Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings
Abstract
:1. Introduction
2. Results
2.1. Characterization of Chitooligosaccharide Derivatives
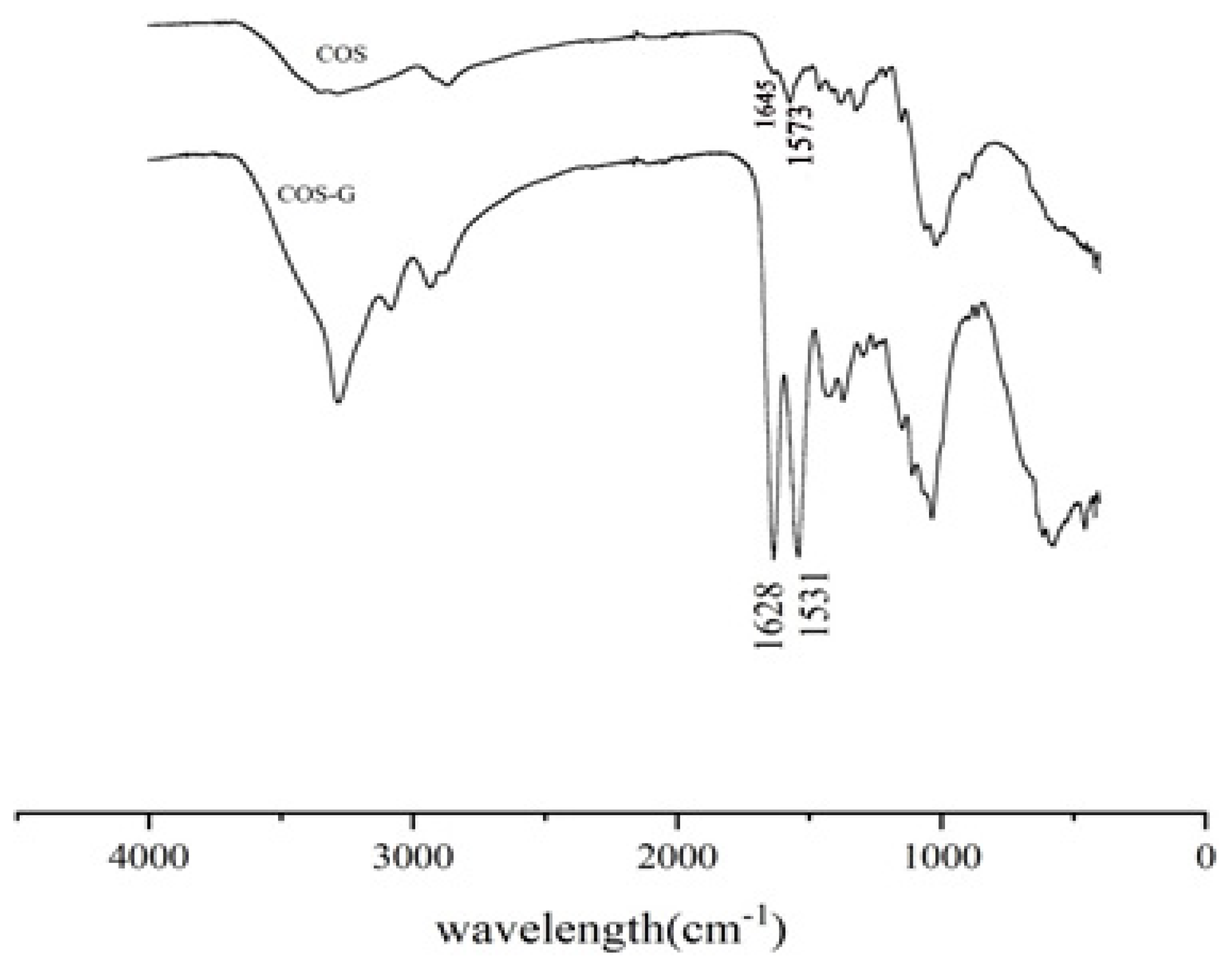
2.1.1. FT-IR Spectroscopy
2.1.2. NMR Spectroscopy
2.1.3. The Degree of Substitution of the Chitooligosaccharide Derivative
2.2. The Effect of COS-G on Wheat Seedlings under Salt Stress
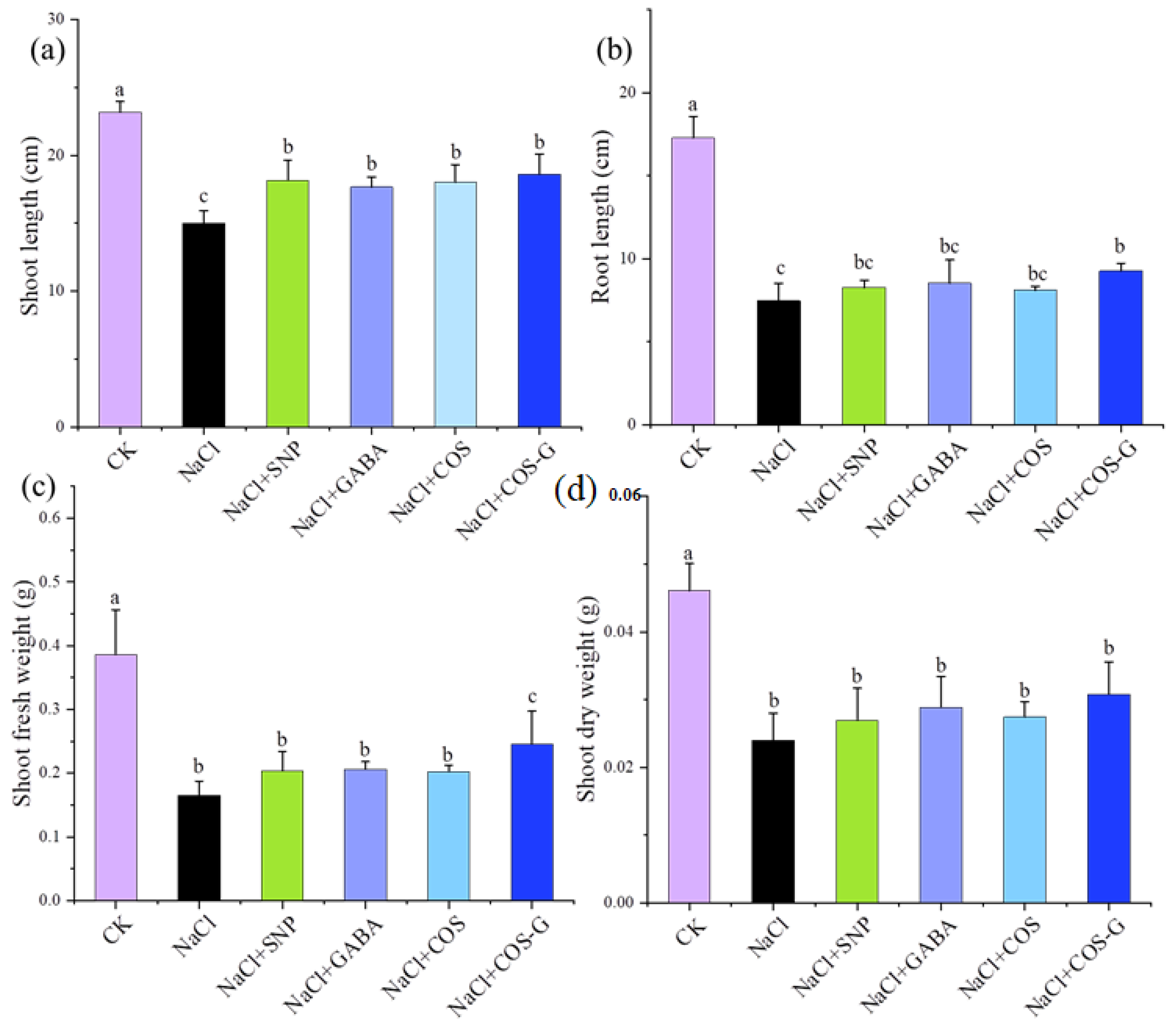
2.2.1. Plant Biomass Accumulation
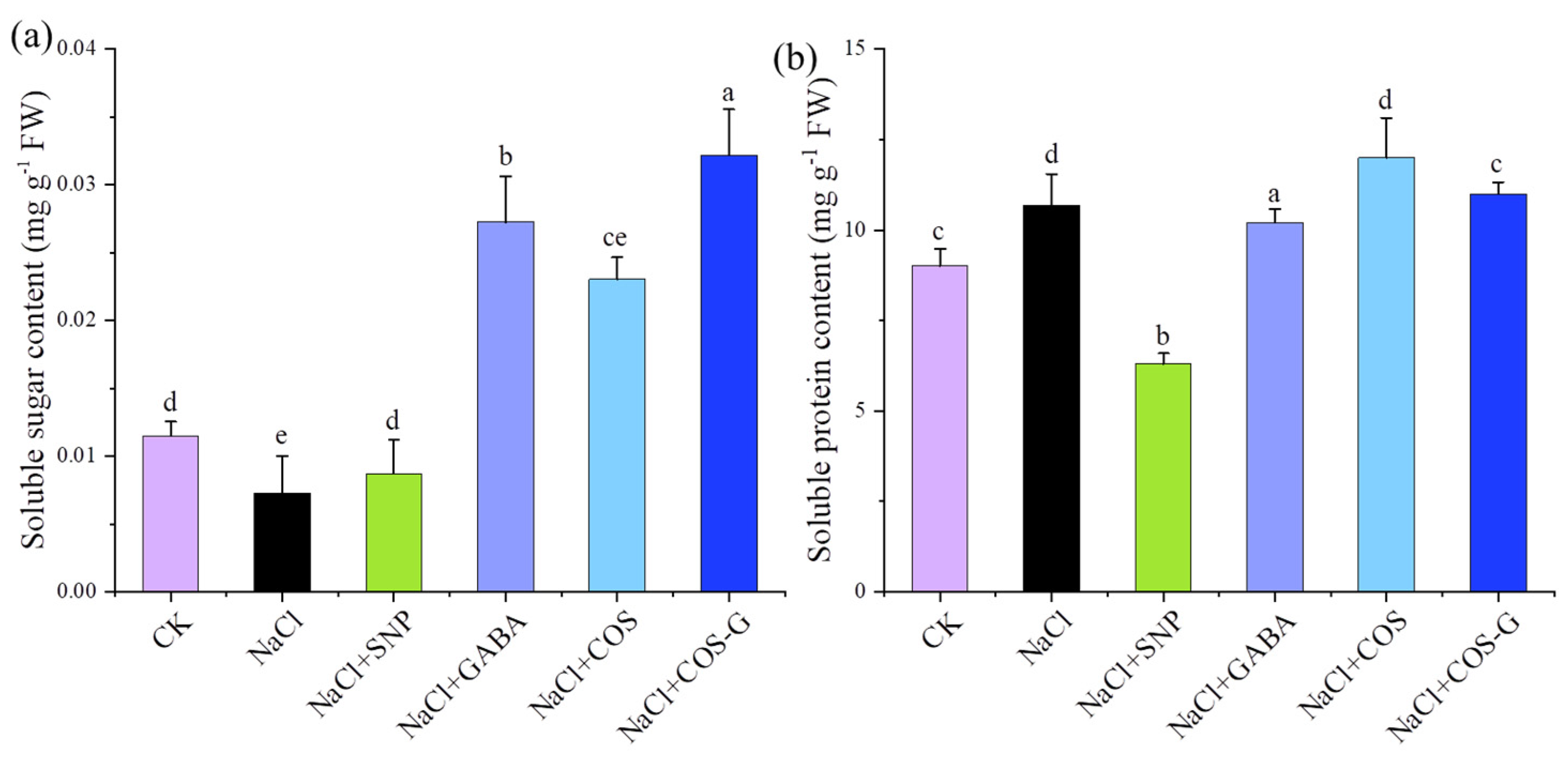
2.2.2. Soluble Sugar and Soluble Protein Contents
2.2.3. MDA Contents
2.2.4. O−2 Production Rate and H2O2 Contents
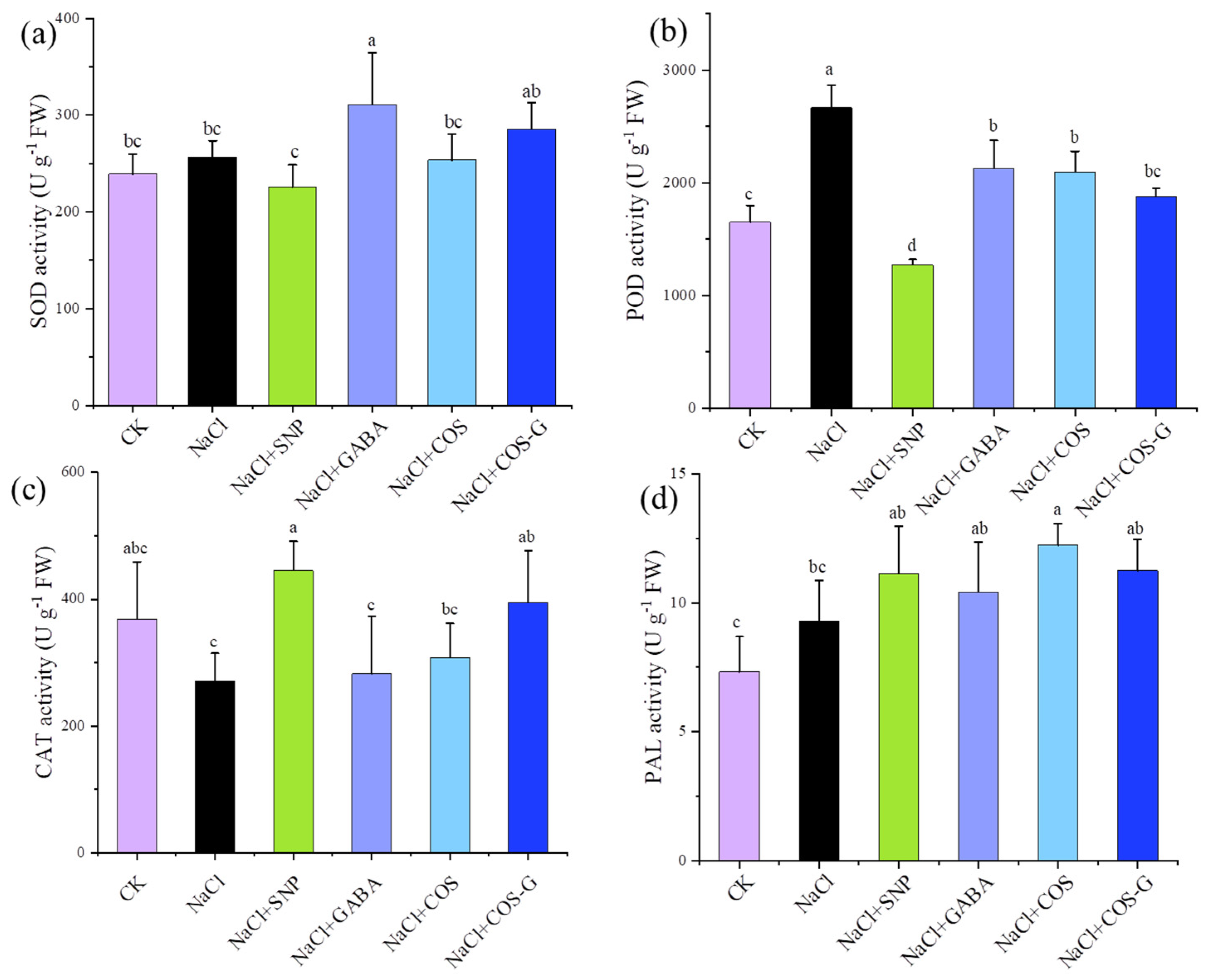
2.2.5. Antioxidant Enzyme Activities
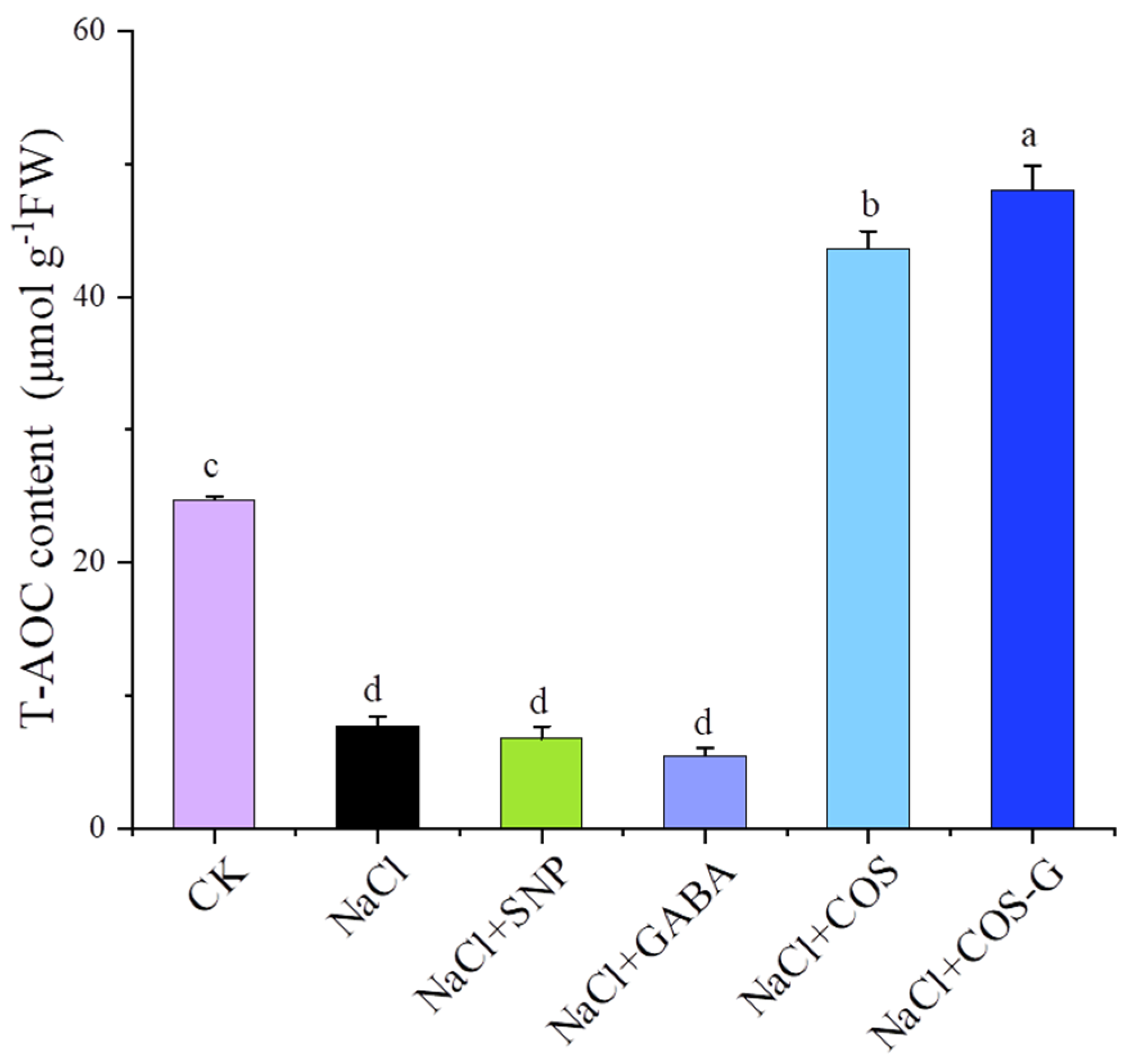
2.2.6. Total Antioxidant Capacity (T-AOC)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Chitooligosaccharide Derivative (COS-G)
4.3. Effect of COS-G on Wheat Seedlings under Salt Stress
4.3.1. Seed Dipping Treatment
4.3.2. Seedling Development
4.4. Determination of Relevant Physiological Indicators of Seedlings
4.4.1. Determination of the MDA Content
4.4.2. Determination of the ROS
4.4.3. Determination of the Osmoregulatory Substance Content
4.4.4. Determination of Antioxidant Enzyme Activity
