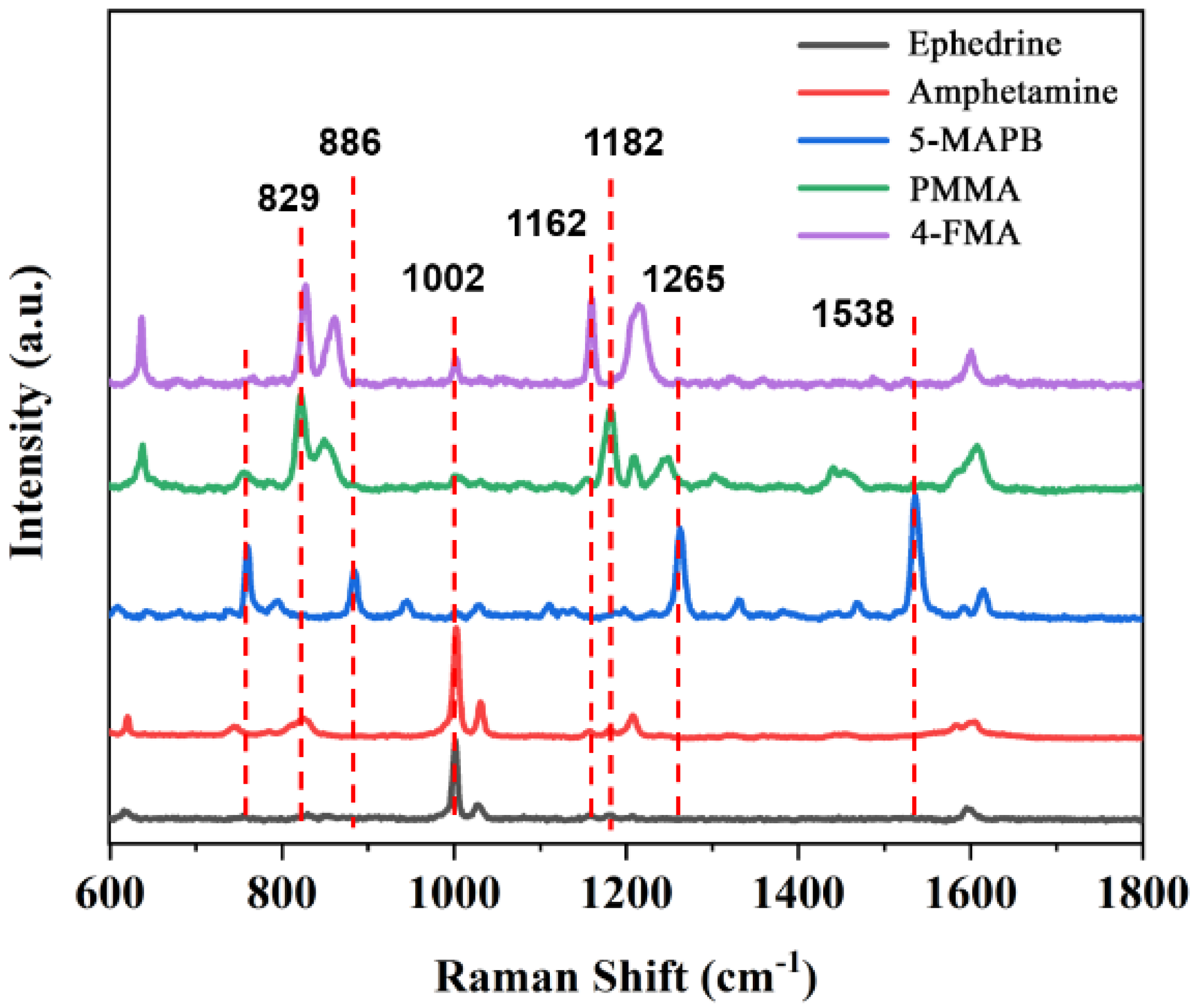
2.2. SERS Properties of the Sponge-like and Granular Porous Ag Cubes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Concave AgCl Cube
3.3. Preparation of Granular Porous Ag Cubes
3.4. Preparation of Sponge-like Porous Ag Cube
3.5. Instrumentation and Characterization
3.6. The SERS Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langille, M.R.; Zhang, J.; Mirkin, C.A. Plasmon-Mediated Synthesis of Heterometallic Nanorods and Icosahedra. Angew. Chem. 2011, 123, 3605–3609. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.Z.; Lu, Y.X.; Pan, W.F.; Yu, D.D.; Zhou, J.G. One-pot synthesis of hollow hydrangea Au nanoparticles as a dual catalyst with SERS activity for in situ monitoring of a reduction reaction. RSC Adv. 2019, 9, 10314–10319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Fan, Z.; Zhang, Z.C.; Niu, W.X.; Li, C.L.; Yang, N.L.; Chen, B.; Zhang, H. Two-Dimensional Metal Nano-materials: Synthesis, Properties, and Applications. Chem. Rev. 2018, 3, 6409–6455. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Sun, W.; Li, Y.; Deng, C.; Qian, H. High catalytic activity of supported Au nanoparticles assisted with the surface selective adsorption. J. Nanoparticle Res. 2019, 21, 146–156. [Google Scholar] [CrossRef]
- Lin, X.; Lin, S.; Liu, Y.; Gao, M.; Zhao, H.; Liu, B.; Hasi, W.; Wang, L. Facile Synthesis of Monodisperse Silver Nanospheres in Aqueous Solution via Seed-Mediated Growth Coupled with Oxidative Etching. Langmuir 2018, 34, 6077–6084. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Wu, F.; Liu, H.; Fang, J. Size-tunable uniform gold octahedra: Fast synthesis, characterization, and plasmonic properties. RSC Adv. 2017, 7, 18601–18608. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wan, D.; Xie, S.; Xia, X.; Huang, C.Z.; Xia, Y. Synthesis of Silver Octahedra with Controlled Sizes and Optical Properties via Seed-Mediated Growth. ACS Nano 2013, 7, 4586–4594. [Google Scholar] [CrossRef]
- Lin, Y.; Zhang, Y.-J.; Yang, W.-M.; Dong, J.-C.; Fan, F.-R.; Zhao, Y.; Zhang, H.; Bodappa, N.; Tian, X.-D.; Yang, Z.-L.; et al. Size and dimension dependent surface-enhanced Raman scattering properties of well-defined Ag nanocubes. Appl. Mater. Today 2019, 14, 224–232. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, L.; Zou, Y.; Song, Z.; Jin, S. Highly reproducible SERS sensor based on self-assembled Au nanocubic monolayer film for sensitive and quantitative detection of glutathione. Appl. Surf. Sci. 2021, 540, 148381. [Google Scholar] [CrossRef]
- Lin, H.X.; Lei, Z.C.; Jiang, Z.Y.; Hou, C.P.; Liu, D.Y.; Xu, M.M.; Tian, Z.Q.; Xie, Z.X. Supersaturation-Dependent Sur-face Structure Evolution: From Ionic, Molecular to Metallic Micro/Nanocrystals. J. Am. Chem. Soc. 2013, 135, 9311–9314. [Google Scholar] [CrossRef] [PubMed]
