Abstract
Molecular imaging probes enable the early and accurate detection of disease-specific biomarkers and facilitate personalized treatment of many chronic diseases, including cancer. Among current clinically used functional imaging modalities, positron emission tomography (PET) plays a significant role in cancer detection and in monitoring the response to therapeutic interventions. Several preclinical and clinical studies have demonstrated the crucial involvement of cyclooxygenase-2 (COX-2) isozyme in cancer development and progression, making COX-2 a promising cancer biomarker. A variety of COX-2-targeting PET radioligands has been developed based on anti-inflammatory drugs and selective COX-2 inhibitors. However, many of those suffer from non-specific binding and insufficient metabolic stability. This article highlights examples of COX-2-targeting PET radioligands labelled with the short-lived positron emitter 18F, including radiosynthesis and PET imaging studies published in the last decade (2012–2021).
1. Introduction
Cyclooxygenase (COX) enzymes play a pivotal role in the metabolism of arachidonic acid and regulate the biosynthesis of various prostanoids under normal and pathological conditions [1,2,3,4,5,6]. COX is known to exist in two main isoforms: COX-1, COX-2. Both isoforms have similar structures and show the same cyclooxygenase and peroxidase activity to produce prostaglandin H2 (PGH2), an important precursor for the biosynthesis of other prostaglandins, prostacyclin and thromboxanes. However, COX-1 and COX-2 induction, regulation, expression, and localization sites are different [7,8,9].
COX-1 isozyme is encoded by the ptgs1 gene. It is regarded as a housekeeping enzyme due to its constitutive expression in most tissues. COX-1 is involved in the biosynthesis of prostanoids required for the regulation of epithelial cytoprotection and other homeostatic cell and tissue functions [4,10]. Upregulation of COX-1 is reported in several cancers [11,12,13,14] and brain disorders associated with neuroinflammation [15,16,17,18,19]. High levels of COX-1 are observed in ovarian [12,20], prostate [21], breast [22], and cervical [23] cancer. Therefore, in addition to non-selective classical non-steroidal anti-inflammatory drugs (NSAIDs: acetylsalicylic acid, ibuprofen, flurbiprofen, naproxen, indomethacin, diclofenac, mefenamic acid, piroxicam, etc.), recently, COX-1-selective inhibitors [11,24], fluorescence imaging probes [25,26,27,28] and PET radioligands [25,26,29,30,31,32] introduced COX-1 as a promising biomarker for imaging and treatment of cancers and neuroinflammatory diseases. Selected examples of COX-1 targeting fluorescent and PET imaging probes are shown in Figure 1. Recently, to follow up the development of COX-1 targeted radioligand ([11C]PS13), Taddei et al. synthesized an 18F-labeled version of PS13, [18F]PS13, by following two labeling approaches based on the nucleophilic addition of [18F]fluoride ion to gem-difluorovinyl precursors [33]. Despite low molar activity, the [18F]PS13 radioligand was evaluated for COX-1 PET imaging in monkeys.
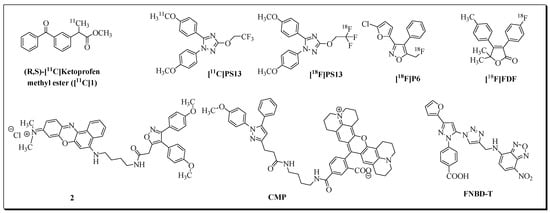
Figure 1.
PET and fluorescent imaging probes for COX-1 isozyme.
In contrast to the constitutively expressed COX-1 isozyme, the COX-2 isoform is primarily an inducible enzyme. The expression of COX-2 is induced by numerous stimuli such as proinflammatory cytokines, lipopolysaccharides, growth factors (fibroblast growth factor, platelet-derived growth factor, epidermal growth factor), hormones, and oncogenes, resulting in the production of prostaglandins (PGs) in inflamed tissues [4,34]. COX-2 is involved in several inflammatory diseases, types of cancer [35,36,37,38,39,40,41,42,43,44,45], and neurodegenerative pathways [45,46,47,48]. COX-2 selective inhibitors (Coxibs) were introduced to attenuate the gastrointestinal toxicity-related side effects of non-selective NSAIDs. COX-2 plays a crucial role in tumorigenicity, angiogenesis, metastasis, and apoptosis-resistance in cancer, including colorectal, pancreatic, brain, and breast cancer. Moreover, COX-2 is frequently upregulated in cancer, making COX-2 a promising biomarker for cancer diagnosis and therapy, as demonstrated by the development of various fluorescent [49,50,51,52,53] and radioimaging probes over the last decades [54,55,56,57]. COX-2-targeting fluorescent probes mainly consist of a fluorescent group attached to known anti-inflammatory drugs. Selected examples of COX-2 fluorescent imaging probes are presented in Figure 2.

Figure 2.
Selected examples of fluorescent probes for COX-2 imaging.
There has also been significant progress in the development of COX-2-targeting radioligands for imaging inflammation, cancer, and neurological disorders [54,55,56,57]. Over the last decades, a variety of radionuclide-based imaging agents have been developed by the incorporation of radioisotopes such as 11C, 18F, 99mTc, 123I, and 125I into NSAIDs and related compounds [55,56,58,59,60,61,62,63,64,65,66,67,68,69,70,71]. Selected examples of PET radioligands for COX-2 imaging are presented in Figure 3
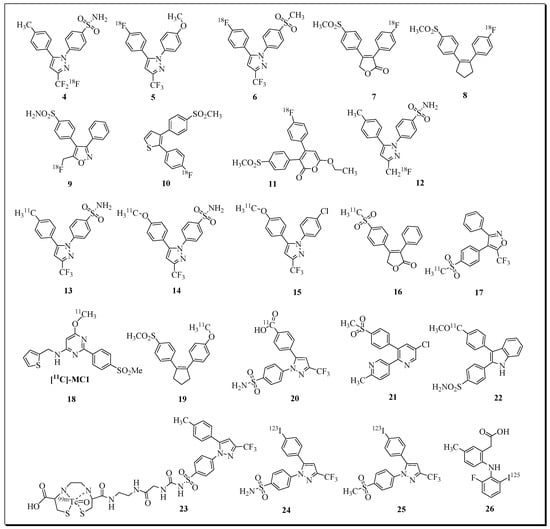
Figure 3.
Selected examples of radioligands for COX-2 imaging.
A major focus involves 18F-labeled radioligands, due to their favourable half-life (t1/2 = 109.8 min), ease of production, the availability of a variety of radiofluorination methods, and better imaging characteristics of the short-lived positron emitter 18F.
This review article primarily covers 18F-labeled radioligands reported in the last decade for targeting COX-2. A particular emphasis is on reporting radiochemistry and PET imaging data.
2. 2012
The development of a series of 3-diarylsubstituted indole-based inhibitors was reported by Hu et al. [72]. The highly potent and selective COX-2 inhibitor 3-(4-fluorophenyl)-2-(4-methylsulfonyl-phenyl)-1H-indole (Ki(COX-2) = 20 nM; Ki(COX-1) >10 µM) was selected for the development of a novel COX-2 radioligand for PET [73]. In 2012, Kniess et al. described an innovative procedure for the radiosynthesis of 3-(4-[18F]fluorophenyl)-2-(4-methylsulfonylphenyl)-1H-indole [18F]-4 (Figure 4).

Figure 4.
Radiosynthesis of [18F]27 via McMurry coupling. Reagents: (i) [18F]fluoride, K222/K2CO3, DMF; (ii) TiCl4, Zn, THF.
The radiosynthesis involved the direct radiofluorination of a non-cyclized trimethylamino triflate precursor using [18F]KF and Kryptofix (K222). The final compound was obtained via McMurry cyclization chemistry using zinc and titanium tetrachloride in an automated radiosynthesis process, including HPLC purification and solid-phase extraction. The radiosynthesis of [18F]27 was accomplished within 80 min in 10% total decay-corrected yield starting from [18F]fluoride. The radiochemical purity was >98%, and the molar activity reached 74–91 GBq/mol.
Kniess et al. examined the COX-1 and COX-2 inhibition profiles of compound 27. The in vitro binding assay results revealed that compound 27 is a moderately potent and selective COX-2 inhibitor (COX-2 IC50 = 1.2 μM, COX-1 IC50 = 6.6 μM, SI = 5.5).
