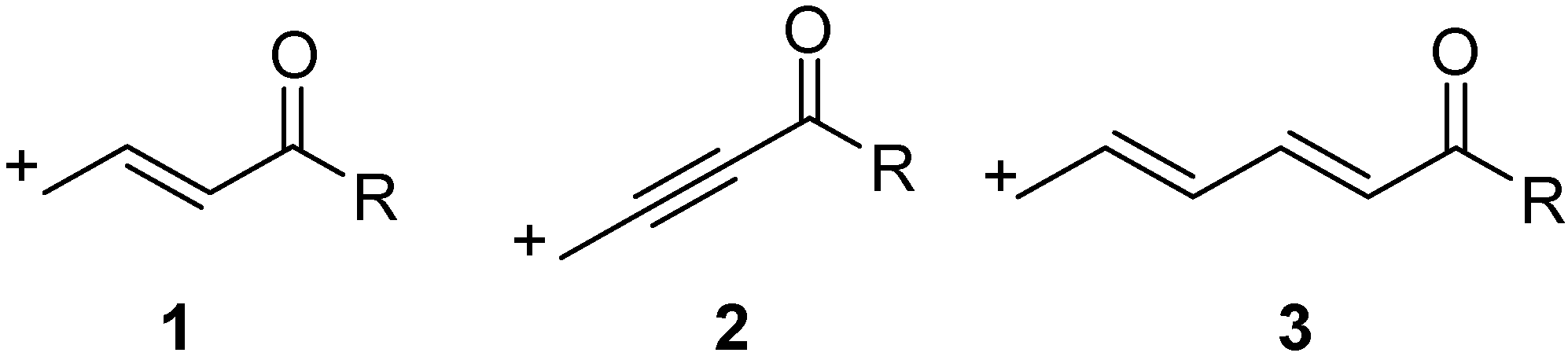
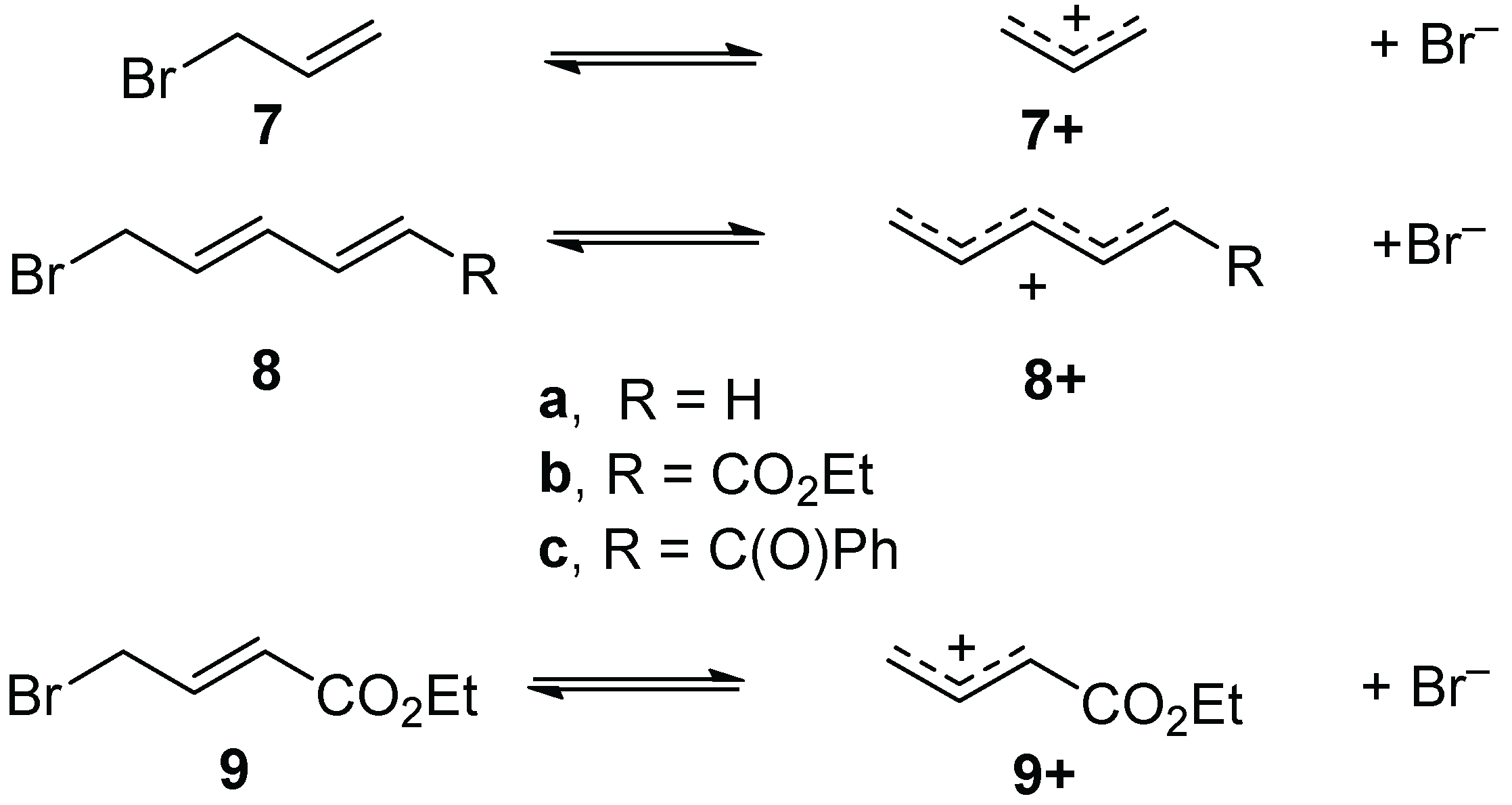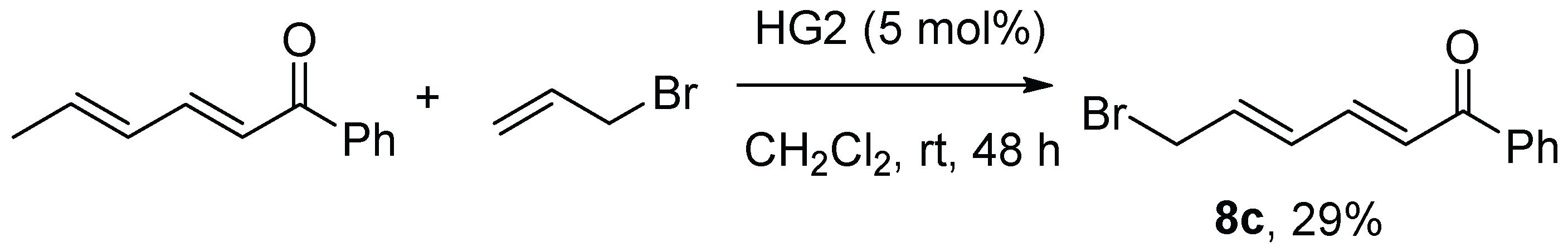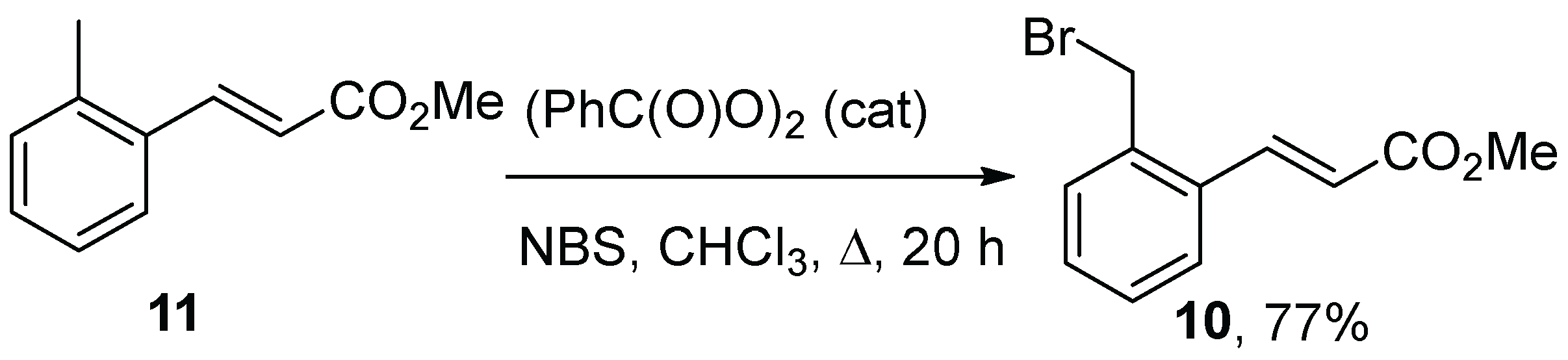2. Results
The viability of direct generation of ε-carbonyl cations was initially addressed computationally, using DFT calculations employing the B3LYP functional and 6-311++G(d,p) basis set. The allyl bromide (
7) to allyl cation (
7+) transformation was the benchmark with which to compare results, as the viability of experimentally verified allyl cation synthetic chemistry has been established, most notably with indium(III) and related catalysts [
25,
26,
27,
28]. Compared to this was ionization of 5-bromo-1,3-pentadiene (
8a) to give pentadienyl cation (
8a+), and the analogous ionizations of ethyl 6-bromosorbate (
8b), 6-bromo-1-phenyl-2,4-butan-1-one (
8c). In addition, the ethyl 4-bromocrotonate (
9) to γ-carbonyl cation species
9+ transformation was included, as an example of a process that has proven difficult experimentally (
Scheme 1,
Table 1).
The results of the calculations were promising. The ionization energy of
8a to dienyl cation
8a+ was unsurprisingly the most favored, the process being 16.8 kcal/mol lower in energy than allyl cation generation. Somewhat to our surprise, the ionizations of the ε-carbonyl cation precursors
8b and
8c also were found to be favored substantially (by 10.9 kcal and 12.7 kcal, respectively), relative to the process with allyl bromide. Finally, the analogous ionization of ethyl 4-bromocrotonate was found to be 6.9 kcal/mol higher in energy than that of allyl bromide, consistent with the difficulty in discrete generation of γ-carbonyl cations. As a result of these findings, we chose to test these observations with an experiment. Given the notably mild conditions reported in the group 13 catalyzed electrophilic reactions of allyl bromides [
