3.1. Physicochemical Properties
The
n-hexane extracted seed oil was assessed for its potential as a feedstock for biodiesel production. In this respect, various physicochemical properties and fatty acid profile of oil were determined. Based on its fatty acid content and properties, base-catalyzed transesterification was implemented to prepare fatty acid methyl esters using sodium hydroxide as a catalyst. The physicochemical properties of seed oil can be seen in
Table 3.
The seeds were dried in an oven for 3 h to reduce the humidity, which was up to 8%. The oil content from dried seeds was 16% (
w/
w), excellent for a biodiesel feedstock. The FFA content (%) of the oil (1.97%) indicates that it is possible to use base-catalyzed transesterification without pretreatment. The kinematic viscosity was 29.5 mm
2/s, in agreement with the composition of the fatty acids (
Table 4).
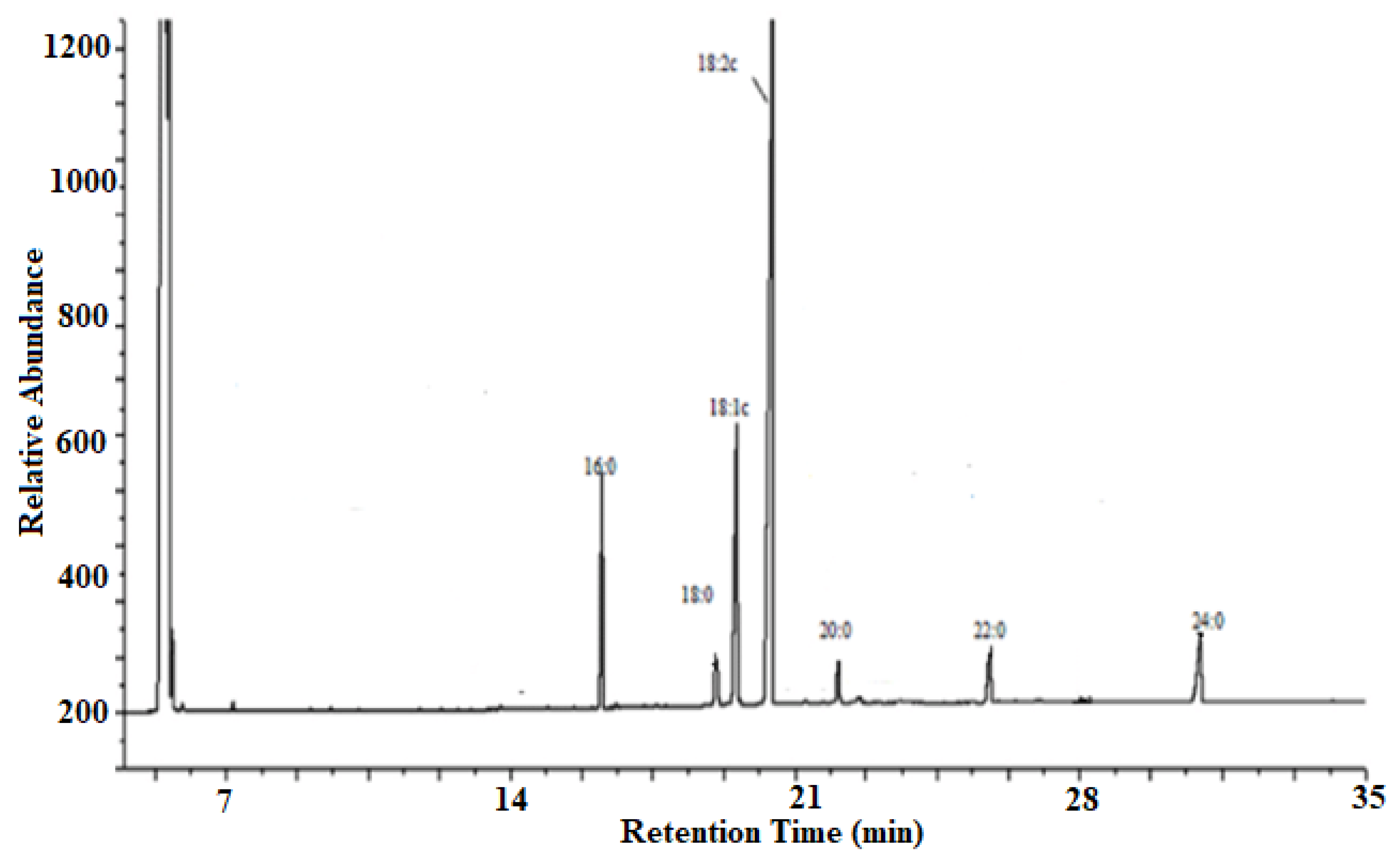
The seed oil consisted of unsaturated fatty acids such as oleic acid (14.52%, C18:1) and linoleic acid (61.51%, C18:2) and saturated fatty acids such as palmitic acid (9.90%, C16:0), stearic acid (2.22%, C18:0), Eicosanoic acid (1.50%, C20:0), Behenic acid (3.90%, C22:0), and Tetracosanoic acid (6.45%, C24:0). Therefore, unsaturated fatty acids contribute more (76.03%) than the saturated fatty acids (23.97%).
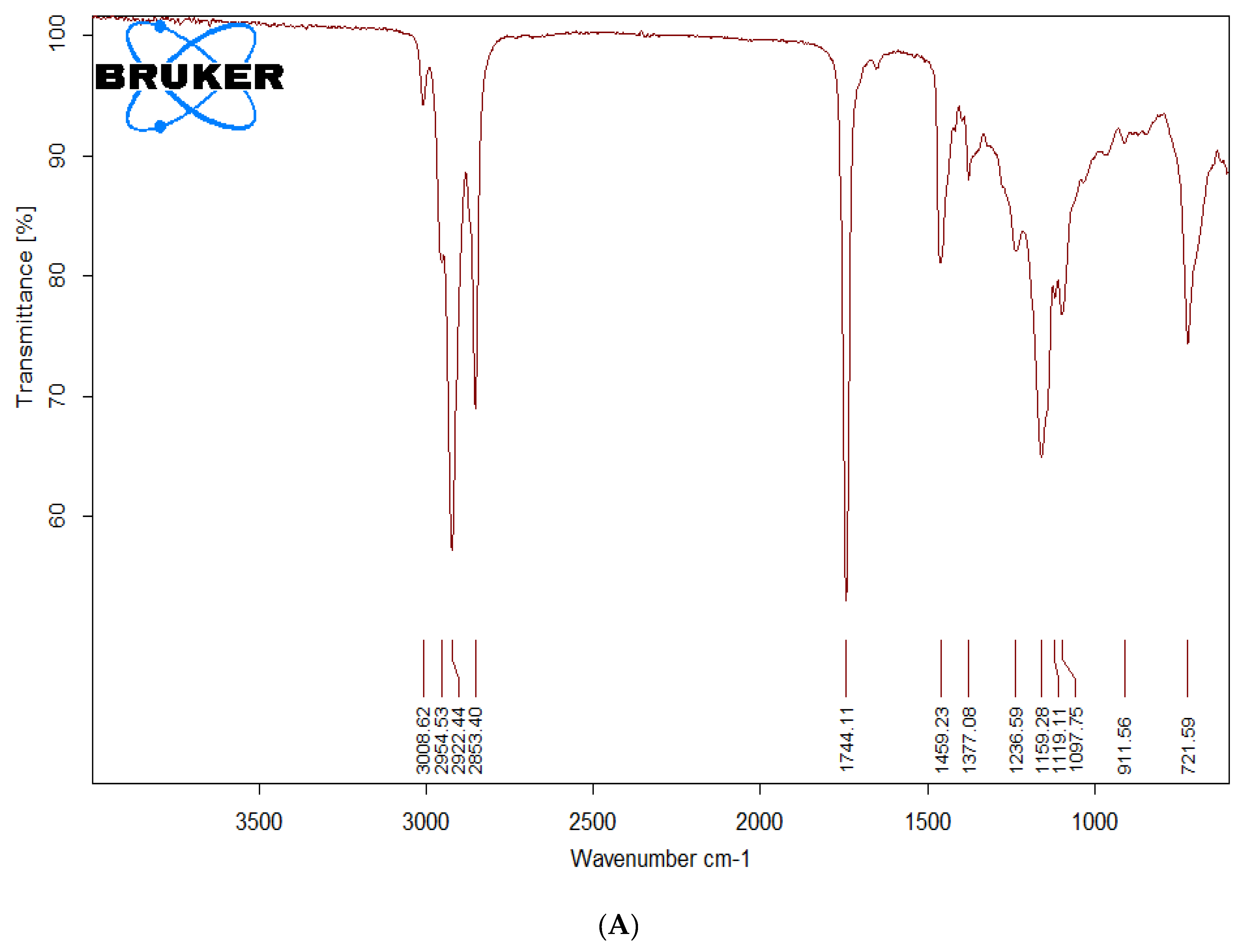
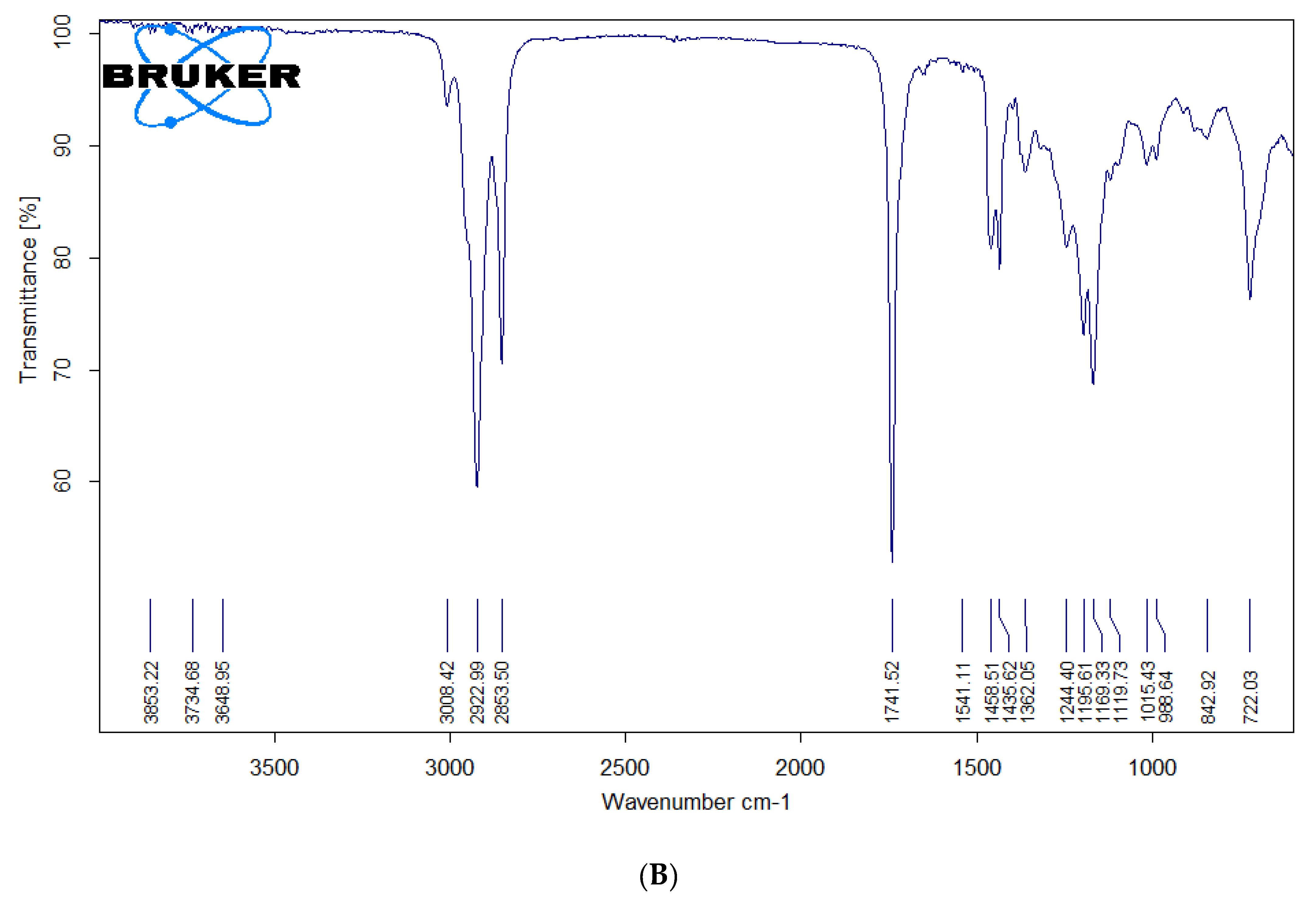
The formation of biodiesel was inferred from FTIR spectroscopy by comparison of spectra of precursor as well as transesterified product. As the precursor and final product are both esters differing only in the alkoxy moiety, it is quite rational to expect FTIR spectra of both the samples to be quite similar. However, there are few minor differences which can be used to differentiate the two. The region of C=O stretching typical of esters (1800–1700 cm
−1) is quite similar in precursor oil and transesterified product. The main spectral region that allows for chemical discrimination between precursor oil and the corresponding methyl ester is specifically the finger print region. The asymmetric stretching of –CH
3 appearing typically at 1446 cm
−1 is characteristic for the methyl ester (i.e., biodiesel) and is absent in the FTIR spectra of precursor oil. The O–CH