In vivo PET imaging was performed in HT-29-tumor-bearing mice. During dynamic small animal PET studies, no substantial tumor accumulation of [18F]-3 was observed (Figure 5). Most of the radioactivity was found to accumulate in the liver, small intestine, and kidney, and a small amount of activity (SUV ~ 1) was noticed in the brain and the blood pool. It was concluded that [18F]27 is unsuitable for in vivo PET imaging of COX-2 in mice with xenotransplanted HT-29 tumors.
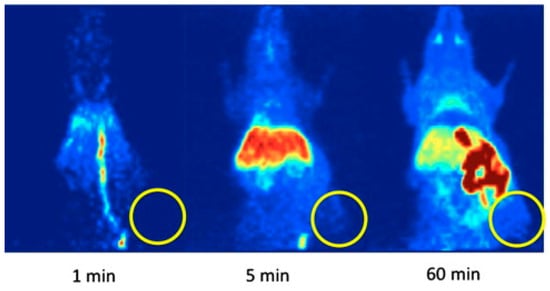
Figure 5.
Representative PET images of a HT-29-tumor-bearing mouse (maximum intensity projections) at 1, 5, and 60 min after a single intravenous injection of [18F]27 (circle: position of the subcutaneously xenotransplanted tumor). Figure readapted with permission from publisher [74].
3. 2013
In 2013, our group reported the synthesis and in vitro evaluation of a series of trifluoromethyl-substituted pyrimidines as COX-2 inhibitors. Three fluorobenzyl-substituted pyrimidine derivatives were further advanced as 18F-labeled radioligands ([18F]28a (18F-Pyricoxib), [18F]29a, and [18F]30a) [75]. Radiotracers [18F]28a and [18F]29a were synthesized using 4-[18F]fluorobenzylamine ([18F]FBA) as a building block (Figure 6). The radiosynthesis and HPLC purification of [18F]28a were accomplished within 95 min, and the decay-corrected radiochemical yield based on [18F]FBA was 27 ± 11%. The total synthesis time for radioligand [18F]29a was 110 min, including HPLC purification. Radioligand [18F]29a was isolated in decay-corrected radiochemical yields of 23% ± 1%.
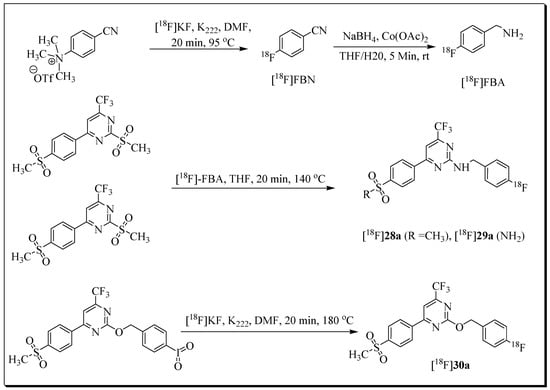
Figure 6.
Radiosynthesis of [18F]28a [18F]29a, and [18F]30a.
The radiosynthesis [18F]30a was accomplished using iodylaryl derivative as the labelling precursor, leading to a radiochemical yield of 5 to 10% (as determined by radio-TLC).
The radiotracers were tested in COX-2-expressing human HCA-7 colorectal cancer cells, and in vitro specificity was examined using COX-2-negative HCT-116 cells, and by blocking studies with COX-2 inhibitors [76,77]
As in the cell uptake studies results in HCA-7 cells, the in vivo studies with [18F]28a (18F-Pyricoxib) showed uptake in HCA-7 tumors (T/M ratio of 2.25 after 4 h p.i.) (Figure 7). A reduced uptake of [18F]28a in the tumor was noticed upon pre-treatment with the COX-2 selective inhibitor celecoxib (Figure 8) [76,77]. However, a similar radioactivity uptake profile was observed for 18F-Pyricoxib during studies on HCT-116-tumor-bearing mice, (SUV2h 0.93 ± 0.06 (HCT-116; n = 3) versus 1.09 ± 0.13 (HCA-7; n = 3). The radiopharmacological profile of [18F]28a was superior to radioligands [18F]29a and [18F]30a [77].

Figure 7.
Left: PET/CT image (coronal slice) 2 h after injection of [18F]Pyricoxib into an HCA-7-tumor-bearing NIH-III mouse. An amount of 3.5% HSA was added as a carrier protein to the final injection solution. Right: Time-activity curves for tumor uptake of [18F]Pyricoxib and its clearance from muscle tissue over 4 h post injection. Data are shown as mean ± SD from seven dynamic PET experiments. Figure readapted with permission from publisher [78].

Figure 8.
Top: transaxial, coronal, and sagittal PET images at 60 min p.i. of [18F]Pyricoxib into HCA-7-tumor-bearing NIH-III mouse (control); bottom: transaxial, coronal, and sagittal PET images at 60 min p.i. of [18F]Pyricoxib into HCA-7-tumor-bearing NIH-III mouse (pre-treated with 2 mg of celecoxib 60 min before radiotracer administration; no HSA added). Figure readapted with permission from publisher [76].
In 2013, two radiolabeled COX-2 selective inhibitors, [11C]celecoxib and [11C] rofecoxib, were assessed as PET tracers for imaging COX-2-normal and ischemic mouse brains [66]. From a series of experiments, including in vitro autoradiography and in vivo PET assays, the authors concluded that [11C]celecoxib is not a suitable COX-2 radioligand for in vitro and in vivo assays, whereas [11C]rofecoxib is useful for in vitro assays of COX-2.
4. 2014
No 18F-labelled COX-2 imaging probes were reported in 2014. However, Perrone et al. reported the development of [18F]-P6 for PET imaging of COX-1 expression in human OVCAR-3 (ovarian cancer) tumor xenografts (Figure 1) [79]. Radioligand [18F]-P6 was prepared in 18% radiochemical yield, and PET/CT imaging analyses showed that radiotracer [18F]-P6 accumulated in COX-1 expressing OVCAR 3 tumors. In addition to this study, a few review articles highlighting applications of PET radioligands were published in 2014 [70,74].
5. 2015
The discovery of a fluorescent probe, Celecoxib-NBD [51], in our lab led to the development of a new series of fluorine-containing cyclooxygenase-2 (COX-2) inhibitors [80]. Based on the favourable COX-2 inhibition profile, compound N-(4-fluorobenzyl)-4-(5-p-tolyl-3-trifluoromethylpyrazol- 1-yl)benzenesulfonamide 31 (IC50 = 0.36 µM, SI > 277) was selected for 18F labelling and PET imaging studies. Radioligand [18F]31 was evaluated in the human colorectal cancer cell line HCA-7 (Figure 9) [81].
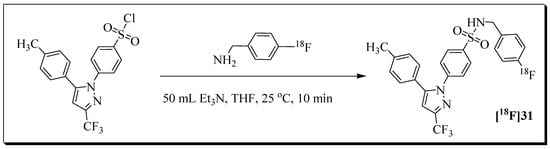
Figure 9.
Radiosynthesis of radioligand [18F]31 from labelling precursor and 4-[18F]fluorobenzylamine ([18F]FBA).
The 18F building block [18F]fluorobenzylamine ([18F]FBA) was used for the radiosynthesis of radioligand [18F]31. The decay-corrected radiochemical yield based on [18F]FBA was 20%, and the molar activity was greater than 40 GB/µmol. The total synthesis time, including HPLC purification, was 85 min.
Although the normalized cellular uptake of [18F]31 in COX-2-positive HCA-7 cells was high (450% radioactivity per mg protein after 60 min), no noticeable blocking effect was observed by pre-treatment with known COX-2 inhibitors (celecoxib, rofecoxib). It was suggested that the uptake and retention of [18F]31 in HCA- 7 cells is associated with unknown non-COX-2 targets. PET imaging of radioligand [18F]31 in HCA-7-tumor-bearing NIH-III mice revealed an SUVmax in HCA-7 tumors of 0.40 ±0.07 (n = 3) after 10 min p.i. (Figure 10). At the same time point, the muscle uptake was 0.29 ± 0.08 (n = 3); therefore, the tumor-to-muscle ratio was only 1.4, providing poor imaging contrast. An analysis of the time–activity curves for radioactivity uptake in the tumor suggested that radioligand [18F]31 was not trapped in tumor tissue. Therefore, radioligand [18F]31 is unsuitable for COX-2 imaging.

Figure 10.
