Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis
Abstract
1. Introduction
2. Results
2.1. Chemical Composition of G18.EE and Its Fractions
2.2. G18.EE Displays Radical Scavenging Activity
2.3. Antitumoral Activity of G18.EE and Its Fractions on Melanoma Cells
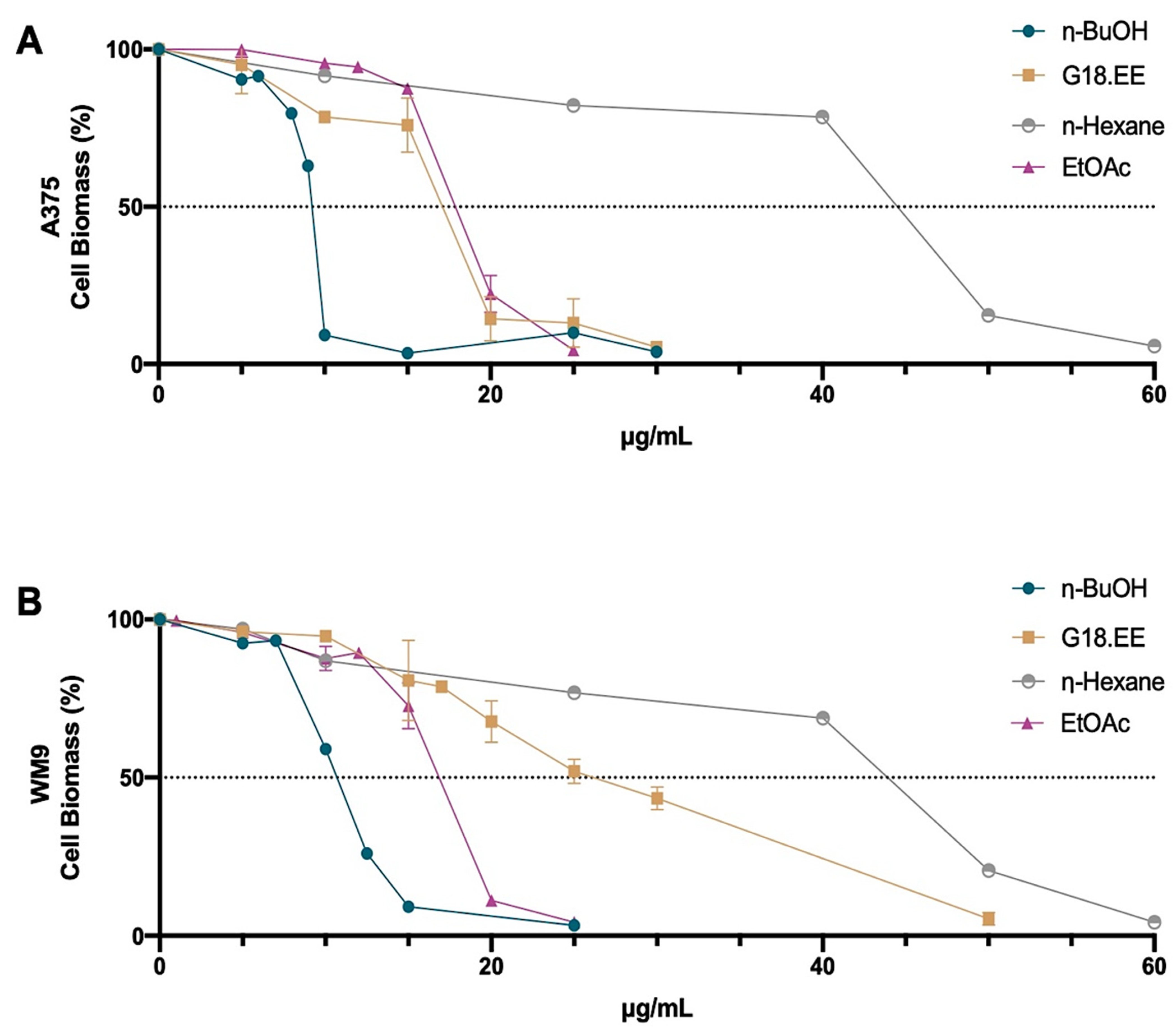
2.3.1. G18.EE and Its Fractions Decreased Melanoma Cell Viability in a Dose-Dependent Manner
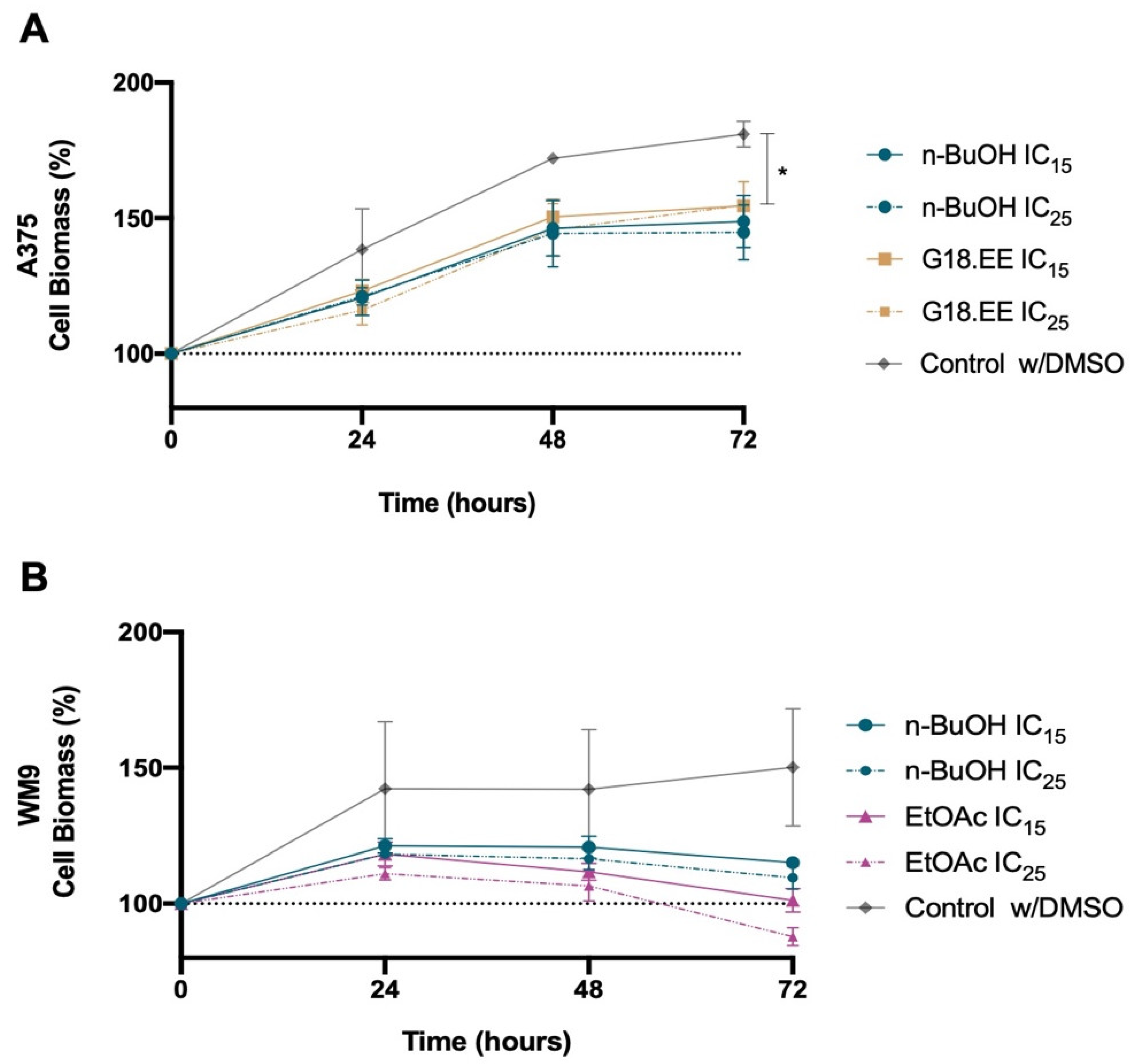
2.3.2. Melanoma Cell Viability for IC15 and IC25 Intermediate Concentrations of G18.EE and Its Fractions
2.3.3. G18.EE and Its Fractions (n-BuOH and EtOAc) Promote Mitochondrial ROS Production in Melanoma Cells
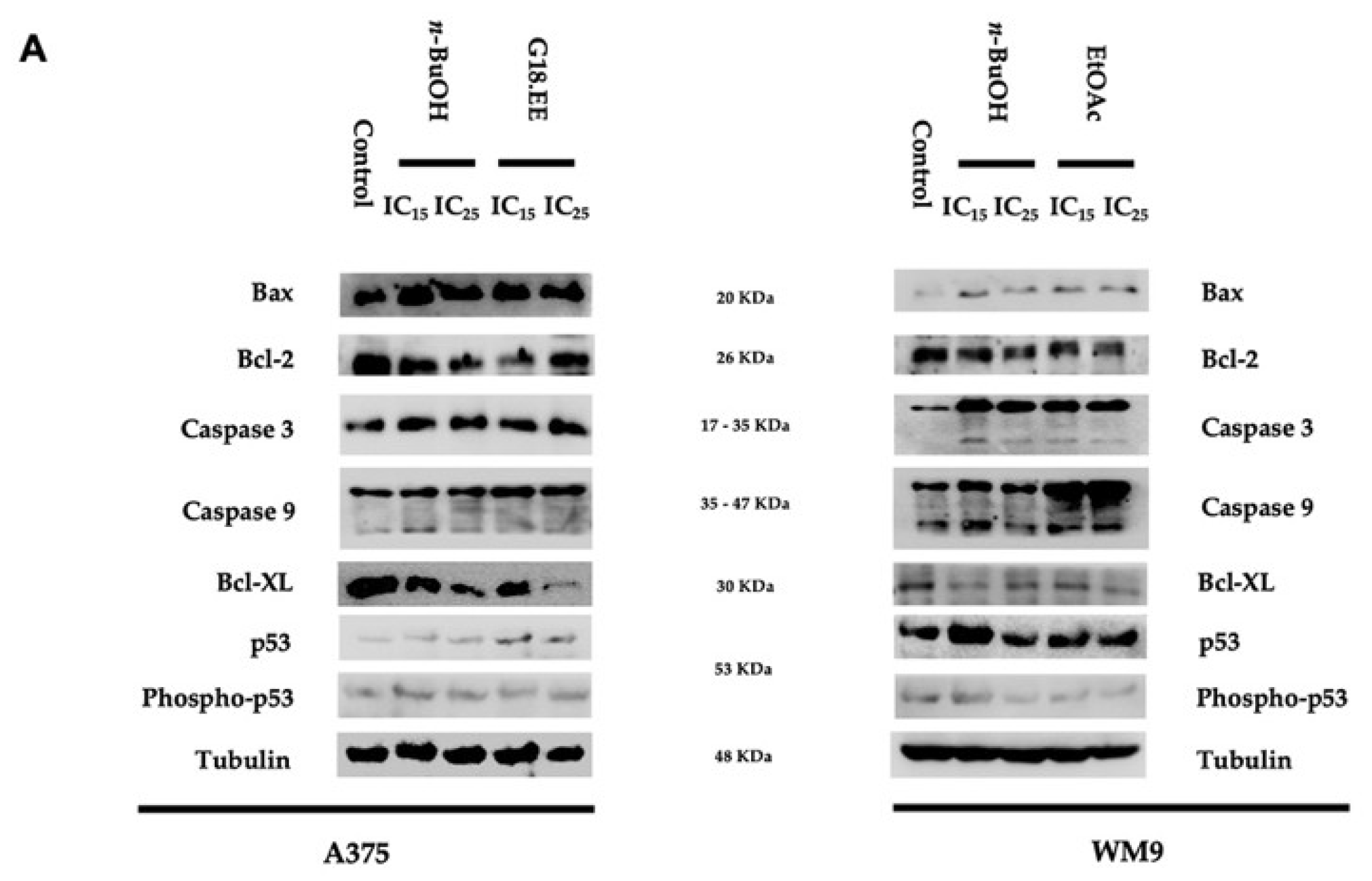
2.3.4. G18.EE, n-BuOH, and EtOAc Treatments Induce Apoptosis in Melanoma Cell Lines
3. Discussion
4. Materials and Methods
4.1. Propolis Sample
4.2. Preparation of Propolis Extract
4.3. Fractionation of Gerês Propolis from 2018 (G18)
4.4. Analysis of Phenolic Compounds from the Propolis Extract and Its Fractions
4.5. Cell Lines and Culture Conditions
4.6. Cell Viability Assay
4.7. Protein Extraction
4.8. Western Blot
4.9. Evaluation of Antioxidant Activity of Propolis Extract and Its Fractions
4.9.1. DPPH Radical Scavenging Activity
4.9.2. ABTS Radical Scavenging Activity
4.9.3. ROS Production and Mitochondrial Membrane Potential
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin Cancer and New Treatment Perspectives: A Review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Chang, Y.A.; Chen, Y.J.; Sung, H.M.; Bogeski, I.; Su, H.L.; Hsu, Y.L.; Wang, H.M.D. The Roles of Extracellular Vesicles in Malignant Melanoma. Cells 2021, 10, 2740. [Google Scholar] [CrossRef] [PubMed]
- Hogue, L.; Harvey, V.M. Basal Cell Carcinoma, Squamous Cell Carcinoma, and Cutaneous Melanoma in Skin of Color Patients. Dermatol. Clin. 2019, 37, 519–526. [Google Scholar] [CrossRef]
- World Health Organization. Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 8 March 2022).
- Bandarchi, B.; Ma, L.; Navab, R.; Seth, A.; Rasty, G. From Melanocyte to Metastatic Malignant Melanoma. Dermatol. Res. Pract. 2010, 2010, 583748. [Google Scholar] [CrossRef]
