Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis
Abstract
:1. Introduction
2. Results
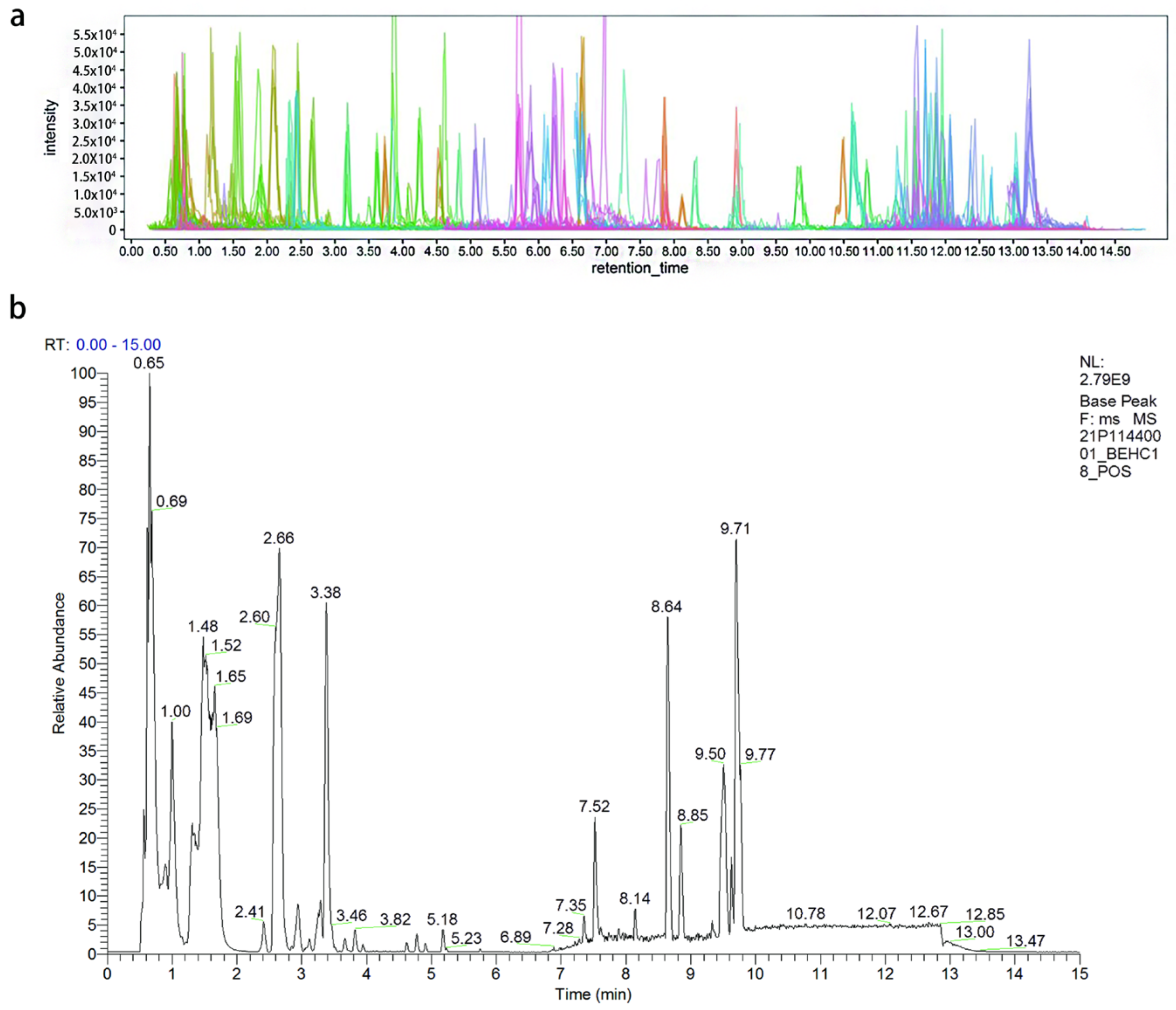
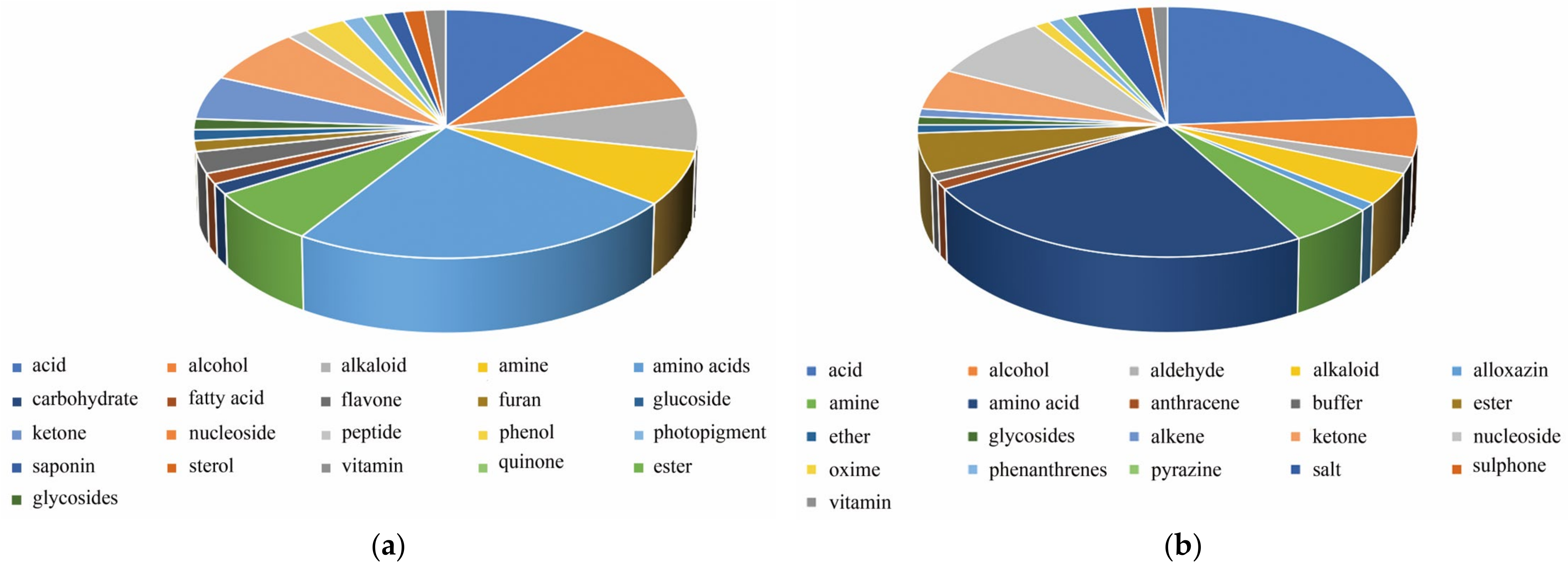
2.1. Metabolite Identification
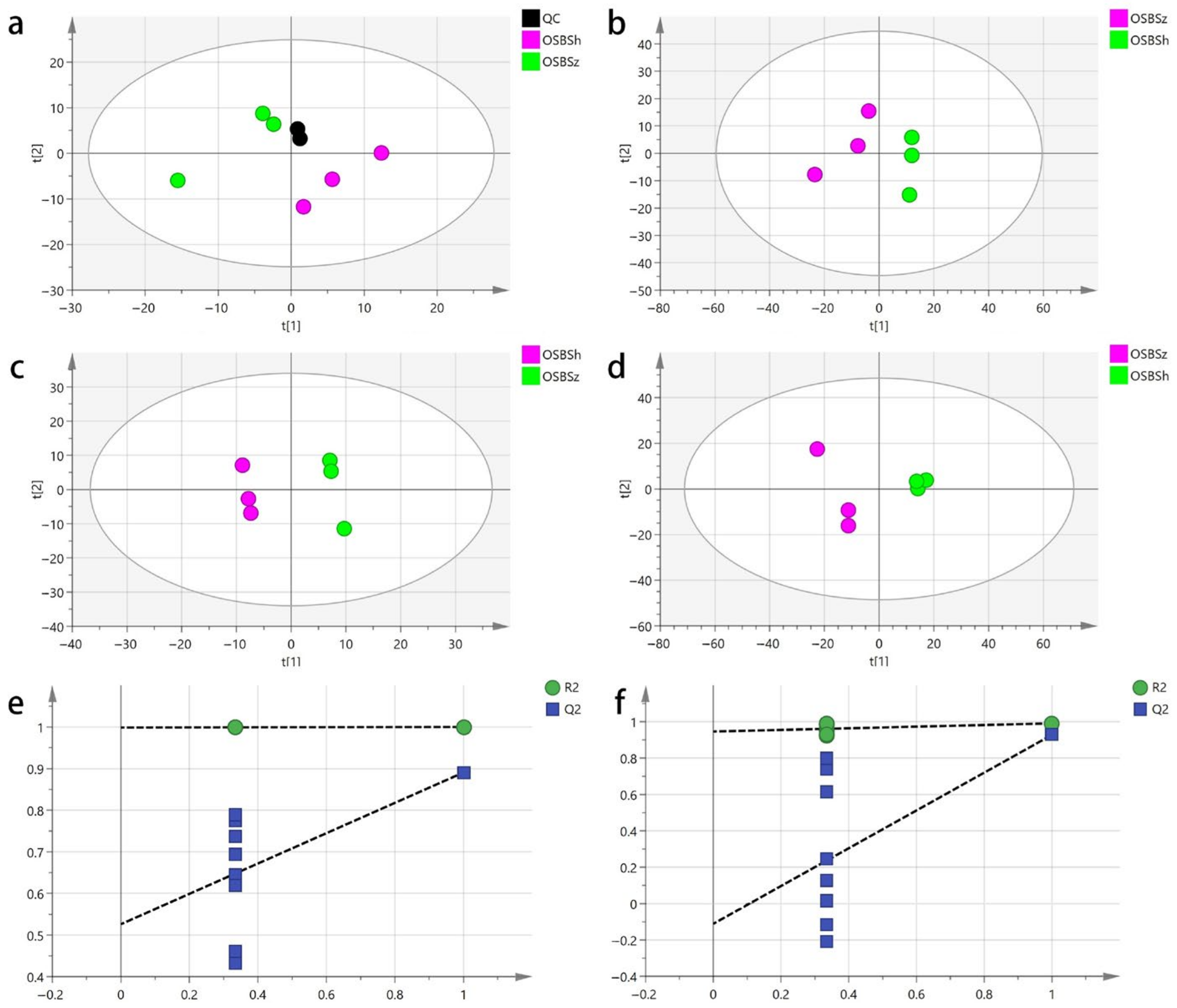
2.2. Principal Component Analysis
2.3. Partial Least Squares Discriminant Analysis
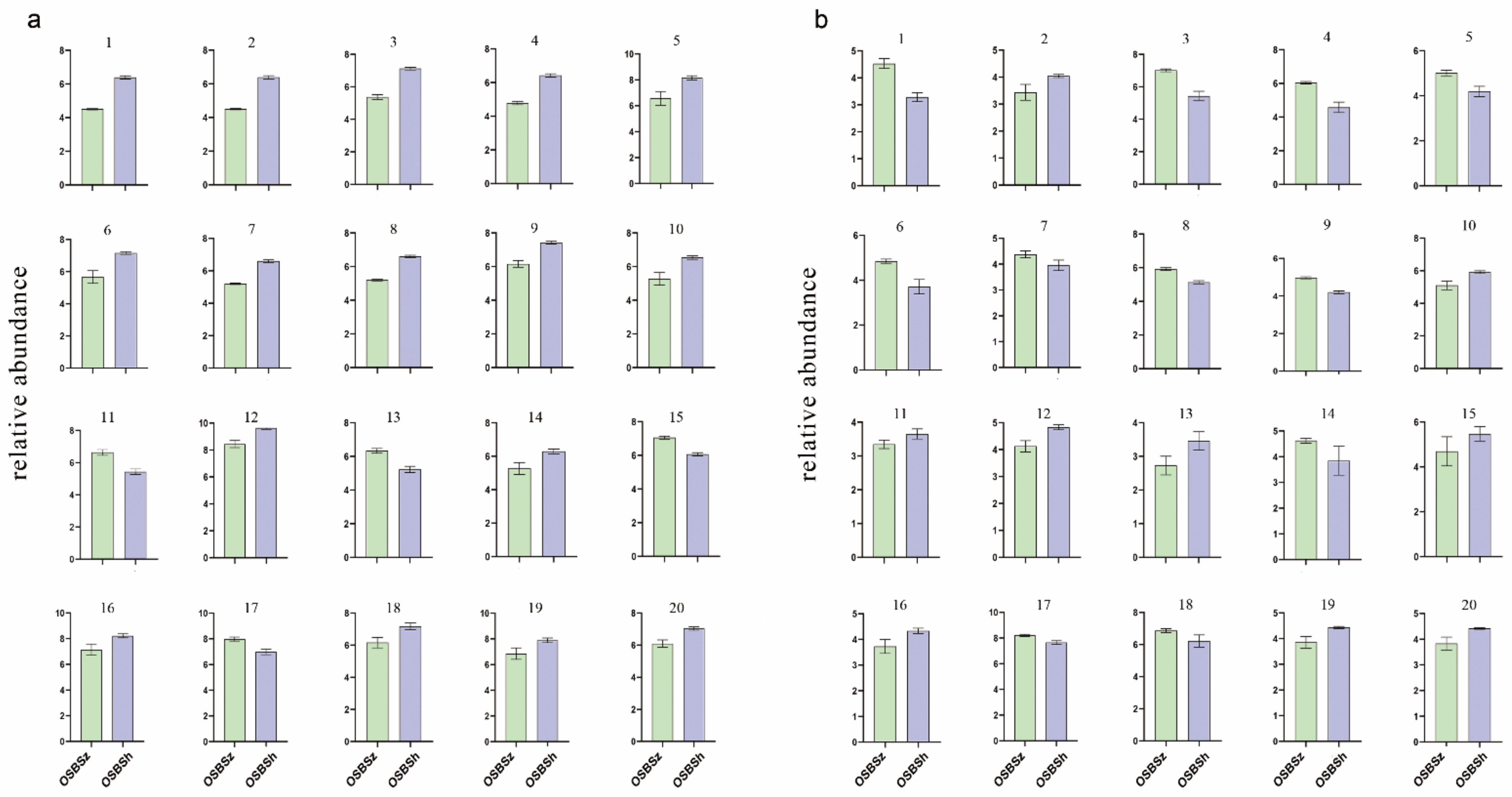
2.4. Screening of Differentially Expressed Metabolites
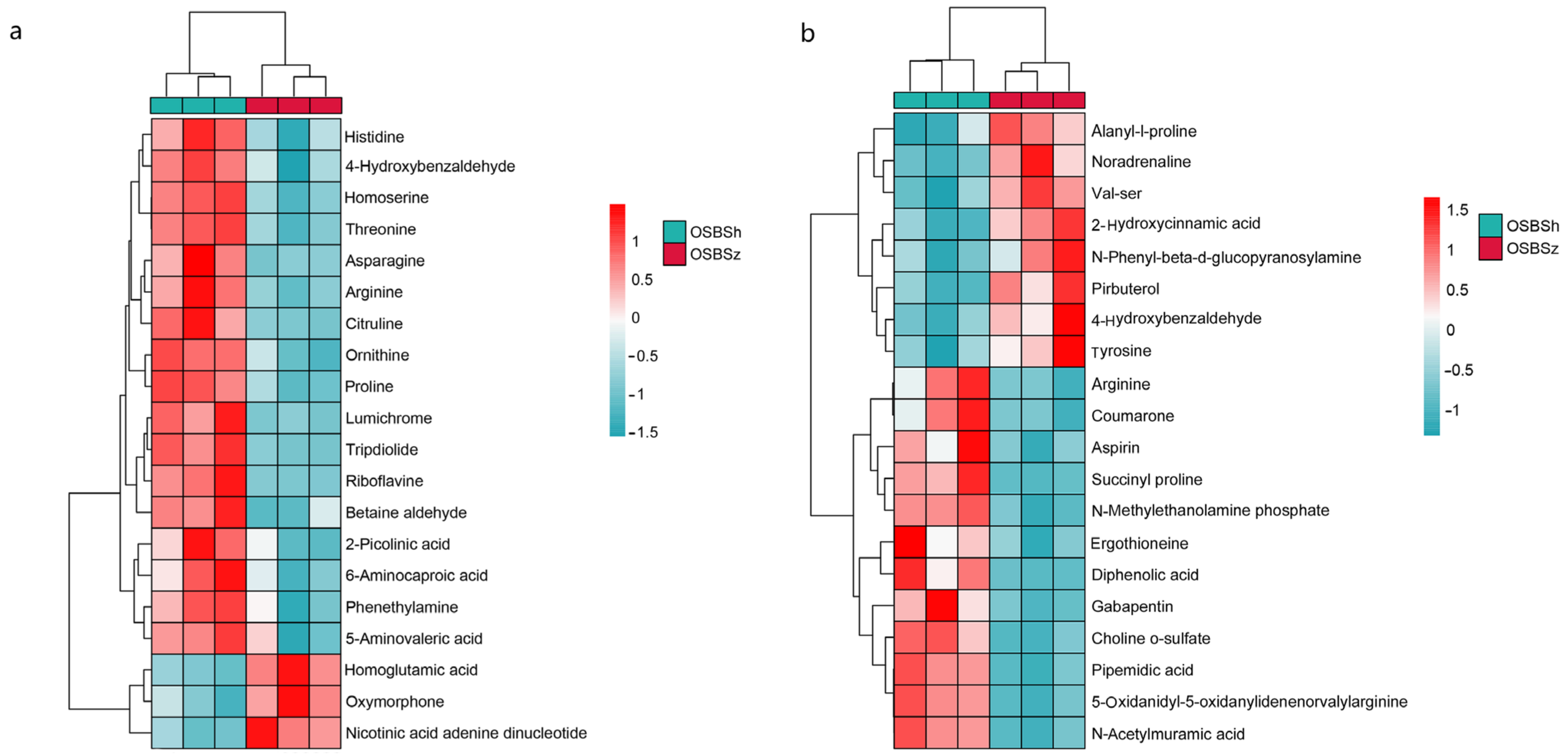
2.5. Hierarchical Cluster Analysis
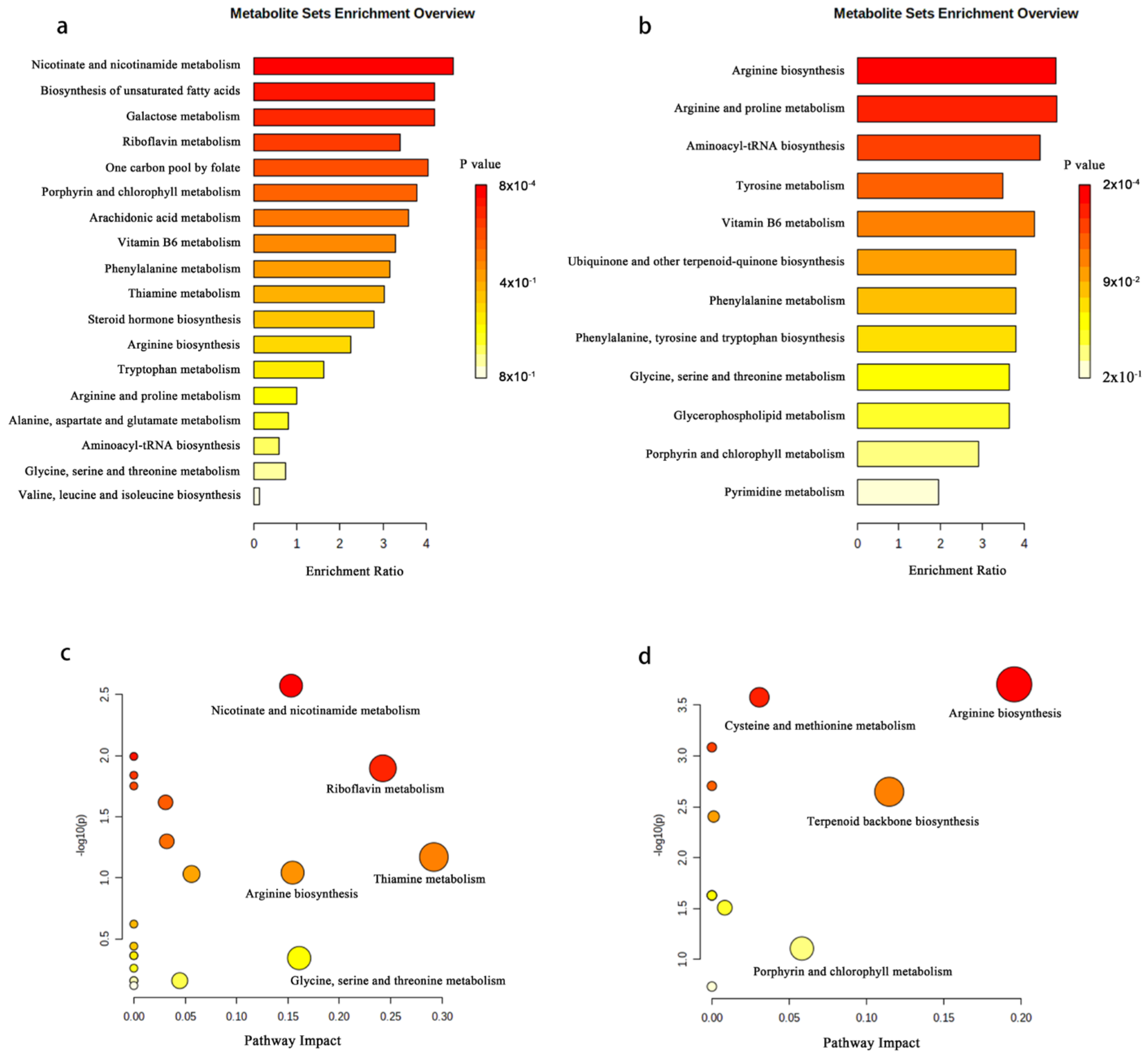
2.6. Analysis of Differentially Expressed Metabolite Pathways and Enrichment Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Metabolite Extraction
4.3. UHPLC-MS Conditions
4.4. Data Processing and Statistical Analysis
4.5. Metabolite Identification and Analysis of Differentially Expressed Metabolites
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sung, G.H.; Hywel-Jones, N.L.; Sung, J.M.; Luangsa-Ard, J.J.; Shrestha, B.; Spatafora, J.W. Phylogenetic Classification of Cordyceps and the Clavicipitaceous Fungi. Stud. Mycol. 2007, 57, 5–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.I.; Hsu, J.H.; Li, T.J.; Yeh, S.H.; Chen, C.C. Safety Assessment of Hea-Enriched Cordyceps Cicadae Mycelia on the Central Nervous System (Cns), Cardiovascular System, and Respiratory System in Icr Male Mice. Food Sci. Nutr. 2021, 9, 4905–4915. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.H.; Yap, C.A.; Razif, M.F.M.; Ng, S.T.; Tan, C.S.; Fung, S.Y. Antioxidant and Cytotoxic Effects and Identification of Ophiocordyceps Sinensis Bioactive Proteins Using Shotgun Proteomic Analysis. Food Technol. Biotechnol. 