Maximum intensity projection (MIP), as well as coronal and transaxial PET images at 10 and 60 min p.i. of radioligand [18F]31 in HCA-7-tumor-bearing (left flank) NIH-III mice (injected activity = 2.89 MBq) under isoflurane anesthesia. Figure readapted with permission from publisher [81].
6. 2016
A novel set of diaryl-substituted heterocycles containing a tricyclic dihydropyrrolo[3,2,1-hi]indole and pyrrolo[3,2,1-hi]indole core structure were designed to improve the COX-2-inhibitory activity of the previously reported compound IND [78]. From this series of compounds, two promising COX-2 selective inhibitors, DHPI (IC50 COX-2: 0.15 mM, SI = >666) and PI (IC50 COX-2: 0.04 mM, SI = >2500) were selected for 18F-labelling and PET imaging studies (Figure 11) [82]. Gassner et al. described the radiosynthesis of [18F]DHPI and [18F]PI. [18F]DHPI was prepared in two steps, involving the [18F]fluorination of trimethylammonium labelling precursor under mild conditions followed by McMurry cyclization in the presence of TiCl4 and zinc.

Figure 11.
Radiosynthesis route leading to [18F]DHPI. [18F]Fluorination of indoline precursor 32 under optimized conditions forms intermediate [18F]33, which is subsequently cyclized to [18F]DHPI by McMurry reaction. The [18F]fluorination of indole precursor 35 furnished only side product [18F]37 instead of [18F]36, so the McMurry reaction was not feasible.
The automated synthesis afforded a decay-corrected radiochemical yield of 16% for radioligand [18F]DHPI. The total synthesis time was 110 min, and the molar activity was 45–106 GBq/mmol.
A series of cell uptake studies in five human cell lines which significantly differed in their COX-2 expression levels were conducted to confirm the COX-2-specific cell uptake of [18F]DHPI. However, blocking experiments with the COX-2 inhibitor celecoxib did not result in a significant change in the cellular uptake of [18F]DHPI. These cellular uptake study results are indicative of non-specific binding of [18F]DHPI. Additional radiopharmacological experiments were conducted in female A205-tumor-bearing NMRI nu/nu mice. Consistent with cellular uptake studies, radioligand [18F]DHPI uptake could also not be blocked in COX-2-positive A2058 tumors after pre-injection of celecoxib.
In another study, indomethacin, a known COX-1/2 inhibitor, was conjugated with zwitterionic phosphonium aryltrifluoroborates for radiolabeling with 18F. The radiolabeling of these novel indomethacin conjugates was achieved by innovative 18F-19F isotopic exchange chemistry [83].
Briefly, phosphonium trifluoroborate/indomethacin conjugates 38/39 were dissolved in DMSO and incubated with irradiated [18O]water containing 18F at 75 °C for 10 min. Radioligands [18/19F]38 and [18/19F]39 were obtained in 95.1% and 93.5% radiochemical yield, respectively (Figure 12). The study was focused on radiosynthesis, and PET imaging experiments were not reported.

Figure 12.
Scheme showing the radiolabeling of 38 and 39 by isotopic exchange in an aqueous solution.
7. 2017
Lebedev et al. utilized electrochemical radiofluorination chemistry for the radiosynthesis of 18F-labelled COX-2 inhibitor 18F-40 (COX-2 IC50 = 1.5 nM) [84]. The radiolabeling occurred directly on a heteroaromatic ring. The electrochemical 18F-labelling was performed using a radioelectrochemical synthesizer built in-house. Briefly, radiolabeling involved 18F drying on a cartridge and pushing precursor through the cartridge into a Teflon reactor. The reaction mixture was electrolyzed at ambient temperature using 1.0 mm Pt wire electrodes. The electrolysis and fluorination in the cell were performed for 70 min using an Autolab PGSTAT204. The slurry was subjected to purification, followed by treatment with HCl in a pre-heated reactor for 15 min.
The radiosynthesis of 18F-40 was completed in 4 h, and the radioligand was prepared in 0.8–2% decay-corrected radiochemical yield. The molar activity was approximately 3 Ci/mmol (Figure 13).

Figure 13.
The reaction scheme of the electrochemical radiosynthesis of COX-2 inhibitor 18F-40.
The authors observed a COX-2-dependent cell uptake of 18F-40 in LPS-treated RAW264.7 macrophage-like cells. A reduction of radioligand uptake was detected when cells were pre-treated with the selective COX-2 inhibitor celecoxib. In vivo metabolism and PET imaging studies with radioligand 18F-40 in healthy mice showed no retention in bones over 2 h, which is indicative of no radiodefluorination in vivo. The radioligand crossed the blood–brain barrier and showed excretion mainly through the hepatobiliary pathway. Radioligand 18F-40 also displayed rapid blood clearance and high metabolic stability in vivo. Overall, radioligand 18F-40 possesses favourable characteristics suitable for PET imaging of COX-2.
Yeh et al. reported the development of m-[18F]fluorofenbufen ester boronopinacol (m-[18F]FFBPin) for boron neutron capture therapy (BNCT) of aggressive cholangiocarcinoma (CCA) that overexpresses COX-2 enzyme [85]. Electrophilic radiofluorination chemistry of protected fenbufen boronopinacol (FBPin) 44 was accomplished using CH3COO[18F]F. This radiofluorination reaction produced two products, the desired product meta-[18F]fluorofenbufen pinacol (m-[18F]FFBPin) and ortho-[18F] fluorofenbufen pinacol (o-[18F]FFBPin), in a radiochemical yield of 8% at a molar activity of 15 MBq/μmol (Figure 14).
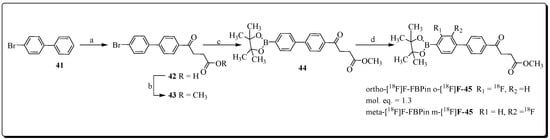
Figure 14.
Preparation of boronofenbufen analogs O- and m-[18F]-45. Reagents and condition (a) dihydrofuran-2,5-dione, AlCl3; (b) CH3OH/H2SO4, 96% over two steps; (c) Bis (pinacolato)diboron, AcOK, Fe(C5H4)2(PPh2)2PdCl2, DMF, 85%; (d) CH3COO[18F]F/CF3COOH, 8%.
Radioligand m-[18F]FFBPin showed moderate COX-2 inhibition and selectivity (COX-2 IC50 = 0.33 ± 0.24 μM, COX-1 IC50 = 0.91 ± 0.68 Μm) in a competitive binding assay. The assessment of radiotracer m-[18F]FFBPin in a COX-2 overexpressing CCA tumor rat model showed a maximum tumor uptake at 10 min; afterwards, radioligand m-[18F]FFBPin was washed out from tumor tissue over 60 min. No reduction of m-[18F]FFBPin uptake levels was noticed in the presence of FBPin 4 (4 mg) during blocking studies.
During BNCT investigations, the CCA rats treated with FBPin (20–30 mg) showed a reduction in tumor size. This therapeutic outcome of FBPin is encouraging and suggests that novel boron-containing COX-2 inhibitors should be further explored for BNCT.
8. 2018
Elie et al. synthesized and evaluated a series of (2,3-di(het)arylated (aza)indazole-based compounds as novel COX-2 inhibitors. Compound 4-[3-(4-fluorophenyl)indazol-2-yl]benzene-sulfonamide (49, IC50 = 0.409 µM) was identified as the lead candidate for radiolabeling with 18F, and subsequent PET imaging in a rat model of neuroinflammation was conducted [80]. The radiosynthesis of [18F]49 followed three different radiolabeling strategies. The first approach failed and encompassed direct radiofluorination chemistry starting from a nitro labelling precursor. Second, 18F labelling was performed using an iodonium salt precursor. However, this approach also failed and yielded only minimal amounts of radioligand [18F]49.
Finally, with the use of boronic ester-based precursor 53, the automated radiosynthesis of [18F]49 was accomplished in 46% decay-corrected radiochemical yield within 20 min (Figure 15).

Figure 15.
