Facile Preparation of Porous Carbon Derived from Pomelo Peel for Efficient Adsorption of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PPPC
2.3. Material Characterizations
2.4. Adsorption and Regeneration Experiments
3. Results and Discussion
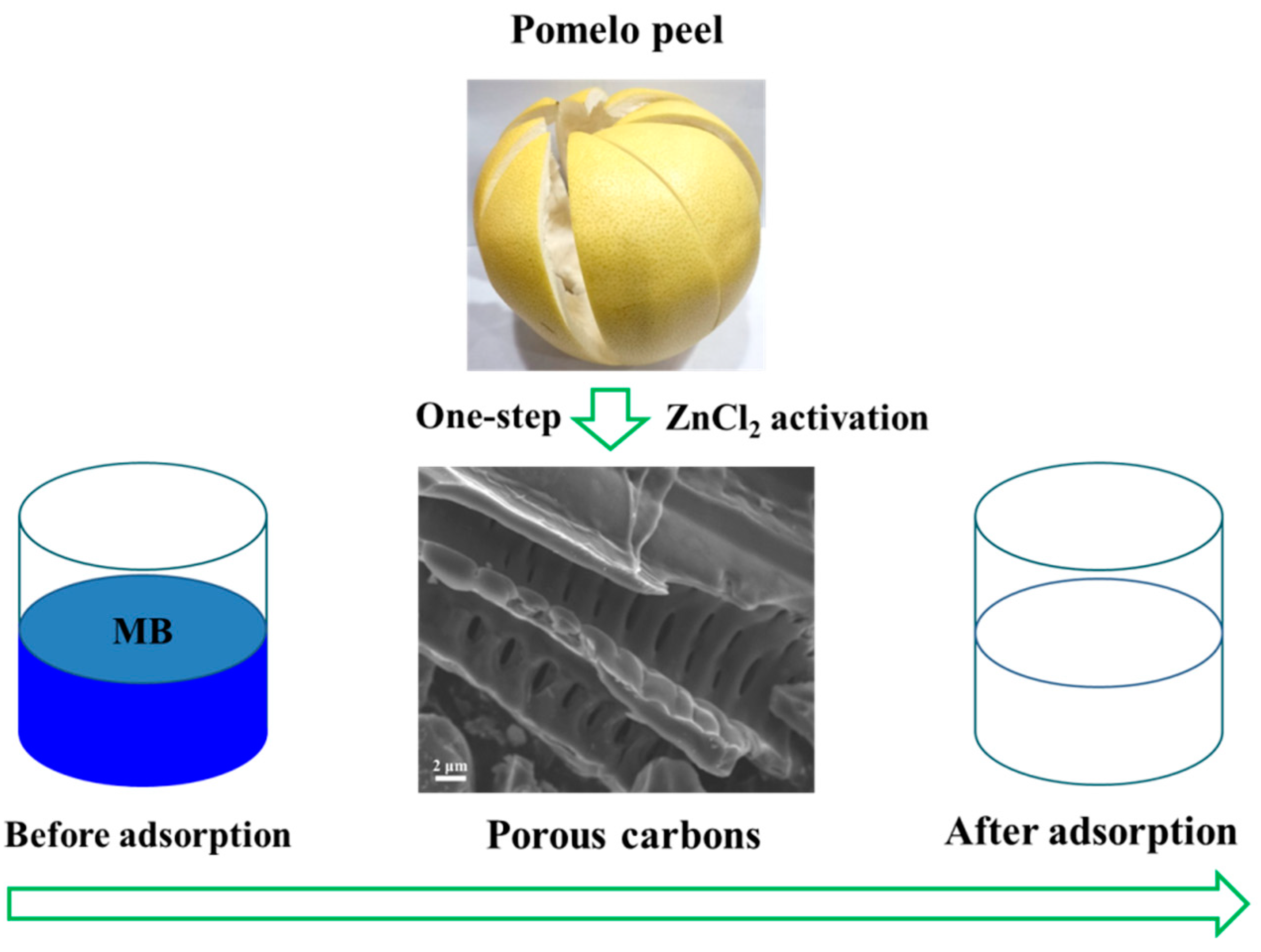
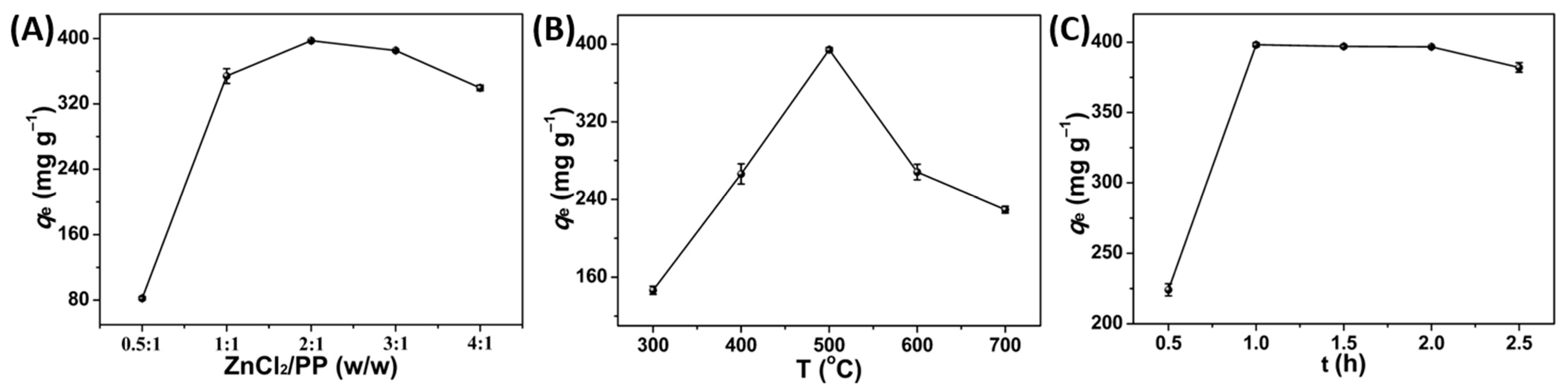
3.1. Synthesis of PPPC
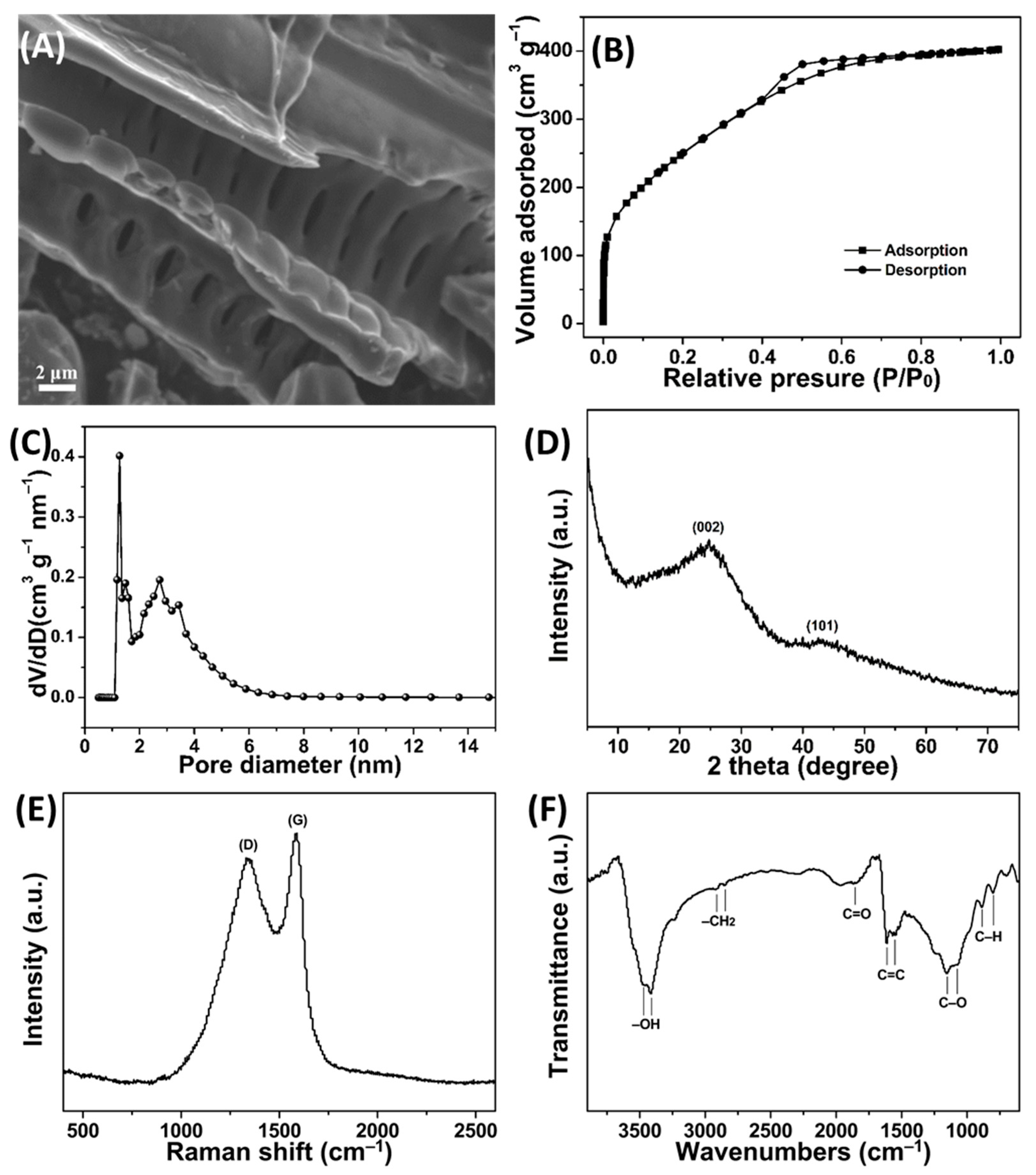
3.2. Characterizations of PPPC
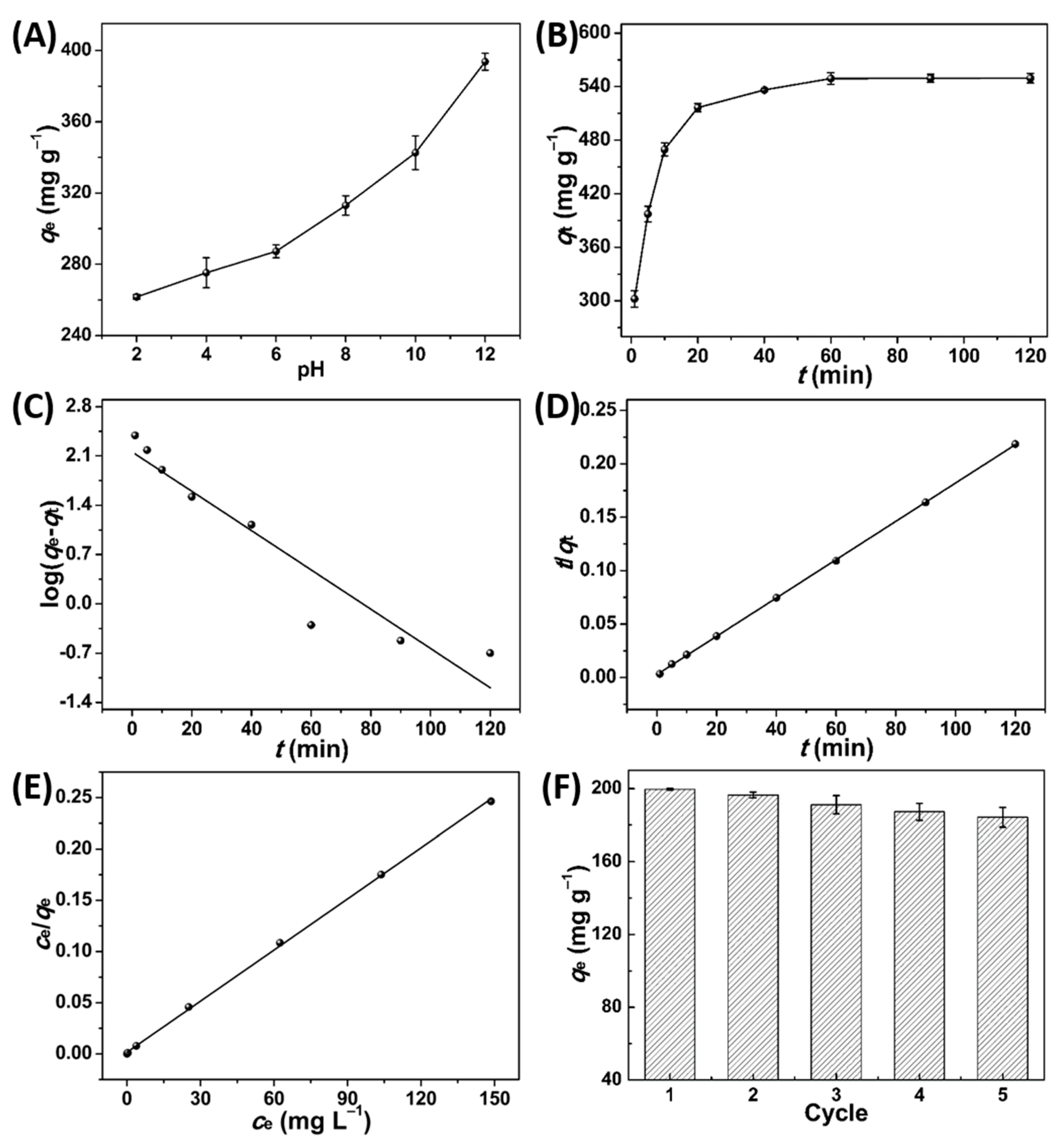
3.3. Adsorption Performance of PPPC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, K.; Wang, T.; Zhai, L.; Wu, W.; Dong, S.; Gao, S.; Mao, L. Adsorption behavior and mechanism of Fe-Mn binary oxide nanoparticles: Adsorption of methylene blue. J. Colloid Interface Sci. 2019, 539, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Rezakazemi, M.; Shirazian, S. Lignin-chitosan blend for methylene blue removal: Adsorption modeling. J. Mol. Liq. 2019, 274, 778–791. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Tang, J.; Lu, H.; Liu, Y. Ginger straw waste-derived porous carbons as effective adsorbents toward methylene blue. Molecules 2019, 24, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Zeng, G.; Chen, A.; Huang, Z.; Peng, M.; Huang, T.; Chen, G. Graphene hybridized polydopamine-kaolin composite as effective adsorbent for methylene blue removal. Compos. Part B Eng. 2019, 161, 141–149. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Yan, L.; Wang, G.; Liu, A. Recent advances in the synthesis of spherical and nanoMOF-derived multifunctional porous carbon for nanomedicine applications. Coordin. Chem. Rev. 2019, 391, 69–89. [Google Scholar] [CrossRef]