- Prieto-Granada, C.; Howe, N.; McCardle, T. Melanoma pathology. In Melanoma; Oxford University Press: Oxford, UK, 2015; Volume 1012, pp. 10–30. [Google Scholar] [CrossRef]
- Khaddour, K.; Maahs, L.; Avila-Rodriguez, A.M.; Maamar, Y.; Samaan, S.; Ansstas, G. Melanoma Targeted Therapies beyond Braf-Mutant Melanoma: Potential Druggable Mutations and Novel Treatment Approaches. Cancers 2021, 13, 5847. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global Surveillance of Trends in Cancer Survival 2000–14 (CONCORD-3): Analysis of Individual Records for 37,513,025 Patients Diagnosed with One of 18 Cancers from 322 Population-Based Registries in 71 Countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted Agents and Immunotherapies: Optimizing Outcomes in Melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef]
- Tas, F. Metastatic Behavior in Melanoma: Timing, Pattern, Survival, and Influencing Factors. J. Oncol. 2012, 2012, 1–9. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Simeone, E.; Festino, L.; Vanella, V.; Strudel, M.; Ascierto, P.A. MEK Inhibitors in the Treatment of Metastatic Melanoma and Solid Tumors. Am. J. Clin. Dermatol. 2017, 18, 745–754. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.A.; Fisher, D.E. The Melanoma Revolution: From UV Carcinogenesis to a New Era in Therapeutics. Science 2014, 346, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The Role of BRAF V600 Mutation in Melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current State of Melanoma Diagnosis and Treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Tanda, E.T.; Vanni, I.; Boutros, A.; Andreotti, V.; Bruno, W.; Ghiorzo, P.; Spagnolo, F. Current State of Target Treatment in BRAF Mutated Melanoma. Front. Mol. Biosci. 2020, 7, 154. [Google Scholar] [CrossRef]