- Madasu, M.; Hsieh, P.-L.; Chen, Y.-J.; Huang, M.H. Formation of Silver Rhombic Dodecahedra, Octahedra, and Cubes through Pseudomorphic Conversion of Ag2O Crystals with Nitroarene Reduction Activity. ACS Appl. Mater. Interfaces 2019, 11, 38039–38045. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, J.; Yun, Q.; Wang, C.; Ruan, Q.; Kan, C. Realization of red plasmon shifts by the selective etching of Ag nanorods. CrystEngComm 2020, 22, 7870–7876. [Google Scholar] [CrossRef]
- Brown, M.; Wiley, B.J. Bromide Causes Facet-Selective Atomic Addition in Gold Nanorod Syntheses. Chem. Mater. 2020, 15, 6410–6415. [Google Scholar] [CrossRef]
- Guo, W.; Johnston-Peck, A.C.; Zhang, Y.; Hu, Y.; Huang, J.; Wei, W.D. Cooperation of Hot Holes and Surface Adsorbates in Plasmon-Driven Anisotropic Growth of Gold Nanostars. J. Am. Chem. Soc. 2020, 142, 10921–10925. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Yang, Y.; Xue, J.; Zhang, F.; Sun, J.; Xu, H.; Jiang, T. Synthesis of Photothermally Stable Triangular Silver Nanoplates for SERS Applications, Photokilling of Bacteria. ChemNanoMat 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Qin, Y.; Pan, W.; Yu, D.; Lu, Y.; Wu, W.; Zhou, J. Stepwise evolution of Au micro/nanocrystals from an octahedron into a truncated ditetragonal prism. Chem. Commun. 2018, 54, 3411–3414. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, Y.; Yu, D.; Zhou, J. Controllable synthesis of Au nanocrystals with systematic shape evolution from an octahedron to a truncated ditetragonal prism and rhombic dodecahedron. CrystEngComm 2019, 21, 5602–5609. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, R.; Zhang, D.; You, H.; Dai, Y.; Liu, W.; Fang, J. Shape-Controlled Hierarchical Flowerlike Au Nanostructure Microarrays by Electrochemical Growth for Surface-Enhanced Raman Spectroscopy Application. Anal. Chem. 2020, 92, 9838–9846. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhai, M.; Xu, H. Controllable synthesis of sea urchin-like gold nanoparticles and their optical characteristics. Appl. Surf. Sci. 2019, 498, 143864. [Google Scholar] [CrossRef]
- Song, C.; Dou, Y.; Yuwen, L.; Sun, Y.; Dong, C.; Li, F.; Yang, Y.; Wang, L. A gold nanoflower-based traceable drug delivery system for intracellular SERS imaging-guided targeted chemo-phototherapy. J. Mater. Chem. B 2018, 6, 3030–3039. [Google Scholar] [CrossRef] [PubMed]
- You, H.J.; Ji, Y.T.; Wang, L.; Yang, S.C.; Yang, Z.M.; Fang, J.X.; Song, X.P.; Ding, B.J. Interface synthesis of gold meso-crystals with highly roughened surfaces for surface-enhanced Raman spectroscopy. J. Mater. Chem. 2012, 22, 1998–2006. [Google Scholar] [CrossRef]
- Jiang, Y.; He, Y.; Gao, H. Recent progress in porous intermetallics: Synthesis mechanism, pore structure, and material properties. J. Mater. Sci. Technol. 2021, 74, 89–104. [Google Scholar] [CrossRef]
- Lv, H.; Xu, D.; Sun, L.; Liu, B. Surfactant Design Strategy for One-Pot Seedless Synthesis of Hollow Mesoporous AuAg Alloy Nanospheres. J. Phys. Chem. Lett. 2020, 11, 5777–5784. [Google Scholar] [CrossRef]
