Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood
Abstract
:1. Introduction
2. Results
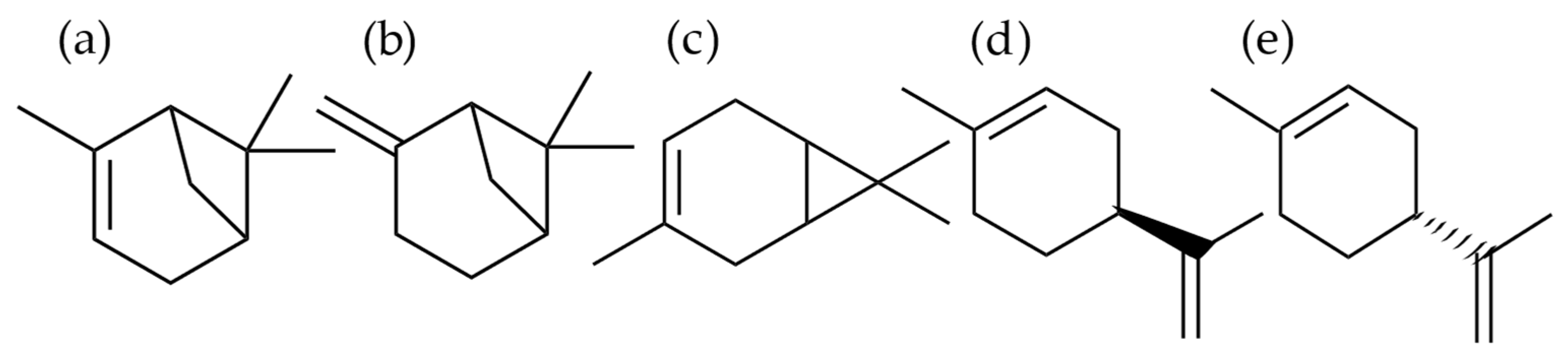
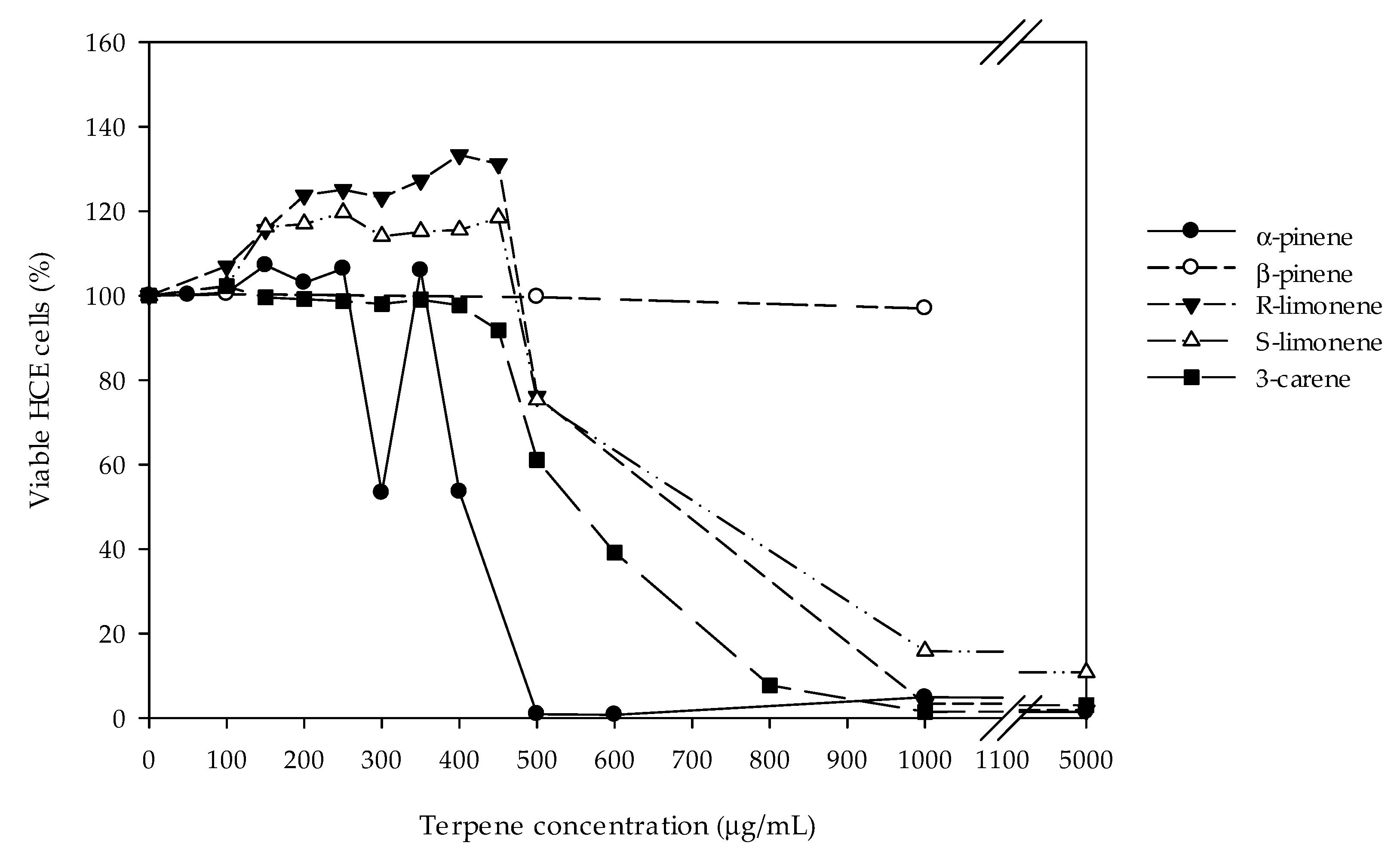
2.1. Cytotoxic and Oxidative-Stress Protective Effects of Monoterpenes in HCE-Cell Cultures
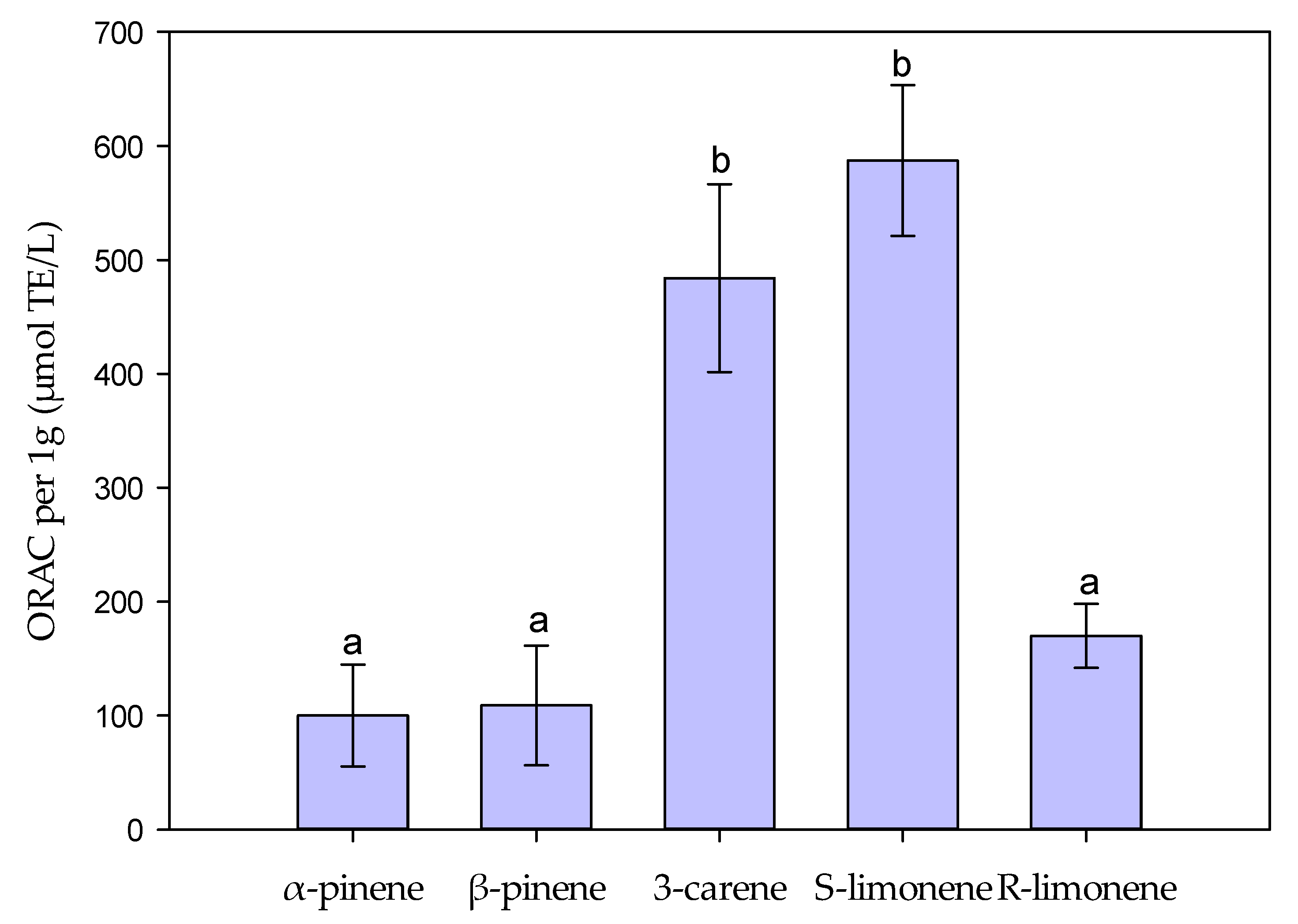
2.2. Antioxidant Properties of Monoterpenes Measured by ORAC and H2O2 Scavenging Assays
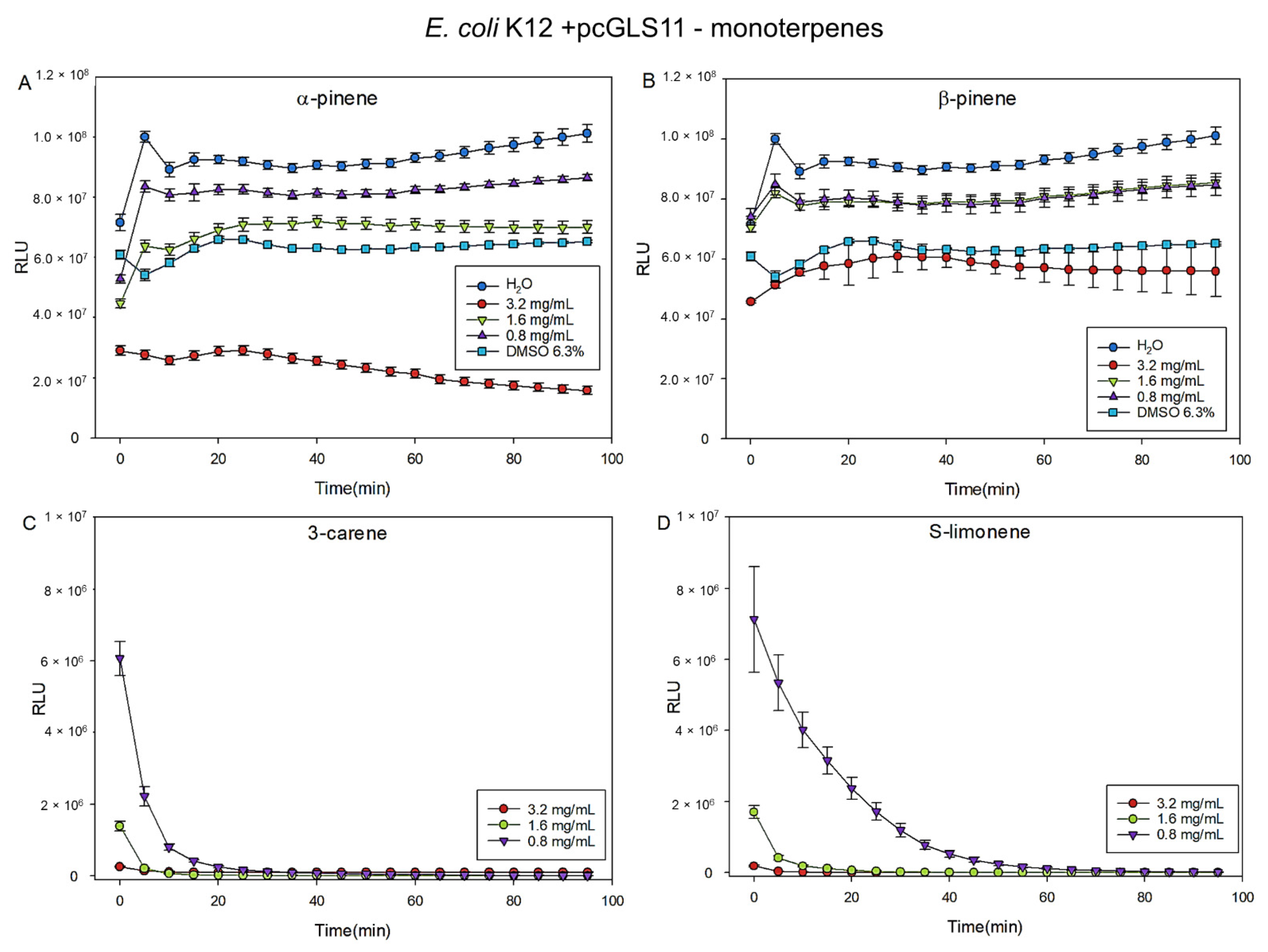
2.3. Antibacterial Efficacy of Monoterpenes Measured by MIC-Test and Biosensors (E. coli K12+pcGLS11 and S. aureus RN4220+pAT19)
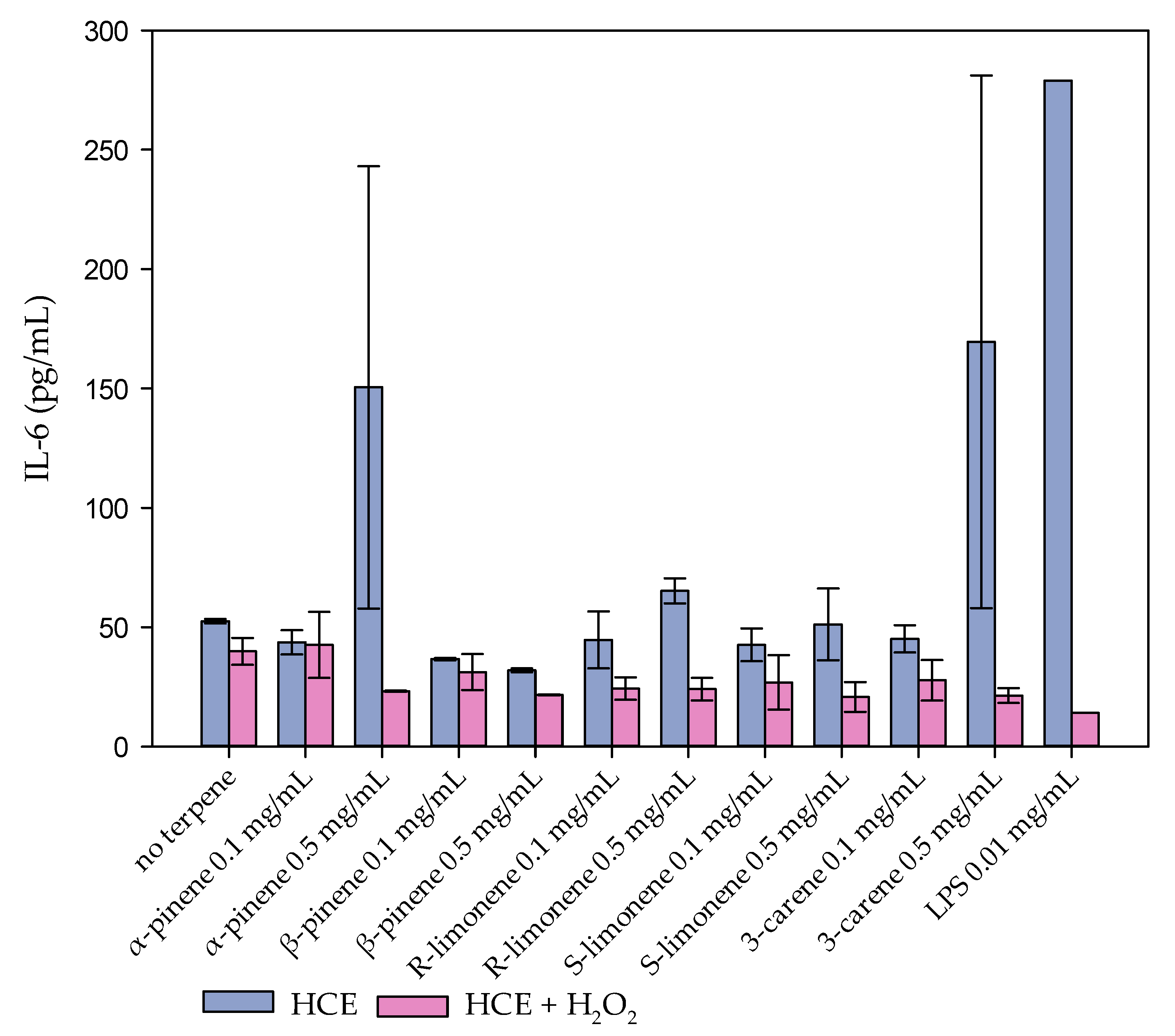
3. Discussion
4. Materials and Methods
4.1. Cell Model Tests: HCE Cell Model and IL-6
4.2. In Vitro Antioxidant Assays: ORAC and H2O2 Scaveging
4.3. Antibacterial Efficacy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scutaru, A.M.; Witterseh, T. Risk Mitigation for Indoor Air Quality using the Example of Construction Products–Efforts Towards a Harmonization of the Health-Related Evaluation in the EU. Int. J. Hyg. Environ. Health 2020, 229, 113588. [Google Scholar] [CrossRef] [PubMed]
- Riess, M.; Lottes, R. Development of harmonised European standards for measuring emissions from construction products in CEN from the perspective of environmental organisations environmental organisations (CEN TC 351—Assessment of release of dangerous substances under the Construction Products Directive (CPD)—Emission into indoor air and release to soil, surface water and ground water). Elin Rev. 2009, 1, 28–30. [Google Scholar] [CrossRef]