2021, 59, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Hsu, T.H.; Lee, C.H.; Lo, H.C. Fruiting Bodies of Chinese Caterpillar Mushroom, Ophiocordyceps Sinensis (Ascomycetes) Alleviate Diabetes-Associated Oxidative Stress. Int. J. Med. Mushrooms 2020, 22, 15–29. [Google Scholar] [CrossRef]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps Sinensis Polysaccharides Modulate Intestinal Mucosal Immunity and Gut Microbiota in Cyclophosphamide-Treated Mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar]
- Peng, Y.; Huang, K.; Shen, L.; Tao, Y.Y.; Liu, C.H. Cultured Mycelium Cordyceps Sinensis Allevi¬Ates Ccl4-Induced Liver Inflammation and Fibrosis in Mice by Activating Hepatic Natural Killer Cells. Acta Pharmacol. Sin. 2016, 37, 204–216. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Tao, T.; Zhang, J.; Su, A.; Zhao, L.; Chen, H.; Hu, Q. Comparison of Effects on Colitis-Associated Tumorigenesis and Gut Microbiota in Mice between Ophiocordyceps Sinensis and Cordyceps Militaris. Phytomedicine 2021, 90, 153653. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.F.; Zhang, Z.M.; Yao, H.Y.; Guan, Y.; Zhu, J.P.; Zhang, L.H.; Jia, Y.L.; Wang, R.W. Cardiovascular Protection and Antioxidant Activity of the Extracts from the Mycelia of Cordyceps Sinensis Act Partially Via Adenosine Receptors. Phytother. Res. 2013, 27, 1597–1604. [Google Scholar] [CrossRef]