Reagents and conditions: (a) corresponding boronic acid (1.2 equiv), Cs2CO3 (3.0 equiv), Pd(PPh3)4 (0.1 equiv), dioxane, 150 °C, lW, 1 h, 42 53%, 25 69%; (b) [K/K222]+ 18F−, DMF, 130 °C, lW, 20 min; (c) ICl (2.0 equiv), CH2Cl2, 0 °C then t.a., 1.5 h, 99%; (d) n-Bu6Sn2 (3.3 equiv), Pd(PPh3)4 (0.1 equiv), dioxane, 90 °C, 2 h, 33%; (e) Moser reagent (1.5 equiv), CH3CN/CH2Cl2 (1/1), r.t., 18 h, 73%; (f) [K/K222]+ 18F−, DMF, Cu(OTf)2 cat, 100 °C, lW, 10 min; (g) 1,4-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzene (3.0 equiv), Na2CO3 (6.5 equiv.), Pd(PPh3)4 (0.02 equiv), dioxane, 100 °C, lW, 40 min, 52%; (h) KOTf; K2CO3, [18F]KF, Cu(OTf)2 cat., DMF, pyridine, 130 °C, 20 min.
In vivo PET imaging of radioligand [18F]49 in a rodent model of neuroinflammation showed uptake in several cerebral regions (cerebellum, striatum, cortex, hippocampus). However, no significant increase of [18F]49 uptake in inflamed brain regions was noticed.
High levels of non-specific binding of [18F]49 was confirmed by blocking studies with COX-2 inhibitor, as no reduced uptake of radioligand [18F]49 was observed in peripheral tissues and organs. Overall, radioligand [18F]49 was unsuccessful for PET imaging of COX-2 in models of neuroinflammation.
Cortes-Salva et al. described the synthesis and COX-2 inhibition analysis of a series of 2(4-methyl-sulfonylphenyl)pyrimidine-based compounds [86]. Among eleven COX-2 inhibitors, three compounds (6-methoxy-2-(4-(methylsulfonyl)phenyl-N-(thiophen-2ylmethyl)pyrimidin-4-amine (55), 6-fluoromethyl analogue (57), and 6-(2-fluoroethoxy) analogue (56)) were selected for radiolabeling based on their favourable COX-2-inhibitory profile, selectivity, and lipophilicity. Radioligand [11C]55, was prepared in 1.85–3.11 GBq yield upon reacting precursor 54 with [11C]iodomethane under basic conditions for 5 min. The molar activity was 204–492 GBq/μmol (Figure 16).

Figure 16.
Radiosyntheses of [11C]55, [18F]56, and [18F]57. Reagents and conditions: (i) [11C]CH3I, DMF, TBAH, RT, 5 min; (ii) [18F]FCH2CH2Br, DMF, Cs2CO3, 18-crown-6, 110 °C, 15 min; (iii) [18F]FCD2Br, Cs2CO3, 18-crown-6, 110 °C, 15 min.
The radiofluorination of precursor 54 was performed with 2-[18F]fluoro-1-bromoethane, and radioligand [18F]57 was obtained in 8.4% radiochemical yields within 15 min at a molar activity of 60 GBq/μmol. Labeling precursor 54 was also used for the preparation of radioligand [d2-18F]56. Compound 54 was reacted with double deuterated agent [d2-18F]fluorobromomethane in the presence of Cs2CO3, and 18-crown-6 at 110 °C for 15 min. Radioligand [d2-18F]56 was isolated in 27% yield at a molar activity of 66 GBq/μmol. In summary, the authors identified promising PET radioligands for imaging COX-2, and established several methods for radiosynthesis. No PET imaging data using the novel radioligands have been reported.
In 2018, Chang et al. reported the synthesis and evaluation of ortho-[18F]fluorocelecoxib for PET imaging of COX-2 in a cholangiocarcinoma (CAA) model [87]. Celecoxib was reacted with a CH3COOF/[18F]CH3COOF mixture for 25 min, and the radioligand ortho-[18F]F-58 was obtained in 9% radiochemical yield (Figure 17). Following the COX-1/2 inhibition analyses of ortho-[18F]F-58 (COX-2 IC50 = 39.0 nM, COX-1 = 24.5 mM), the radioligand was tested for COX-2 uptake in CAA cells and tumor models. PET studies demonstrated the accumulation of ortho-[18F]F-58 in CAA tumors, although no reduction in activity levels could be observed during blocking experiments with celecoxib.

Figure 17.
Preparation of ortho-[18F]F-58.
9. 2019
In 2019, no reports related to 18F based COX-2 targeting PET probes were published. However, Uddin et al. reported the development of radioligand [18F]FDF for PET imaging of COX-1 in an ovarian cancer model [32]. The authors described the identification of COX-1 selective inhibitor 3-(4-fluorophenyl)-5,5- dimethyl-4-(p-tolyl)furan-2(5H)-one (FDF), possessing acceptable in vivo stability, plasma half-life, and pharmacokinetic properties for its application as an imaging agent.
[18F]FDF radiosynthesis was tested with several reaction conditions, and ultimately the radioligand was obtained via Lewis acid-catalyzed nucleophilic aromatic deiodo[18F]fluorination reaction using tetrabutylammonium 18F-fluoride and labelling precursor 59 (Figure 18).
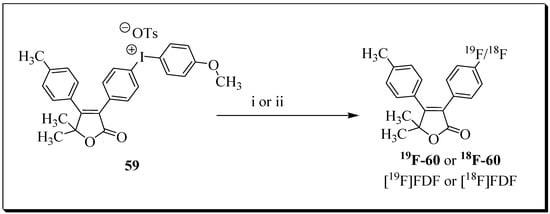
Figure 18.
Synthesis of [18F]FDF: (i) [19F]-Bu4NF, m-CPBA, DMSO, 80 °C, 30 min; (ii) Bu4NHCO3, 18F-fluoride, m-CPBA, DMSO, 80 °C, 30 min.
Radioligand [18F]FDF was obtained in 7.4% decay-corrected radiochemical yield [n = 14 batches] at a molar activity of 578.8 Ci/mmol with 99.9% radiochemical purity. The overall synthesis time was 50 min. PET imaging studies with the radioligand [18F]FDF in COX-1-overexpressing ovarian cancer models in mice showed COX-1-mediated uptake in the tumors compared to normal tissues. The study presented radioligand [18F]FDF as a promising lead compound for further preclinical and clinical development.
10. 2020
Kumar et al. reported the synthesis and evaluation of pyrimidine-based radiotracer [18F]6-fluoro-2 (4-(methylsulfonyl)phenyl)-N-(thiophen-2-ylmethyl)pyrimidin-4-amine ([18F]FMTP) [88]. The radiotracer was synthesized by the replacement of chloride substituent with 18F on position 6 of the pyrimidine ring of labelling precursor. Radioligand [18F]FMTP was isolated in 35 ± 5% radiochemical yield with >99% radiochemical purity and a molar activity of 92.5 ± 18.5 GBq/μmol (n = 10) (Figure 19).

Figure 19.
Synthesis of FMTP and radiosynthesis of [18F]FMTP.
COX-2 binding affinity was screened by cell uptake experiments in COX-2 positive BxPC3 cells and COX-2 negative PANC1 cells. More uptake of radioligand [18F]FMTP was noticed in BxPC3 cells compared to PANC1 cells. FMTP was more effective during blocking experiments than celecoxib for blocking radioligand uptake in BxPC3 cells.
PET imaging studies with radioligand [18F]FMTP in normal mice demonstrated that the radioligand was able to cross the blood–brain barrier. However, the radiotracer also showed rapid brain wash-out within the first few minutes. An approximately two times higher uptake of radioligand [18F]FMTP in the brain was noticed in an LPS-induced neuroinflammation model compared with PBS-treated mice.
The preliminary studies of the radioligand showed promising first results, and additional radiopharmacological studies are needed to validate and understand the COX-2-specific uptake in the neuroinflammation model.
Our lab described the in situ click chemistry generation of a highly potent and selective COX-2 inhibitor (COX-2 IC50 = 90 nM, COX-1 IC50 > 100 μM) [89]. The lead compound was advanced for 18F-labelling and PET imaging studies [90]. The Cu-mediated late-stage radiofluorination reaction with boronic acid pinacol ester-based precursor afforded [18F]triacoxib in radiochemical yields of 72% (decay corrected) within 90 min, and the molar activity exceeded 90 GBq/μmol (Figure 20). During metabolic stability analysis, ∼90% of [18F]triacoxib was found intact after 60 min p.i. The radiotracer showed significant uptake in COX-2 overexpressing HCA-7 cells, but blocking studies with nonradioactive triacoxib suggest the occurrence of unidentified, nonspecific cellular uptake of [18F]triacoxib in HCA-7 cells. In pre-clinical PET imaging studies (Figure 21), tumor uptake of [18F]triacoxib was noticed, and the pre-treatment celecoxib (2 mg) reduced [18F]triacoxib tumor uptake by ∼20% at 60 min p.i. (SUV). It was suggested that the remaining ∼80% of radiotracer uptake and retention in HCA-7 tumors is related to the nonspecific binding mechanisms, including lipophilicity, lysosomal trapping, and off-target binding not related to COX-2. Therefore, it was recommended that additional in vivo experiments should be performed to understand the nonspecific binding mechanisms of [18F]triacoxib.