- Yu, M.; Han, Y.; Li, J.; Wang, L. CO2-activated porous carbon derived from cattail biomass for removal of malachite green dye and application as supercapacitors. Chem. Eng. J. 2017, 317, 493–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, G.; Li, C.; Wang, X.; Li, A. Templating synthesis of hierarchical porous carbon from heavy residue of tire pyrolysis oil for methylene blue removal. Chem. Eng. J. 2020, 390, 124398. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Zhang, W.; Zhou, Z.; Li, C.M. γ-Fe2O3 nanocrystals-anchored macro/meso-porous graphene as a highly efficient adsorbent toward removal of methylene blue. J. Colloid Interface Sci. 2016, 476, 200–205. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived porous carbons as a superior adsorbent toward removal of methylene blue. J. Solid State Chem. 2016, 243, 101–105. [Google Scholar] [CrossRef]
- Wong, K.T.; Eu, N.C.; Ibrahim, S.; Kim, H.; Yoon, Y.; Jang, M. Recyclable magnetite-loaded palm shell-waste based activated carbon for the effective removal of methylene blue from aqueous solution. J. Clean. Prod. 2016, 115, 337–342. [Google Scholar] [CrossRef]
- Rashid, R.; Jawad, A.; Azlan, M.; Ishak, M.; Kasim, N. FeCl3-activated carbon developed from coconut leaves: Characterization and application for methylene blue removal. Sains Malays. 2018, 47, 603–610. [Google Scholar] [CrossRef]
- Pathania, D.; Sharma, S.; Singh, P. Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast. Arab. J. Chem. 2017, 10, S1445–S1451. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Fakudze, S.; Zhang, Y.; Ma, R.; Shang, Q.; Chen, J.; Liu, C.; Chu, Q. Co-hydrothermal carbonization of pomelo peel and PVC for production of hydrochar pellets with enhanced fuel properties and dechlorination. Energy 2022, 239, 122350. [Google Scholar] [CrossRef]
