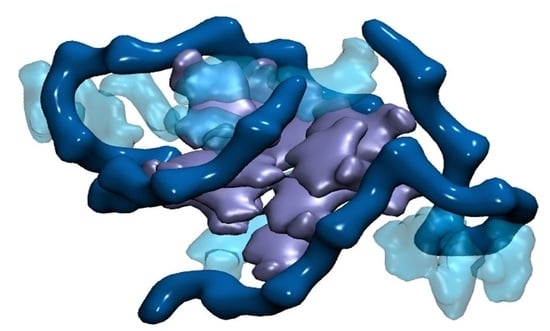
Never Cared for What They Do: High Structural Stability of Guanine-Quadruplexes in the Presence of Strand-Break Damage
Abstract
1. Introduction
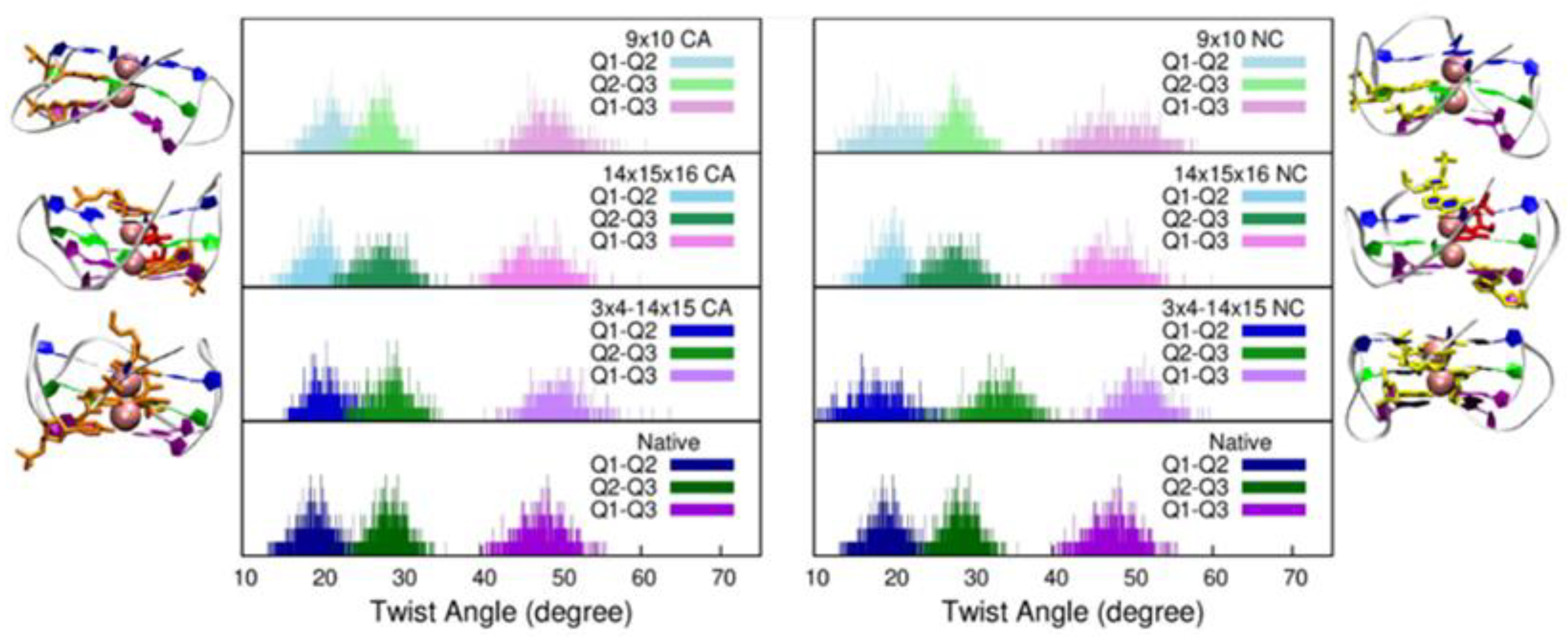
2. Results
3. Discussion and Conclusions
4. Materials and Methods
4.1. Force Field for Non-Standard Nucleotides
4.2. Molecular Dynamics Simulations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burge, S.; Parkinson, G.N.; Hazel, P.; Todd, A.K.; Neidle, S. Quadruplex DNA: Sequence, topology and structure. Nucleic Acids Res. 2006, 34, 5402–5415. [Google Scholar] [CrossRef] [PubMed]
- Majee, P.; Kumar Mishra, S.; Pandya, N.; Shankar, U.; Pasadi, S.; Muniyappa, K.; Nayak, D.; Kumar, A. Identification and characterization of two conserved G-quadruplex forming motifs in the Nipah virus genome and their interaction with G-quadruplex specific ligands. Sci. Rep. 2020, 10, 1477. [Google Scholar] [CrossRef] [PubMed]
- Metifiot, M.; Amrane, S.; Litvak, S.; Andreola, M.L. G-quadruplexes in viruses: Function and potential therapeutic applications. Nucleic Acids Res. 2014, 42, 12352–12366. [Google Scholar] [CrossRef]
- Hognon, C.C.; Miclot, T.; Iriepa, C.G.; Francés-Monerris, A.; Grandemange, S.; Terenzi, A.; Marazzi, M.; Barone, G.; Monari, A.; Garcia-Iriepa, C.; et al. Role of RNA Guanine Quadruplexes in Favoring the Dimerization of SARS Unique Domain in Coronaviruses. J. Phys. Chem. Lett. 2020, 11, 5661–5667. [Google Scholar] [CrossRef] [PubMed]
- Panera, N.; Tozzi, A.; Alisi, A. The G-quadruplex/helicase world as a potential antiviral approach against COVID-19. Drugs 2020, 80, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Vonrhein, C.; Smart, O.S.; Bricogne, G.; Bollati, M.; Kusov, Y.; Hansen, G.; Mesters, J.R.; Schmidt, C.L.; Hilgenfeld, R. The SARS-Unique Domain (SUD) of SARS coronavirus contains two macrodomains that bind G-quadruplexes. PLoS Pathog. 2009, 5, e1000428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qin, G.; Niu, J.; Wang, Z.; Wang, C.; Ren, J.; Qu, X. Targeting RNA G-Quadruplex in SARS-CoV-2: A Promising Therapeutic Target for COVID-19? Angew. Chem. Int. Ed. 2021, 60, 432–438. [Google Scholar] [CrossRef]
- Miclot, T.; Hognon, C.; Bignon, E.; Terenzi, A.; Marazzi, M.; Barone, G.; Monari, A. Structure and Dynamics of RNA Guanine Quadruplexes in SARS-CoV-2 Genome. Original Strategies against Emerging Viruses. J. Phys. Chem. Lett. 2021, 12, 10277–10283. [Google Scholar] [CrossRef]
- Abiri, A.; Lavigne, M.; Rezaei, M.M.; Nikzad, S.S.; Zare, P.; Mergny, J.-L.L.; Rahimi, H.-R.R.; Peyman, Z.; Mergny, J.-L.L.; Rahimi, H.-R.R. Unlocking G-quadruplexes as antiviral targets. Pharmacol. Rev. 2021, 73, 897–923. [Google Scholar] [CrossRef]
- Ruggiero, E.; Richter, S.N. Survey and summary G-quadruplexes and G-quadruplex ligands: Targets and tools in antiviral therapy. Nucleic Acids Res. 2018, 46, 3270–3283. [Google Scholar] [CrossRef]