4.4.5. Determination of the Total Antioxidant Enzyme Capacity (T-AOC)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butcher, K.; Wick, A.F.; De Sutter, T.; Chatterjee, A.; Harmon, J. Soil salinity: A threat to global food security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Hassan, W.M.; Al-Suhaibani, N.A.; Refay, Y.; Abdella, K.A. Comparative performance of multivariable agro-physiological parameters for detecting salt tolerance of wheat cultivars under simulated saline field growing conditions. Front. Plant Sci. 2017, 8, 435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A.; Läuchli, A. Approaches to increasing the salt tolerance of wheat and other cereals. J. Exp. Bot. 2006, 57, 1025–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiology 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Guan, Y.-J.; Hu, J.; Wang, X.-J.; Shao, C.-X. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, N.; Ahmad, R. The activity of antioxidant enzymes in response to salt stress in safflower (Carthamus tinctorius L.) and sunflower (Helianthus annuus L.) seedlings raised from seed treated with chitosan. J. Sci. Food Agric. 2013, 93, 1699–1705. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Pongprayoon, W.; Roytrakul, S.; Pichayangkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Quitadamo, F.; De Simone, V.; Beleggia, R.; Trono, D. Chitosan-induced activation of the antioxidant defense system counteracts the adverse effects of salinity in durum wheat. Plants 2021, 10, 1365. [Google Scholar] [CrossRef] [PubMed]