Figure 20.
Synthesis of [18F]triacoxib.

Figure 21.
Transaxial and sagittal PET projection of PET images of [18F]triacoxib at 60 min p.i. in the same mouse after two consecutive days under control (left) and blocking (right) conditions. Statistical analysis of the blocking effect with 2 mg celecoxib at 60 min p.i. (middle). Data are presented as individual points with the mean ± SEM from n = 4 dynamic PET experiments. ** p < 0.01. Figure readapted with permission from publisher [90].
Laube et al. described the radiosynthesis and evolution of three methylsulfonyl-substituted celecoxib derivatives, [18F]66a,b and [D2,18F]66a [91]. The three radiolabeled compounds were synthesized through a reaction of [18F]fluoride with tosylated precursors 65a,b and [D2]65a under standard radiolabeling condition in an automated radiosynthesizer (Figure 22). Based on their insufficient COX-2 inhibition potency, the three compounds were not forwarded for in vivo studies in tumor-xenograft-bearing mice. However, in vivo evaluation of biodistribution in healthy mice indicated that the three compounds possess similar pharmacokinetic properties. Upon 5 min p.i., the highest initial radioactivity concentration was observed in liver, adrenals, and brown as well as white adipose tissue. In contrast, dynamic PET studies indicated that [18F]66b is eliminated more rapidly than [18F]66a or [D2,18F]66a. An enhancement in metabolic stability was noticed for deuterated compound and a decrease in 18F-defluorination was noticed in the order [18F]66a > [18F]66b > [D2,18F]66a.
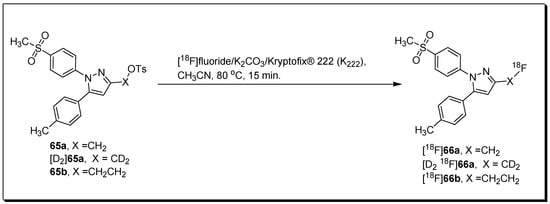
Figure 22.
Radiosynthesis of [18F]66a,b and [D2,18F]66a.
11. 2021
The encouraging outcome of a study focused on neuroinflammation imaging with the radioligand [11C]MPbP (4’-[11C]methoxy-5-propyl-1,10-biphenyl-2-ol) inspired the design and synthesis of a new set of honokiol analogs [92]. All four compounds were tested for their anti-inflammatory activities and compared with celecoxib. Compound (4’-(2-fluoroethoxy)-2-hydroxy-5-propyl-1,10-biphenyl) F-IV (COX-2 IC50 = 0.09 µM, SI = 48) was selected for radiolabeling and evaluation in a rat model of LPS-induced neuroinflammation. Radioligand (4’-(2-[18F]fluoro-ethoxy)-2-hydroxy-5-propyl-1,10-biphenyl) [18F]F-IV was synthesized by radioalkylation reaction of 2-[18F]fluoro-1-bromoethane [18F]FEB with labelling precursor 65 (Figure 23).
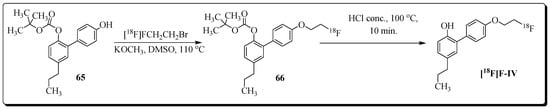
Figure 23.
Radiosynthesis of [18F]F-IV.
Radioligand [18F]F-IV was prepared in radiochemical yield of 35% at a molar activity of 35–40 GBq/µmol. Assessment of the ex vivo biodistribution profile of radioligand [18F]F-IV in LPS-treated and normal Wistar rats showed that the radioligand was able to penetrate the BBB, as high brain uptake was observed with a peak value of 2.21 ± 0.64 and 2.09 ± 0.65 (%ID/g) in the pons and medulla, respectively, at 10 min post-injection. Moreover, blocking experiments with the COX-2-selective inhibitor celecoxib showed a significant reduction of [18F]F-IV uptake in almost all extracted organs and tissues of LPS rats. The most prominent reduction (20–32%) in radioactivity uptake was observed in the brain (pons and medulla), heart, lung, and kidney. Overall, the outcome of preliminary studies presents [18F]F-IV as a promising candidate for COX-2-targeting PET imaging of neuroinflammation.
12. Conclusions
PET imaging of COX-2 has emerged as an exciting strategy for studying and understanding the role of COX-2 in inflammatory diseases and cancer.
This review summarizes the literature describing the synthesis and evaluation of COX-2-targeting PET radioligands over the last decade. The radiosynthesis of 18F-labelled COX-2 radioligands has benefited from recent developments in 18F radiochemistry, particularly late-stage radiofluorination. Many 18F-labelled COX-2-targeting radioligands with favourable PET imaging characteristics are based on popular selective COX-2 inhibitors like celecoxib. Instalment of 18F was either on an aromatic ring or an aliphatic side chain. In addition, recent advancements in [18F]CF3 radiochemistry should now open new opportunities for designing and preparing COX-2-targeting radioligands at high molar activity using CF3-group-containing compounds like celecoxib or mavacoxib. Moreover, complementary imaging techniques like optical imaging have also helped to test novel COX-2 inhibitors and to select promising candidates for radiolabeling with 18F.
Despite the challenges associated with high unspecific binding and metabolic stability, the preclinical data of several COX-2-targeting PET imaging agents look promising. The best candidates should be advanced for clinical testing to fully assess the potential and usefulness of COX-2 imaging with PET in the clinic.
Author Contributions
Conceptualization, J.K., A.B. and F.W.; investigation and writing-original draft preparation, J.K., A.B. and F.W.; data collection, J.K., A.B. and F.W.; visualization, J.K., A.B. and F.W.; writing, review and editing, J.K., A.B. and F.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We gratefully acknowledge the Dianne and Irving Kipnes Foundation, the Canadian Institute for Health Research (CIHR), and the National Science and Engineering Research Council of Canada (NSERC) and Alberta Cancer Foundation (ACF) for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- van der Donk, W.A.; Tsai, A.L.; Kulmacz, R.J. The cyclooxygenase reaction mechanism. Biochemistry 2002, 41, 15451–15458. [Google Scholar] [CrossRef] [PubMed]
- Blobaum, A.L.; Marnett, L.J. Structural and functional basis of cyclooxygenase inhibition. J. Med. Chem. 2007, 50, 1425–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marnett, L.J. The COXIB experience: A look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase Isozymes: The Biology of Prostaglandin Synthesis and Inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the Cyclooxygenase Pathways of Prostanoid Biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouzer, C.A.; Marnett, L.J. Structural and chemical biology of the interaction of cyclooxygenase with substrates and non-steroidal anti-inflammatory drugs. Chem. Rev. 2020, 120, 7592–7641. [Google Scholar] [CrossRef]
- Smith, W.L.; Garavito, R.M.; DeWitt, D.L. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J. Biol. Chem. 1996, 271, 33157–33160. [Google Scholar] [CrossRef] [Green Version]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Iyashiro, J.M.; Penning, T.D.; Seibert, K. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Pannunzio, A.; Coluccia, M. Cyclooxygenase-1 (COX-1) and COX-1 Inhibitors in Cancer: A Review of Oncology and Medicinal Chemistry Literature. Pharmaceuticals 2018, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.A.; Tejada, L.V.; Tong, B.J.; Das, S.K.; Morrow, J.D.; Dey, S.K.; DuBois, R.N. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003, 63, 906–911. [Google Scholar]