- Ruggiero, E.; Zanin, I.; Terreri, M.; Richter, S.N. G-quadruplex targeting in the fight against viruses: An update. Int. J. Mol. Sci. 2021, 22, 10984. [Google Scholar] [CrossRef] [PubMed]
- Asamitsu, S.; Takeuchi, M.; Ikenoshita, S.; Imai, Y.; Kashiwagi, H.; Shioda, N. Perspectives for applying g-quadruplex structures in neurobiology and neuropharmacology. Int. J. Mol. Sci. 2019, 20, 2884. [Google Scholar] [CrossRef] [PubMed]
- Shioda, N.; Yabuki, Y.; Yamaguchi, K.; Onozato, M.; Li, Y.; Kurosawa, K.; Tanabe, H.; Okamoto, N.; Era, T.; Sugiyama, H.; et al. Targeting G-quadruplex DNA as cognitive function therapy for ATR-X syndrome. Nat. Med. 2018, 24, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Thombre, R.; Shah, Y.; Latanich, R.; Wang, J. G-Quadruplexes as pathogenic drivers in neurodegenerative disorders. Nucleic Acids Res. 2021, 49, 4816–4830. [Google Scholar] [CrossRef]
- Paeschke, K.; Capra, J.A.; Zakian, V.A. DNA Replication through G-Quadruplex Motifs Is Promoted by the Saccharomyces cerevisiae Pif1 DNA Helicase. Cell 2011, 145, 678–691. [Google Scholar] [CrossRef]
- Prorok, P.; Artufel, M.; Aze, A.; Coulombe, P.; Peiffer, I.; Lacroix, L.; Guédin, A.; Mergny, J.-L.; Damaschke, J.; Schepers, A.; et al. Involvement of G-quadruplex regions in mammalian replication origin activity. Nat. Commun. 2019, 10, 3274. [Google Scholar] [CrossRef]
- Yuan, L.; Tian, T.; Chen, Y.; Yan, S.; Xing, X.; Zhang, Z.; Zhai, Q.; Xu, L.; Wang, S.; Weng, X.; et al. Existence of G-quadruplex structures in promoter region of oncogenes confirmed by G-quadruplex DNA cross-linking strategy. Sci. Rep. 2013, 3, 01811. [Google Scholar] [CrossRef]
- Huppert, J.L.; Balasubramanian, S. G-quadruplexes in promoters throughout the human genome. Nucleic Acids Res. 2007, 35, 2105. [Google Scholar] [CrossRef][Green Version]
- Lago, S.; Nadai, M.; Cernilogar, F.M.; Kazerani, M.; Domíniguez Moreno, H.; Schotta, G.; Richter, S.N. Promoter G-quadruplexes and transcription factors cooperate to shape the cell type-specific transcriptome. Nat. Commun. 2021, 12, 3885. [Google Scholar] [CrossRef]
- Robinson, J.; Raguseo, F.; Nuccio, S.P.; Liano, D.; Di Antonio, M. DNA G-quadruplex structures: More than simple roadblocks to transcription? Nucleic Acids Res. 2021, 49, 8419–8431. [Google Scholar] [CrossRef]
- Xue, Y.; Kan, Z.Y.; Wang, Q.; Yao, Y.; Liu, J.; Hao, Y.H.; Tan, Z. Human telomeric DNA forms parallel-stranded intramolecular G-quadruplex in K+ solution under molecular crowding condition. J. Am. Chem. Soc. 2007, 129, 11185–11191. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Seimiya, H. Revisiting Telomere Shortening in Cancer. Cells 2019, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Neidle, S. Human telomeric G-quadruplex: The current status of telomeric G-quadruplexes as therapeutic targets in human cancer: G-quadruplexes as cancer drug targets. FEBS J. 2010, 277, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.A.; Upton, H.E.; Vogan, J.M.; Collins, K. Telomerase Mechanism of Telomere Synthesis. Annu. Rev. Biochem. 2017, 86, 439–460. [Google Scholar] [CrossRef]