- Richter, H.O.; Sundin, S.; Long, J. Visually Deficient Working Conditions and Reduced Work Performance in Office Workers: Is it Mediated by Visual Discomfort? Int. J. Ind. Ergon. 2019, 72, 128. [Google Scholar] [CrossRef]
- Nättinen, J.; Jylhä, A.; Aapola, U.; Mäkinen, P.; Beuerman, R.; Pietilä, J.; Vaajanen, A.; Uusitalo, H. Age-associated changes in human tear proteome. Clin. Proteom. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, M.; Masalin-Weijo, M.; Kalliokoski, P.; Pasanen, P. Comparison of VOC Emissions between Air-Dried and Heat-Treated Norway Spruce (Picea Abies), Scots Pine (Pinus Sylvesteris) and European Aspen (Populus Tremula) Wood. Atmos. Environ. 2010, 44, 5028–5033. [Google Scholar] [CrossRef]
- Muilu-Mäkelä, R.; Kilpeläinen, P.; Kitunen, V.; Harju, A.; Venäläinen, M.; Sarjala, T. Indoor storage time affects the quality and quantity of volatile monoterpenes emitted from softwood timber. Holzforschung 2021, 75, 945–956. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.-S. Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. CFR-Code of Federal Regulations Title 21; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2012. [Google Scholar]