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The Genus Cordyceps: An Extensive Review of Its Traditional Uses, Phytochemistry and Pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced Development in Chemical Analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef]
- Yang, X.Y.; Luo, X.; Lei, L. Analysis and Comparison of Adenosine Content in Different Parts of Cordyceps Sinensis. Straits Pharm. 2021, 33, 48–50. [Google Scholar]
- Xia, M.C.; Cai, L.; Xu, F.; Zhan, Q.; Feng, J.; Guo, C.; Li, Q.; Li, Z. Whole-Body Chemical Imaging of Cordyceps Sinensis by Tof-Sims to Visualize Spatial Differentiation of Ergosterol and Other Active Components. Soc. Sci. Electron. Publ. 2022, 177, 107303. [Google Scholar] [CrossRef]
- Li, R.F.; Zhou, X.B.; Zhou, H.X.; Yang, Z.F.; Jiang, H.M.; Wu, X.; Li, W.J.; Qiu, J.J.; Mi, J.N.; Chen, M.; et al. Novel Fatty Acid in Cordyceps Suppresses Influenza a (H1n1) Virus-Induced Proinflammatory Response through Regulating Innate Signaling Pathways. ACS Omega 2021, 6, 1505–1515. [Google Scholar] [CrossRef]
- Wu, D.T.; Cheong, K.L.; Wang, L.Y.; Lv, G.P.; Ju, Y.J.; Feng, K.; Zhao, J.; Li, S.P. Characterization and Discrimination of Polysaccharides from Different Species of Cordyceps Using Saccharide Mapping Based on Pace and Hptlc. Carbohydr. Polym. 2014, 103, 100–109. [Google Scholar] [CrossRef]
- Meusinger, R. Qualitative and Quantitative Determination of Oxygenates in Gasoline Using 1h Nmr Spectroscopy. Anal. Chim. Acta 1999, 391, 277–288. [Google Scholar] [CrossRef]
- Qian, Z.M.; Wu, Z.; Huang, Q.; Wang, C.X.; Tan, G.Y.; Li, W.J.; Sun, W.Y.; Lv, G.P.; Gao, H. Development of an Eco-Friendly and Fast Hplc Method for Quantitative Analysis of Four Nucleosides in Cordyceps and Related Products. Chin. J. Nat. Med. 2021, 19, 954–960. [Google Scholar] [CrossRef]
