Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia
Abstract
1. Introduction
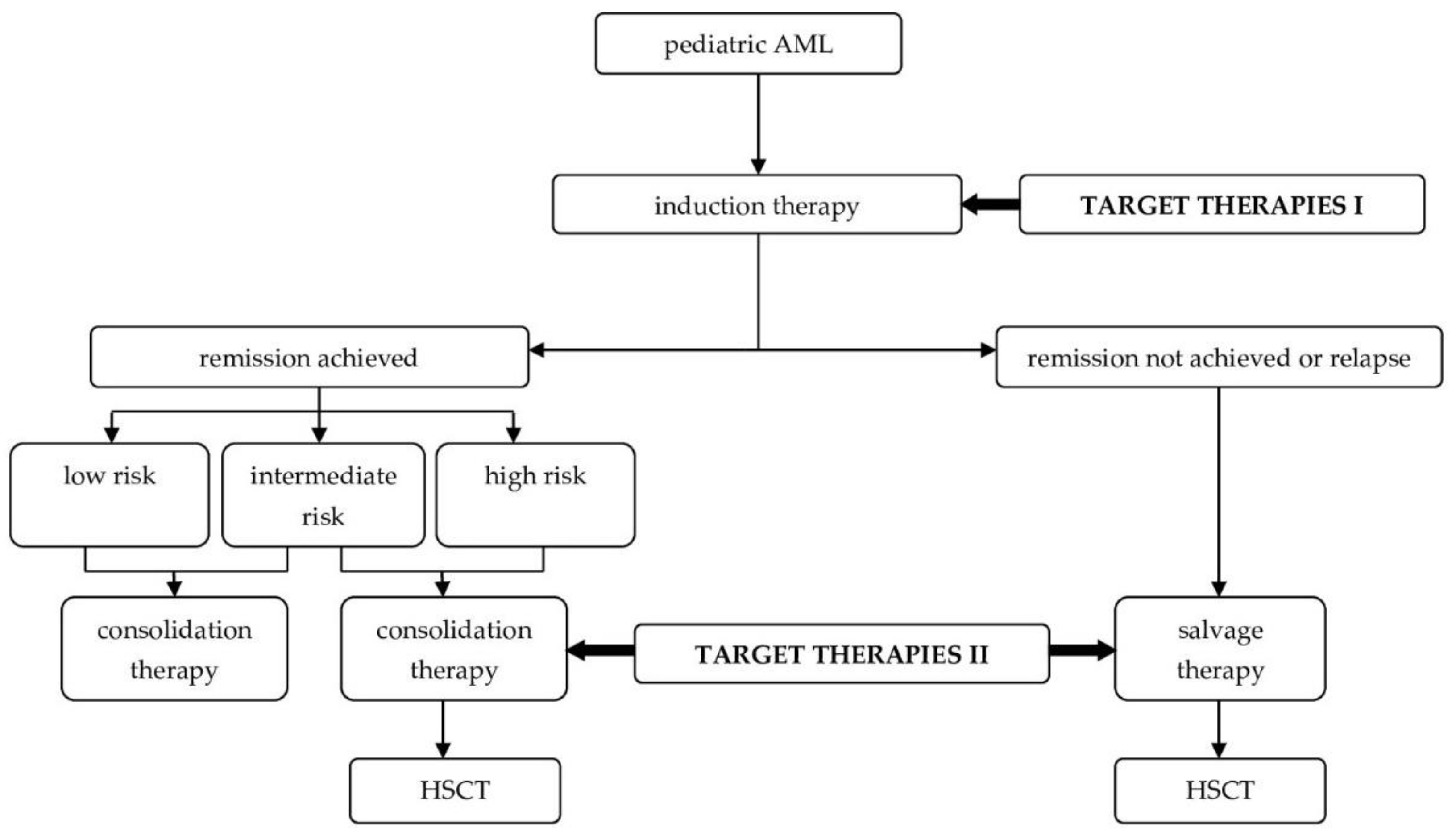
2. An Innovative Approach in the Treatment of Pediatric Acute Myeloid Leukemia
2.1. Signaling Molecule Inhibitors
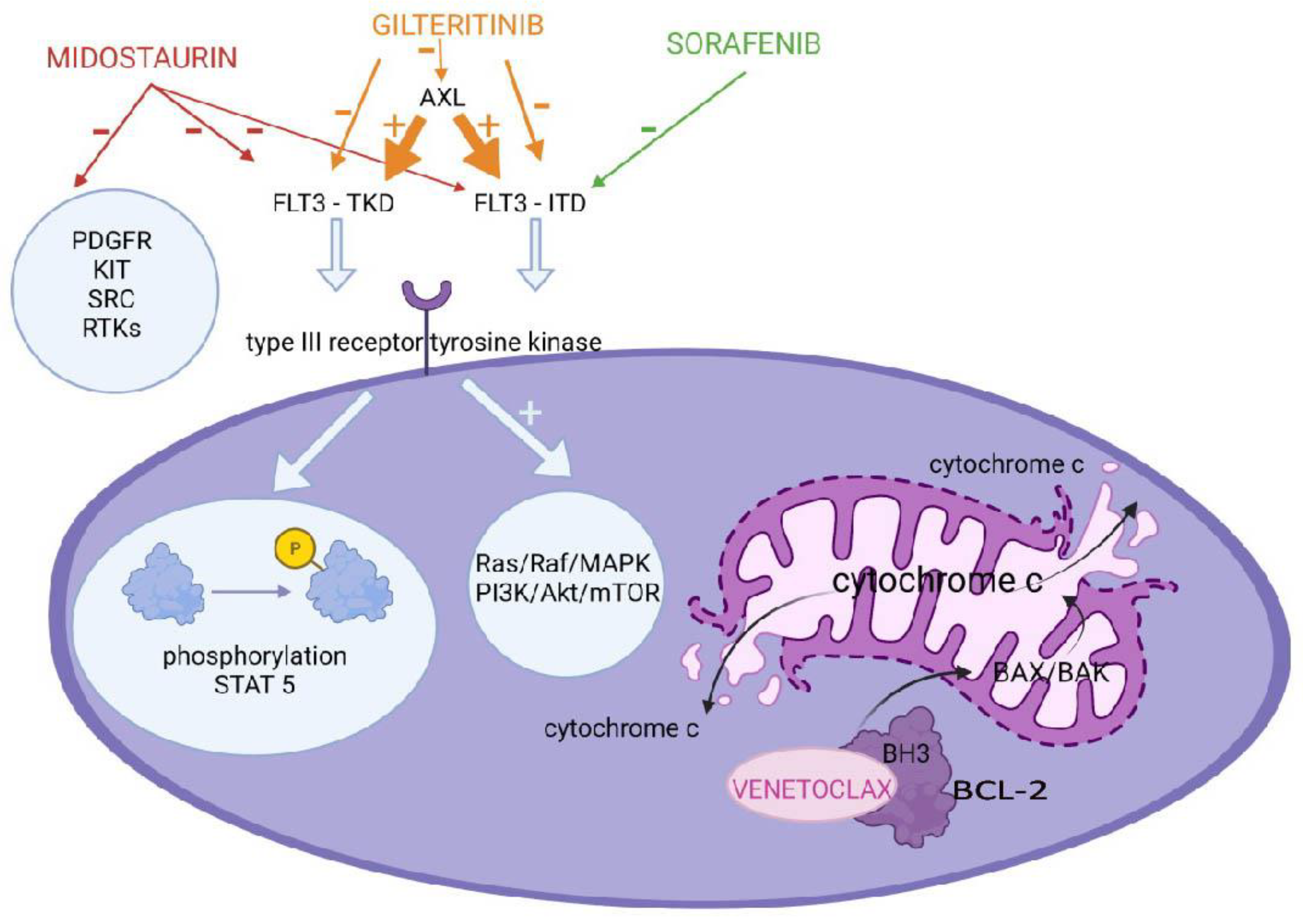
2.1.1. FLT3 Inhibitors
2.1.2. Sub-Analysis of Molecular Therapies for AML with Other Frequent Genetic Mutations
2.1.3. BCL-2 Inhibitors
2.2. Epigenetic Modifier Inhibitors
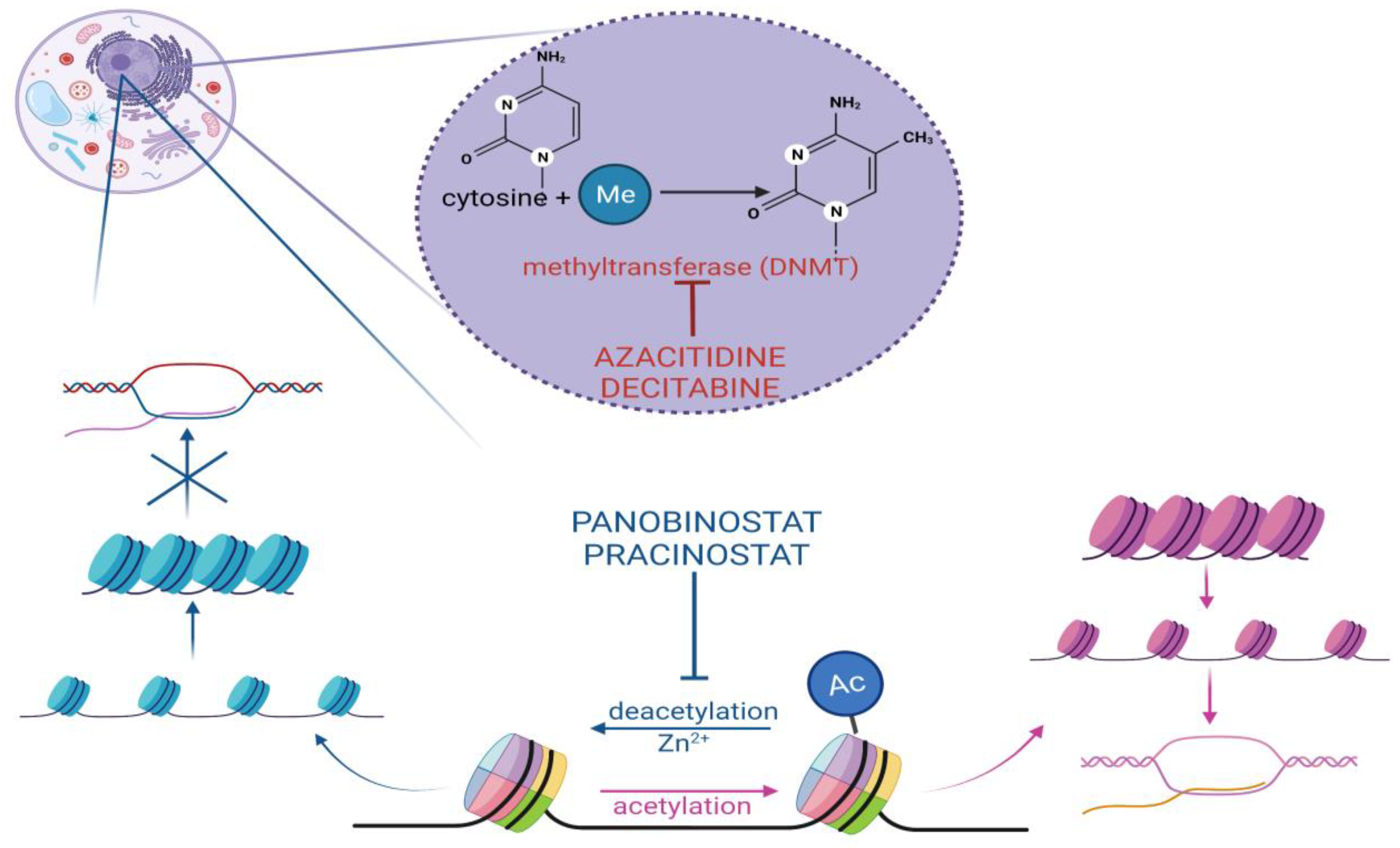
2.2.1. Hypomethylating Agents
2.2.2. Histone Deacetylase Inhibitors
2.3. Immunotherapy
2.3.1. Drug–Antibody Conjugates
2.3.2. Chimeric Antigen Receptor T Cells
2.4. Future Directions
2.4.1. Bispecific and Trispecific Antibodies
2.4.2. Paradigm Shift
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADCs | Antibody–drug conjugates |
| AE | adverse event |
| alloHSCT | allogeneic hematopoietic stem cell transplantation |
| AML | acute myeloid leukemia |
| AML-BFM | Acute Myeloid Leukemia Berlin-Frankfurt-Münster studies |
| ATAC | Assay for Transposase Accessible Chromatin |
| AZA | azacitidine |
| BCL-2 | B-cell leukaemia/lymphoma 2 |
| BiKEs | bispecific killer engager antibodies |
| BiTEs | bispecific T-engaging antibodies |
| CAR | chimeric antigen receptor |
| COG | Childhood Oncology Group |
| CR | complete remission |
| CRi | complete remission with incomplete blood count recovery |
| DAC | decitabine |
| DARTs | dual affinity retargets antibodies |
| DCOG | Dutch Childhood Oncology Group |
| DETs | differentially expressed protein-coding transcripts |
| DLT | dose-limiting toxicity |
| DNMT | DNA methyltransferase |