- Gastaldello, G.H.; Cazeloto, A.C.V.; Ferreira, J.C.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Zoccal, K.F.; Tefé-Silva, C. Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines 2021, 8, 20. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Propolis: A Review. Bee World 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Miranda-Gonçalves, V.; Ferreira, A.M.; Cardoso, S.M.; Sobral, A.J.F.N.; Almeida-Aguiar, C.; Baltazar, F. Antitumoural and Antiangiogenic Activity of Portuguese Propolis in in Vitro and in Vivo Models. J. Funct. Foods 2014, 11, 160–171. [Google Scholar] [CrossRef]
- Cruz, M.; Antunes, P.; Paulo, L.; Ferreira, A.M.; Cunha, A.; Almeida-Aguiar, C.; Oliveira, R. Antioxidant and Dual Dose-Dependent Antigenotoxic and Genotoxic Properties of an Ethanol Extract of Propolis. RSC Adv. 2016, 6, 49806–49816. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical Composition, Biological Properties and Therapeutic Activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evidence-Based Complement. Altern. Med. 2015, 2015, 1–29. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phyther. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent Advances in Chemistry and Plant Origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Advances in the Propolis Chemical Composition between 2013 and 2018: A Review. eFood 2019, 1, 24. [Google Scholar] [CrossRef]
- Pagliarone, A.C.; Orsatti, C.L.; Búfalo, M.C.; Missima, F.; Bachiega, T.F.; Júnior, J.P.A.; Sforcin, J.M. Propolis Effects on Pro-Inflammatory Cytokine Production and Toll-like Receptor 2 and 4 Expression in Stressed Mice. Int. Immunopharmacol. 2009, 9, 1352–1356. [Google Scholar] [CrossRef]
- Velazquez, C.; Navarro, M.; Acosta, A.; Angulo, A.; Dominguez, Z.; Robles, R.; Robles-Zepeda, R.; Lugo, E.; Goycoolea, F.M.; Velazquez, E.F.; et al. Antibacterial and Free-Radical Scavenging Activities of Sonoran Propolis. J. Appl. Microbiol. 2007, 103, 1747–1756. [Google Scholar] [CrossRef]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, Antifungal and Antiviral Activity of Propolis of Different Geographic Origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef]
- Silici, S.; Koç, N.A.; Ayangil, D.; Çankaya, S. Antifungal Activities of Propolis Collected by Different Races of Honeybees against Yeasts Isolated from Patients with Superficial Mycoses. J. Pharmacol. Sci. 2005, 99, 39–44. [Google Scholar] [CrossRef]