- Kim, Y.W.; Kim, M.J.; Chung, B.Y.; Bang, D.Y.; Lim, S.K.; Choi, S.M.; Lim, D.S.; Cho, M.C.; Yoon, K.; Kim, H.S.; et al. Safety Evaluation and Risk Assessment of d-Limonene. J. Toxicol. Environ. Health Part B Crit. Rev. 2013, 16, 17–38. [Google Scholar] [CrossRef]
- Ochocka, J.R.; Asztemborska, M.; Sybilska, D.; Langa, W. Determination of Enantiomers of Terpenic Hydrocarbons in Essential Oils Obtained from Species of Pinus and Abies. Pharm. Biol. 2002, 40, 395–399. [Google Scholar] [CrossRef]
- Kim, J.; Dinh, T.; Oh, H.; Son, Y.; Ahn, J.; Song, K.; Choi, I.; Park, C.; Suzlejko, J.; Kim, K. The Potential Benefits of Therapeutic Treatment using Gaseous Terpenes at Ambient Low Levels. Appl. Sci. 2019, 9, 4507. [Google Scholar] [CrossRef] [Green Version]
- Ganjitabar, H.; Singh, D.P.; Chapman, R.; Gardner, A.; Minns, R.S.; Powis, I.; Reid, K.L.; Vredenborg, A. The Role of the Intermediate State in Angle-Resolved Photoelectron Studies using (2 + 1) Resonance-Enhanced Multiphoton Ionization of the Chiral Terpenes, A-Pinene and 3-Carene. Mol. Phys. 2020, 119, 1–14. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. Pisoschi 2015 the Role of Antioxidants in the Chemistry of Oxidative Stress—A Review Elsevier Enhanced Reader. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Noacco, N.; Rodenak-Kladniew, B.; de Bravo, M.G.; Castro, G.R.; Islan, G.A. Simple colorimetric method to determine the in vitro antioxidant activity of different monoterpenes. Anal. Biochem. 