| EFS | event-free survival |
| FLT3-ITD | internal tandem duplication mutations in the juxtamembrane domain |
| FLT3-TKD | deletion in the tyrosine kinase domain |
| GO | Gemtuzumabozogamicin |
| HDACIs | histone deacetylase inhibitors |
| HDAC | Histone deacetylase |
| HLE | half-life extended |
| HMAs | hypomethylating agents |
| MRD | minimal residual disease |
| nd | no data |
| NIH | The National Institutes of Health |
| OS | overall survival |
| PDX | patient-derived xenografts |
| PPLLSG | Polish Pediatric Leukemia and Lymphoma Study Group |
| RFS | relapse-free survival |
| RTK | receptor tyrosine kinase |
| scFv | single-chain variable fragment |
| SD | standard deviation |
| SIRT | sirtuin protein family |
| SJCRH | St. Jude Children’s Research Hospital |
| TAA | tumor-associated antigen |
| Tregs | regulatory T cells |
| TriKEs | trispecific killer engager antibodies |
| TSG | tumor suppressor genes |
| WHO | World Health Organizatiοn |
Appendix A
| Drug | FDA Approval for Any Indication | Year of FDA Approval in AML |
|---|---|---|
| Midostaurin | Yes | 2017 |
| Gilteritinib | Yes | 2018 |
| Sorafenib | Yes | - |
| Venetoclax | Yes | 2018 |
| Azacitidine | Yes | 2020 |
| Decitabine | Yes | - |
| Panobinostat | Yes | - |
| Pracinostat | No | |
| Gemtuzumabozogamicin | Yes | 2017 |
| Camidanlumabtesirine | No | |
| CAR T cells | Yes | - |
| BiTEs | Yes | - |
| Drug | Trial 1 | Variable Used for Outcome Assessment | |||||
|---|---|---|---|---|---|---|---|