- Sforcin, J.M. Propolis and the Immune System: A Review. J. Ethnopharmacol. 2007, 113, 1–14. [Google Scholar] [CrossRef]
- Gekker, G.; Hu, S.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 Activity of Propolis in CD4+ Lymphocyte and Microglial Cell Cultures. J. Ethnopharmacol. 2005, 102, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phyther. Res. 2010, 24 (Suppl. 1), S20–S28. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phyther. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.S.; Cunha, A.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Constancy of the Bioactivities of Propolis Samples Collected on the Same Apiary over Four Years. Food Res. Int. 2019, 119, 622–633. [Google Scholar] [CrossRef] [PubMed]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological Activities of Portuguese Propolis: Protection against Free Radical-Induced Erythrocyte Damage and Inhibition of Human Renal Cancer Cell Growth in Vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Edrada Ebel, R.; Paget, T.; de Koning, H.P.; et al. The Chemical Characterization of Nigerian Propolis Samples and Their Activity Against Trypanosoma Brucei. Sci. Rep. 2017, 7, 923. [Google Scholar] [CrossRef]
- Libério, S.A.; Pereira, A.L.A.; Araújo, M.J.A.M.; Dutra, R.P.; Nascimento, F.R.F.; Monteiro-Neto, V.; Ribeiro, M.N.S.; Gonçalves, A.G.; Guerra, R.N.M. The Potential Use of Propolis as a Cariostatic Agent and Its Actions on Mutans Group Streptococci. J. Ethnopharmacol. 2009, 125, 1–9. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evidence-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef]
- Chen, J.; Long, Y.; Han, M.; Wang, T.; Chen, Q.; Wang, R. Water-Soluble Derivative of Propolis Mitigates Scopolamine-Induced Learning and Memory Impairment in Mice. Pharmacol. Biochem. Behav. 2008, 90, 441–446. [Google Scholar] [CrossRef]
- Yildirim, Z.; Hacievliyagil, S.; Kutlu, N.O.; Aydin, N.E.; Kurkcuoglu, M.; Iraz, M.; Durmaz, R. Effect of Water Extract of Turkish Propolis on Tuberculosis Infection in Guinea-Pigs. Pharmacol. Res. 2004, 49, 287–292. [Google Scholar] [CrossRef]