25,
26,
27,
28], we chose to pursue the analogous approach for ε-carbonyl cations.
The ester- and phenyl ketone-substituted dienyl bromides,
8b–
8c, were chosen as substrates. Ethyl 6-bromohexadienoate (ethyl 6-bromosorbate,
8b) was obtained by literature radical bromination of ethyl sorbate [
29]. Phenyl ketone
8c was prepared from 1-phenyl-2,4-butadienone [
30], by HG-II-induced cross metathesis with allyl bromide (
Scheme 2) [
31].
In addition, a third substrate chosen for the study was
10, employing an aryl spacer rather than one of the alkene spacers between the ester and bromide. Compound
10 was prepared by the radical bromination of cinnamate ester derivative
11 (
10, 77%) (
Scheme 3), itself being prepared by the Wittig reaction of
o-tolualdehyde [
32].
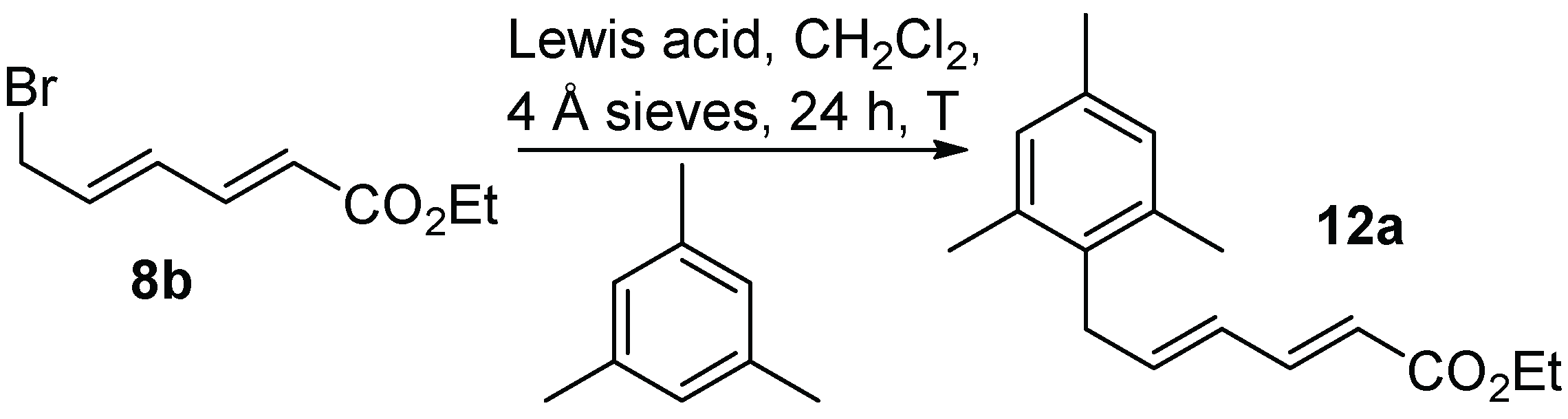
Experimental work began with ethyl 6-bromohexadienoate (ethyl 6-bromosorbate,
8b). Test reactions were undertaken with mesitylene (5 equiv) as the nucleophile, and catalytic amounts (10 mol%) of Lewis acids CuCl, SnCl
4, InCl
3, GaCl
3, and BiI
3, in CH
2Cl
2 with 4 Å molecular sieves (
Table 2,
Scheme 4). CuCl and BiI
3 afforded no product and minimal amounts of product, respectively. Conversely, GaCl
3, InCl
3, and SnCl
4 gave more significant amounts of conversion to
12a over 24 h, although small amounts of starting material remained. Repetition of the reactions at reflux afforded complete starting material consumption, but also gave some polar decomposition byproduct. Ultimately, GaCl
3 at room temperature proved to be the most successful Lewis acid, giving
12a in a 68% yield. Reducing the amount of GaCl
3 to 5 mol% decreased the yield noticeably (47%), while an increase to 15 mol% made a negligible difference (67% yield). Omission of the 4 Å molecular sieves also gave a decrease in the yield of
12a (51%, 58% brsm).