- Perrone, M.G.; Luisi, O.; De Grassi, A.; Ferorelli, S.; Cormio, G.; Scilimati, A. Translational Theragnosis of Ovarian Cancer: Where do we stand? Curr. Med. Chem. 2020, 27, 5675–5715. [Google Scholar] [CrossRef]
- Wilson, A.J.; Fadare, O.; Beeghly-Fadiel, A.; Son, D.S.; Liu, Q.; Zhao, S.; Saskowski, J.; Uddin, M.J.; Daniel, C.; Crews, B.; et al. Aberrant over-expression of COX-1 intersects multiple pro-tumorigenic pathways in high-grade serous ovarian cancer. Oncotarget 2015, 25, 21353–21368. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Bueno, B.; Serrats, J.; Sawchenko, P.E. Cerebrovascular cyclooxygenase-1, expression, regulation, and role in hypothalamic-pituitary-adrenal axis activation by inflammatory stimuli. J. Neurosci. 2009, 29, 12970–12981. [Google Scholar] [CrossRef]
- Yermakova, A.V.; Rollins, J.; Callahan, L.M.; Rogers, J.; O’Banion, M.K. Cyclooxygenase-1 in human Alzheimer and control brain: Quantitative analysis of expression by microglia and CA3 hippocampal neurons. J. Neuropathol. Exp. Neurol. 1999, 58, 1135–1146. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar] [CrossRef]
- Choi, S.H.; Bosetti, F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Aid, S.; Silva, A.C.; Candelario-Jalil, E.; Choi, S.H.; Rosenberg, G.A.; Bosetti, F. Cyclooxygenase-1 and -2 differentially modulate lipopolysaccharide-induced blood-brain barrier disruption through matrix metalloproteinase activity. J. Cereb. Blood Flow Metab. 2010, 30, 370–380. [Google Scholar] [CrossRef]
- Daikoku, T.; Tranguch, S.; Trofimova, I.N.; Dinulescu, D.M.; Jacks, T.; Nikitin, A.Y.; Connolly, D.C.; Dey, S.K. Cyclooxygenase-1 is overexpressed in multiple genetically engineered mouse models of epithelial ovarian cancer. Cancer Res. 2006, 66, 2527–2531. [Google Scholar] [CrossRef] [Green Version]
- Kirschenbaum, A.; Klausner, A.P.; Le, P.; Unger, P.; Yao, S.; Liu, X.-H.; Levine, A.C. Expression of cyclooxygenase-1 and cyclooxygenase- 2 in the human prostate. Urology 2000, 56, 671–676. [Google Scholar] [CrossRef]
- Hwang, D.; Scollard, D.; Byrne, J.; Levine, E. Expression of cyclooxygenase- 1 and cyclooxygenase-2 in human breast cancer. J. Natl. Cancer Inst. 1998, 90, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.J.; Katz, A.A.; Howard, B.; Soeters, R.P.; Millar, R.P.; Jabbour, H.N. Cyclooxygenase-1 is up-regulated in cervical carcinomas: Autocrine/paracrine regulation of cyclooxygenase-2, prostaglandin E receptors, and angiogenic factors by cyclooxygenase-1. Cancer Res. 2002, 62, 424–432. [Google Scholar] [PubMed]
- Vitale, P.; Perrone, M.G.; Malerba, P.; Lavecchia, A.; Scilimati, A. Selective COX-1 inhibition as a target of theranostic novel diarylisoxazoles. Eur. J. Med. Chem. 2014, 74, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Scilimati, A.; Ferorelli, S.; Iaselli, M.C.; Miciaccia, M.; Pati, M.L.; Fortuna, C.G.; Aleem, A.M.; Marnett, L.J.; Perrone, M.G. Targeting COX-1 by mofezolac-based fluorescent probes for ovarian cancer detection. Eur. J. Med. Chem. 2019, 179, 16–25. [Google Scholar] [CrossRef]
- Malerba, P.; Crews, B.C.; Ghebreselasie, K.; Daniel, C.K.; Jashim, E.; Aleem, A.M.; Salam, R.A.; Marnett, L.J.; Uddin, M.J. Targeted Detection of Cyclooxygenase-1 in Ovarian Cancer. ACS Med. Chem. Lett. 2020, 11, 1837–1842. [Google Scholar] [CrossRef]
- Di Nunno, L.; Vitale, P.; Scilimati, A.; Tacconelli, S.; Patrignani, P. Novel synthesis of 3,4-diarylisoxazole analogues of valdecoxib: Reversal cyclooxygenase-2 selectivity by sulfonamide group removal. J. Med. Chem. 2004, 47, 4881–4890. [Google Scholar] [CrossRef]
- Kaur, J.; Bhardwaj, A.; Wuest, F. Development of Fluorescence Imaging Probes for Labeling COX-1 in Live Ovarian Cancer Cells. ACS Med. Chem. Lett. 2021, 12, 798–804. [Google Scholar]
- Shukuri, M.; Takashima-Hirano, M.; Tokuda, K.; Takashima, T.; Matsumura, K.; Inoue, O.; Doi, H.; Suzuki, M.; Watanabe, Y.; Onoe, H. In vivo expression of cyclooxygenase-1 in activated microglia and macrophages during neuroinflammation visualized by PET with 11C-ketoprofen methyl ester. J. Nucl. Med. 2011, 52, 1094–1101. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Shrestha, S.; Cortes-Salva, M.Y.; Jenko, K.J.; Zoghbi, S.S.; Morse, C.L.; Innis, R.B.; Pike, V.W. 3-Substituted 1,5-Diaryl-1H-1,2,4-triazoles as Prospective PET Radioligands for Imaging Brain COX-1 in Monkey. Part 2: Selection and Evaluation of [11C]PS13 for Quantitative Imaging. ACS Chem. Neurosci. 2018, 9, 2620–2627. [Google Scholar] [CrossRef]
- Imanishi, J.; Morita, Y.; Yoshimi, E.; Kuroda, K.; Masunaga, T.; Yamagami, K.; Kuno, M.; Hamachi, E.; Aoki, S.; Takahashi, F.; et al. Pharmacological profile of FK881 (ASP6537), a novel potent and selective cyclooxygenase-1 inhibitor. Biochem. Pharmacol. 2011, 82, 746–754. [Google Scholar] [CrossRef]
- Uddin, M.J.; Wilson, A.J.; Crews, B.C.; Malerba, P.; Uddin, M.I.; Kingsley, P.J.; Ghebreselasie, K.; Daniel, C.K.; Nickels, M.L.; Tantawy, M.N.; et al. Discovery of Furanone-Based Radiopharmaceuticals for Diagnostic Targeting of COX-1 in Ovarian Cancer. ACS Omega 2019, 4, 9251–9261. [Google Scholar] [CrossRef] [Green Version]
- Seibert, K.; Zhang, Y.; Leahy, K.; Hauser, S.; Masferrer, J.; Perkins, W.; Lee, L.; Isakson, P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA 1994, 91, 12013. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Fan, X.M. Role of cyclooxygenase-2 in gastric cancer development and progression. World J. Gastroenterol. 2013, 19, 7361–7368. [Google Scholar] [CrossRef]
- Cathcart, M.C.; O’Byrne, K.J.; Reynolds, J.V.; O’Sullivan, J.; Pidgeon, G.P. COX-derived prostanoid pathways in gastrointestinal cancer development and progression: Novel targets for prevention and intervention. Biochim. Biophys. Acta 2012, 1825, 49–63. [Google Scholar] [CrossRef]
- Glover, J.A.; Hughes, C.M.; Cantwell, M.M.; Murray, L.J. A systematic review to establish the frequency of cyclooxygenase-2 expression in normal breast epithelium, ductal carcinoma in situ, microinvasive carcinoma of the breast and invasive breast cancer. Br. J. Cancer 2011, 105, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Parida, S.; Mandal, M. Inflammation induced by human papillomavirus in cervical cancer and its implication in prevention. Eur. J. Cancer Prev. 2014, 23, 432–448. [Google Scholar] [CrossRef]
- Kaminska, K.; Szczylik, C.; Lian, F.; Czarnecka, A.M. The role of prostaglandin E2 in renal cell cancer development: Future implications for prognosis and therapy. Future Oncol. 2014, 10, 2177–2187. [Google Scholar] [CrossRef]
- Gakis, G. The role of inflammation in bladder cancer. Adv. Exp. Med. Biol. 2014, 816, 183–196. [Google Scholar]