2 stretching of glycerol group (mono-, di- and triglycerides) appearing at 1377 cm
−1 is only present in oil spectrum and is expected to be absent in methyl ester derivative of oil. The peak around 1196 cm
−1 corresponds to O–CH
3 stretching, which is typical of biodiesel, and is absent in precursor oil. The asymmetric axial stretching of HO–CH
2− moiety appears in the range of 1075–1100 cm
−1. These peaks are present only in the spectrum of oil and are absent in that of biodiesel. The specific fingerprint region is between 400 cm
−1 and 1500 cm
−1 in IR spectrum [
19].
Figure 2 represents the FTIR spectra of the precursor oil (A) and the biodiesel (B) prepared by transesterification of the
T. indica seed oil.
3.2. Optimal Process Parameters
Table 5 shows the experimental yields of
T. indica methyl esters (TIMEs) from transesterification of seed oil, which were calculated using the L
9 orthogonal array. The table also includes the computed SNR and the average mean SNR values. As reported previously, the current study’s target necessitated adopting the ‘larger the better’ SNR model. Experiment 6 had the highest mean yield (93.17%) and SNR (39.41). On the other hand, Experiment 1 recorded the lowest mean yield (61.33%), with an SNR of (35.75). However, the set of parameters corresponding to maximum yield may not be the optimum set of parameters.
Table 6 shows the SNR
L (level mean SNRs) values for each experimental variable at each level listed. For example, the SNR
L for parameter A at level 1 has been calculated as ‘36.51’ using SNR values from Experiments No. 1, 2, and 3. At level 2, it has been calculated as ‘38.88’ using SNR values from Experiments No. 4, 5, and 6, and so on. The SNR
L values for each parameter at various levels demonstrate its impact on TIMEs yield. The higher the effect of a particular parameter at a given level, the higher the value of SNR
L. The maximum value of SNR
L, which is directly related to the maximum yield of TIMEs, corresponds to the optimum level of every parameter. The optimum levels for parameters A, B, and C were 1, 2, and 3, respectively, corresponding to the methanol-to-oil ratio of 6:1, an amount of catalyst 1.5% (wt./wt.), and a time of reaction of 2 h.