The characterization of 12a was most clearly defined from the 1H NMR spectrum, which revealed a doublet (J = 15.4 Hz) at 5.77 ppm (Hα), a doublet of doublets (J = 15.4, 11.0 Hz) at 7.30 ppm (Hβ), a doublet of doublets (J = 15.2, 11.0 Hz) at 6.02 ppm (Hγ), and doublet of triplets (J = 15.2, 5.7 Hz) at 6.23 ppm (Hδ), indicative of the conjugated diene of (E, E-) geometry resulting from ε-substitution. A small amount (<5% of the mixture) of isomeric material was co-eluted with the main product. Most of the 1H NMR spectral resonances are obscured by the dominant isomer due to the similar 1H spectral features, but with the Hε methylene observable as a doublet of doublets (J = 7.4, 1.5 Hz) at 3.65 ppm, and with the Hβ observable as a doublet of doublets (J = 15.1, 11.6 Hz) at 7.85 ppm, we have assigned this minor compound as the (2E, 4Z)-isomer of 12a.
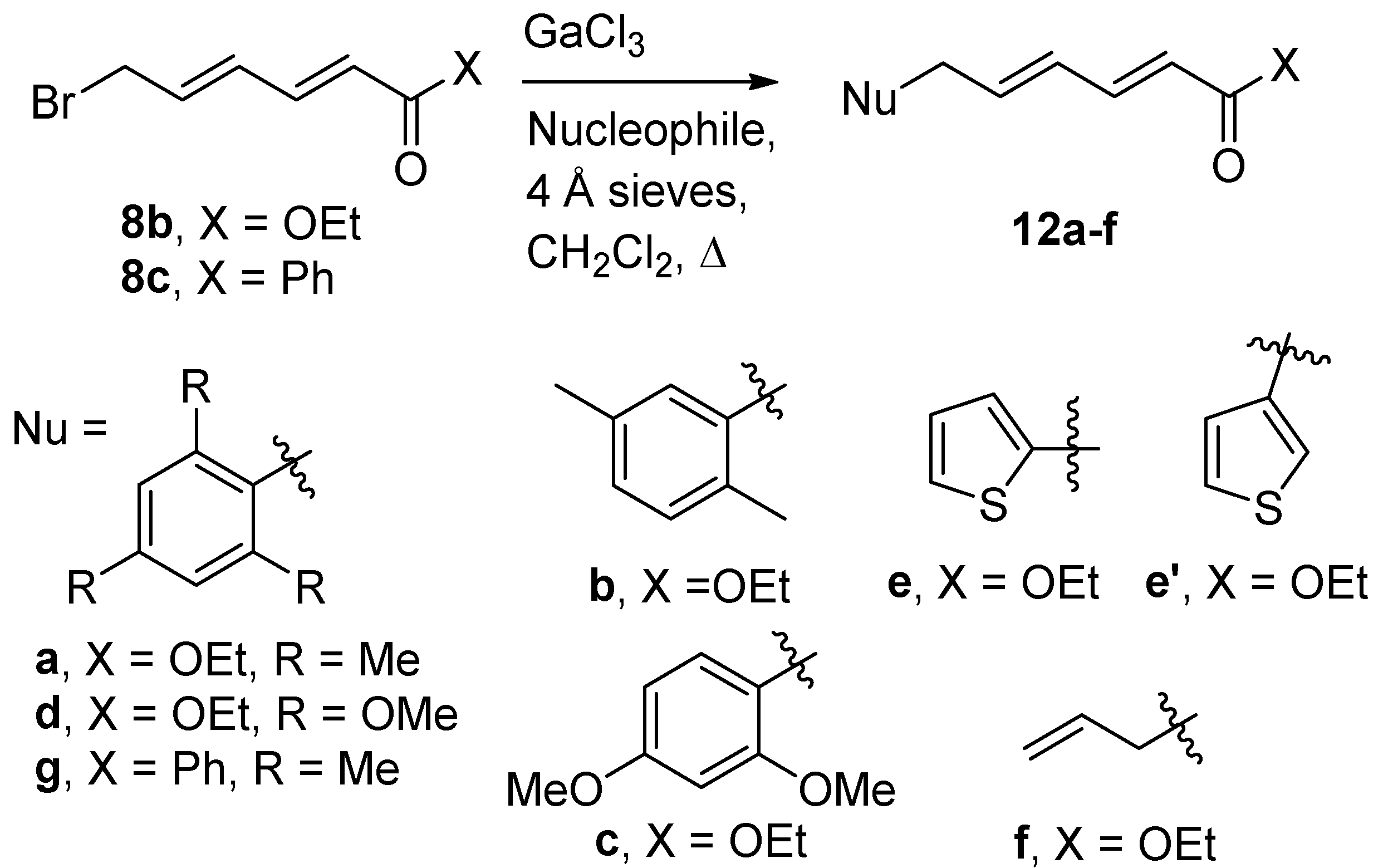
These conditions were adopted for other arene nucleophiles, with the exception that the yields were found to be, in general, superior for other nucleophiles at reflux (
Scheme 5,
Table 3).
p-Xylene, under analogous conditions, gave a modest yield of
12b at rt (33% yield, 54% brsm), but better yields (65%) at reflux. 1,3-Dimethoxybenzene gave
12c in 56% yield at reflux, while 1,3,5-trimethoxybenzene required 20 mol% GaCl
3 for complete conversion, giving
12d in 51% yield. Thiophene gave a 63% yield of product, as a 72:28 mixture C-2 (
12e) and C-3 (
12e′) substitutions. With allyltrimethylsilane, no condensation product was observed with 10 mol% GaCl
3. Switching the catalyst to InCl
3 was much more successful; 10 mol% InCl
3 in CH
2Cl
2 at reflux gave approximately 80% conversion and 53% of
12f, while 20 mol% InCl
3 gave
12f in a 66% yield. Finally, the phenyl ketone
8c and mesitylene with GaCl
3 at reflux gave
12g in a 50% yield.