- Geng, W.; Li, Z.; Hassan, M.J.; Peng, Y. Chitosan regulates metabolic balance, polyamine accumulation, and Na+ transport contributing to salt tolerance in creeping bentgrass. BMC Plant Biol. 2020, 20, 506. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Vo, T.P.K.; Tran, T.D. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym. 2011, 84, 751–755. [Google Scholar]
- Li, Y.Y.; Zhang, Q.Q.; Ou, L.N.; Ji, D.Z.; Liu, T.; Lan, R.M.; Li, X.Y.; Jin, L.H. Response to the cold stress signaling of the tea plant (Camellia sinensis) elicited by chitosan oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gu, W.; Meng, Y.; Xie, T.; Li, L.; Li, J.; Wei, S. γ-aminobutyric acid imparts partial protection from salt stress injury to maize seedlings by improving photosynthesis and upregulating osmoprotectants and antioxidants. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Ji, J.; Yue, J.; Xie, T.; Chen, W.; Du, C.; Chang, E.; Chen, L.; Jiang, Z.; Shi, S. Roles of γ-aminobutyric acid on salinity-responsive genes at transcriptomic level in poplar: Involving in abscisic acid and ethylene-signalling pathways. Planta 2018, 248, 675–690. [Google Scholar] [CrossRef]
- Shi, S.-Q.; Shi, Z.; Jiang, Z.-P.; Qi, L.-W.; Sun, X.-M.; Li, C.-X.; Liu, J.-F.; Xiao, W.-F.; Zhang, S.-G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010, 33, 149–162. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-aminobutyric acid (gaba) alleviates salt stress damage during seeds germination of white clover associated with Na+/K+ transportation, dehydrins accumulation, and stress-related genes expression in white clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef]