| Median Overall Survival (Months) | Median Event-Free Survival (Months) | 5-Year Event-Free Survival (%) | 5-Year Relapse-Free Survival (%) | Complete Remission with Full or Partial Hematologic Recovery (%) | Median Relapse-Free Survival (Months) | ||
| Midostaurin | RATIFY | 74.7 vs. 25.6 2 | 8.2 vs. 3.0 2 | 45 vs. 34 | - | - | - |
| Sorafenib | SORAML | - | - | 41 vs. 27 2 | 53 vs. 36 2 | - | - |
| Gilteritinib | ADMIRAL | 9.3 vs. 5.6 2 | 2.8 vs. 0.7 2 | - | - | 34 vs. 15.3 2 | - |
| Venetoclax | NCT03069352 | 8.4 vs. 4.1 2 | 4.7 vs. 2 2 | - | - | 48 vs. 13 2 | - |
| Azacitidine | QUAZAR | 24.7 vs. 14.8 2 | - | - | - | - | 10.2 vs. 4.8 |
References
- Cancer Stat Facts: Leukemia—Acute Myeloid Leukemia (AML). Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 24 April 2022).
- NCCR*Explorer: An Interactive Website for NCCR Cancer Statistics, National Cancer Institute. Available online: https://NCCRExplorer.ccdi.cancer.gov/ (accessed on 24 April 2022).
- Creutzig, U.; van den Heuvel-Eibrink, M.M.; Gibson, B.; Dworzak, M.N.; Adachi, S.; de Bont, E.; Harbott, J.; Hasle, H.; Johnston, D.; Kinoshita, A.; et al. Diagnosis and Management of Acute Myeloid Leukemia in Children and Adolescents: Recommendations from an International Expert Panel. Blood 2012, 120, 3187–3205. [Google Scholar] [CrossRef]
- World Health Organization. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. In World Health Organization Classification of Tumours, Revised 4th ed.; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 129–173. ISBN 978-92-832-4494-3. [Google Scholar]
- Leukemia and Lymphoma Society. Available online: https://www.lls.org/leukemia/acute-myeloid-leukemia/diagnosis/aml-subtypes (accessed on 26 April 2022).