- Jiang, B.; Song, H.; Kang, Y.; Wang, S.; Wang, Q.; Zhou, X.; Kani, K.; Guo, Y.; Ye, J.; Li, H.; et al. A mesoporous non-precious metal boride system: Synthesis of mesoporous cobalt boride by strictly controlled chemical reduction. Chem. Sci. 2020, 11, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Wu, E.; Zhang, Q.; Xie, A.; Yang, W.K.; Peng, C.; Hou, J.; He, Y.X.; Zhang, B.K.; Deng, L.F. Synthesis of hollow echinus-like Au@PdAgNSs decorated reduced graphene oxide as an excellent electrocatalyst for enhanced ethanol electrooxidation. J. Alloy. Comp. 2019, 789, 174–182. [Google Scholar] [CrossRef]
- Odziomek, M.; Bahri, M.; Boissiere, C.; Sanchez, C.; Lassalle-Kaiser, B.; Zitolo, A.; Ersen, O.; Nowak, S.; Tard, C.; Giraud, M.; et al. Aerosol synthesis of thermally stable porous noble metals and alloys by using bi-functional templates. Mater. Horizons 2020, 7, 541–550. [Google Scholar] [CrossRef]
- Kim, T.H.; Hasani, A.; Van Quyet, L.; Kim, Y.; Park, S.Y.; Lee, M.G.; Sohn, W.; Nguyen, T.P.; Choi, K.S.; Kim, S.Y.; et al. NO2 sensing properties of porous Au-incorporated tungsten oxide thin films prepared by solution process. Sens. Actuators B Chem. 2019, 286, 512–520. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, R.; Yang, P.; Lu, L.; Shen, L.; Tao, J.; Liu, Z.; Zhao, P. An Excellent Electrochemical Sensor Based on Highly Porous Gold Film Modified Gold Electrode for Detecting Quercetin in Food and Medicine. J. Electrochem. Soc. 2020, 167, 047514. [Google Scholar] [CrossRef]
- Liu, K.; Bai, Y.; Zhang, L.; Yang, Z.; Fan, Q.; Zheng, H.; Yin, Y.; Gao, C. Porous Au–Ag Nanospheres with High-Density and Highly Accessible Hotspots for SERS Analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, G.; Zhang, S.; Dai, Y.; Ghafoor, S.; Huang, W.; Zu, Z.; Lu, Y. A porous Au–Ag hybrid nanoparticle array with broadband absorption and high-density hotspots for stable SERS analysis. Nanoscale 2019, 11, 9587–9592. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, X.; Yin, Z.; Liu, Y.; Zhao, J.; Quan, Z. Ordered mesoporous silver superstructures with SERS hot spots. Chem. Commun. 2019, 55, 7982–7985. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Wu, Y.; Zhang, H.; Xiang, J.; Sun, Y.; Zhang, T.; Men, D. Green and rapid synthesis of porous Ag submicrocubes via Ag3PO4 templates for near-infrared surface-enhanced Raman scattering with high accessibility. J. Alloy. Compd. 2020, 820, 153107. [Google Scholar] [CrossRef]
- Liu, G.; Li, Y.; Duan, G.; Wang, J.; Changhao, L.; Cai, W. Tunable Surface Plasmon Resonance and Strong SERS Performances of Au Opening-Nanoshell Ordered Arrays. ACS Appl. Mater. Interfaces 2012, 4, 1–5. [Google Scholar] [CrossRef]
- Garoli, D.; Calandrini, E.; Giovannini, G.; Hubarevich, A.; Caligiuri, V.; De Angelis, F. Nanoporous gold metamaterials for high sensitivity plasmonic sensing. Nanoscale Horiz. 2019, 4, 1153–1157. [Google Scholar] [CrossRef]