- Mishra, S.; Chaudhary, R.; Singh, S.; Kota, S.; Misra, H.S. Guanine quadruplex DNA regulates gamma radiation response of genome functions in the radioresistant bacterium deinococcus radiodurans. J. Bacteriol. 2019, 201, e00154. [Google Scholar] [CrossRef]
- Saranathan, N.; Vivekanandan, P. G-Quadruplexes: More Than Just a Kink in Microbial Genomes. Trends Microbiol. 2019, 27, 148–163. [Google Scholar] [CrossRef]
- Pratviel, G.; Meunier, B. Guanine oxidation: One- and two-electron reactions. Chem. A Eur. J. 2006, 12, 6018–6030. [Google Scholar] [CrossRef]
- Steenken, S.; Jovanovic, S.V. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J. Am. Chem. Soc. 1997, 119, 617–618. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Fleming, A.M.; Burrows, C.J. Interplay of Guanine Oxidation and G-Quadruplex Folding in Gene Promoters. J. Am. Chem. Soc. 2020, 142, 1115–1136. [Google Scholar] [CrossRef]
- Miclot, T.; Corbier, C.; Terenzi, A.; Hognon, C.; Grandemange, S.; Barone, G.; Monari, A. Forever Young: Structural Stability of Telomeric Guanine Quadruplexes in the Presence of Oxidative DNA Lesions **. Chem. A Eur. J. 2021, 27, 8865–8874. [Google Scholar] [CrossRef] [PubMed]
- Bielskute, S.; Plavec, J.; Podbevšek, P. Impact of Oxidative Lesions on the Human Telomeric G-Quadruplex. J. Am. Chem. Soc. 2019, 141, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Szalai, V.A.; Singer, M.J.; Thorp, H.H. Site-specific probing of oxidative reactivity and telomerase function using 7,8-dihydro-8-oxoguanine in telomeric DNA. J. Am. Chem. Soc. 2002, 124, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Vorlícková, M.; Tomasko, M.; Sagi, A.J.; Bednarova, K.; Sagi, J. 8-Oxoguanine in a quadruplex of the human telomere DNA sequence. FEBS J. 2012, 279, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Burrows, C.J.; Muller, J.G. Oxidative nucleobase modifications leading to strand scission. Chem. Rev. 1998, 98, 1109–1151. [Google Scholar] [CrossRef]
- Driessens, N.; Versteyhe, S.; Ghaddhab, C.; Burniat, A.; De Deken, X.; Van Sande, J.; Dumont, J.E.; Miot, F.; Corvilain, B. Hydrogen peroxide induces DNA single- and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocr. Relat. Cancer 2009, 16, 845–856. [Google Scholar] [CrossRef]
- Henner, W.D.; Rodriguez, L.O.; Hecht, S.M.; Haseltine, W.A. gamma Ray induced deoxyribonucleic acid strand breaks. 3’ Glycolate termini. J. Biol. Chem. 1983, 258, 711–713. [Google Scholar] [CrossRef]