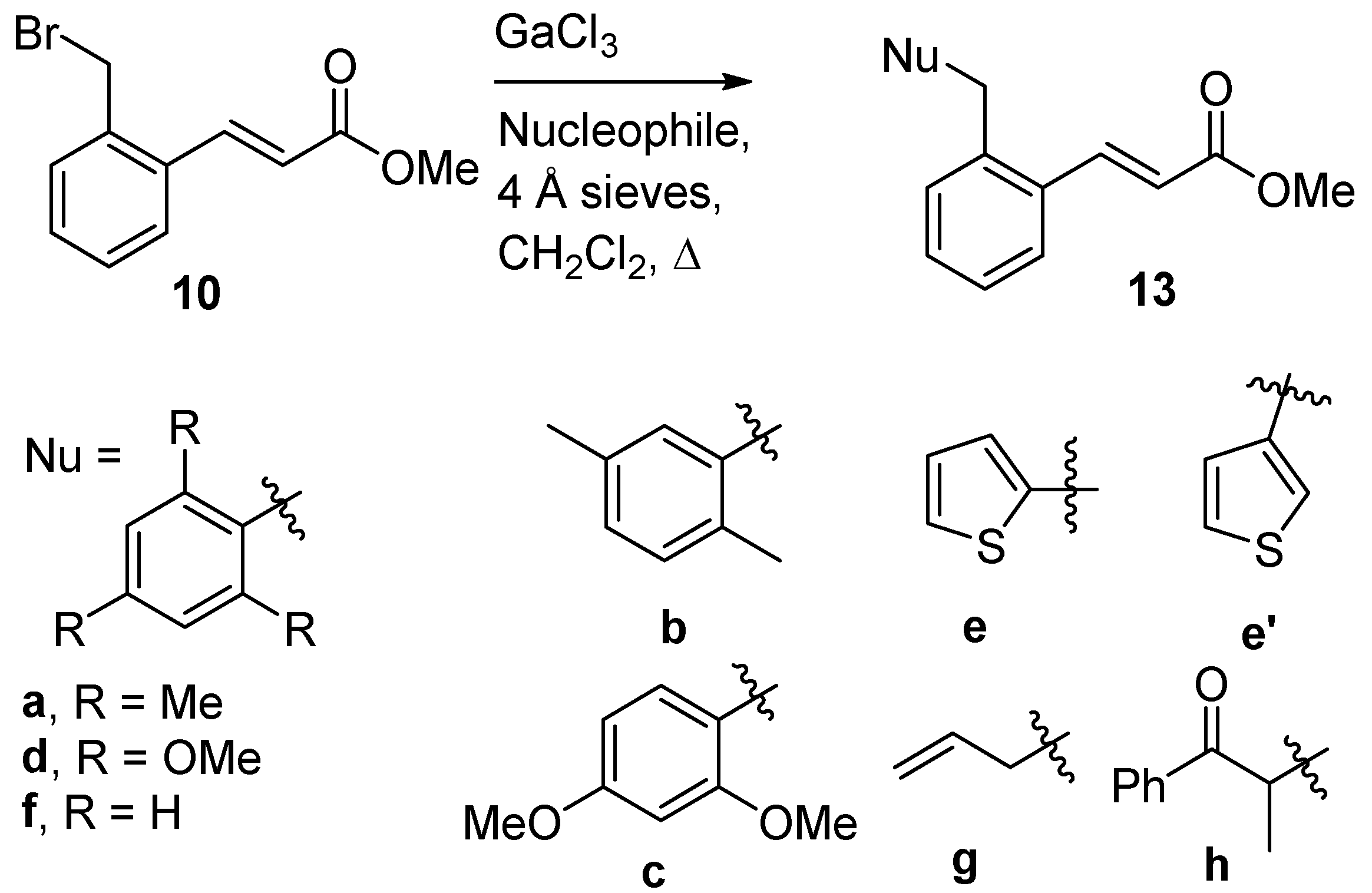
The benzylic bromide analogue,
10, also reacted under the optimized conditions, again at reflux (
Scheme 6,
Table 4). Mesitylene afforded
13a in a 73% yield, with no evidence of even trace amounts of isomeric products present.
p-Xylene (
13b, 76% yield), 1,3-dimethoxybenzene (
13c, 77% yield), and 1,3,5-trimethoxybenzene (
13d, 75% yield) behaved analogously. Thiophene worked well, again affording an isomeric mixture of C-2 and C-3 substitution products (
13e and
13e′, 92% yield,
13e:
13e′ = 71:29). The aromatic nucleophiles could be extended to benzene itself (
13f, 72% yield), although a greater amount of GaCl
3 catalyst (30 mol%) was required.
The reaction with allyltrimethylsilane was again more difficult than for arene nucleophiles with GaCl3 catalysis. In this case, while 10 mol% GaCl3 showed no significant conversion, 50 mol% GaCl3 gave a 46% yield of 13g. InCl3 again proved to be a superior catalyst with allyltrimethylsilane; 10 mol% of InCl3 afforded a 29% yield of 13g, while raising the catalyst amount to 20 mol% InCl3 gave 13g in 64% (78% brsm). Finally, a switch to higher temperature reaction conditions (1,2-dichloroethane, reflux) demonstrated that propiophenone trimethylsilyl enol ether was also amenable to reaction with 10 (13h, 82% yield) with the use of InCl3 as the catalyst.
4. Materials and Methods
The starting materials and reagents involved in the reactions were purchased from commercial sources, unless otherwise noted. GaCl3 and InCl3 were stored under an inert atmosphere prior to use. Purification of synthesized products was conducted by either column chromatography (using SilaFlash® P60, 230–400 mesh, SiliCycle, Quebec City, QC, Canada), preparative TLC (SiliaPlate, 1000 μm thickness, SiliCycle, Quebec City, QC, Canada) or radial chromatography (Silica gel, 2000 μm thickness, EM Science, Gibbstown, NJ, USA). Analytical thin layer chromatography (TLC) was performed using Silicycle aluminum-backed sheets (SiliCycle, Quebec City, QC, Canada). Dichloromethane and tetrahydrofuran solvents (Sigma-Aldrich Canada, Milton, ON, Canada) were obtained from a solvent purification system. All of the reactions were performed under an atmosphere of nitrogen unless otherwise stated. Prior to reaction, all glassware was dried in an oven at 110 °C for a minimum of one hour and subsequently cooled in a desiccator. Reactions conducted at greater than 25 °C were conducted in a heated oil bath.
All of the NMR spectral analyses were conducted on 300 MHz and 500 MHz spectrometers (Bruker Canada, Milton, ON, Canada) at room temperature in solutions of CDCl
3 (CIL, Andover, MA, USA). The residual CHCl
3 peak was set to 7.27 ppm and 77.0 ppm for the
1H NMR and
13C NMR spectra, respectively.
1H NMR spectral data are listed with units of ppm for peak position (δ) and Hz for coupling constant (
J). The following symbols were used for peak appearance: s, singlet; d, doublet; t, triplet; dd, doublet of doublets; dt, doublet of triplets; q, quartet; m, multiplet. The
1H and
13C NMR spectra are available in the
Supplementary Materials. The IR analysis was conducted on an ATR infrared (FTIR) spectrometer (Bruker Canada, Milton, ON, Canada). For IR spectra listed in the characterization of compounds and the absorption peaks with the greatest functional group relevance are reported in wavenumbers (cm
−1). High resolution mass spectrometry results were obtained by direct insertion probe on a Waters Xevo G2-XS Time-of-Flight Mass Spectrometer (Waters, Toronto, ON, Canada) in ASAP(+) mode at the University of Windsor Mass Spectrometry lab. The computational calculations were conducted with Gaussview 5.0.9 and B3LYP/6-311++G(d,p) to optimize the structures studied, both with and without solvation in dichloromethane. Final coordinates are available in the
Supplementary Materials.