2018, 555, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul. Immunol. Inflamm. 2017, 26, 37–50. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Narazaki, M.; Kishimoto, T. Targeting Interleukin-6 Signaling in Clinic. Immunity 2019, 50, 1007–1023. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Ahn, C.; Kim, J.; Park, M.; Kim, S.R.; Lee, S.; Jeung, E. Anti-Inflammatory Effects of Natural Volatile Organic Compounds from Pinus Koraiensis and Larix Kaempferi in Mouse Model. J. Biomed. Res. 2019, 33, 343–350. [Google Scholar]
- Bai, J.; Zheng, Y.; Wang, G.; Liu, P. Protective Effect of D-Limonene against Oxidative Stress-Induced Cell Damage in Human Lens Epithelial Cells via the p38 Pathway. Oxid. Med. Cell. Longev. 2015, 2016, 5962832. [Google Scholar] [CrossRef]
- Han, Y.; Sun, Z.; Chen, W. Antimicrobial Susceptibility and Antibacterial Mechanism of Limonene Against Listeria Monocytogenes. Molecules 2019, 25, 33. [Google Scholar] [CrossRef] [Green Version]
- Shu, H.; Chen, H.; Wang, X.; Hu, Y.; Yun, Y.; Zhong, Q.; Chen, W.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of 3-Carene against Brochothrix thermosphacta and Pseudomonas fluorescens. Molecules 2019, 24, 