- Bi, R.; Yue, L.; Niazi, S.; Khan, I.M.; Sun, D.; Wang, B.; Wang, Z.P.; Jiang, Q.X.; Xia, W.S. Facile synthesis and antibacterial activity of geraniol conjugated chitosan oligosaccharide derivatives. Carbohydr. Polym. 2021, 251, 117099. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; He, X.; Zhang, X.; Xing, R.; Li, P. Effect of sulfated chitooligosaccharides on wheat seedlings (Triticum aestivum L.) under salt stress. J. Agric. Food Chem. 2016, 64, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Marchi, C.S.; Liu, H.; Lavernia, E.J.; Rangel, R.H.; Sickinger, A.; Muehlberger, E. Numerical analysis of the deformation and solidification of a single droplet impinging onto a flat substrate. J. Mater. Sci. 1993, 28, 3313–3321. [Google Scholar] [CrossRef]
- Atangana, E.; Chiweshe, T.T.; Roberts, H. Modification of novel chitosan-starch cross-linked derivatives polymers: Synthesis and characterization. J. Polym. Environ. 2019, 27, 979–995. [Google Scholar] [CrossRef]
- Luo, Y.; Ling, Y.; Wang, X.; Han, Y.; Zeng, X.; Sun, R. Maillard reaction products from chitosan-xylan ionic liquid solution. Carbohydr. Polym. 2013, 98, 835–841. [Google Scholar] [CrossRef]