- Tocmo, R.; Pena-Fronteras, J.; Calumba, K.F.; Mendoza, M.; Johnson, J.J. Valorization of pomelo (Citrus grandis Osbeck) peel: A review of current utilization, phytochemistry, bioactivities, and mechanisms of action. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1969–2012. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Zhang, H.; Liu, Q.; Li, R.; Li, B.; Wang, J. Three-dimensional hierarchical and interconnected honeycomb-like porous carbon derived from pomelo peel for high performance supercapacitors. J Solid State Chem. 2017, 257, 64. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhang, L.; Tian, Y.; Gui, G.; Yang, S. A cost-effective porous carbon derived from pomelo peel for the removal of methyl orange from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 191. [Google Scholar] [CrossRef]
- Olivaresmarin, M.; Fernandezgonzalez, C.; Maciasgarcia, A.; Gomezserrano, V. Preparation of activated carbon from cherry stones by chemical activation with ZnCl2. Appl. Surf. Sci. 2006, 252, 5967–5971. [Google Scholar] [CrossRef]
- Wang, H.; Yan, T.; Shen, J.; Zhang, J.; Shi, L.; Zhang, D. Efficient removal of metal ions by capacitive deionization with straw waste derived graphitic porous carbon nanosheets. Environ. Sci. Nano 2020, 7, 317–326. [Google Scholar] [CrossRef]
- Zhu, G.; Liu, Q.; Cao, F.; Qin, Q.; Jiao, M. Silkworm cocoon derived N, O-codoped hierarchical porous carbon with ultrahigh specific surface area for efficient capture of methylene blue with exceptionally high uptake: Kinetics, isotherm, and thermodynamics. RSC Adv. 2019, 9, 33872–33882. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, M.; Li, J.; Li, J.; Xu, J.; Wang, X. Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J. Mater Chem. 2014, 2, 4391–4397. [Google Scholar] [CrossRef]
- Xian, F.; Gao, L.; Zhang, Z.; Zhang, H.; Dong, S.; Cui, G. N,P dual-doped multi-wrinkled nanosheets prepared from the egg crude lecithin as the efficient metal-free electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2019, 476, 76–83. [Google Scholar] [CrossRef]