- Rubnitz, J.E.; Crews, K.R.; Pounds, S.; Yang, S.; Campana, D.; Gandhi, V.V.; Raimondi, S.C.; Downing, J.R.; Razzouk, B.I.; Pui, C.-H.; et al. Combination of Cladribine and Cytarabine Is Effective for Childhood Acute Myeloid Leukemia: Results of the St Jude AML97 Trial. Leukemia 2009, 23, 1410–1416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lange, B.J.; Smith, F.O.; Feusner, J.; Barnard, D.R.; Dinndorf, P.; Feig, S.; Heerema, N.A.; Arndt, C.; Arceci, R.J.; Seibel, N.; et al. Outcomes in CCG-2961, a Children’s Oncology Group Phase 3 Trial for Untreated Pediatric Acute Myeloid Leukemia: A Report from the Children’s Oncology Group. Blood 2008, 111, 1044–1053. [Google Scholar] [CrossRef]
- Creutzig, U.; Ritter, J.; Zimmermann, M.; Hermann, J.; Gadner, H.; Sawatzki, D.B.; Niemeyer, C.; Schwabe, D.; Selle, B.; Boos, J.; et al. Idarubicin Improves Blast Cell Clearance during Induction Therapy in Children with AML: Results of Study AML-BFM 93. Leukemia 2001, 15, 348–354. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.A.; Russell, S.J.; Vowels, M.R.; Oswald, C.M.; Tiedemann, K.; Shaw, P.J.; Lockwood, L.; Teague, L.; Rice, M.; Marshall, G.M. Results of Consecutive Trials for Children Newly Diagnosed with Acute Myeloid Leukemia from the Australian and New Zealand Children’s Cancer Study Group. Blood 2002, 100, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Hills, R.K.; Milligan, D.W.; Goldstone, A.H.; Prentice, A.G.; McMullin, M.-F.; Duncombe, A.; Gibson, B.; Wheatley, K. Attempts to Optimize Induction and Consolidation Treatment in Acute Myeloid Leukemia: Results of the MRC AML12 Trial. J. Clin. Oncol. 2010, 28, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Rubnitz, J.E.; Inaba, H.; Dahl, G.; Ribeiro, R.C.; Bowman, W.P.; Taub, J.; Pounds, S.; Razzouk, B.I.; Lacayo, N.J.; Cao, X.; et al. Minimal Residual Disease-Directed Therapy for Childhood Acute Myeloid Leukaemia: Results of the AML02 Multicentre Trial. Lancet Oncol. 2010, 11, 543–552. [Google Scholar] [CrossRef]
- Krock, B.; Oberley, M.J. Molecular Genetics of Pediatric Acute Myeloid Leukemia. Clin. Lab. Med. 2021, 41, 497–515. [Google Scholar] [CrossRef]
- Quessada, J.; Cuccuini, W.; Saultier, P.; Loosveld, M.; Harrison, C.J.; Lafage-Pochitaloff, M. Cytogenetics of Pediatric Acute Myeloid Leukemia: A Review of the Current Knowledge. Genes 2021, 12, 924. [Google Scholar] [CrossRef]
- Reinhardt, D.; Antoniou, E.; Waack, K. Pediatric Acute Myeloid Leukemia—Past, Present, and Future. J. Clin. Med. 2022, 11, 504. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Dou, H.; Yang, Z.; Bi, J.; Huang, Y.; Lu, L.; Yu, J.; Bao, L. Cytogenetic and Mutational Analysis and Outcome Assessment of a Cohort of 284 Children with de Novo Acute Myeloid Leukemia Reveal Complex Karyotype as an Adverse Risk Factor for Inferior Survival. Mol. Cytogenet. 2021, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Elgarten, C.W.; Aplenc, R. Pediatric Acute Myeloid Leukemia: Updates on Biology, Risk Stratification, and Therapy. Curr. Opin. Pediatr. 2020, 32, 57–66. [Google Scholar] [CrossRef]
- Waack, K.; Schneider, M.; Walter, C.; Creutzig, U.; Klusmann, J.-H.; Rasche, M.; Boztug, H.; Jansen, K.; Escherich, G.; Frühwald, M.; et al. Improved Outcome in Pediatric AML—the AML-BFM 2012 Study. Blood 2020, 136, 12–14. [Google Scholar] [CrossRef]
- Czogała, M.; Balwierz, W.; Pawińska-Wąsikowska, K.; Książek, T.; Bukowska-Strakova, K.; Czogała, W.; Sikorska-Fic, B.; Matysiak, M.; Skalska-Sadowska, J.; Wachowiak, J.; et al. Advances in the First Line Treatment of Pediatric Acute Myeloid Leukemia in the Polish Pediatric Leukemia and Lymphoma Study Group from 1983 to 2019. Cancers 2021, 13, 4536. [Google Scholar] [CrossRef]
- Aplenc, R.; Meshinchi, S.; Sung, L.; Alonzo, T.; Choi, J.; Fisher, B.; Gerbing, R.; Hirsch, B.; Horton, T.; Kahwash, S.; et al. Bortezomib with Standard Chemotherapy for Children with Acute Myeloid Leukemia Does Not Improve Treatment Outcomes: A Report from the Children’s Oncology Group. Haematologica 2020, 105, 1879–1886. [Google Scholar] [CrossRef]
- Rubnitz, J.E.; Lacayo, N.J.; Inaba, H.; Heym, K.; Ribeiro, R.C.; Taub, J.; McNeer, J.; Degar, B.; Schiff, D.; Yeoh, A.E.-J.; et al. Clofarabine Can Replace Anthracyclines and Etoposide in Remission Induction Therapy for Childhood Acute Myeloid Leukemia: The AML08 Multicenter, Randomized Phase III Trial. J. Clin. Oncol. 2019, 37, 2072–2081. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Litsenburg, R.R.L.; Haas, V.; Dors, N.; den Heuvel-Eibrink, M.M.; Knops, R.R.G.; Tissing, W.J.E.; Versluys, B.A.; Zwaan, C.M.; Kaspers, G.J.L. Causes of Early Death and Treatment-related Death in Newly Diagnosed Pediatric Acute Myeloid Leukemia: Recent Experiences of the Dutch Childhood Oncology Group. Pediatr. Blood Cancer 2020, 67. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.M.K.; Mills, M.L.; Bittencourt-Silvestre, J.; Keeshan, K. Insights into the Molecular Profiles of Adult and Paediatric Acute Myeloid Leukaemia. Mol. Oncol. 2021, 15, 2253–2272. [Google Scholar] [CrossRef] [PubMed]