- Yang, F.Q.; Feng, K.; Zhao, J.; Li, S.P. Analysis of Sterols and Fatty Acids in Natural and Cultured Cordyceps by One-Step Derivatization Followed with Gas Chromatography-Mass Spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 1172–1178. [Google Scholar] [CrossRef]
- Gong, Y.X.; Li, S.P.; Li, P.; Liu, J.J.; Wang, Y.T. Simultaneous Determination of Six Main Nucleosides and Bases in Natural and Cultured Cordyceps by Capillary Electrophoresis. J. Chromatogr. A 2004, 1055, 215–221. [Google Scholar] [CrossRef]
- Xie, C.; Xu, N.; Shao, Y.; He, Y. Using Ft-Nir Spectroscopy Technique to Determine Arginine Content in Fermented Cordyceps Sinensis Mycelium. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 971–977. [Google Scholar] [CrossRef]
- Lu, Y.; Zhi, Y.; Miyakawa, T.; Tanokura, M. Metabolic Profiling of Natural and Cultured Cordyceps by Nmr Spectroscopy. Sci. Rep. 2019, 9, 7735. [Google Scholar] [CrossRef]
- Hu, H.; Xiao, L.; Zheng, B.; Wei, X.; Ellis, A.; Liu, Y.M. Identification of Chemical Markers in Cordyceps Sinensis by Hplc-Ms/Ms. Anal. Bioanal. Chem. 2015, 407, 8059–8066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiehn, O. Metabolic Networks of Cucurbita Maxima Phloem. Phytochemistry 2003, 62, 875–886. [Google Scholar] [CrossRef]