- Banneville, A.-S.; Tour, C.B.d.l.; Hognon, C.; Colletier, J.-P.; Teulon, J.-M.; Roy, A.L.; Pellequer, J.-L.; Monari, A.; Dehez, F.; Confalonieri, F.; et al. Structural and functional characterization of DdrC, a novel DNA damage-induced nucleoid associated protein involved in DNA compaction. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hennertg, W.; Grunbergsl, S.; Haseltinell, W. Sites and Structure of Gamma Radiation-induced DNA Strand Breaks. J. Biol. Chem. 1982, 257, 11750–11754. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Hagen, U.; Schön-Bopp, A.; Schulte-Frohlinde, D. Radiation-Induced Strand Breaks in DNA: Chemical and Enzymatic Analysis of End Groups and Mechanistic Aspects. Adv. Radiat. Biol. 1981, 9, 109–142. [Google Scholar]
- Lauková, L.; Konečná, B.; Janovičová, L.; Vlková, B.; Celec, P. Deoxyribonucleases and their applications in biomedicine. Biomolecules 2020, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Haber, J.E. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb. Perspect. Biol. 2014, 6, a016428. [Google Scholar] [CrossRef] [PubMed]
- Tomkinson, A.E.; Della-Maria, J.A. DNA Ligases: Mechanism and Functions. In Encyclopedia of Biological Chemistry, 2nd ed.; Academic Press: London, UK, 2013; pp. 28–32. ISBN 9780123786319. [Google Scholar]
- Tomkinson, A.E.; Vijayakumar, S.; Pascal, J.M.; Ellenberger, T. DNA ligases: Structure, reaction mechanism, and function. Chem. Rev. 2006, 106, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Didenko, V.V. 5′OH DNA breaks in apoptosis and their labeling by topoisomerase-based approach. Methods Mol. Biol. 2011, 682, 77–87. [Google Scholar]
- Saito, Y.; Hikita, H.; Nozaki, Y.; Kai, Y.; Makino, Y.; Nakabori, T.; Tanaka, S.; Yamada, R.; Shigekawa, M.; Kodama, T.; et al. DNase II activated by the mitochondrial apoptotic pathway regulates RIP1-dependent non-apoptotic hepatocyte death via the TLR9/IFN-β signaling pathway. Cell Death Differ. 2019, 26, 470–486. [Google Scholar] [CrossRef]
- Chappell, C.; Hanakahi, L.A.; Karimi-Busheri, F.; Weinfeld, M.; West, S.C. Involvement of human polynucleotide kinase in double-strand break repair by non-homologous end joining. EMBO J. 2002, 21, 2827–2832. [Google Scholar] [CrossRef]
- Karimi-Busheri, F.; Lee, J.; Tomkinson, A.E.; Weinfeld, M. Repair of DNA strand gaps and nicks containing 3′-phosphate and 5′-hydroxyl termini by purified mammalian enzymes. Nucleic Acids Res. 1998, 26, 4395–4400. [Google Scholar] [CrossRef]
- Das, U.; Chakravarty, A.K.; Remus, B.S.; Shuman, S. Rewriting the rules for end joining via enzymatic splicing of DNA 3′-PO4 and 5′-OH ends. Proc. Natl. Acad. Sci. USA 2013, 110, 20437–20442. [Google Scholar] [CrossRef]
- Schmier, B.J.; Shuman, S. Deinococcus radiodurans HD-Pnk, a nucleic acid end-healing enzyme, abets resistance to killing by ionizing radiation and mitomycin C. J. Bacteriol. 2018, 200, e00151. [Google Scholar] [CrossRef]