4.1. 6-Bromo-1-phenyl-2,4-hexadienone (8c)
A procedure for synthesis of similar compounds had previously been reported, [
31] so this procedure was adapted to use on 1-phenyl-2,4-hexadienone. To a solution of 1-phenyl-2,4-hexadienone (0.2287 g, 1.33 mmol) and allyl bromide (0.56 mL, 6.6 mmol, 5 equiv.) in dichloromethane (40 mL) were added to the Hoveyda-Grubbs II catalyst (0.021 g, 0.034 mmol, 2.5 mol%). After stirring under N
2 for 24 h, another portion of Hoveyda Grubbs II catalyst (0.021 g, 0.034 mmol, 2.5 mol%) was added. After 48 h total, the solvent was evaporated under reduced pressure and the product was subjected to flash chromatography (5:1 PE:Et
2O) to yield
8c as a yellow solid (0.0982 g, 29%).
IR (neat) λ
max 3024, 2921, 2856, 1660, 1261, 1003, 693, and 590 cm
−1;
1H NMR (00 MHz, CDCl
3) δ 7.95 (d,
J = 8.7 Hz, 2H), 7.58 (apparent t,
J = 7.4 Hz, 1H), 7.49 (apparent t,
J = 7.6 Hz, 2H), 7.39 (dd,
J = 15.1, 11.0 Hz, 1H), 7.02 (d,
J = 15.1 Hz, 1H), 6.53 (dd,
J = 15.0 Hz, 11.0 Hz, 1H), 6.36 (m, 1H), and 4.07 (d,
J = 7.7 Hz, 2H);
13C NMR (125 MHz, CDCl
3) δ 190.2, 142.5, 137.8, 132.9, 132.6, 128.6, 128.4, 127.0, and 31.3; the
HRMS m/e for C
12H
11BrO calculated (M + 1)
+ 251.0072, found 251.0068.
4.2. Methyl 3-[2-(Bromomethyl)phenyl]acrylate (10)
Bromination was conducted with methods derived from those described by Snead [
34]. Methyl 3-(2-methylphenyl)acrylate
11 (1.1761 g, 4.2 mmol) and N-bromosuccinimide (1.6947 g, 9.522 mmol) were heated to reflux in chloroform (35 mL). Once at reflux, benzoyl peroxide (0.1670 g, 0.6894 mmol) was added. The reaction was stirred at reflux for 20 h, then cooled, filtered through Celite
® (Sigma-Aldrich Canada, Milton, ON, Canada) and concentrated under reduced pressure. The residue was then subjected to flash chromatography (10:1 petroleum ether: Et
2O) and 0.8078 g (77%) of light yellow solid product
10 was obtained. The mp was 84.5–85.5 °C.
IR (neat) λ
max 3030, 2950, 1700, 1431, 1078, and 599 cm
−1;
1H NMR (300 MHz, CDCl
3) δ 8.03 (d,
J = 15.9 Hz, 1H), 7.53 (m, 1H), 7.30 (m, 3H), 6.40 (d,
J = 15.9 Hz, 1H), 4.54 (s, 2H), and 3.78 (s, 3H);
13C NMR (75 MHz, CDCl
3) δ 166.7, 140.5, 136.4, 133.4, 130.5, 130.1, 129.1, 127.0, 120.4, 51.6, and 30.4; the
HRMS m/e for C
11H
11BrO
2 calculated (M + 1)
+ 255.0021, and found 255.0019.
4.3. Ethyl 6-(2,4,6-Trimethylphenyl)-2,4-hexadienoate (12a)
To a suspension of GaCl3 (0.009 g, 0.05 mmol, 10 mol%) and 4Å molecular sieves (ca. 0.4 g), CH2Cl2 (6 mL) was added to mesitylene (0.37 mL, 2.67 mmol, 5 equiv.) and 8b (0.1161 g, 0.5299 mmol) at room temperature. The reaction was stirred under N2 and monitored by TLC for 26 h. Following removal of volatiles under reduced pressure and flash chromatography (10:1 PE:Et2O), 12a (0.0902 g, 68%) was isolated as a yellow oil. This compound was also made by methods outlined below in General Procedure 1, where the reaction was brought to reflux for 22 h after the reagents were added. This afforded the product 12a in a 63% yield. IR (neat) λmax 2975, 2919, 2861, 1709, 1638, and 1130 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.30 (dd, J = 15.4 Hz, 11.0 Hz, 1H), 6.90 (s, 2H), 6.23 (dt, J = 15.2 Hz, 5.7 Hz, 1H), 6.02 (dd, J = 15.2 Hz, 11.0 Hz, 1H), 5.77 (d, J = 15.4 Hz, 1H), 4.21 (q, J = 7.2 Hz, 2H), 3.51 (d, J = 5.7 Hz, 2H), 2.31 (s, 3H), 2.28 (s, 6H), and 1.31 (t, J = 7.1 Hz, 3H); 13C NMR (125 MHz, CDCl3) δ 167.0, 144.5, 141.0, 136.5, 135.8, 131.9, 128.9, 128.1, 119.6, 60.0, 32.5, 20.8, 19.7, and 14.2; the HRMS m/e for C17H22O2 calculated (M + 1)+ 259.1698, and found 259.1691.
4.4. Ethyl 6-(2,5-Dimethylphenyl)-2,4-hexadienoate (12b)
General Procedure 1. To a suspension of GaCl3 (0.004 g, 0.02 mmol, 10 mol%) and 4Å molecular sieves (ca. 0.4 g), CH2Cl2 (6 mL) was added to para-xylene (0.14 mL, 1.1 mmol, 5 equiv.) and 8b (0.048 g, 0.22 mmol) at room temperature. The mixture was heated to reflux, stirred under N2 and monitored by TLC for 23 h. Following removal of volatiles under reduced pressure and flash chromatography (5:1 PE:Et2O), 12b (0.0349 g, 65%) was isolated as a yellow oil. This compound was also prepared where the reaction was stirred at room temperature for 23 h, and the yield of product 12b was 34%. IR (neat) λmax 2979, 2925, 1710, 1640, 1131, 1000, and 810 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.29 (dd, J = 15.3 Hz, 10.5 Hz, 1H), 7.06 (d, J = 7.5 Hz, 1H), 6.96 (m, 2H), 6.26 (dt, J = 15.3 Hz, 6.0 Hz, 1H), 6.12 (dd, J = 15.9 Hz, 10.5 Hz, 1H), 5.80 (d, J = 15.0 Hz, 1H), 4.20 (q, J = 7.2 Hz, 2H), 3.46 (d, J = 6.3 Hz, 2H), 2.31 (s, 3H), 2.24 (s, 3H), and 1.29 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 167.0, 144.4, 141.6, 136.5, 135.4, 132.9, 130.0, 129.8, 128.9, 127.1, 119.7, 60.0, 36.6, 20.7, 18.7, and 14.1; the HRMS m/e for C16H20O2 calculated (M + 1)+ 245.1550, and found 245.1539.