- Matich, E.K.; Chavez Soria, N.G.; Aga, D.S.; Atilla-Gokcumen, G.E. Applications of Metabolomics in Assessing Ecological Effects of Emerging Contaminants and Pollutants on Plants. J. Hazard Mater. 2019, 373, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farag, M.; Edrada-Ebel, R. Isolation of Anticancer and Anti-Trypanosome Secondary Metabolites from the Endophytic Fungus Aspergillus Flocculus Via Bioactivity Guided Isolation and Ms Based Metabolomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1106–1107, 71–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfender, J.L.; Marti, G.; Thomas, A.; Bertrand, S. Current Approaches and Challenges for the Metabolite Profiling of Complex Natural Extracts. J. Chrom. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Creek, D.J.; Dunn, W.B.; Fiehn, O.; Griffin, J.L.; Hall, R.D.; Lei, Z.; Mistrik, R.; Neumann, S.; Schymanski, E.L.; Sumner, L.W.; et al. Metabolite Identification: Are You Sure? And How Do Your Peers Gauge Your Confidence? Metab. Sumner 2014, 10, 350–353. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, Z.J. A High-Throughput Targeted Metabolomics Workflow for the Detection of 200 Polar Metabolites in Central Carbon Metabolism. Methods Mol. Biol. 2019, 1859, 263–1874. [Google Scholar] [PubMed]
- t’Kindt, R.; De Veylder, L.; Storme, M.; Deforce, D.; Van Bocxlaer, J. Lc-Ms Metabolic Profiling of Arabidopsis Thaliana Plant Leaves and Cell Cultures: Optimization of Pre-Lc-Ms Procedure Parameters. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 871, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Verpoorte, R. Sample Preparation for Plant Metabolomics. Phytochem. Anal. 2010, 21, 4–13. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; Bastos, M.L.; Guedes de Pinho, P. Metabolomics Analysis for Biomarker Discovery: Advances and Challenges. Curr. Med. Chem. 2013, 20, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Li, S.; Zhong, X.; Wang, H.; Liu, X. Comparative Metabolic Profiling of Ophiocordyceps Sinensis and Its Cultured Mycelia Using Gc-Ms. Food Res. Int. 2020, 134, 109241. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, K.; Wang, Z.; Wang, S.; Xu, F. Comparison of Metabolism Substances in Cordyceps Sinensis and Cordyceps Militaris Cultivated with Tussah Pupa Based on Lc-Ms. J. Food Biochem. 2021, 45, e13735. [Google Scholar] [CrossRef]
- Sato, K.; Tatsunami, R.; Wakame, K. Epalrestat Suppresses Inflammatory Response in Lipopolysaccharide-Stimulated Raw264.7 Cells. Allergol. Immunopathol. 2021, 49, 1–8. [Google Scholar] [CrossRef]
- Alvi, Z.; Akhtar, M.; Mahmood, A.; Ur-Rahman, N.; Nazir, I.; Sadaquat, H.; Ijaz, M.; Syed, S.K.; Waqas, M.K.; Wang, Y. Enhanced Oral Bioavailability of Epalrestat Sbe(7)-Β-Cd Complex Loaded Chitosan Nanoparticles: Preparation, Characterization and in-Vivo Pharmacokinetic Evaluation. Int. J. Nanomed. 2021, 16, 8353–8373. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Rinaldi, G. Riboflavin in Neurological Diseases: A Narrative Review. Clin. Drug Investig. 2021, 41, 513–527. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s Disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Wu, J.; Wu, B.; Tang, C.; Zhao, J. Analytical Techniques and Pharmacokinetics of Gastrodia Elata Blume and Its Constituents. Molecules 2017, 22, 1137. [Google Scholar] [CrossRef] [Green Version]