- McMurry, H.; Fletcher, L.; Traer, E. IDH Inhibitors in AML—Promise and Pitfalls. Curr. Hematol. Malig. Rep. 2021, 16, 207–217. [Google Scholar] [CrossRef]
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 Mutations in Acute Myeloid Leukemia: Therapeutic Paradigm beyond Inhibitor Development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef]
- Daver, N.; Venugopal, S.; Ravandi, F. FLT3 Mutated Acute Myeloid Leukemia: 2021 Treatment Algorithm. Blood Cancer J. 2021, 11, 104. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Voso, M.T.; Larson, R.A.; Jones, D.; Marcucci, G.; Prior, T.; Krauter, J.; Heuser, M.; Lavorgna, S.; Nomdedeu, J.; Geyer, S.M.; et al. Midostaurin in Patients with Acute Myeloid Leukemia and FLT3-TKD Mutations: A Subanalysis from the RATIFY Trial. Blood Adv. 2020, 4, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Zwaan, C.M.; Söderhäll, S.; Brethon, B.; Luciani, M.; Rizzari, C.; Stam, R.W.; Besse, E.; Dutreix, C.; Fagioli, F.; Ho, P.A.; et al. A Phase 1/2, Open-label, Dose-escalation Study of Midostaurin in Children with Relapsed or Refractory Acute Leukaemia. Br. J. Haematol. 2019, 185, 623–627. [Google Scholar] [CrossRef]
- Hoffmeister, L.M.; Orhan, E.; Walter, C.; Niktoreh, N.; Hanenberg, H.; von Neuhoff, N.; Reinhardt, D.; Schneider, M. Impact of KMT2A Rearrangement and CSPG4 Expression in Pediatric Acute Myeloid Leukemia. Cancers 2021, 13, 4817. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.A.; Guest, E.; Alonzo, T.A.; Gerbing, R.B.; Loken, M.R.; Brodersen, L.E.; Kolb, E.A.; Aplenc, R.; Meshinchi, S.; Raimondi, S.C.; et al. GemtuzumabOzogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results from the Phase III Children’s Oncology Group Trial AAML0531. J. Clin. Oncol. 2021, 39, 3149–3160. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, W.-J.; Zhou, D.-H.; Fang, J.-P.; Mishra, S.; Chai, L.; Xu, L.-H. Wilms Tumor 1 Mutations Are Independent Poor Prognostic Factors in Pediatric Acute Myeloid Leukemia. Front. Oncol. 2021, 11, 632094. [Google Scholar] [CrossRef]
- Augsberger, C.; Hänel, G.; Xu, W.; Pulko, V.; Hanisch, L.J.; Augustin, A.; Challier, J.; Hunt, K.; Vick, B.; Rovatti, P.E.; et al. Targeting Intracellular WT1 in AML with a Novel RMF-Peptide-MHC-Specific T-Cell Bispecific Antibody. Blood 2021, 138, 2655–2669. [Google Scholar] [CrossRef]
- Kreutmair, S.; Pfeifer, D.; Waterhouse, M.; Takács, F.; Graessel, L.; Döhner, K.; Duyster, J.; Illert, A.L.; Frey, A.-V.; Schmitt, M.; et al. First-in-Human Study of WT1 Recombinant Protein Vaccination in Elderly Patients with AML in Remission: A Single-Center Experience. Cancer Immunol. Immunother. 2022. [Google Scholar] [CrossRef]
- Gonzales, F.; Barthélémy, A.; Peyrouze, P.; Fenwarth, L.; Preudhomme, C.; Duployez, N.; Cheok, M.H. Targeting RUNX1 in Acute Myeloid Leukemia: Preclinical Innovations and Therapeutic Implications. Expert Opin. Ther. Targets 2021, 25, 299–309. [Google Scholar] [CrossRef]
- Katagiri, S.; Chi, S.; Minami, Y.; Fukushima, K.; Shibayama, H.; Hosono, N.; Yamauchi, T.; Morishita, T.; Kondo, T.; Yanada, M.; et al. Mutated KIT Tyrosine Kinase as a Novel Molecular Target in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2022, 23, 4694. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Serve, H.; Noppeney, R.; Hanoun, M.; Krug, U.; Baldus, C.D.; Brandts, C.H.; Kunzmann, V.; Einsele, H.; Krämer, A.; et al. Sorafenib or Placebo in Patients with Newly Diagnosed Acute Myeloid Leukaemia: Long-Term Follow-up of the Randomized Controlled SORAML Trial. Leukemia 2021, 35, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.; Bodo, J.; Hill, B.T.; Hsi, E.D.; Almasan, A. Targeting BCL-2 in B-Cell Malignancies and Overcoming Therapeutic Resistance. Cell Death Dis. 2020, 11, 941. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A.; Hou, J.-Z.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined with Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results from a Phase Ib/II Study. J. Clin. Oncol. 2019, 37, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for Newly Diagnosed AML Ineligible for Intensive Chemotherapy: A Phase 3 Randomized Placebo-Controlled Trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef]