- Chung, Y.C.; Yeh, J.Y.; Tsai, C.F. Antibacterial characteristics and activity of water-soluble chitosan derivatives prepared by the maillard reaction. Molecules 2011, 16, 8504–8514. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef] [Green Version]
- Amjad, M.; Akhtar, J.; Anwar-Ul-Haq, M.; Riaz, M.A.; Saqib, Z.A.; Murtaza, B.; Naeem, M.A. Effectiveness of potassium in mitigating the salt-induced oxidative stress in contrasting tomato genotypes. J. Plant Nutr. 2016, 39, 1926–1935. [Google Scholar] [CrossRef]
- Reginato, M.A.; Castagna, A.; Furlan, A.; Castro, S.; Ranieri, A.; Luna, V. Physiological responses of a halophytic shrub to salt stress by Na2SO4 and NaCl: Oxidative damage and the role of polyphenols in antioxidant protection. Aob. Plants. 2014, 6, plu042. [Google Scholar] [CrossRef]
- Bandeoglu, E.; Eyidogan, F.; Yucel, M.; Oktem, H.A. Antioxidant responses of shoots and roots of lentil to NaCl-salinity stress. Plant Growth Regul. 2004, 42, 69–77. [Google Scholar] [CrossRef]
- Ding, H.N.; Ma, D.Y.; Huang, X.; Hou, J.F.; Wang, C.Y.; Xie, Y.X.; Wang, Y.H.; Qin, H.X.; Guo, T.C. Exogenous hydrogen sulfide alleviates salt stress by improving antioxidant defenses and the salt overly sensitive pathway in wheat seedlings. Acta Physiol. Plant. 2019, 41, 1–11. [Google Scholar] [CrossRef]
- Zhao, G.M.; Han, Y.; Sun, X.; Li, S.H.; Shi, Q.M.; Wang, C.H. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crops Prod. 2015, 64, 175–181. [Google Scholar]