- Clemmensen, C.; Smajilovic, S.; Smith, E.P.; Woods, S.C.; Bräuner-Osborne, H.; Seeley, R.J.; D’Alessio, D.A.; Ryan, K.K. Oral L-Arginine Stimulates Glp-1 Secretion to Improve Glucose Tolerance in Male Mice. Endocrinology 2013, 154, 3978–3983. [Google Scholar] [CrossRef] [Green Version]
- Chantarawong, W.; Kuncharoen, N.; Tanasupawat, S.; Chanvorachote, P. Lumichrome Inhibits Human Lung Cancer Cell Growth and Induces Apoptosis Via a P53-Dependent Mechanism. Nutr. Cancer 2019, 71, 1390–1402. [Google Scholar] [CrossRef]
- Gil-Ortiz, F.; Ramón-Maiques, S.; Fernández-Murga, M.L.; Fita, I.; Rubio, V. Two Crystal Structures of Escherichia Coli N-Acetyl-L-Glutamate Kinase Demonstrate the Cycling between Open and Closed Conformations. J. Mol. Biol. 2010, 399, 476–490. [Google Scholar] [CrossRef]
- Raza, M.F.; Wang, Y.; Cai, Z.; Bai, S.; Yao, Z.; Awan, U.A.; Zhang, Z.; Zheng, W.; Zhang, H. Gut Microbiota Promotes Host Resistance to Low-Temperature Stress by Stimulating Its Arginine and Proline Metabolism Pathway in Adult Bactrocera Dorsalis. PLoS Pathog. 2020, 16, e1008441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatovskaia, K.V.; Seliverstov, A.V.; Liubetskiĭ, V.A. Attenuation Regulation of Amino Acid and Amino Acyl-Trna Biosynthetic Operons in Bacteria: Comparative Genomics Analysis. Mol. Biol. 2010, 44, 140–151. [Google Scholar]
- Shi, X.; Wang, S.; Jasbi, P.; Turner, C.; Hrovat, J.; Wei, Y.; Liu, J.; Gu, H. Database-Assisted Globally Optimized Targeted Mass Spectrometry (Dgot-Ms): Broad and Reliable Metabolomics Analysis with Enhanced Identification. Anal. Chem. 2019, 91, 13737–13745. [Google Scholar] [CrossRef]