- Zou, J.; Dai, Y.; Liu, D.; Wang, S.; Zhou, L.; Zhou, Y. Synthesis of carboxyl-functionalized magnetic nanoparticle for the removal of methylene blue. Colloid Surf. A Physicochem. Eng. Asp. 2019, 572, 58–66. [Google Scholar]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Singh, R.; Pal, D.; Mathur, A.; Singh, A.; Krishnan, M.A.; Chattopadhyay, S. An efficient pH sensitive hydrogel, with biocompatibility and high reusability for removal of methylene blue dye from aqueous solution. React. Funct. Polym. 2019, 144, 104346. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Huang, W.; Cao, B. Selective adsorption and separation of organic dyes in aqueous solutions by hydrolyzed PIM-1 microfibers. Chem. Eng. Res. Des. 2016, 109, 76–85. [Google Scholar] [CrossRef]
- Dai, J.; Sun, J.; Xie, A.; He, J.; Li, C.; Yan, Y. Designed preparation of 3D hierarchically porous carbon material via solvothermal route and in situ activation for ultrahigh-efficiency dye removal: Adsorption isotherm, kinetics and thermodynamics characteristics. RSC Adv. 2016, 6, 3446–3457. [Google Scholar] [CrossRef]
- Nasrullah, A.; Saad, B.; Bhat, A.; Khan, A.S.; Danish, M.; Isa, M.H.; Naeem, A. Mangosteen peel waste as a sustainable precursor for high surface area mesoporous activated carbon: Characterization and application for methylene blue removal. J. Clean. Prod. 2019, 211, 1190–1200. [Google Scholar] [CrossRef]
- Shen, X.; Huang, P.; Li, F.; Wang, X.; Yuan, T.; Sun, R. Compressive alginate sponge derived from seaweed biomass resources for methylene blue removal from wastewater. Polymers 2019, 11, 961. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Zhang, L.; Ma, A.; Xia, H.; Peng, J.; Li, C.; Shu, J. Comparison of activated carbon and iron/cerium modified activated carbon to remove methylene blue from wastewater. J. Environ. Sci. 2018, 65, 92–102. [Google Scholar] [CrossRef]