- Elmets, C.A.; Ledet, J.J.; Athar, M. Cyclooxygenases: Mediators of UV-induced skin cancer and potential targets for prevention. J. Investig. Dermatol. 2014, 134, 2497–2502. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.A.; Carvalho, J.F.; van der Waal, I. An overview on the expression of cyclooxygenase-2 in tumors of the head and neck. Oral Oncol. 2009, 45, e124–e128. [Google Scholar] [CrossRef]
- Ramon, S.; Woeller, C.F.; Phipps, R.P. The influence of Cox-2 and bioactive lipids on hematological cancers. Curr. Angiogenes. 2013, 2, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Hoozemans, J.J.M.; Rozemuller, J.M.; van Haastert, E.S.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase-1 and-2 in the different stages of Alzheimer’s disease pathology. Curr. Pharm. Des. 2008, 14, 1419–1427. [Google Scholar] [CrossRef]
- Bartels, A.L.; Leenders, K.L. Cyclooxygenase and Neuroinflammation in Parkinson’s Disease Neurodegeneration. Curr. Neuropharmacol. 2010, 8, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Ho, L.; Purohit, D.; Haroutunian, V.; Luterman, J.D.; Willis, F.; Naslund, J.; Buxbaum, J.D.; Mohs, R.C.; Aisen, P.S.; Pasinetti, G.M. Neuronal cyclooxygenase 2 expression in the hippocampal formation as a function of the clinical progression of Alzheimer disease. Arch. Neurol. 2001, 58, 487–492. [Google Scholar] [CrossRef]
- Minghetti, L. Cyclooxygenase-2 in inflammatory and Degenerative Brain Diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Crews, B.C.; Blobaum, A.L.; Kingsley, P.J.; Gorden, D.L.; McIntyre, J.O.; Matrisian, L.; Subbaramaiah, M.K.; Dannenberg, A.J.; Piston, D.W.; et al. Selective Visualization of Cyclooxygenase-2 in Inflammation and Cancer by Targeted Fluorescent Imaging Agents. Cancer Res. 2010, 70, 3618–3627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Fan, J.; Wang, J.; Dou, B.; Zhou, F.; Cao, J.; Qu, J.; Cao, Z.; Zhao, W.; Peng, X. Fluorescence Discrimination of Cancer from Inflammation by Molecular Response to COX-2 Enzymes. J. Am. Chem. Soc. 2013, 135, 17469–17475. [Google Scholar] [CrossRef]
- Gurram, B.; Zhang, S.; Li, M.; Li, H.; Xie, Y.; Cui, H.; Du, J.; Fan, J.; Wang, J.; Peng, X. Celecoxib conjugated fluorescent probe for identification and discrimination of cyclooxygenase-2 enzyme in cancer cells. Anal. Chem. 2018, 90, 5187–5193. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kaur, J.; Wuest, F.; Knaus, E.E. Fluorophore-labeled cyclooxygenase-2 inhibitors for the imaging of cyclooxygenase-2 overexpression in cancer: Synthesis and biological studies. ChemMedChem 2014, 9, 109–240. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Bhardwaj, A.; Wuest, F. In Cellulo Generation of Fluorescent Probes for Live-Cell Imaging of Cylooxygenase-2. Chemistry 2021, 27, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 Inhibitors for Non-Invasive Visualization of COX-2 Expression and Activity—A Critical Update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prabhakaran, J.; Molotkov, A.; Mintz, A.; Mann, J.J. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules 2021, 26, 3208. [Google Scholar] [CrossRef]
- Dagallier, C.; Avry, F.; Touchefeu, Y.; Buron, F.; Routier, S.; Chérel, M.; Arlicot, N. Development of PET Radioligands Targeting COX-2 for Colorectal Cancer Staging, a Review of in vitro and Preclinical Imaging Studies. Front. Med. 2021, 8, 675209. [Google Scholar] [CrossRef]
- Tietz, O.; Marshall, A.; Wuest, M.; Wang, M.; Wuest, F. Radiotracers for molecular imaging of cyclooxygenase-2 (COX-2) enzyme. Curr. Med. Chem. 2013, 20, 4350–4369. [Google Scholar] [CrossRef]
- Baecker, D.; Obermoser, V.; Kirchner, E.A.; Hupfauf, A.; Kircher, B.; Gust, R. Fluorination as tool to improve bioanalytical sensitivity and COX-2-selective antitumor activity of cobalt alkyne complexes. Dalton Trans. 2019, 48, 15856–15868. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tago, T.; Toyohara, J.; Saito, Y.; Yamamoto, F. Radiosynthesis and in Vivo and ex Vivo Evaluation of Isomeric [11C]methoxy Analogs of Nimesulide as Brain Cyclooxygenase-2-Targeted Imaging Agents. Biol. Pharm. Bull. 2022, 45, 94–103. [Google Scholar] [CrossRef]
- Carpinelli, A.; Rainone, P.; Belloli, S.; Reale, A.; Cappelli, A.; Germano, G.; Murtaj, V.; Coliva, A.; Di Grigoli, G.; Valeri, A.; et al. Radiosynthesis and Preclinical Evaluation of 11 C-VA426, a Cyclooxygenase-2 Selective Ligand. Contrast Media Mol. Imaging 2019, 2019, 5823261. [Google Scholar] [CrossRef] [Green Version]
- Prabhakaran, J.; Underwood, M.; Zanderigo, F.; Simpson, N.R.; Cooper, A.R.; Matthew, J.; Rubin-Falcone, H.; Parsey, R.V.; Mann, J.J.; Dileep Kumar, J.S. Radiosynthesis and in vivo evaluation of [11C]MOV as a PET imaging agent for COX-2. Bioorg. Med. Chem. Lett. 2018, 28, 2432–2435. [Google Scholar] [CrossRef]
- Taddei, C.; Cheryl, L.; Morse, C.L.; Kim, M.-J.; Liow, J.-S.; Santamaria, J.M.; Zhang, A.; Manly, L.S.; Zanotti-Fregonara, P.; Gladding, R.L.; et al. Synthesis of [18F]PS13 and Evaluation as a PET Radioligand for Cyclooxygenase-1 in Monkey. ACS Chem. Neurosci. 2021, 12, 517–530. [Google Scholar] [CrossRef]
- Kim, M.-J.; Shrestha, S.S.; Cortes, M.; Singh, P.; Morse, C.; Liow, J.-S.; Gladding, R.L.; Brouwer, C.; Henry, K.; Gallagher, E.; et al. Evaluation of two potent and selective PET radioligands to image COX-1 and COX-2 in rhesus monkeys. J. Nucl. Med. 2018, 59, 1907–1912. [Google Scholar] [CrossRef]
- Kumar, J.; Zanderigo, F.; Prabhakaran, J.; Rubin-Falcone, H.; Parsey, R.V.; Mann, J.J. In vivo evaluation of [11C]TMI, a COX-2 selective PET tracer, in baboons. Bioorg. Med. Chem. Lett. 2018, 28, 3592–3595. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.S.; Yu, Z.X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET measurement of cyclooxygenase-2 using a novel radioligand: Upregulation in primate neuroinflammation and first-in-human study. J. Neuroinflammation 2020, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, J.H.; Juarez Anaya, F.; Hong, J.; Miller, W.; Telu, S.; Singh, P.; Cortes, M.Y.; Henry, K.; Tye, G.L.; et al. First-in-human evaluation of [11C]PS13, a novel PET radioligand, to quantify cyclooxygenase-1 in the brain. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 3143–3151. [Google Scholar] [CrossRef]
- Ji, B.; Kumata, K.; Onoe, H.; Kaneko, H.; Zhang, M.R.; Seki, C.; Ono, M.; Shukuri, M.; Tokunaga, M.; Minamihisamatsu, T.; et al. Assessment of radioligands for PET imaging of cyclooxygenase-2 in an ischemic neuronal injury model. Brain Res. 2013, 1533, 152–162. [Google Scholar] [CrossRef]
- Erfani, M.; Sharifzadeh, S.; Doroudi, A.; Shafiei, M. Labeling and evaluation of 99mTc-tricarbonyl-meloxicam as a preferential COX-2 inhibitor for inflammation imaging. J. Labelled. Comp. Radiopharm. 2016, 59, 284–290. [Google Scholar] [CrossRef]