3246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vainio-Kaila, T.; Hänninen, T.; Kyyhkynen, A.; Ohlmeyer, M.; Siitonen, A.; Rautkari, L. Effect of volatile organic compounds from Pinus sylvestris and Picea abies on Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae and Salmonella enterica serovar Typhimurium. Holzforschung 2017, 71, 905–912. [Google Scholar] [CrossRef]
- Wolkoff, P. External eye symptoms in indoor environments. Indoor Air 2016, 27, 246–260. [Google Scholar] [CrossRef]
- Wolkoff, P.; Nielsen, G.D. Effects by inhalation of abundant fragrances in indoor air—An overview. Environ. Int. 2017, 101, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Risholm-Sundman, M.; Lundgren, M.; Vestin, E.; Herder, P. Emissions of acetic acid and other volatile organic compounds from different species of solid wood. Holz als Roh- und Werkst. 1998, 56, 125–129. [Google Scholar] [CrossRef]
- Sumitomo, K.; Akutsu, H.; Fukuyama, S.; Minoshima, A.; Kukita, S.; Yamamura, Y.; Sato, Y.; Hayasaka, T.; Osanai, S.; Funakoshi, H.; et al. Conifer-Derived Monoterpenes and Forest Walking. Mass Spectrom. 2015, 4, A0042. [Google Scholar] [CrossRef] [Green Version]
- Araki-Sasaki, K.; Ohashi, Y.; Sasabe, T.; Hayashi, K.; Watanabe, H.; Tano, Y.; Handa, H. An SV40-Immortalized Human Corneal Epithelial Cell Line and Its Characterization. Investig. Ophthalmol. Vis. Sci. 1995, 36, 614–621. [Google Scholar]