- Odat, N.A.; Al Tawaha, A.R.M.; Hasan, M.; Imran; Amanullah; Al Tawaha, A.R.M.; Thangadurai, D.; Sangeetha, J.; Rauf, A.; Khalid, S.; et al. Seed priming with chitosan alleviates salinity stress by improving germination and early growth parameters in common vetch (Vicia sativa). IOP Conf. Ser. Earth Environ. Sci. 2021, 788, 12–15. [Google Scholar] [CrossRef]
- Turk, H. Chitosan-induced enhanced expression and activation of alternative oxidase confer tolerance to salt stress in maize seedlings. Plant Physiol. Biochem. 2019, 141, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.L.; Xue, Q.Z. Effects of chitosan coating on seed germination and salt-tolerance of seedlings in hybrid rice (Oryza sativa L.). Acta Agron. Sin. 2002, 28, 803–808. [Google Scholar]
- El-Samad, H.M.A.; Shaddad, M.A.K. Salt tolerance of soybean cultivars. Biol. Plant. 1997, 39, 263–269. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Ahanger, M.A.; Egamberdieva, D.; Wijaya, L.; Alam, P. Salicylic Acid (SA) induced alterations in growth, biochemical attributes and antioxidant enzyme activity in faba bean (Vicia faba L.) seedlings under NaCl toxicity. Russ. J. Plant Physiol. 2018, 65, 104–114. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. GABA enhances physio-biochemical metabolism and antioxidant capacity of germinated hulless barley under NaCl stress. J. Plant Physiol. 2018, 231, 192–201. [Google Scholar] [CrossRef]