- Liu, G.; Li, K.; Zhang, Y.; Du, J.; Ghafoor, S.; Lu, Y. A facile periodic porous Au nanoparticle array with high-density and built-in hotspots for SERS analysis. Appl. Surf. Sci. 2020, 527, 146807. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Q.; Liu, G.; Cai, W. 4-Mercaptophenylboronic acid modified Au nanosheets-built hollow sub-microcubes for active capture and ultrasensitive SERS-based detection of hexachlorocyclohexane pesticides. Sens. Actuators B Chem. 2019, 293, 63–70. [Google Scholar] [CrossRef]
- Lu, Y.; Qin, Y.; Yu, D.; Zhou, J. Stepwise Evolution of AgCl Microcrystals from Octahedron into Hexapod with Mace Pods and their Visible Light Photocatalytic Activity. Crystals 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Gatemala, H.; Thammacharoen, C.; Ekgasit, S.; Pienpinijtham, P. 3D nanoporous Ag microstructures fabricated from AgCl microcrystal templates via concerted oxidative etching/re-deposition and galvanic replacement. CrystEngComm 2016, 18, 6664–6672. [Google Scholar] [CrossRef]
- Bai, S.; Serien, D.; Hu, A.; Sugioka, K. 3D Microfluidic Surface-Enhanced Raman Spectroscopy (SERS) Chips Fabricated by All-Femtosecond-Laser-Processing for Real-Time Sensing of Toxic Substances. Adv. Funct. Mater. 2018, 28, 17062621. [Google Scholar] [CrossRef]
- Wang, C.; Xu, X.; Qiu, G.; Ye, W.; Li, Y.; Harris, R.A.; Jiang, C. Group-Targeting SERS Screening of Total Benzodiazepines Based on Large-Size (111) Faceted Silver Nanosheets Decorated with Zinc Oxide Nanoparticles. Anal. Chem. 2021, 93, 3403–3410. [Google Scholar] [CrossRef] [PubMed]
- Ekaterina, V.; Alfredo, T.F.; Velazquez-Salazar, J.J. Surface-enhanced Raman scattering of N-acetylneuraminic acid on silver nanoparticle surface. J. Raman Spectrosc. 2015, 45, 730–735. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Merrick, J.P.; Moran, D.; Radom, L. An Evaluation of Harmonic Vibrational Frequency Scale Factors. J. Phys. Chem. A 2007, 111, 11683–11700. [Google Scholar] [CrossRef]
- Li, B.; Wang, T.; Su, Q.; Wu, X.; Dong, P. Fabrication of Au Nanorods by the Oblique Angle Deposition Process for Trace Detection of Methamphetamine with Surface-Enhanced Raman Scattering. Sensors 2019, 19, 3742. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Mo, F.; Yao, S.; Wu, Y.; He, Y.; Yao, W. Facile Synthesis of Porous Ag Crystals as SERS Sensor for Detection of Five Methamphetamine Analogs. Molecules 2022, 27, 3939. https://doi.org/10.3390/molecules27123939
Qin Y, Mo F, Yao S, Wu Y, He Y, Yao W. Facile Synthesis of Porous Ag Crystals as SERS Sensor for Detection of Five Methamphetamine Analogs. Molecules. 2022; 27(12):3939. https://doi.org/10.3390/molecules27123939
Chicago/Turabian StyleQin, Yazhou, Fan Mo, Sen Yao, Yuanzhao Wu, Yingsheng He, and Weixuan Yao. 2022. "Facile Synthesis of Porous Ag Crystals as SERS Sensor for Detection of Five Methamphetamine Analogs" Molecules 27, no. 12: 3939. https://doi.org/10.3390/molecules27123939
APA StyleQin, Y., Mo, F., Yao, S., Wu, Y., He, Y., & Yao, W. (2022). Facile Synthesis of Porous Ag Crystals as SERS Sensor for Detection of Five Methamphetamine Analogs. Molecules, 27(12), 3939. https://doi.org/10.3390/molecules27123939