- Suarez, F.; Levitt, M.D. Abdominal Symptoms and Lactose: The Discrepancy between Patients’ Claims and the Results of Blinded Trials. Am. J. Clin. Nutr. 1996, 64, 251–252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Conte, F.L.; Tasca, K.I.; Santiago, K.B.; de Oliveira Cardoso, E.; Romagnoli, G.G.; de Assis Golim, M.; Braz, A.M.M.; Berretta, A.A.; do Rosário de Souza, L.; Sforcin, J.M. Propolis Increases Foxp3 Expression and Lymphocyte Proliferation in HIV-Infected People: A Randomized, Double Blind, Parallel-Group and Placebo-Controlled Study. Biomed. Pharmacother. 2021, 142, 111984. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Fernandes Júnior, A.; Lopes, C.A.M.; Funari, S.R.C.; Bankova, V. Seasonal Effect of Brazilian Propolis on Candida Albicans and Candida Tropicalis. J. Venom. Anim. Toxins 2001, 7, 139–144. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant Activity of Propolis of Various Geographic Origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Almaraz-Abarca, N.; da Graça Campos, M.; Ávila-Reyes, J.A.; Naranjo-Jiménez, N.; Herrera Corral, J.; González-Valdez, L.S. Antioxidant Activity of Polyphenolic Extract of Monofloral Honeybee-Collected Pollen from Mesquite (Prosopis Juliflora, Leguminosae). J. Food Compos. Anal. 2007, 20, 119–124. [Google Scholar] [CrossRef]
- Farooqui, T.; Farooqui, A.A. Molecular Mechanism Underlying the Therapeutic Activities of Propolis: A Critical Review. Curr. Nutr. Food Sci. 2010, 6, 186–199. [Google Scholar] [CrossRef]
- Valença, I.; Morais-Santos, F.; Miranda-Gonçalves, V.; Ferreira, A.M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Disturbs Glycolytic Metabolism of Human Colorectal Cancer in Vitro. BMC Complement. Altern. Med. 2013, 13, 184. [Google Scholar] [CrossRef]
- Sforcin, J.M.; Bankova, V. Propolis: Is There a Potential for the Development of New Drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Sheng, J.; Zhou, J.; Wang, L.; Xu, J.; Hu, Q. Antioxidant Activity of Ethanol and Petroleum Ether Extracts from Brazilian Propolis. Eur. Food Res. Technol. 2007, 225, 249–253. [Google Scholar] [CrossRef]