- Ganzel, C.; Ram, R.; Gural, A.; Wolach, O.; Gino-Moor, S.; Vainstein, V.; Nachmias, B.; Apel, A.; Koren-Michowitz, M.; Pasvolsky, O.; et al. Venetoclax Is Safe and Efficacious in Relapsed/Refractory AML. Leuk. Lymphoma 2020, 61, 2221–2225. [Google Scholar] [CrossRef]
- Winters, A.C.; Maloney, K.W.; Treece, A.L.; Gore, L.; Franklin, A.K. Single-center Pediatric Experience with Venetoclax and Azacitidine as Treatment for Myelodysplastic Syndrome and Acute Myeloid Leukemia. Pediatr. Blood Cancer 2020, 67, e28398. [Google Scholar] [CrossRef]
- Place, A.E.; Goldsmith, K.; Bourquin, J.-P.; Loh, M.L.; Gore, L.; Morgenstern, D.A.; Sanzgiri, Y.; Hoffman, D.; Zhou, Y.; Ross, J.A.; et al. Accelerating Drug Development in Pediatric Cancer: A Novel Phase I Study Design of Venetoclax in Relapsed/Refractory Malignancies. Future Oncol. 2018, 14, 2115–2129. [Google Scholar] [CrossRef]
- Figueroa, M.E.; Lugthart, S.; Li, Y.; Erpelinck-Verschueren, C.; Deng, X.; Christos, P.J.; Schifano, E.; Booth, J.; van Putten, W.; Skrabanek, L.; et al. DNA Methylation Signatures Identify Biologically Distinct Subtypes in Acute Myeloid Leukemia. Cancer Cell 2010, 17, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Straube, J.; Lane, S.W.; Vu, T. Optimizing DNA Hypomethylating Therapy in Acute Myeloid Leukemia and Myelodysplastic Syndromes. BioEssays News Rev. Mol. Cell. Dev. Biol. 2021, 43, e2100125. [Google Scholar] [CrossRef] [PubMed]
- Greve, G.; Schüler, J.; Grüning, B.A.; Berberich, B.; Stomper, J.; Zimmer, D.; Gutenkunst, L.; Bönisch, U.; Meier, R.; Blagitko-Dorfs, N.; et al. Decitabine Induces Gene Derepression on Monosomic Chromosomes: In Vitro and In Vivo Effects in Adverse-Risk Cytogenetics AML. Cancer Res. 2021, 81, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Döhner, H.; Pocock, C.; Montesinos, P.; Afanasyev, B.; Dombret, H.; Ravandi, F.; Sayar, H.; Jang, J.-H.; Porkka, K.; et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020, 383, 2526–2537. [Google Scholar] [CrossRef] [PubMed]
- Pommert, L.; Schafer, E.S.; Malvar, J.; Gossai, N.; Florendo, E.; Pulakanti, K.; Heimbruch, K.; Stelloh, C.; Chi, Y.; Sposto, R.; et al. Decitabine and Vorinostat with FLAG Chemotherapy in Pediatric Relapsed/Refractory AML: Report from the Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) Consortium. Am. J. Hematol. 2022, 97, 613–622. [Google Scholar] [CrossRef]
- Cheng, S.; Xiao, P.; Wang, J.; Li, Z.; Gao, L.; Zheng, J.; Hu, Y.; Ding, X.; Ling, J.; Lu, Q.; et al. Decitabine Combined with Minimally Myelosuppressive Therapy for Induction of Remission in Pediatric High-Risk Acute Myeloid Leukemia with Chromosome 5q Deletion: A Report of Three Cases. Int. J. Hematol. 2022. [Google Scholar] [CrossRef]
- San José-Enériz, E.; Gimenez-Camino, N.; Agirre, X.; Prosper, F. HDAC Inhibitors in Acute Myeloid Leukemia. Cancers 2019, 11, 1794. [Google Scholar] [CrossRef]
- Ungerstedt, J. Epigenetic Modifiers in Myeloid Malignancies: The Role of Histone Deacetylase Inhibitors. Int. J. Mol. Sci. 2018, 19, 3091. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Walker, A.R.; Schlenk, R.F.; Sierra, J.; Medeiros, B.C.; Ocio, E.M.; Röllig, C.; Strickland, S.A.; Thol, F.; Valera, S.; et al. Safety and Efficacy of Oral Panobinostat plus Chemotherapy in Patients Aged 65 Years or Younger with High-Risk Acute Myeloid Leukemia. Leuk. Res. 2019, 85, 106197. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Abaza, Y.; Takahashi, K.; Medeiros, B.C.; Arellano, M.; Khaled, S.K.; Patnaik, M.; Odenike, O.; Sayar, H.; Tummala, M.; et al. Pracinostat plus Azacitidine in Older Patients with Newly Diagnosed Acute Myeloid Leukemia: Results of a Phase 2 Study. Blood Adv. 2019, 3, 508–518. [Google Scholar] [CrossRef]
- Pan, T.; Qi, J.; You, T.; Yang, L.; Wu, D.; Han, Y.; Zhu, L. Addition of Histone Deacetylase Inhibitors Does Not Improve Prognosis in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia Compared with Hypomethylating Agents Alone: A Systematic Review and Meta-Analysis of Seven Prospective Cohort Studies. Leuk. Res. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Karol, S.E.; Cooper, T.M.; Mead, P.E.; Crews, K.R.; Panetta, J.C.; Alexander, T.B.; Taub, J.W.; Lacayo, N.J.; Heym, K.M.; Kuo, D.J.; et al. Safety, Pharmacokinetics, and Pharmacodynamics of Panobinostat in Children, Adolescents, and Young Adults with Relapsed Acute Myeloid Leukemia. Cancer 2020, 126, 4800–4805. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, U.; Parakh, S.; Gan, H.K.; Scott, A.M. Antibody–Drug Conjugates for Cancer Therapy. Molecules 2020, 25, 4764. [Google Scholar] [CrossRef]
- Stokke, J.L.; Bhojwani, D. Antibody–Drug Conjugates for the Treatment of Acute Pediatric Leukemia. J. Clin. Med. 2021, 10, 3556. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Wang, E.S. Novel Therapies for AML: A Round-up for Clinicians. Expert Rev. Clin. Pharmacol. 2020, 13, 1389–1400. [Google Scholar] [CrossRef]