- Jin, Q.; Li, Y.; Yang, D.; Cui, J. Chitosan-derived three-dimensional porous carbon for fast removal of methylene blue from wastewater. RSC Adv. 2018, 8, 1255–1264. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Wei, X.; Liu, G.; Mu, M.; Ma, X.; Gao, Y.; Zong, Z. CO2-hierarchical activated carbon prepared from coal gasification residue: Adsorption equilibrium, isotherm, kinetic and thermodynamic studies for methylene blue removal. Chin. J. Chem. Eng. 2020, 28, 1694–1700. [Google Scholar] [CrossRef]
- Ladshaw, A.; Yiacoumi, S.; Lin, R.; Nan, Y.; Tavlarides, L.L.; Tsouris, C. A mechanistic modeling framework for gas-phase adsorption kinetics and fixed-bed transport. AIChE J. 2017, 63, 5029–5043. [Google Scholar] [CrossRef]
- Li, X.; Han, D.; Xie, J.; Wang, Z.; Gong, Z.; Li, B. Hierarchical porous activated biochar derived from marine macroalgae wastes (Enteromorpha prolifera): Facile synthesis and its application on Methylene Blue removal. RSC Adv. 2018, 8, 29237–29247. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, D.; Xu, J.; Zhang, L.; Zhang, X. Graphene oxide gel-derived, free-Standing, hierarchically porous carbon for high-capacity and high-rate rechargeable LiO2 batteries. Adv. Funct. Mater. 2012, 22, 3699–3705. [Google Scholar] [CrossRef]
- Kim, K.; Park, S. Synthesis and electrochemical performance of well-balanced mesopore/micropore contained carbons by activation-free method. Electrochem. Commun. 2012, 22, 89–92. [Google Scholar] [CrossRef]
- Xu, F.; Cai, R.; Zeng, Q.; Zou, C.; Wu, D.; Li, F.; Liu, X.; Liang, Y.; Fu, R. Fast ion transport and high capacitance of polystyrene-based hierarchical porous carbon electrode material for supercapacitors. J. Mater. Chem. 2011, 21, 1970–1976. [Google Scholar] [CrossRef]
