Study on the Correlation between the Protein Profile of Lupin Milk and Its Cheese Production Compared with Cow’s Milk
Abstract
1. Introduction
2. Results and Discussion
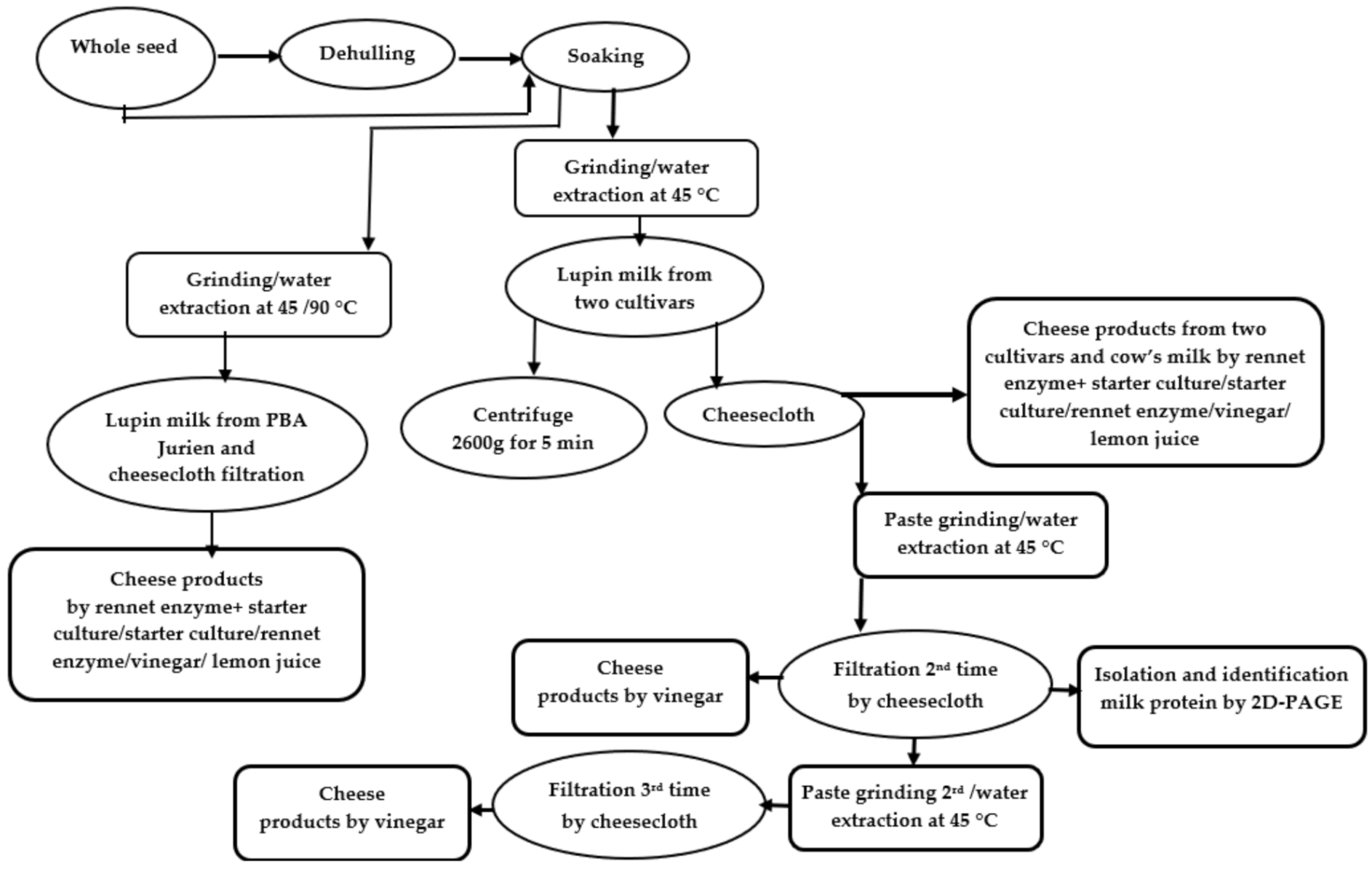
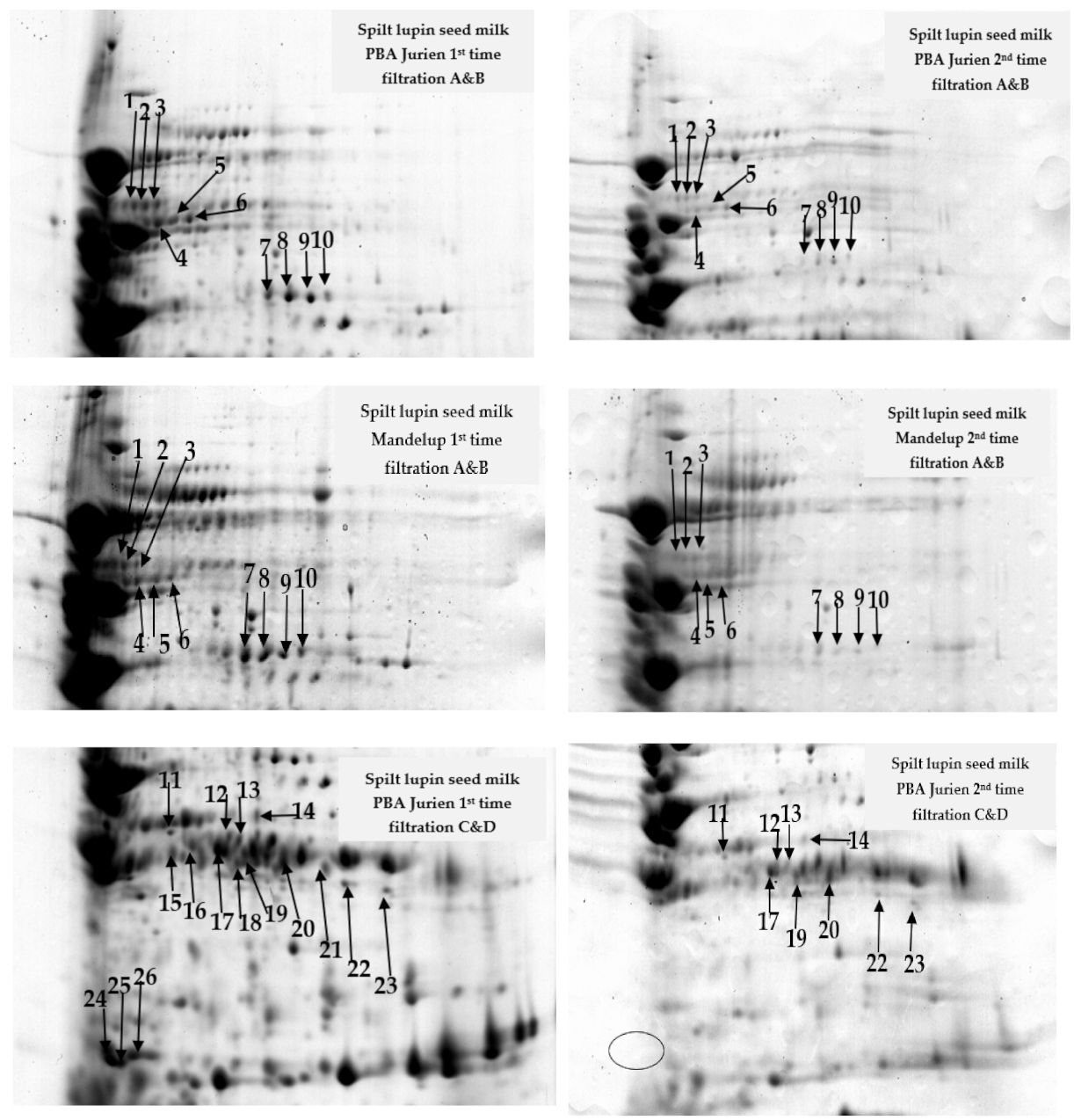
2.1. Protein Profile of Lupin Milk Based on Number of Cheesecloth Filtration
2.2. Cheese Production by Different Coagulation Methods
2.3. Effect of Water Temperature during Grinding of Split Lupin on the Yield and Quality of Lupin Cheese
2.4. Correlation between Lupin Protein and Cheese Preparation Based on Type and Number of Filtrations
3. Materials and Methods
3.1. Plant Materials
3.2. Methods
3.2.1. Preparation of Lupin Milk
3.2.2. Preparation of Protein Samples for 2D-PAGE
3.2.3. Determination of the Acetic Acid Level in Vinegar, and the Citric Acid Level in Lemon Juice
3.2.4. Preparation of the Starter Culture Lactic Acid Bacteria
3.2.5. The Lupin Cheesemaking Process from Different Coagulation Methods
3.2.6. Lupin Cheesemaking by Vinegar with Respect to Number of Filtrations
3.2.7. Data Analysis
3.2.8. Curd Yield Determination
3.2.9. Chemical Analysis
3.2.10. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, R.; Johnson, S.; Baxter, A.; Ball, M. Lupin kernel fibre-enriched foods beneficially modify serum lipids in men. Eur. J. Clin. Nutr. 2005, 59, 325–333. [Google Scholar] [CrossRef]
- Lee, Y.P.; Mori, T.A.; Sipsas, S.; Barden, A.; Puddey, I.B.; Burke, V.; Hall, R.S.; Hodgson, J.M. Lupin-enriched bread increases satiety and reduces energy intake acutely. Am. J. Clin. Nutr. 2006, 84, 975–980. [Google Scholar] [CrossRef]
- Hondelmann, W. The lupin—Ancient and modern crop plant. Theor. Appl. Genet. 1984, 68, 1–9. [Google Scholar] [CrossRef]
- Department of Agriculture and Food Government of Western Australia. Australian Sweet Lupin A very Healthy Asset; Department of Agriculture and Food Government of Western Australia: Australia, 2007.