- Obi, I.; Rentoft, M.; Singh, V.; Jamroskovic, J.; Chand, K.; Chorell, E.; Westerlund, F.; Sabouri, N. Stabilization of G-quadruplex DNA structures in Schizosaccharomyces pombe causes single-strand DNA lesions and impedes DNA replication. Nucleic Acids Res. 2020, 48, 10998–11015. [Google Scholar] [CrossRef]
- van Kregten, M.; Tijsterman, M. The repair of G-quadruplex-induced DNA damage. Exp. Cell Res. 2014, 329, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Vartak, S.V.; Dahal, S.; Kumari, S.; Desai, S.S.; Gopalakrishnan, V.; Choudhary, B.; Raghavan, S.C. G-quadruplex Structures Contribute to Differential Radiosensitivity of the Human Genome. iScience 2019, 21, 288–307. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Rocca, R.; Palazzesi, F.; Amato, J.; Costa, G.; Ortuso, F.; Pagano, B.; Randazzo, A.; Novellino, E.; Alcaro, S.; Moraca, F.; et al. Folding intermediate states of the parallel human telomeric G-quadruplex DNA explored using Well-Tempered Metadynamics. Sci. Rep. 2020, 10, 3176. [Google Scholar] [CrossRef] [PubMed]
- Hognon, C.; Gebus, A.; Barone, G.; Monari, A. Human DNA telomeres in presence of oxidative lesions: The crucial role of electrostatic interactions on the stability of guanine quadruplexes. Antioxidants 2019, 8, 337. [Google Scholar] [CrossRef] [PubMed]
- Bignon, E.; Gattuso, H.; Morell, C.; Dehez, F.; Georgakilas, A.G.; Monari, A.; Dumont, E. Correlation of bistranded clustered abasic DNA lesion processing with structural and dynamic DNA helix distortion. Nucleic Acids Res. 2016, 44, 8588–8599. [Google Scholar] [CrossRef]
- Gattuso, H.; Durand, E.; Bignon, E.; Morell, C.; Georgakilas, A.G.; Dumont, E.; Chipot, C.; Dehez, F.; Monari, A. Repair Rate of Clustered Abasic DNA Lesions by Human Endonuclease: Molecular Bases of Sequence Specificity. J. Phys. Chem. Lett. 2016, 7, 3760–3765. [Google Scholar] [CrossRef]
- Moon, J.; Han, J.H.; Kim, D.Y.; Jung, M.; Joon, K.; Kim, S.; Kim, S.K. Effects of deficient of the Hoogsteen base-pairs on the {G}-quadruplex stabilization and binding mode of a cationic porphyrin. Biochem. Biophys. Rep. 2015, 2, 29–35. [Google Scholar] [CrossRef]
- Largy, E.; Mergny, J.L.; Gabelica, V. Role of Alkali Metal Ions in G-Quadruplex Nucleic Acid Structure and Stability. Met. Ions Life Sci. 2016, 16, 203–258. [Google Scholar]
- Galindo-Murillo, R.; Robertson, J.C.; Zgarbová, M.; Šponer, J.; Otyepka, M.; Jurečka, P.; Cheatham, T.E. Assessing the Current State of Amber Force Field Modifications for DNA. J. Chem. Theory Comput. 2016, 12, 4114–4127. [Google Scholar] [CrossRef]
- Dans, P.D.; Ivani, I.; Hospital, A.; Portella, G.; González, C.; Orozco, M. How accurate are accurate force-fields for B-DNA? Nucleic Acids Res. 2017, 45, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09. Gaussian 09 Revis. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009; Available online: https://gaussian.com/glossary/g09/ (accessed on 25 April 2022).