- Lambert, J.; Pautas, C.; Terré, C.; Raffoux, E.; Turlure, P.; Caillot, D.; Legrand, O.; Thomas, X.; Gardin, C.; Gogat-Marchant, K.; et al. Gemtuzumab Ozogamicin for de Novo Acute Myeloid Leukemia: Final Efficacy and Safety Updates from the Open-Label, Phase III ALFA-0701 Trial. Haematologica 2019, 104, 113–119. [Google Scholar] [CrossRef]
- Gamis, A.S.; Alonzo, T.A.; Meshinchi, S.; Sung, L.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Kahwash, S.B.; Heerema-McKenney, A.; Winter, L.; et al. Gemtuzumab Ozogamicin in Children and Adolescents With De Novo Acute Myeloid Leukemia Improves Event-Free Survival by Reducing Relapse Risk: Results from the Randomized Phase III Children’s Oncology Group Trial AAML0531. J. Clin. Oncol. 2014, 32, 3021–3032. [Google Scholar] [CrossRef]
- Goldberg, A.D.; Atallah, E.; Rizzieri, D.; Walter, R.B.; Chung, K.-Y.; Spira, A.; Stock, W.; Tallman, M.S.; Cruz, H.G.; Boni, J.; et al. Camidanlumab Tesirine, an Antibody-Drug Conjugate, in Relapsed/Refractory CD25-Positive Acute Myeloid Leukemia or Acute Lymphoblastic Leukemia: A Phase I Study. Leuk. Res. 2020, 95, 106385. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yu, S.-F.; del Rosario, G.; Leong, S.R.; Lee, G.Y.; Vij, R.; Chiu, C.; Liang, W.-C.; Wu, Y.; Chalouni, C.; et al. An Anti–CLL-1 Antibody–Drug Conjugate for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 1358–1368. [Google Scholar] [CrossRef]
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-Targeting Antibody-Drug Conjugate, IMGN632, Designed to Eradicate AML While Sparing Normal Bone Marrow Cells. Blood Adv. 2018, 2, 848–858. [Google Scholar] [CrossRef]
- Epperly, R.; Gottschalk, S.; Velasquez, M. Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond. Children 2020, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Sadelain, M.; Brentjens, R.; Rivière, I. The Basic Principles of Chimeric Antigen Receptor Design. Cancer Discov. 2013, 3, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Rahman, H.S.; Al-Obaidi, Z.M.J.; Jalil, A.T.; Abdelbasset, W.K.; Suksatan, W.; Dorofeev, A.E.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; et al. Novel CAR T Therapy Is a Ray of Hope in the Treatment of Seriously Ill AML Patients. Stem Cell Res. Ther. 2021, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- Lonetti, A.; Pession, A.; Masetti, R. Targeted Therapies for Pediatric AML: Gaps and Perspective. Front. Pediatr. 2019, 7, 463. [Google Scholar] [CrossRef]
- Rotiroti, M.C.; Buracchi, C.; Arcangeli, S.; Galimberti, S.; Valsecchi, M.G.; Perriello, V.M.; Rasko, T.; Alberti, G.; Magnani, C.F.; Cappuzzello, C.; et al. Targeting CD33 in Chemoresistant AML Patient-Derived Xenografts by CAR-CIK Cells Modified with an Improved SB Transposon System. Mol. Ther. 2020, 28, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, L.; Li, Z.; Nalin, A.P.; Dai, H.; Xu, T.; Yin, J.; You, F.; Zhu, M.; Shen, W.; et al. First-in-Man Clinical Trial of CAR NK-92 Cells: Safety Test of CD33-CAR NK-92 Cells in Patients with Relapsed and Refractory Acute Myeloid Leukemia. Am. J. Cancer Res. 2018, 8, 1083–1089. [Google Scholar]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol. Res. 2019, 7, 100–112. [Google Scholar] [CrossRef]
- Driouk, L.; Gicobi, J.K.; Kamihara, Y.; Rutherford, K.; Dranoff, G.; Ritz, J.; Baumeister, S.H.C. Chimeric Antigen Receptor T Cells Targeting NKG2D-Ligands Show Robust Efficacy Against Acute Myeloid Leukemia and T-Cell Acute Lymphoblastic Leukemia. Front. Immunol. 2020, 11, 580328. [Google Scholar] [CrossRef]
- Atilla, P.A.; McKenna, M.K.; Tashiro, H.; Srinivasan, M.; Mo, F.; Watanabe, N.; Simons, B.W.; Stevens, A.M.; Redell, M.S.; Heslop, H.E.; et al. Modulating TNFα Activity Allows Transgenic IL15-Expressing CLL-1 CAR T Cells to Safely Eliminate Acute Myeloid Leukemia. J. Immunother. Cancer 2020, 8, e001229. [Google Scholar] [CrossRef]
- Zhang, H.; Gan, W.-T.; Hao, W.-G.; Wang, P.-F.; Li, Z.-Y.; Chang, L.-J. Successful Anti-CLL1 CAR T-Cell Therapy in Secondary Acute Myeloid Leukemia. Front. Oncol. 2020, 10, 685. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04010877 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04662294 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT05023707 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04835519 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04265963 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04762485 (accessed on 21 April 2022).
- Available online: https://www.clinicaltrials.gov/ct2/show/NCT04351022 (accessed on 21 April 2022).