4.5. Ethyl 6-(2,4-Dimethoxyphenyl)-2,4-hexadienoate (12c)
General Procedure 1 was carried out with GaCl3 (0.005 g, 0.030 mmol, 10 mol%), 1,3-dimethoxybenzene (0.20 mL, 1.5 mmol, 5 equiv.) and 8b (0.0653 g, 0.298 mmol). The reaction was monitored by TLC for 23 h under reflux and N2, and after purification by flash chromatography (3:1 PE:Et2O), 12c (0.0460 g, 56%) was isolated as a yellow oil. IR (neat) λmax 2935, 2837, 1708, 1207, 1155, 1132, and 1035 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.28 (dd, J = 10.8 Hz, 5.1 Hz, 1H), 7.00 (d, J = 7.5 Hz, 1H), 6.50 (m, 2H), 6.21 (m, 2H), 5.78 (d, J = 15.3 Hz, 1H), 4.19 (q, J = 7.2 Hz, 2H), 3.80 (s, 6H), 3.42 (d, J = 6.6 Hz, 2H), and 1.29 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 167.1, 159.5, 157.9, 144.8, 142.6, 129.9, 128.4, 119.3, 103.8, 98.4, 59.9, 55.2, 32.8, and 14.1; the HRMS m/e for C16H20O4 calculated (M + 1)+ 277.1440, and found 277.1440.
4.6. Ethyl 6-(2,4,6-Trimethoxyphenyl)-2,4-hexadienoate (12d)
General Procedure 1 was carried out with GaCl3 (0.010 g, 0.057 mmol, 20 mol%), 1,3,5-trimethoxybenzene (0.2521 g, 1.499 mmol, 5 equiv.) and 8b (0.0629 g, 0.287 mmol). The reaction was monitored by TLC for 24 h under reflux and N2, and after purification by flash chromatography (3:1 PE:Et2O), 12d (0.0446 g, 51%) was isolated as a beige solid, and the mp was 69–70.5 °C. IR (neat) λmax 2941, 2837, 1697, 1595, and 1149 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.25 (dd, J = 15.3 Hz, 11.0 Hz, 1H), 6.22 (dt, J = 15.1, 6.4 Hz, 1H), 6.15 (s, 2H), 6.10 (m, 2H), 5.74 (d, J = 15.3 Hz, 1H), 4.17 (q, J = 7.2 Hz, 2H), 3.82 (s, 3H), 3.80 (s, 6H), 3.43 (d, J = 6.4 Hz, 2H), and 1.27 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 167.3, 159.8, 158.7, 145.5, 143.2, 127.6, 118.8, 107.6, 90.6, 60.0, 55.7, 55.3, 26.1, and 14.3; the HRMS m/e for C17H22O5 calculated (M + 1)+ 307.1545, and found 307.1539.
4.7. Ethyl 6-(2-Thienyl)-2,4-hexadienoate (12e) and Ethyl 6-(3-thienyl)-2,4-hexadienoate (12e′)
General Procedure 1 was carried out with GaCl3 (0.004 g, 0.02 mmol, 10 mol%), thiophene (0.17 mL, 2.1 mmol, 10 equiv.) and 8b (0.0476 g, 0.217 mmol). The reaction was monitored by TLC for 23 h under reflux and N2, and after purification by flash chromatography (4:1 PE:Et2O), an 12e/12e′ mixture (0.0306 g, 63%) was isolated as a yellow oil. The product contained a 72:28 12e:12e′ based on 1H NMR spectral integration of the resonances at 3.70 ppm (12e), and 3.52 ppm (12e′) corresponding to the hydrogen atoms bonded to the sp3 carbon adjacent to the thiophene, but these two compounds were not able to be separated. IR (neat) λmax 2980, 2934, 1707, 1253, and 1131 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.25–7.34 (m, 1H), 7.18 (d, J = 5.1 Hz, 1H), 6.96 (dd, 5.1, 3.5 Hz, 1H), 6.83 (m, 1H), 6.19–6.31 (m, 2H), 5.86 (d, J = 15.0 Hz, 1H), 4.21 (q, J = 7.2 Hz, 2H), 3.70 (d, J = 5.7 Hz, 2H), and 1.30 (t, J = 7.2 Hz, 3H). Resonances from minor product 12e′ were observed at: δ 6.98 (m, 1H), 6.93 (dd, J = 4.9, 1.2 Hz, 1H), 5.83 (d, J = 15.3 Hz, 1H), and 3.52 (d, J = 5.4 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 167.0, 144.0, 141.3, 140.6, 129.5, 127.0, 125.8, 125.1, 124.0, 120.8, 60.3, 33.1, and 14.3. Resonances from minor product 12e′ were observed at: δ 167.1, 144.3, 139.0, 129.3, 128.1, 125.8, 121.2, 120.3, 60.2, and 33.7; the HRMS m/e for C12H14O2S calculated (M + 1)+ 223.0793, and found 223.0797.
4.8. Ethyl 2,4,6-Nonatrienoate (12f)
A mixture of InCl3 (0.0127 g, 20 mol%), 4Å molecular sieves, 8b (0.0633 g, 0.289 mmol) and allyltrimethylsilane (0.23 mL, 5 equiv) in CH2Cl2 (7 mL) were heated to reflux under N2 for 14 h. Following a conventional workup, preparative TLC (7.5:1 hexanes: Et2O) afforded 12f (0.0343 g, 66%) as a faintly tan oil. IR (neat) λmax 2980, 2928, 1712, 1253, 1136, and 998 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.25 (dd, J = 15.4 Hz, 10.5 Hz, 1H), 6.19 (d of ½ AB, J = 10.5, 15.2 Hz, 1H), 6.11 (t of ½ AB, J = 6.5, 15.2 Hz, 1H), 5.73–5.84 (m, 2H), 5.03 (dd, J = 17.1, 1.6 Hz, 1H), 4.99 (dd, J = 10.2, 1.6 Hz, 1H), 4.19 (q, J = 7.2 Hz, 2H), 2.27 (m, 2H), 2.19 (m, 2H), and 1.28 (t, J = 7.2 Hz, 3H). Resonances from the minor (2E, 4Z) isomer can be observed at 6.89 (dd, J = 15.7, 7.5 Hz, 1H), 5.73 (m, 1H), 5.10 (d, J = 10.3 Hz, 1H), and 4.18 (obscured q, J = 7.1 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 167.2, 144.8, 143.3, 137.4, 128.7, 119.5, 115.3, 60.1, 32.7, 32.2, and 14.3; the HRMS m/e for C11H16O2 calculated (M + 1)+ 181.1228, and found 181.1228.