- Yang, H.; Dong, Y.; Du, H.; Shi, H.; Peng, Y.; Li, X. Antioxidant Compounds from Propolis Collected in Anhui, China. Molecules 2011, 16, 3444–3455. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Zhelev, Z.; Aoki, I.; Bakalova, R.; Higashi, T. Overproduction of Reactive Oxygen Species—Obligatory or Not for Induction of Apoptosis by Anticancer Drugs. Chin. J. Cancer Res. 2016, 28, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portu-guese propolis by LC-MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Falcão, S.I.; Vilas-Boas, M.; Estevinho, L.M.; Barros, C.; Domingues, M.R.M.; Cardoso, S.M. Phenolic characterization of Northeast Portuguese propolis: Usual and unusual compounds. Anal. Bioanal. Chem. 2010, 396, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.-G. Chemical Composition of European Propolis: Expected and Unexpected Results. Z. Nat. 2002, 56, 530–533. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant Properties, Total Phenols and Pollen Analysis of Propolis Samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Falcão, S.I.; Freire, C.; Vilas-Boas, M. A Proposal for Physicochemical Standards and Antioxidant Activity of Portuguese Propolis. J. Am. Oil Chem. Soc. 2013, 90, 1729–1741. [Google Scholar] [CrossRef]
- Freitas, A.S.; Cunha, A.; Oliveira, R.; Almeida-Aguiar, C. Propolis Antibacterial and Antioxidant Synergisms with Gentamicin and Honey. J. Appl. Microbiol. 2022, 132, 2733–2745. [Google Scholar] [CrossRef]
- Da Cruz, F.B.; Martins Nascimento, D.H.; Martins, N.; Ferreira, J.D.F.; Silveira, D.; Yris Maria, B.F. Antioxidant Activity of Apis Mellifera Bee Propolis: A Review. J. Nat. Prod. Discov. 2022, 1, 1–44. [Google Scholar] [CrossRef]
- Falcão, S.I.; Calhelha, R.C.; Touzani, S.; Lyoussi, B.; Ferreira, I.C.F.R.; Vilas-Boas, M. In Vitro Interactions of Moroccan Propolis Phytochemical’s on Human Tumor Cell Lines and Anti-Inflammatory Properties. Biomolecules 2019, 9, 315. [Google Scholar] [CrossRef] [PubMed]