- Siddiqui, D.M.H.; Alamri, S.; Al-Khaishany, M.; Khan, M.; Al-Amri, A.; Ali, H.; Alaraidh, I.; Alsahli, A. Exogenous melatonin counteracts NaCl-induced damage by regulating the antioxidant system, proline and carbohydrates metabolism in tomato seedlings. Int. J. Mol. Sci. 2019, 20, 353. [Google Scholar] [CrossRef] [Green Version]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef]
- Mi, Y.W.; Wang, G.X.; Gong, C.W.; Cai, Z.P.; Wu, W.G. Effects of salt stress on growth and physiology of Isatis indigotica seedlings. Acta Prataculturae Sin. 2018, 27, 43–51. [Google Scholar]
- Wang, W.-B.; Kim, Y.-H.; Lee, H.-S.; Kim, K.-Y.; Deng, X.-P.; Kwak, S.-S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Gerami, M.; Majidian, P.; Ghorbanpour, A.; Alipour, Z. Stevia rebaudiana Bertoni responses to salt stress and chitosan elicitor. Physiol. Mol. Biol. Plants 2020, 26, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wan, Y.; Zhao, M.; Liu, Y.; Zhang, S. Preparation and characterization of antimicrobial chitosan-N-arginine with different degrees of substitution. Carbohydr. Polym. 2011, 83, 144–150. [Google Scholar] [CrossRef]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants—Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhausser, S.M. A method for routine measurements of total sugar and starch content in woody plant tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumbdhindsa, P.; Thorpe, T.A. Leaf senescence—Correlated with increased levels of membrane-permeability and lipid-peroxidation, and decreased levels of superoxide-dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Dhindsa, R.S.; Matowe, W. Drought tolerance in two mosses: Correlated with enzymatic defence against lipid peroxidation. J. Exp. Bot. 1981, 32, 79–91. [Google Scholar] [CrossRef] [Green Version]



| Treatment | NaCl (mmol/L) | Concentration |
|---|---|---|
| CK | 0 | 0 |
| NaCl | 100 | 0 |
| SNP | 100 | 0.08 mmol/L |
| GABA | 100 | 500 mg/L |
| COS | 100 | 500 mg/L |
| COS-G | 100 | 500 mg/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, S.; Yan, M. Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings. Molecules 2022, 27, 3068. https://doi.org/10.3390/molecules27103068
Wang W, Liu S, Yan M. Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings. Molecules. 2022; 27(10):3068. https://doi.org/10.3390/molecules27103068
Chicago/Turabian StyleWang, Wenyun, Song Liu, and Mingyan Yan. 2022. "Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings" Molecules 27, no. 10: 3068. https://doi.org/10.3390/molecules27103068
APA StyleWang, W., Liu, S., & Yan, M. (2022). Synthesis of γ-Aminobutyric Acid-Modified Chitooligosaccharide Derivative and Enhancing Salt Resistance of Wheat Seedlings. Molecules, 27(10), 3068. https://doi.org/10.3390/molecules27103068