- Peng, X.; Huang, D.; Odoomwubah, T.; Fu, D.; Huang, J.; Qin, Q. Adsorption of anionic and cationic dyes on ferromagnetic ordered mesoporous carbon from aqueous solution: Equilibrium, thermodynamic and kinetics. J. Colloid Interf. Sci. 2014, 430, 272–282. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, Y.; Hao, J.; Liu, Y.; Li, W.; Li, J. N and P co-functionalized three-dimensional porous carbon networks as efficient metal-free electrocatalysts for oxygen reduction reaction. Carbon 2017, 122, 64–73. [Google Scholar] [CrossRef]
- Wang, Z.; Tan, Y.; Yang, Y.; Zhao, X.; Liu, Y.; Niu, L.; Tichnell, B.; Kong, L.; Kang, L.; Liu, Z.; et al. Pomelo peels-derived porous activated carbon microsheets dual-doped with nitrogen and phosphorus for high performance electrochemical capacitors. J. Power Sources 2018, 378, 499–510. [Google Scholar] [CrossRef]
- Basaleh, A.A.; Almalack, M.H.; Saleh, T.A. Methylene Blue removal using polyamide-vermiculite nanocomposites: Kinetics, equilibrium and thermodynamic study. J. Environ. Chem. Eng. 2019, 7, 103107. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus pectin derived ultrasmall Fe3O4@C nanoparticles as a high-performance adsorbent toward removal of methylene blue. J. Mol. Liq. 2016, 222, 995–1002. [Google Scholar] [CrossRef]
- Liu, F.; Zou, H.; Hu, J.; Liu, H.; Peng, J.; Chen, Y.; Lu, F.; Huo, Y. Fast removal of methylene blue from aqueous solution using porous soy protein isolate based composite beads. Chem. Eng. J. 2016, 287, 410–418. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotox. Environ. Safe. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Lu, Q.; Li, Q.; Zhang, J.; Li, J.; Lu, J. Facile mesoporous template-assisted hydrothermal synthesis of ordered mesoporous magnesium silicate as an efficient adsorbent. Appl. Surf. Sci. 2016, 360, 889–895. [Google Scholar] [CrossRef]