- Benguedouar, L.; Lahouel, M.; Gangloff, S.C.; Durlach, A.; Grange, F.; Bernard, P.; Antonicelli, F. Ethanolic Extract of Algerian Propolis and Galangin Decreased Murine Melanoma Tumor Progression in Mice. Anticancer Agents Med. Chem. 2016, 16, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Cisilotto, J.; Sandjo, L.P.; Faqueti, L.G.; Fernandes, H.; Joppi, D.; Biavatti, M.W.; Creczynski-Pasa, T.B. Cytotoxicity Mechanisms in Melanoma Cells and UPLC-QTOF/MS2 Chemical Characterization of Two Brazilian Stingless Bee Propolis: Uncommon Presence of Piperidinic Alkaloids. J. Pharm. Biomed. Anal. 2018, 149, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Sun, L.; Chen, X.P.; Zhang, D.S. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, S.C. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Sousa, J.F.; Torrieri, R.; Silva, R.R.; Pereira, C.G.; Valente, V.; Torrieri, E.; Peronni, K.C.; Martins, W.; Muto, N.; Francisco, G.; et al. Novel Primate-Specific Genes, RMEL 1, 2 and 3, with Highly Restricted Expression in Melanoma, Assessed by New Data Mining Tool. PLoS ONE 2010, 5, e13510. [Google Scholar] [CrossRef]
- AlQathama, A.; Ezuruike, U.F.; Mazzari, A.L.D.A.; Yonbawi, A.; Chieli, E.; Prieto, J.M. Effects of Selected Nigerian Medicinal Plants on the Viability, Mobility, and Multidrug-Resistant Mechanisms in Liver, Colon, and Skin Cancer Cell Lines. Front. Pharmacol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Honavar, M.; Pinheiro, C.; Martinho, O.; Pires, M.M.; Pinheiro, C.; Cordeiro, M.; Bebiano, G.; Costa, P.; Palmeirim, I.; et al. Monocarboxylate Transporters (MCTs) in Gliomas: Expression and Exploitation as Therapeutic Targets. Neuro-Oncology 2013, 15, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Mitra, K.; Uddin, N. Total Phenolics, Flavonoids, Proanthrocyanidins, Ascorbic Acid Contents and In-Vitro Antioxidant Activities of Newly Developed Isolated Soya Protein. Discourse J. Agric. Food Sci. 2014, 2, 160–168. [Google Scholar]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic Antioxidants from Clonal Oregano (Origanum vulgare) with Antimicrobial Activity against Helicobacter Pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Miranda-Gonçalves, V.; Cardoso-Carneiro, D.; Valbom, I.; Cury, F.P.; Silva, V.A.; Granja, S.; Reis, R.M.; Baltazar, F.; Martinho, O. Metabolic Alterations Underlying Bevacizumab Therapy in Glioblastoma Cells. Oncotarget 2017, 8, 103657–103670. [Google Scholar] [CrossRef] [PubMed]