3.3. Analysis of Variance (ANOVA)
The highest percentage contribution is furnished by the molar ratio of alcohol to oil, i.e., 75.9%, which is in agreement with findings of Akhtar et al. [
13], for the production of biodiesel from cantaloupe seed oil, wherein the contribution of this experimental variable was even higher. Similarly, a small contribution was furnished by the reaction time which again complies with the above mentioned study. Furthermore, these percentage contributions are in correspondence with the variation in their respective SNRL values [
20]. The computed sum of squares (SS) for each parameter and the paramaters’ percentage contributions are shown in
Table 7. These findings aid in determining the most critical parameter with the most significant impact on the TIMEs yield. The ‘molar ratio of alcohol’ used to transform oil to methyl esters has the greatest effect (75.9%), followed by catalyst amount in relation to oil (20.7%) and reaction time (3.37%). This could be linked to minor variations in SNR
L values for each parameter at three levels. In other words, the % contribution of a parameter (∆SNR) to the mean yield of the final product is directly determined by the difference between the minimum and maximum SNR
L values for that parameter. These parameters can be ranked using ∆SNR calculations, with the highest rank going to the parameter with the highest value of ∆SNR. According to the data in
Table 6 and
Table 7, the ‘alcohol to oil molar ratio’ ranks first, followed by the catalyst amount and reaction time. In three replicate trials, the optimal levels of all parameters were used to assess the percentage yield of TIMEs. The average biodiesel yield was 93.5%, similar to the result obtained in Experiment No. 6. As for optimization of biodiesel production using the Taguchi method, the amount of catalyst, molar ratio of alcohol to oil, and reaction time and temperature of reaction were influencing parameters [
20].
3.4. Fuel Properties of Methyl Esters
Table 8 shows the different fuel characteristics/properties of biodiesel obtained from the transesterification of seed oil. The kinematic viscosity was determined to be 5.4 mm
2/s, critical in the spraying of fuel and the formulation of mixtures and combustion procedure. However, high kinematic viscosity interferes with the injection process, causing improper atomization of the fuel. Thus, it should be kept within limits defined in biodiesel standards such as ASTM D6751. The flash point (FP) was discovered to be 180 °C, which is an essential feature of fuel in terms of transportation and storage protection. The fuel volatility is also linked to flash point, a significant factor in starting and warming an engine. Low fuel volatility combined with high viscosity causes weak engine start-up (cold), ignition delay, and misfire [
21]. The pour point and cloud point of a liquid fuel were −2 and 5 °C, which correspondingly determine a fuel’s cold-weather consistency.
An acid value greater than 3% causes various operational issues such as pump corrosion and deposit formation. The acid value of the prepared biodiesel was 0.31 mg KOH/g, which is within acceptable limits. CN measures fuel ignition delay, a period between fuel injection and combustion. The ignition delay decreases with increasing cetane number, allowing the main combustion process to extend (diffusion-controlled combustion). The CN of prepared biodiesel is 47, which is well within acceptable limits. If the CN of fuel is greater than 65, it can ignite in a short time and at a great distance from the injector, allowing it to overheat, resulting in cooked particles and blocking of the injector nozzle. The Cu-strip corrosion, the one remaining characteristic, was also found to be within defined limits. Thus,
T. indica seed oil can be used as a possible feedstock to synthesize biodiesel, since all of the fuel properties are within ASTM D6751 limits (
Table 8).