- Tabata, R.; Chi, S.; Yuda, J.; Minami, Y. Emerging Immunotherapy for Acute Myeloid Leukemia. Int. J. Mol. Sci. 2021, 22, 1944. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Zeidan, A.M.; Bewersdorf, J.P. BiTEs, DARTS, BiKEs and TriKEs—Are Antibody Based Therapies Changing the Future Treatment of AML? Life 2021, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Jitschin, R.; Saul, D.; Braun, M.; Tohumeken, S.; Völkl, S.; Kischel, R.; Lutteropp, M.; Dos Santos, C.; Mackensen, A.; Mougiakakos, D. CD33/CD3-Bispecific T-Cell Engaging (BiTE®) Antibody Construct Targets Monocytic AML Myeloid-Derived Suppressor Cells. J. Immunother. Cancer 2018, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, C.; Volta, L.; Rinaldi, F.; Murer, P.; Myburgh, R.; Manz, M.G.; Neri, D. Development of a Novel Fully-Human Anti-CD123 Antibody to Target Acute Myeloid Leukemia. Leuk. Res. 2019, 84, 106178. [Google Scholar] [CrossRef]
- Brauchle, B.; Goldstein, R.L.; Karbowski, C.M.; Henn, A.; Li, C.-M.; Bücklein, V.L.; Krupka, C.; Boyle, M.C.; Koppikar, P.; Haubner, S.; et al. Characterization of a Novel FLT3 BiTE Molecule for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2020, 19, 1875–1888. [Google Scholar] [CrossRef]
- Chaudhury, S.; O’Connor, C.; Cañete, A.; Bittencourt-Silvestre, J.; Sarrou, E.; Prendergast, Á.; Choi, J.; Johnston, P.; Wells, C.A.; Gibson, B.; et al. Age-Specific Biological and Molecular Profiling Distinguishes Paediatric from Adult Acute Myeloid Leukaemias. Nat. Commun. 2018, 9, 5280. [Google Scholar] [CrossRef]
| Risk | Molecular and Cytogenetic Abnormalities | Frequency in Childhood AML | References |
|---|---|---|---|
| Favorable | CEBPA gene single or double mutations | 4–9% | [12] |
| t(15;17)(q24;q21); PML::RARA | 5–10% | [12,13] | |
| t(8;21)(q22;q22); RUNX1::RUNX1T1 | 15% | ||
| inv(16)(p13q22) or t(16;16)(p13;q22); CBFB::MYH11 | 10–15% | ||
| NPM1 | 4% | [12] | |
| t(16;21)(q24;q22); RUNX1::CBFA2T3 | 0.2% | [13,14] | |
| Intermediate | del(7q) | 3% | [13] |
| t(9;11)(p22;q23); KMT2A::AF9(MLLT3) | 6–9% | ||
| t(11;19)(q23;p13.1); KMT2A::ELL | 1–2% | ||
| t(11;19)(q23;p13.3); KMT2A::ENL(MLLT1) | 1% | ||
| t(10;11)(p12;q14); PICALM::MLLT10 | <1% | ||
| t(3;5)(q25;q35); NPM1::MLF1 | <0.5% | ||
| t(8;16)(p11;p13); KAT6A::CREBBP | <1% | ||
| t(1;22)(p13;q13); RBM15::MKL1 | 0.3% | ||
| Adverse | Complex karyotype | 8–17% | [13,15] |
| Monosomy 5, del(5q) | 1.2% | [13] | |
| Monosomy 7 | 3% | ||
| FLT3-ITD | 10–20% | [16] | |
| t(10;11)(p12;q23) or ins(10;11) (p12;q23q13); KMT2A::AF10(MLLT10) | 2–3% | [13] | |
| t(6;11)(q27;q23); KMT2A::AF6(MLLT4) | 1–2% | ||
| t(4;11)(q21;q23); KMT2A::AFF1 | - | [17] | |
| t(9;11)(p21;q23) KMT2A::MLLT3 | - | ||
| t(5;11)(q35;p15); NUP98::NSD1 | 3–4% | [13] | |
| t(11;12)(p15;p13) NUP98::KMD5A | 1–2% | ||
| t(7;12)(q36;p13); ETV6, MNX1 | 1% | ||
| inv(3)(q21q26.2) or t(3;3)(q21;q26.2); GATA2, EVI1(MECOM) | 2% | ||
| t(6;9)(p22;q34); DEK::NUP214 | <2% | [12,13] | |
| t(16;21)(p11;q22); FUS::ERG | 0.4% | [13] | |
| inv(16)(p13q24); CBFA2T3::GLIS2 | 2–3% | ||
| t(9;22)(q34;q11); BCR::ABL1 | 0.6% | ||
| Discussed | Monosomal karyotype | 3–5% | |
| Trisomy 8 | 10–14% | ||
| FLT3-TKD | 7% | [16] | |
| KIT gene | <5% | ||
| No significance | Hyperdiploidy (48~49–65 chr.) | 11% | [13] |
| According to cryptic CA or to mutations | Normal karyotype | 20–26% |
| Study Group | Study | Period | Patients | Probability of 3-Years/5-Years OS ± SD (%) | Probability of 3-Years/5-Years EFS ± SD (%) | Relapses (%) | Early Deaths | Deaths from Toxicities | Reference |
|---|---|---|---|---|---|---|---|---|---|
| AML-BFM | AML-BFM 2012 1 | 2012–2018 | 164 | 82 ± 3/nd | 69 ± 4/nd | 22 | n = 9 | n = 6 3.7% | [14,17] |
| PPLLSG | AML-BFM 2012 1 | 2015–2019 | 131 | 75 ± 5/nd | 67 ± 5/nd | 17 | n = 8 | n = 3 8% | [18] |
| COG | AAML1031 2 | 2011–2016 | 1097 | 65.4 ± 3.1/nd | 45.9 ± 3.2/nd | 47.2 ± 3.2 | nd | 11.85 ± 5.2% | [19] |
| SJCGH | AML08 3 | 2008–2017 | 285 | 74.8/nd | 52.9/nd | 21 | n = 4 | nd | [20] |
| DCOG | ANLL-97/ MRC AML-12 4 | 1998–2002 | 118 | nd/57 ± 5 | nd/45 ± 5 | 45 | n = 6 | n = 1 | [21] |
| AML-15 5 | 2002–2009 | 60 | nd/61 ± 6 | nd/49 ± 7 | 43 | n = 4 | n = 1 | ||
| DB AML-01 6 | 2009–2014 | 67 | nd/72 ± 6 | nd/48 ± 6 | 43 | n = 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obszański, P.; Kozłowska, A.; Wańcowiat, J.; Twardowska, J.; Lejman, M.; Zawitkowska, J. Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia. Molecules 2022, 27, 3911. https://doi.org/10.3390/molecules27123911
Obszański P, Kozłowska A, Wańcowiat J, Twardowska J, Lejman M, Zawitkowska J. Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia. Molecules. 2022; 27(12):3911. https://doi.org/10.3390/molecules27123911
Chicago/Turabian StyleObszański, Piotr, Anna Kozłowska, Jakub Wańcowiat, Julia Twardowska, Monika Lejman, and Joanna Zawitkowska. 2022. "Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia" Molecules 27, no. 12: 3911. https://doi.org/10.3390/molecules27123911
APA StyleObszański, P., Kozłowska, A., Wańcowiat, J., Twardowska, J., Lejman, M., & Zawitkowska, J. (2022). Molecular-Targeted Therapy of Pediatric Acute Myeloid Leukemia. Molecules, 27(12), 3911. https://doi.org/10.3390/molecules27123911