4.9. 6-(2,4,6-Trimethylphenyl)-1-phenyl-2,4-hexadienone (12g)
To a suspension of GaCl3 (0.003 g, 0.02 mmol, 10 mol%), and 4Å molecular sieves (ca. 0.4 g), CH2Cl2 (6 mL) was added to mesitylene (0.12 mL, 0.86 mmol, 5 equiv.) and 8c (0.0438 g, 0.17 mmol) at room temperature. The reaction was heated to reflux, stirred under N2 and monitored by TLC for 20 h. Following the removal of volatiles under reduced pressure and flash chromatography (10:1 PE:Et2O), 12g (0.0251 g, 50%) was isolated as a yellow oil. IR (neat) λmax 3000, 2917, 2851, 1660, 1587, 1000, 693 cm−1; 1H NMR (300 MHz, CDCl3) δ 7.92 (d, J = 7.2 Hz, 2H), 7.55 (m, 1H), 7.36–7.50 (m, 3H), 6.90 (s, 2H), 6.83 (d, J = 15.0 Hz, 1H), 6.37 (dt, J = 15.0 Hz, 5.7 Hz, 1H), 6.10 (dd, J = 15.0 Hz, 11.1 Hz, 1H), 3.54 (d, J = 5.1 Hz, 2H), 2.30 (s, 3H), and 2.27 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 190.8, 144.9, 143.0, 138.2, 136.5, 135.9, 132.5, 131.9, 129.0, 128.9, 128.5, 128.3, 124.0, 32.8, 20.9, and 19.8; the HRMS m/e for C21H22O calculated (M + 1)+ 291.1749, and found 291.1745.
4.10. Methyl 3-[2-(2,4,6-Trimethylbenzyl)phenyl]acrylate (13a)
General procedure 2. To a suspension of GaCl3 (0.004 g, 0.02 mmol, 10 mol%), and 4Å molecular sieves (ca. 0.4 g), CH2Cl2 (6 mL) was added to mesitylene (0.15 mL, 5 equiv.) and 10 (0.0532 g, 0.210 mmol) at room temperature. The reaction was heated to reflux, stirred under N2 and monitored by TLC for 24 h. Following removal of volatiles under reduced pressure and chromatography (5:1 PE:Et2O), 13a (0.0449 g, 73%) was obtained as a beige solid; mp was 81.5–83.0 °C. IR (neat) λmax 3056, 2969, 2948, 2915, 1713, 1164, 982, and 760 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.24 (d, J = 15.6 Hz, 1H), 7.59 (dd, J = 6.9 Hz, 2.1 Hz, 1H), 7.14–7.23 (m, 2H), 6.93 (s, 2H), 6.60 (d, J = 7.8 Hz, 1H), 6.44 (d, J = 15.9 Hz, 1H), 4.10 (s, 2H), 3.86 (s, 3H), 2.32 (s, 3H), and 2.15 (s, 6H); 13C NMR (125 MHz, CDCl3) δ 167.4, 142.2, 139.1, 137.2, 135.9, 133.4, 132.7, 130.3, 128.9, 127.2, 126.5, 126.3, 119.5, 51.7, 31.8, 20.9, and 19.9; the HRMS m/e for C20H23O2 calculated (M + 1)+ 295.1698, and found 295.1699.
4.11. Methyl 3-[2-(2,5-Dimethylbenzyl)phenyl]acrylate (13b)
General procedure 2 was carried out with GaCl3 (0.004 g, 0.02 mmol, 10 mol%), para-xylene (0.13 mL, 5 equiv.) and 10 (0.0540 g, 0.213 mmol). The reaction was monitored by TLC for 21 h under reflux and N2, and after evaporation under reduced pressure and purification by flash chromatography (5:1 PE:Et2O), 13b (0.0452 g, 76%) was obtained as a faintly yellow solid; mp was 51.0–53.0 °C. IR (neat) λmax 3015, 2949, 2923, 2892, 1714, 1172, 1015, 977, and 765 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.03 (d, J = 15.9 Hz, 1H), 7.61 (dd, J = 7.5 Hz, 1.5 Hz, 1H), 7.24–7.32 (m, 2H), 7.10, (d, J = 7.8 Hz, 1H), 6.98 (m, 2H), 6.75 (s, 1H), 6.38 (d, J = 15.9 Hz, 1H), 4.07 (s, 2H), 3.81 (s, 3H), 2.26 (s, 3H), and 2.23 (s, 3H); 13C NMR (125 MHz, CDCl3) δ 167.3, 142.4, 139.8, 137.7, 135.5, 133.5, 133.3, 130.4, 130.13, 130.11, 129.9, 127.2, 126.7, 126.6, 119.5, 51.7, 36.2, 21.0, and 19.1; the HRMS m/e for C19H21O2 calculated (M + 1)+ 281.1541, and found 281.1544.
4.12. Methyl 3-[2-(2,4-Dimethoxybenzyl)phenyl]acrylate (13c)
General procedure 2 was carried out with GaCl3 (0.003 g, 0.02 mmol, 10 mol%), 1,3-dimethoxybenzene (0.11 mL, 0.84 mmol, 5 equiv.) and 10 (0.0445 g, 0.175 mmol). The reaction was monitored by TLC for 22 h under reflux and N2, and after evaporation under reduced pressure and purification by flash chromatography (4:1 PE:Et2O), 13c (0.0423 g, 77%) was isolated as a light yellow viscous oil. IR (neat) λmax 2934, 2878, 2837, 1716, 1241, 1114, and 1036 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.11 (d, J = 15.9 Hz, 1H), 7.58 (dd, J = 7.5 Hz, 1.2 Hz, 1H), 7.20–7.34 (m, 2H), 7.17 (dd, J = 6.0 Hz, 1.2 Hz, 1H), 6.81 (d, J = 8.3 Hz, 1H), 6.48 (d, J = 2.4 Hz, 1H), 6.38 (dd, J = 8.3, 2.4 Hz, 2H), 6.36 (d, J = 15.9 Hz, 1H), 4.03 (s, 2H), 3.83 (s, 3H), 3.80 (s, 3H), and 3.79 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 167.4, 159.5, 157.9, 142.9, 140.7, 133.5, 130.6, 130.3, 130.0, 126.5, 126.4, 121.0, 119.0, 104.0, 98.4, 55.3, 51.6, and 32.1; the HRMS m/e for C19H20O4 calculated (M + 1)+ 313.1440, and found 313.1441.
4.13. Methyl 3-[2-(2,4,6-Trimethoxybenzyl)phenyl]acrylate (13d)
General procedure 2 was carried out with GaCl3 (0.004 g, 0.02 mmol, 10 mol%), 1,3,5-trimethoxybenzene (0.1907 g, 1.134 mmol, 5 equiv.) and 10 (0.0547 g, 0.215 mmol). The reaction was monitored by TLC for 22 h under reflux and N2, and after evaporation under reduced pressure and purification by flash chromatography (5:1 PE:Et2O), 13d (0.0552 g, 75%) was obtained as a colorless solid; the mp was 84–85 °C. IR (neat) λmax 2949, 2839, 1702, 1118, 949, and 764 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.43 (d, J = 15.9 Hz, 1H), 7.53 (dd, J = 7.5 Hz, 1.2 Hz, 1H), 7.14–7.25 (m, 2H), 7.11 (m, 1H), 6.37 (d, J = 15.9 Hz, 1H), 6.17 (s, 2H), 4.06 (s, 2H), 3.84 (s, 3H), 3.83 (s, 3H), and 3.77 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 167.7, 159.9, 158.9, 143.8, 141.6, 133.1, 129.7, 129.3, 126.1, 125.8, 118.4, 109.0. 90.5, 55.5, 55.3, 51.5, and 25.5; the HRMS m/e for C20H23O5 calculated (M + 1)+ 343.1545, and found 343.1547.