- Yang, D.J.; Bryant, J.; Chang, J.Y.; Mendez, R.; Oh, C.; Yu, D.; Ito, M.; Azhdarinia, A.; Kohanim, S.; Kim, E.E.; et al. Assessment of cyclooxygense-2 expression with 99m-Tc-labeled celebrex. Anticancer Drugs 2004, 15, 255. [Google Scholar] [CrossRef]
- Tietz, O.; Dzandzi, J.; Bhardwaj, A.; Valliant, J.F.; Wuest, F. Design and synthesis of [(125)I]Pyricoxib: A novel (125)I-labeled cyclooxygenase-2 (COX-2) inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1516–1520. [Google Scholar] [CrossRef]
- Pacelli, A.; Greenman, J.; Cawthorne, C.; Smith, G. Imaging COX-2 expression in cancer using PET/SPECT radioligands: Current status and future directions. J. Labelled. Comp. Radiopharm. 2014, 57, 317–322. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Wuest, F. PET Imaging of Cyclooxygenases in Neuroinflammation. In PET and SPECT of Neurobiological Systems; Springer: Berlin/Heidelberg, Germany, 2021; pp. 265–293. [Google Scholar]
- Hu, W.; Guo, Z.; Chu, F.; Bai, A.; Yi, X.; Cheng, G.; Li, J. Synthesis and biological evaluation of substituted 2-sulfonyl-phenyl-3-phenyl-indoles: A new series of selective COX-2 inhibitors. Bioorg. Med. Chem. 2003, 11, 1153. [Google Scholar] [CrossRef]
- Kniess, T.; Laube, M.; Bergmann, F.; Sehn, F.; Graf, F.; Steinbach, J.; Wuest, F.; Pietzsch, J. Radiosynthesis of a 18F-labeled 2,3-diarylsubstituted indole via McMurry coupling for functional characterization of cyclooxygenase-2 (COX-2) in vitro and in vivo. Bioorg. Med. Chem. 2012, 20, 3410. [Google Scholar] [CrossRef] [PubMed]
- Ory, D.; Celen, S.; Verbruggen, A.; Bormans, G. PET Radioligands for In Vivo Visualization of Neuroinflammation. Curr. Pharm. Des. 2014, 20, 5897–5913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tietz, O.; Sharma, S.K.; Kaur, J.; Way, J.; Marshall, A.; Wuest, M.; Wuest, F. Synthesis of three 18F-labelled cyclooxygenase-2 (COX-2) inhibitors based on a pyrimidine scaffold. Org. Biomol. Chem. 2013, 11, 8052–8064. [Google Scholar] [CrossRef]
- Tietz, O.; Wuest, M.; Marshall, A.; Glubrecht, D.; Hamann, I.; Wang, M.; Bergman, C.; Way, J.D.; Wuest, F. PET imaging of cyclooxygenase-2 (COX-2) in a pre-clinical colorectal cancer model. EJNMMI Res. 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Tietz, O.; Marshall, A.; Bergman, C.; Wuest, M.; Wuest, F. Impact of structural alterations on the radiopharmacological profile of 18F-labeled pyrimidines as cyclooxygenase-2 (COX-2) imaging agents. Nucl. Med. Biol. 2018, 62, 9–17. [Google Scholar] [CrossRef]
- Laube, M.; Gassner, C.; Sharma, S.K.; Gunther, R.; Pigorsch, A.; Konig, J.; Kockerling, M.; Wuest, F.; Pietzsch, J.; Kniess, T. Diaryl-Substituted (Dihydro)pyrrolo[3,2,1-hi]indoles, a Class of Potent COX-2 Inhibitors with Tricyclic Core Structure. J. Org. Chem. 2015, 80, 5611–5624. [Google Scholar] [CrossRef]
- Perrone, M.G.; Malerba, P.; Uddin, J.; Vitale, P.; Panella, A.; Crews, B.C.; Daniel, C.K.; Ghebreselasie, K.; Nickels, M.; Tantawy, M.N.; et al. PET radiotracer [¹⁸F]-P6 selectively targeting COX-1 as a novel biomarker in ovarian cancer: Preliminary investigation. Eur. J. Med. Chem. 2014, 80, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Elie, J.; Vercouillie, J.; Arlicot, N.; Lemaire, L.; Bidault, R.; Bodard, S.; Hosselet, C.; Deloye, J.-B.; Chalon, S.; Emond, P. Design of selective COX-2 inhibitors in the (aza)indazole series. Chemistry, in vitro studies, radiochemistry and evaluations in rats of a [18F] PET tracer. J. Enzyme Inhib. Med. Chem. 2019, 34, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Tietz, O.; Bhardwaj, A.; Marshall, A.; Way, J.; Wuest, M.; Wuest, F. Design, Synthesis, and Evaluation of an (18)F-Labeled Radiotracer Based on Celecoxib-NBD for Positron Emission Tomography (PET) Imaging of Cyclooxygenase-2 (COX-2). ChemMedChem 2015, 10, 1635–1640. [Google Scholar] [CrossRef]
- Gassner, C.; Neuber, C.; Laube, M.; Bergmann, R.; Kniess, T.; Pietzsch, J. Development of a 18F-labeled Diaryl-Substituted Dihydropyrrolo[3,2,1- hi ]indole as Potential Probe for Functional Imaging of Cyclooxygenase-2 with PET. Chem. Sel. 2016, 1, 5812–5820. [Google Scholar]
- Chansaenpak, K.; Wang, M.; Liu, S.; Wu, Z.; Yuan, H.; Conti, P.S.; Li, Z.; Gabba, F.P. Synthesis and in vivo stability studies of [18F]- zwitterionic phosphonium aryltrifluoroborate/ indomethacin conjugates. RSC Adv. 2016, 6, 23126–23133. [Google Scholar] [CrossRef]
- Lebedev, A.; Jiao, J.; Lee, J.; Yang, F.; Allison, N.; Herschman, H.; Sadeghi, S. Radiochemistry on electrodes: Synthesis of an 18F-labelled and in vivo stable COX-2 inhibitor. PLoS ONE 2017, 12, e0176606. [Google Scholar]
- Yeh, C.N.; Chang, C.W.; Chung, Y.H.; Tien, S.W.; Chen, Y.R.; Chen, T.W.; Huang, Y.C.; Wang, H.E.; Chou, Y.C.; Chen, M.H.; et al. Synthesis and characterization of boron fenbufen and its F-18 labeled homolog for boron neutron capture therapy of COX-2 overexpressed cholangiocarcinoma. Eur. J. Pharm. Sci. 2017, 107, 217–229. [Google Scholar] [CrossRef]
- Cortes-Salva, M.Y.; Shrestha, S.; Singh, P.; Morse, C.L.; Jenko, K.J.; Montero Santamaria, J.A.; Zoghbi, S.S.; Innis, R.B.; Pike, V.W. 2-(4-Methylsulfonylphenyl)pyrimidines as Prospective Radioligands for Imaging Cyclooxygenase-2 with PET-Synthesis, Triage, and Radiolabeling. Molecules 2018, 23, 2850. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Yeh, C.N.; Chung, Y.H.; Chen, Y.R.; Tien, S.W.; Chen, T.W.; Farn, S.S.; Huang, Y.C.; Yu, C.S. Synthesis and evaluation of ortho-[18F] fluorocelecoxib for COX-2 cholangiocarcinoma imaging. Drug Des. Dev. Ther. 2018, 12, 1467–1478. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Prabhakaran, J.; Molotkov, A.; Sattiraju, A.; Kim, J.; Doubrovin, M.; Mann, J.J.; Mintz, A. Radiosynthesis and evaluation of [18F]FMTP, a COX-2 PET ligand. Pharmacol. Rep. 2020, 72, 1433–1440. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Kaur, J.; Wuest, M.; Wuest, F. In situ click chemistry generation of cyclooxygenase-2 inhibitors. Atul Bhardwaj. Nat. Commun. 2017, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Litchfield, M.; Wuest, M.; Glubrecht, D.; Wuest, F. Radiosynthesis and Biological Evaluation of [18F]Triacoxib: A New Radiotracer for PET Imaging of COX-2. Mol. Pharm. 2020, 17, 251–261. [Google Scholar] [CrossRef]
- Laube, M.; Gassner, C.; Neuber, C.; Wodtke, R.; Ullrich, M.; Haase-Kohn, C.; Löser, R.; Köckerling, M.; Kopka, K.; Kniess, T.; et al. Deuteration versus ethylation—Strategies to improve the metabolic fate of an 18F-labeled celecoxib derivative. RSC Adv. 2020, 10, 38601–38611. [Google Scholar] [CrossRef]
- Vaulina, D.D.; Stosman, K.I.; Sivak, K.V.; Aleksandrov, A.G.; Viktorov, N.B.; Kuzmich, N.N.; Kiseleva, M.M.; Kuznetsova, O.F.; Gomzina, N.A. Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4’-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging. Molecules 2021, 26, 6630. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).