| DPPH• | ABTS (Absolute Ethanol) | |
|---|---|---|
| EC50 (µg/mL) | EC50 (µg/mL) | |
| G18.EE | 10.90 ± 0.34 | 9.83 ± 0.21 |
| Gallic Acid | 1.21 ± 0.08 | - - - - - |
| Trolox | - - - - - | 3.46 ± 0.22 |
| IC50 (µg/mL) | IC25 (µg/mL) | IC15 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| A375 | WM9 | A375 | WM9 | A375 | WM9 | |
| G18.EE | 16.98 ± 0.93 | 25.03 ± 1.34 | 10.85 ± 0.12 | 15.32 ± 0.14 | 8.88 ± 0.12 | 12.05 ± 0.14 |
| n-hexane | 45.71 ± 1.69 | 39.54 ± 0.17 | 24.79 ± 0.09 | 20.49 ± 0.04 | 19.13 ± 0.11 | 15.48 ± 0.04 |
| EtOAc | 17.12 ± 0.72 | 16.39 ± 0.46 | 12.8 ± 0.03 | 8.56 ± 0.11 | 10.9 ± 0.09 | 6.69 ± 0.15 |
| n-BuOH | 8.14 ± 0.03 | 11.22 ± 1.66 | 6.16 ± 0.10 | 8.08 ± 0.22 | 4.57 ± 0.09 | 6.01 ± 0.11 |
| IC15 (µg/mL) | IC25 (µg/mL) | |
|---|---|---|
| G18.EE | 10 | 13 |
| EtOAc | 8 | 10 |
| n-BuOH | 5 | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, R.D.; Celeiro, S.P.; Barbosa-Matos, C.; Freitas, A.S.; Cardoso, S.M.; Viana-Pereira, M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules 2022, 27, 3533. https://doi.org/10.3390/molecules27113533
Oliveira RD, Celeiro SP, Barbosa-Matos C, Freitas AS, Cardoso SM, Viana-Pereira M, Almeida-Aguiar C, Baltazar F. Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules. 2022; 27(11):3533. https://doi.org/10.3390/molecules27113533
Chicago/Turabian StyleOliveira, Rafaela Dias, Sónia Pires Celeiro, Catarina Barbosa-Matos, Ana Sofia Freitas, Susana M. Cardoso, Marta Viana-Pereira, Cristina Almeida-Aguiar, and Fátima Baltazar. 2022. "Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis" Molecules 27, no. 11: 3533. https://doi.org/10.3390/molecules27113533
APA StyleOliveira, R. D., Celeiro, S. P., Barbosa-Matos, C., Freitas, A. S., Cardoso, S. M., Viana-Pereira, M., Almeida-Aguiar, C., & Baltazar, F. (2022). Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules, 27(11), 3533. https://doi.org/10.3390/molecules27113533