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber biomolecular simulation programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, G.; Lee, M.; Neidle, S. Crystal structure of parallel quadruplexes from human telomeric DNA. Exp. Mol. Pathol. 2002, 417, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Hopkins, C.W.; Le Grand, S.; Walker, R.C.; Roitberg, A.E. Long-time-step molecular dynamics through hydrogen mass repartitioning. J. Chem. Theory Comput. 2015, 11, 1864–1874. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kollman, P.A. Settle: An analytical version of the SHAKE and RATTLE algorithm for rigid water models. J. Comput. Chem. 1992, 13, 952–962. [Google Scholar] [CrossRef]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant pressure molecular dynamics simulation: The Langevin piston method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Tsvetkov, V.; Pozmogova, G.; Varizhuk, A. The systematic approach to describing conformational rearrangements in G-quadruplexes. J. Biomol. Struct. Dyn. 2016, 34, 705–715. [Google Scholar] [CrossRef] [PubMed]
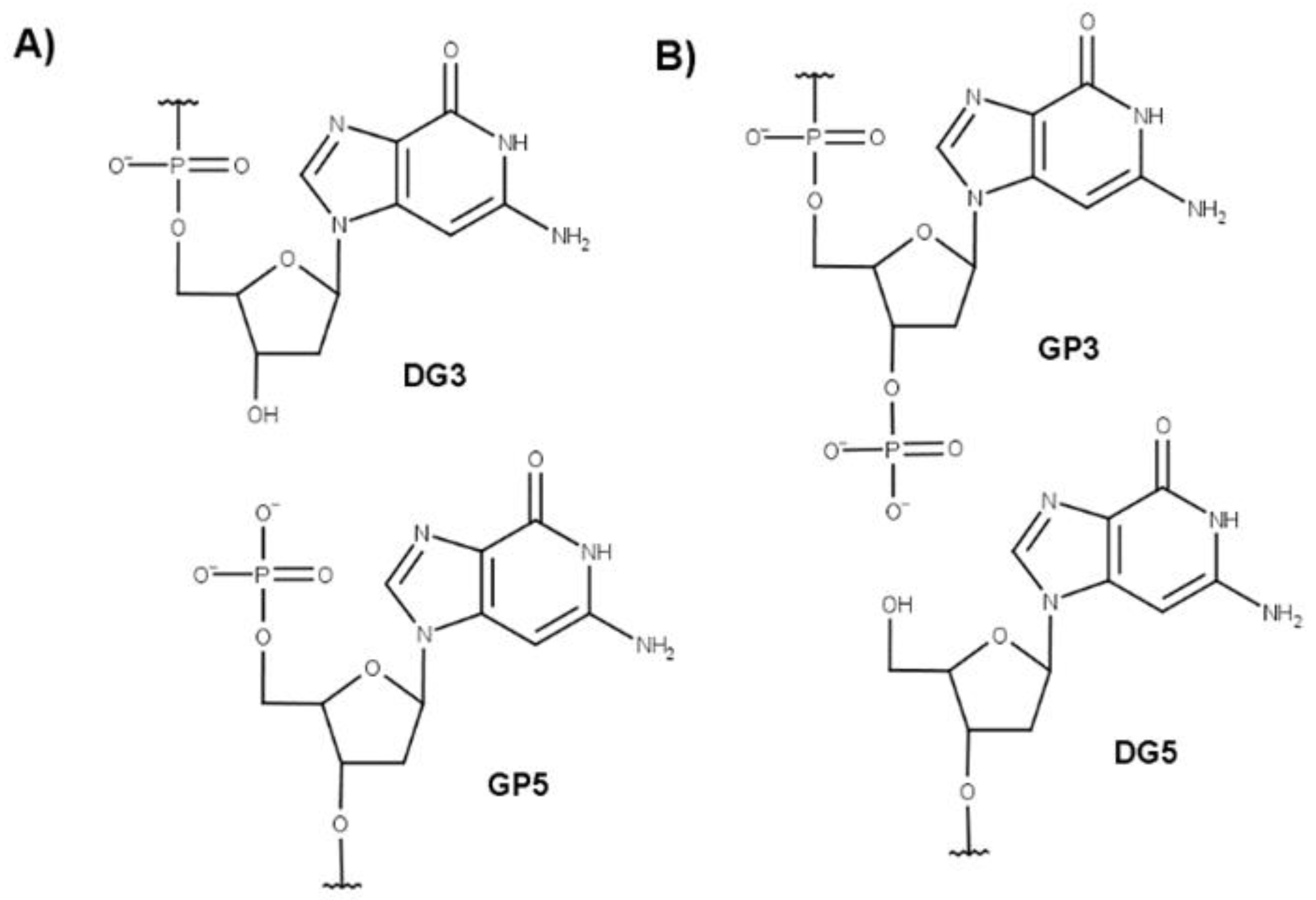


| Strand Break Type | Strand Break Position | Sequence |
|---|---|---|
| Native | A GGG TTA GGG TTA GGG TTA GGG | |
| Single | 2–3 | A G★GG TTA GGG TTA GGG TTA GGG |
| 3–4 | A GG★G TTA GGG TTA GGG TTA GGG | |
| 9–10 | A GGG TTA GG★G TTA GGG TTA GGG | |
| 14–15 | A GGG TTA GGG TTA G★GG TTA GGG | |
| 15–16 | A GGG TTA GGG TTA GG★G TTA GGG | |
| 21–22 | A GGG TTA GGG TTA GGG TTA GG★G | |