Biodiesel has some added benefits when it is compared with traditional diesel fuels because of its lesser pollution, lower toxicity, eco-friendliness, and renewability attributes. Through the process of transesterification, biodiesel could be synthesized from different edible and non-edible sources. Non-edible available resources are normally consumed to prepare biodiesel because of their lack of dependency on the food chain and low cost. Non-edible sources include non-edible vegetable oil, algal oil, animal waste oil, and cooking waste oil. The production mechanism depends upon various factors such as time and temperature of reaction mixture, alcohol-to-oil molar ratio, catalyst type, and catalyst concentration. Through use of alternate technologies, different suitable types of catalyst, and suitable feedstock, the cost of biodiesel could be reduced from an economic perspective. Furthermore, cost could be reduced through some low-cost alternate raw materials, selling of by-products, operational labor cost, optimum operation conditions, certain types of catalysts, and mechanism of reaction. One of the major by-products which is produced during its reaction is crude glycerol, which has yields ranging from 8.0% to 10.1%. This crude glycerol could be used to prepare hydrogen, biopolymers, and ethanol or serve as an additive for fuel via gasification and pyrolysis processes. However, this work is more concerned with transesterification for non-edible reserves of biodiesel preparation along with economical aspects, by-product application, and fuel characteristics. Lastly, process optimum conditions along with economic parameters for biodiesel production should be evaluated as important parameters in order to achieve economic sustainability for biodiesel production [
22].
Various methods for biodiesel production through different feedstocks have been employed in recent past. Increase in the price of petroleum-based fuels and depletion of energy reservoirs has led to the exploitation of nonrenewable resources. Therefore, there is a certain need to look for sustainable and suitable alternatives to traditional fuels. The major attributes for substituted fuel should be renewability, ready availability, and lesser dependency on limited resources, which could result in lesser pollution or no pollution at all. Biodiesel has attracted interest because of its eco-friendly and non-toxic properties. Nano-catalyst technology has also been used in recent years for the synthesis of biodiesel because of its various positive attributes such as re-usability, large surface area, and increased activity [
21].
It is reported that biodiesel prepared from the indigenous plant
Salvadora persica L. seed oil meets the international standards for biodiesel (ASTM D6751) and a one-step transesterification procedure is sufficient for the preparation of biodiesel. The yield of biodiesel was 1.57 g/5 g (31.40 percent by weight), and in-situ transesterification ester content conversion was 97.70%. Density of the biodiesel produced was 0.893 g/mL, the kinematic viscosity was 5.51 mm
2/s, 210 °C was the flash point, CN was 61, and sulfur content was 0.0844%. At 595 °C, full oxidation of biodiesel resulted in a 97.0% weight loss, according to thermal analysis [
23,
24].
Waste-oil-based biodiesel is a better approach to preparing economical biodiesel, but a problem that may arise is that the process of transesterification may be hindered by the presence of much higher quantity of the free fatty acids (FFA). This process involves the consumption of wasted cooking oil in order to prepare good yield of biodiesel. Higher percentages of FFA were reported through acid value (5.49 mg KOH/gm) testing of waste cooking oil. Esterification processes were utilized through various acid-based catalysts such as sulfuric acid, hydrochloric acids and phosphoric acid. Among all, sulfuric-acid-based catalyst was proved to be more effective, because its FFA value was reduced to 88.78% at 59 °C, with oil-to-methanol molar ratio 2.5:1. The process of transesterification was carried out in the presence of alkali-catalyst-like potassium hydroxide, while output of fatty acid methyl ester was a maximum of 94% in the presence of 1.1% catalyst at 510 °C. Biodiesel produced from these reactions was evaluated through different tests such as specific gravity, acid value, cloud point, calorific point, iodine value, break test, CN, saponification value, and pour point. Further biodiesel testing was performed through gas chromatography. Waste-cooking-oil-based biodiesel was best synthesized through an alkali-catalyst-based transesterification process. Through data analysis, it was proved that waste cooking oil could be used as an effective resource for biodiesel production along with lessening environmental pollution for society [
1,
18,
19].