4.14. Methyl 3-[2-(2-Methylthienyl)phenyl]acrylate (13e) and Methyl 3-[2-(3-methylthienyl)phenyl]acrylate (13e′)
General procedure 2 was carried out with GaCl3 (0.003 g, 0.02 mmol, 10 mol%), thiophene (0.075 mL, 0.94 mmol, 5 equiv.) and 10 (0.0465 g, 0.183 mmol). The reaction was monitored by TLC for 20 h under reflux and N2, and after purification by flash chromatography (5:1 PE:Et2O), the 13e/13e′ mixture (0.0437 g, 92% combined) was found as a light yellow oil. Based on 1H NMR integration of the hydrogen atoms bonded to the sp3 carbon adjacent to the thiophene group (4.27 ppm for 13e and 4.10 ppm for 13e′), the product is an inseparable mixture of 13e:13e′ in a ratio of 71:29. IR (neat) λmax 2949, 1711, 1170, 977, 763, 731, and 698 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.02 (d, J = 15.6 Hz, 1H), 7.57 (d, J = 7.5 Hz, 1H), 7.28 (m, 3H), 7.12 (d, J = 5.1 Hz, 1H), 6.88 (m, 1H), 6.72 (d, J = 3.0 Hz, 1H), 6.34 (d, J = 15.9 Hz, 1H), 4.27 (s, 2H), and 3.78 (s, 3H). Most resonances from minor product 13e′ were superimposed on those from 13e, but the following resonances from 13e′ were clearly observed: δ 6.83 (s, 1H), and 4.10 (s, 2H); 13C NMR (75 MHz, CDCl3) δ 167.1, 143.0, 141.9, 139.5, 133.1, 130.3, 130.2, 127.2, 126.8, 126.7, 125.1, 124.0, 119.6, 51.6, and 33.3. Some resonances from minor product 13e′ were superimposed on those from 13e but the following resonances from 13e′ were clearly observed: δ 142.2, 139.8, 133.2, 130.4, 130.1, 128.0, 126.9, 126.6, 125.7, 125.2, 121.4, 119.3, and 33.8; the HRMS m/e for C15H14O2S calculated (M + 1)+ 259.0793, and found 259.0801.
4.15. Methyl 3-[2-Benzylphenyl]acrylate (13f)
General procedure 2 was carried out with GaCl3 (0.0107 g, 0.061 mmol, 30 mol%), benzene (0.25 mL, 14 equiv.) and 10 (0.0518 g, 0.204 mmol). The reaction was monitored by TLC for 30 h under reflux and N2, and following a conventional (CH2Cl2) extractive workup and purification by preparative TLC (7:1 PE:Et2O); 13f (0.0367 g, 72%) was obtained as a faintly tan oil. IR (neat) λmax 3062, 3026, 2950, 1714, 1172, 1634, and 1599 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.06, (d, J = 15.8 Hz, 1H), 7.61 (d, J = 7.5 Hz, 1H), 7.10–7.40 (m, 8H), 6.36 (d, J = 15.8 Hz, 1H), 4.16 (s, 2H), and 3.80 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 167.2, 142.4, 140.14, 140.05, 133.5, 130.8, 130.1, 128.7, 128.5, 126.9, 126.7, 126.2, 119.4, 51.6, and 38.9; MS m/e 252 (M+).
4.16. Methyl 3-[2-(3-Butenyl)phenyl]acrylate (13g)
To a suspension of InCl3 (0.008 g, 0.04 mmol, 20 mol%) and 4Å molecular sieves (ca. 0.4 g), CH2Cl2 (6 mL) was added to allyltrimethylsilane (0.15 mL, 0.94 mmol, 5 equiv.) and 10 (0.0455 g, 0.179 mmol) at room temperature. The reaction was heated to reflux, stirred under N2 and monitored by TLC for 19 h. Following removal of volatiles under reduced pressure and purification by flash chromatography (5:1 PE:Et2O), 13g was isolated as a light beige oil (0.0246 g, 64%, 78% BRMS). Continued elution afforded starting 10 (0.0083 g, 18%) in subsequent fractions. IR (neat) λmax 3066, 2948, 1715, 1169, 979, and 763 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.03 (d, J = 15.9 Hz, 1H), 7.57 (d, J = 7.8 Hz, 1H), 7.32 (m, 1H), 7.18-7.27 (m, 2H), 6.38 (d, J = 15.9 Hz, 1H), 5.87 (m, 1H), 4.97–5.11 (m, 2H), 3.83 (s, 3H), 2.86 (dd, J = 9.7, 6.0 Hz, 2H), and 2.34 (m, 2H); 13C NMR (75 MHz, CDCl3) δ 167.4, 142.3, 141.4, 137.4, 132.9, 130.0, 126.6, 126.5, 119.1, 115.4, 51.7, 35.4, and 32.7; the HRMS m/e for C14H16O2 calculated (M + 1)+ 217.1228, and found 217.1230.
4.17. Methyl 3-(2-(2-Methyl-3-oxo-3-phenylpropylphenyl)acrylate (13h)
To a suspension of InCl3 (0.0065 g, 0.029 mmol, 18 mol%) and 4Å molecular sieves (ca. 0.4 g) in 1,2-dichloroethane (5 mL) were added propiophenone trimethylsilyl enol ether (0.229 g, 1.11 mmol, 6.7 equiv.) and 10 (0.0422 g, 0.165 mmol) at room temperature. The reaction was heated to reflux, stirred under N2 and monitored by TLC for 15 h. Following a conventional extractive (CH2Cl2) workup and purification by preparative TLC (3:1 hexane:Et2O), 13h was isolated as a light beige oil (0.0420 g, 82%). IR (neat) λmax 3061, 2950, 1717, 1681, 1632, and 1597 cm−1; 1H NMR (300 MHz, CDCl3) δ 8.10 (d, J = 15.8 Hz, 1H), 7.88 (m, 2H), 7.55 (m, 2H), 7.45 (m, 2H), 7.17–7.31 (m, 3H), 6.40 (d, J = 15.8 Hz, 1H), 3.84 (s, 3H), 3.72 (m, 1H), 3.30 (dd, J = 14.0, 6.6 Hz, 1H), 1.87 (dd, J = 14.0, 7.8 Hz), and 1.19 (d, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ203.4, 167.2, 142.2, 139.4, 136.3, 133.3, 133.0, 131.2, 130.0, 128.7, 128.2, 127.0, 119.6, 51.7, 42.1, 36.6, and 17.3; MS m/e 508 (M+).