| Double | 2–3–4 | A G★G★G TTA GGG TTA GGG TTA GGG |
| 2–3/14–15 | A G★GG TTA GGG TTA G★GG TTA GGG | |
| 3–4/9–10 | A GG★G TTA GG★G TTA GGG TTA GGG | |
| 3–4/14–15 | A GG★G TTA GGG TTA G★GG TTA GGG | |
| 3–4/15–16 | A GG★G TTA GGG TTA GG★G TTA GGG | |
| 14–15–16 | A GGG TTA GGG TTA G★G★G TTA GGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miclot, T.; Hognon, C.; Bignon, E.; Terenzi, A.; Grandemange, S.; Barone, G.; Monari, A. Never Cared for What They Do: High Structural Stability of Guanine-Quadruplexes in the Presence of Strand-Break Damage. Molecules 2022, 27, 3256. https://doi.org/10.3390/molecules27103256
Miclot T, Hognon C, Bignon E, Terenzi A, Grandemange S, Barone G, Monari A. Never Cared for What They Do: High Structural Stability of Guanine-Quadruplexes in the Presence of Strand-Break Damage. Molecules. 2022; 27(10):3256. https://doi.org/10.3390/molecules27103256
Chicago/Turabian StyleMiclot, Tom, Cécilia Hognon, Emmanuelle Bignon, Alessio Terenzi, Stéphanie Grandemange, Giampaolo Barone, and Antonio Monari. 2022. "Never Cared for What They Do: High Structural Stability of Guanine-Quadruplexes in the Presence of Strand-Break Damage" Molecules 27, no. 10: 3256. https://doi.org/10.3390/molecules27103256
APA StyleMiclot, T., Hognon, C., Bignon, E., Terenzi, A., Grandemange, S., Barone, G., & Monari, A. (2022). Never Cared for What They Do: High Structural Stability of Guanine-Quadruplexes in the Presence of Strand-Break Damage. Molecules, 27(10), 3256. https://doi.org/10.3390/molecules27103256