The sustainability of the supply of fuel dependent on petroleum has received wide attention due to increased use in different industries and petroleum resource depletion. In the global community, market values for crude oil are volatile and uncertain. Additionally, there are also environmental issues rising due to emissions of toxic contaminants and greenhouse gases. Accordingly, it is important to use renewable energy sources, including biodiesel. Biodiesel is primarily created from non-limited resources of nature by a method of transesterification. This offers different benefits; for example, it is nontoxic, biodegradable, and eco-friendly over petro-diesel; the emissions it creates, apart from being healthy, have minimal sulfur and aromatic content [
25].
A daunting challenge is to use sustainable feedstock for alternative fuel production. Since fossil-fuel reserves are rapidly depleting, and the price of crude petroleum is fluctuating, the need to find the importance of new fuel is growing. In addition, renewable fuels must be environmentally sustainable, inexpensive, technically appropriate, and plentiful. The chemical or lipase-catalyzed transesterification of fats and oils to produce biodiesel results in the formation of fatty acid alkyl esters, an environmentally friendly substitute liquid fuel. In addition to its green roots, it has economic as well as environmental advantages. For biodiesel production, animal fats and vegetable oils are important feedstocks. Since the conversion of edible oils into fuels is limited, demand for biodiesel made from non-edible oils is steadily rising. Researchers are therefore searching for hopeful new non-digestible oil sources that can support the formation and use of biodiesel [
26].
Recently, biodiesel has become a genuine potential substitute for petroleum fuels because of a variety of desirable properties and its eco-friendly nature. However, the high cost of raw material that is used for its production is a major block to its economic viability. It has been found that biodiesel can be derived from
Eriobotrya japonica (
E. japonica) seeds oil by alkali-catalyzed transesterification, using the Taguchi method for enhancement of production parameters such as reaction time, catalyst amount, temperature, and alcohol-to-water molar ratio. Maximum production of biodiesel of almost 94.53% was obtained using optimum conditions including alcohol-to-water ratio 6:1, amount of catalyst 1% wt./wt., temperature 50 °C, and reaction time 2 h. The ideal conditions for obtaining 94.52% were found to be the reaction temperature. The catalyst amount (67.32 percent) had the highest contribution, followed by molar alcohol-to-oil ratio (25.51 percent). Significant fuel characteristics of
E. japonia methyl esters formed under optimum conditions within the defined ASTM D6751 limits have been established. Biodiesel may also be considered a prospective replacement for petro-diesel [
18].
Biodiesel is biodegradable and non-toxic, as opposed to petroleum diesel. The need for food competes with the utilization of edible vegetable oils for the formation of biodiesel. As long-term biodiesel sources, the continuously growing price makes biodiesel made from edible vegetable oils uneconomical. Waste vegetable oils, in addition to being a possible primary source for the formation of biodiesel, are considered to be environmental pollution, in addition to being inexpensive and easily accessible [
27,
28,
29,
30,
31]. In the presence of a catalyst, transesterification of vegetable oil is carried out to generate biodiesel. The catalyst can be homogeneous, heterogeneous, enzymatic, or nanoparticles. Homogeneous catalysts are thought to be more efficient than heterogeneous counterparts due to lower mass transfer limitations and higher conversion. Because of the difficulties in separating and purifying biodiesel derived from homogeneously acid/base-catalyzed transesterification of vegetable oils, the emphasis has shifted to non-catalytic supercritical ethanol/methanol. It has been recorded that 95 percent conversion can be achieved using supercritical methanol at a reaction temperature of 350 °C, a methanol/oil molar ratio of 42, and a time of 400 s. In addition to the impact of the process variable, this study discusses and presents the benefits and drawbacks of biodiesel production processes, as well as illustrating the main data set that is currently unavailable, which would improve commercialization and economics and increase biodiesel production versus other energy sources [
27,
31,
32,
33,
34,
35,
36,
37,
38].