- Zha, H.; Cai, Y.; Yin, Y.; Wang, Z.; Li, K.; Zhu, Z.J. Swathtomrm: Development of High-Coverage Targeted Metabolomics Method Using Swath Technology for Biomarker Discovery. Anal. Chem. 2018, 90, 4062–4070. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, M.H.; Gaudin, M.; Lewis, M.R.; Martin, F.P.; Holmes, E.; Nicholson, J.K.; Dumas, M.E. Objective Set of Criteria for Optimization of Sample Preparation Procedures for Ultra-High Throughput Untargeted Blood Plasma Lipid Profiling by Ultra Performance Liquid Chromatography-Mass Spectrometry. Anal. Chem. 2014, 86, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Hou, D.; Zou, W.; Wang, J.; Luo, R.; Wang, M.; Yu, H. Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis. Molecules 2022, 27, 3645. https://doi.org/10.3390/molecules27113645
Zhou J, Hou D, Zou W, Wang J, Luo R, Wang M, Yu H. Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis. Molecules. 2022; 27(11):3645. https://doi.org/10.3390/molecules27113645
Chicago/Turabian StyleZhou, Jinna, Donghai Hou, Weiqiu Zou, Jinhu Wang, Run Luo, Mu Wang, and Hong Yu. 2022. "Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis" Molecules 27, no. 11: 3645. https://doi.org/10.3390/molecules27113645
APA StyleZhou, J., Hou, D., Zou, W., Wang, J., Luo, R., Wang, M., & Yu, H. (2022). Comparison of Widely Targeted Metabolomics and Untargeted Metabolomics of Wild Ophiocordyceps sinensis. Molecules, 27(11), 3645. https://doi.org/10.3390/molecules27113645