- Gan, W.; Shang, X.; Li, X.; Zhang, J.; Fu, X. Achieving high adsorption capacity and ultrafast removal of methylene blue and Pb2+ by graphene-like TiO2@C. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 218–225. [Google Scholar] [CrossRef]
| Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||||||
|---|---|---|---|---|---|---|---|
| c0 | qe,exp | qe,cal | k1 | R2 | qe,cal | k2 | R2 |
| (mg L−1) | (mg g−1) | (mg g−1) | (min−1) | (mg g−1) | (g mg−1 min−1) | ||
| 300 | 549.6 | 143.4 | 0.06 | 0.8973 | 558.7 | 0.0012 | 0.9999 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qm (mg g−1) | b (L mg−1) | R2 | k | 1/n | R2 |
| 602.4 | 1.02 | 0.9995 | 298.33 | 0.17 | 0.8327 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Liu, M.; Zhao, Y.; Liao, Q. Facile Preparation of Porous Carbon Derived from Pomelo Peel for Efficient Adsorption of Methylene Blue. Molecules 2022, 27, 3096. https://doi.org/10.3390/molecules27103096
Zhang W, Liu M, Zhao Y, Liao Q. Facile Preparation of Porous Carbon Derived from Pomelo Peel for Efficient Adsorption of Methylene Blue. Molecules. 2022; 27(10):3096. https://doi.org/10.3390/molecules27103096
Chicago/Turabian StyleZhang, Wenlin, Mingwan Liu, Yuhong Zhao, and Qinhong Liao. 2022. "Facile Preparation of Porous Carbon Derived from Pomelo Peel for Efficient Adsorption of Methylene Blue" Molecules 27, no. 10: 3096. https://doi.org/10.3390/molecules27103096
APA StyleZhang, W., Liu, M., Zhao, Y., & Liao, Q. (2022). Facile Preparation of Porous Carbon Derived from Pomelo Peel for Efficient Adsorption of Methylene Blue. Molecules, 27(10), 3096. https://doi.org/10.3390/molecules27103096