- Wu, W.; Hu, D.; Gao, H.; Chen, M.; Wang, D.; Rosen, R.; McCormick, S.A. Subtoxic Levels Hydrogen Peroxide-Induced Production of Interleukin-6 by Retinal Pigment Epithelial Cells. Mol. Vis. 2010, 16, 1864–1873. [Google Scholar]
- Vesterlund, S.; Paltta, J.; Lauková, A.; Karp, M.; Ouwehand, A.C. Rapid screening method for the detection of antimicrobial substances. J. Microbiol. Methods 2004, 57, 23–31. [Google Scholar] [CrossRef]
- Gminski, R.; Marutzky, R.; Kevekordes, S.; Fuhrmann, F.; Bürger, W.; Hauschke, D.; Ebner, W.; Mersch-Sundermann, V. Sensory irritations and pulmonary effects in human volunteers following short-term exposure to pinewood emissions. J. Wood Sci. 2011, 57, 436–445. [Google Scholar] [CrossRef]
- Jyske, T.; Järvenpää, E.; Kunnas, S.; Sarjala, T.; Raitanen, J.; Mäki, M.; Pastell, H.; Korpinen, R.; Kaseva, J.; Tupasela, T. Sprouts and Needles of Norway Spruce (Picea abies (L.) Karst.) as Nordic Specialty—Consumer Acceptance, Stability of Nutrients, and Bioactivities during Storage. Molecules 2020, 25, 4187. [Google Scholar] [CrossRef] [PubMed]
- Porres-Martínez, M.; González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Major Selected Monoterpenes α-Pinene and 1,8-Cineole found in Salvia Lavandulifolia (Spanish sage) Essential Oil as Regulators of Cellular Redox Balance. Pharm. Biol. 2015, 53, 921–929. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Kanimozhi, G.; Prasad, N.R.; Agilan, B.; Ganesan, M.; Srithar, G. Alpha Pinene Modulates UVA-Induced Oxidative Stress, DNA Damage and Apoptosis in Human Skin Epidermal Keratinocytes. Life Sci. 2018, 212, 150–158. [Google Scholar] [CrossRef]
- Välimaa, A.; Raitanen, J.; Tienahi, J.; Sarjala, T.; Nakayama, E.; Korpinen, R.; Mäkinen, S.; Eklund, P.; Willför, S.; Jyske, T. Enhancement of Norway Spruce Bark Side-Streams: Modification of Bioactive and Protective Properties of Stilbenoid-Rich Extracts by UVA-Irradiation. Ind. Crop. Prod. 2020, 145, 112150. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.S.; Dias, A.; Sharopov, D.; Taheri, F.; Martins, Y.; Baghalpour, N.; et al. Therapeutic Potential of A- and Β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The Effect of Five Common Essential Oil Components on Listeria Monocytogenes Biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.M.; Alviano, C.S.; Alviano, D.S. Biological Activities of A-Pinene and Β-Pinene Enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement. Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Woollard, A.C.S.; Wolff, S.P. Hydrogen Peroxide Production during Experimental Protein Glycation. FEBS Lett. 1990, 268, 69. [Google Scholar] [CrossRef] [Green Version]
- Tienaho, J.; Reshamwala, D.; Sarjala, T.; Kilpeläinen, P.; Liimatainen, J.; Dou, J.; Viherä-Aarnio, A.; Linnakoski, R.; Marjomäki, V.; Jyske, T. Salix spp. Bark Hot Water Extracts show Antiviral, Antibacterial, and Antioxidant Activities-the Bioactive Properties of 16 Clones. Front. Bioeng. Biotechnol. 2021, 9, 797939. [Google Scholar] [CrossRef] [PubMed]
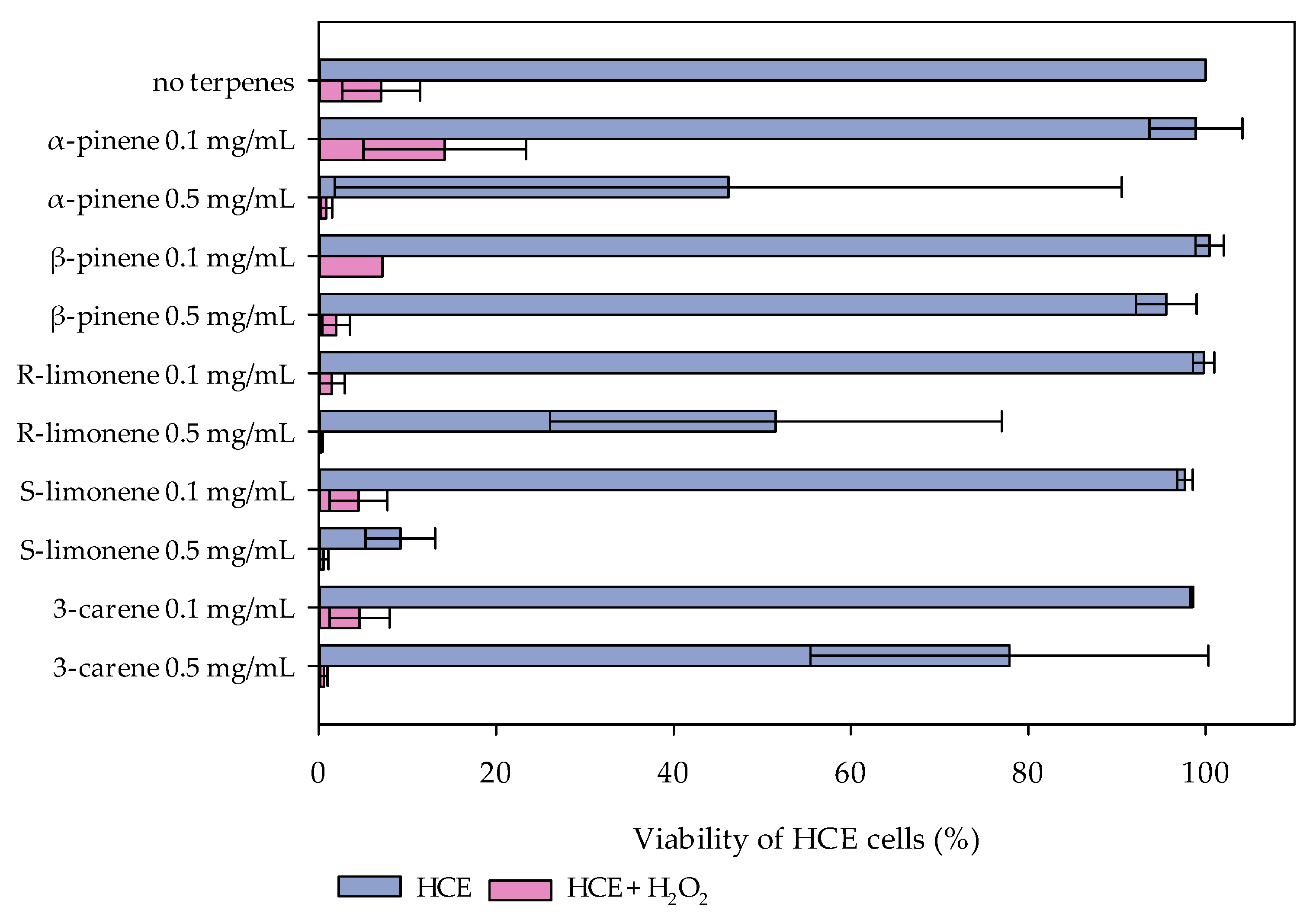

| Terpene | IC50 |
|---|---|
| α-pinene | 401 |
| β-pinene | nd 1 |
| R-limonene | 502 |
| S-limonene | 502 |
| 3-carene | 552 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muilu-Mäkelä, R.; Aapola, U.; Tienaho, J.; Uusitalo, H.; Sarjala, T. Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood. Molecules 2022, 27, 3891. https://doi.org/10.3390/molecules27123891
Muilu-Mäkelä R, Aapola U, Tienaho J, Uusitalo H, Sarjala T. Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood. Molecules. 2022; 27(12):3891. https://doi.org/10.3390/molecules27123891
Chicago/Turabian StyleMuilu-Mäkelä, Riina, Ulla Aapola, Jenni Tienaho, Hannu Uusitalo, and Tytti Sarjala. 2022. "Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood" Molecules 27, no. 12: 3891. https://doi.org/10.3390/molecules27123891
APA StyleMuilu-Mäkelä, R., Aapola, U., Tienaho, J., Uusitalo, H., & Sarjala, T. (2022). Antibacterial and Oxidative Stress-Protective Effects of Five Monoterpenes from Softwood. Molecules, 27(12), 3891. https://doi.org/10.3390/molecules27123891