- Jayasena, V.; Chih, H.J.; Nasar-Abbas, S. Efficient isolation of lupin protein. Food Aust. 2011, 63, 306–309. Available online: http://hdl.handle.net/20.500.11937/22645 (accessed on 19 March 2021).
- Drakos, A.; Doxastakis, G.; Kiosseoglou, V. Functional effects of lupin proteins in comminuted meat and emulsion gels. Food Chem. 2007, 100, 650–655. [Google Scholar] [CrossRef]
- Smith, S.C.; Choy, R.; Johnson, S.K.; Hall, R.S.; Wildeboer-Veloo, A.C.; Welling, G.W. Lupin kernel fiber consumption modifies fecal microbiota in healthy men as determined by rRNA gene fluorescent in situ hybridization. Eur. J. Nutr. 2006, 45, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Aiking, H.; de Boer, J.; Vereijken, J. Sustainable Protein Production and Consumption: Pigs or Peas? Springer Science & Business Media: Dordrecht, The Netherlands, 2006; Volume 45. [Google Scholar]
- Van de Noort, M. Lupin: An important protein and nutrient source. In Sustainable Protein Sources; Academic Press is an imprint of Elsevier: Amsterdam, The Netherlands, 2017; pp. 165–183. [Google Scholar] [CrossRef]
- Xu, J.; Mohamed, A.A. Thermal and rheological properties of Lupinus albus flour. J. Am. Oil Chem. Soc. 2003, 80, 763–766. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S.K. Nutritional, health, and technological functionality of lupin flour addition to bread and other baked products: Benefits and challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Lorrungruang, C.; Sinma, K.; Pantagrud, P.; Wannasirisuk, S.; Mahabandha, K.; Khucharoenphaisan, K. Red Cheese Production from Soymilk by Monascus purpureus and Lactobacillus casei. Res. J. Appl. 2014, 14, 2819–2824. [Google Scholar]
- Adejuyitan, J.; Olanipekun, B.; Moyinwin, O. Production and evaluation of cheese-like product from the blend of soymilk and coconut milk. Arch. Appl. Sci. Res. 2014, 6, 263–266. [Google Scholar]
- Jiménez-Martínez, C.; Hernández-Sánchez, H.; Dávila-Ortiz, G. Production of a yogurt-like product from Lupinus campestris seeds. J. Sci. Food Agric. 2003, 83, 515–522. [Google Scholar] [CrossRef]
- Wilkinson, I. Western Australian Lupin Industry. 2017, DAFWA. Available online: https://www.agric.wa.gov.au/grains-research-development/westernaustralian-lupin-industry (accessed on 2 May 2018).
- Lqari, H.; Vioque, J.; Pedroche, J.; Millán, F. Lupinus angustifolius protein isolates: Chemical composition, functional properties and protein characterization. Food Chem. 2002, 76, 349–356. [Google Scholar] [CrossRef]
- Chatchatee, P.; Järvinen, K.M.; Bardina, L.; Beyer, K.; Sampson, H.A. Identification of IgE-and IgG-binding epitopes on αs1-casein: Differences in patients with persistent and transient cow’s milk allergy. J. Allergy Clin. Immunol. 2001, 107, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Grandison, A.S.; Lewis, M.J. (Eds.) Separation Processes in the Food and Biotechnology Industries: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Al-Saedi, N.; Agarwal, M.; Ma, W.; Islam, S.; Ren, Y. Proteomic characterisation of lupin (Lupinus angustifolius) milk as influenced by extraction techniques, seed coat and cultivars. Molecules 2020, 25, 1782. [Google Scholar] [CrossRef]
- Gladstones, J.S. An historical review of lupins in Australia. In Proceedings of the 1st Australian Lupin Technical Symposium, Perth, Australia, 17–21 October 1994; Dracup, M., Palta, J., Eds.; Department of Agriculture: Perth, Australia, 1994; pp. 1–38. [Google Scholar]
- Martínez-Villaluenga, C.; Frías, J.; Vidal-Valverde, C. Raffinose family oligosaccharides and sucrose contents in 13 Spanish lupin cultivars. Food Chem. 2005, 91, 645–649. [Google Scholar] [CrossRef]
- Murevanhema, Y.Y.; Jideani, V.A. Potential of bambara groundnut (Vigna subterranea (L.) Verdc) milk as a probiotic beverage—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 954–967. [Google Scholar] [CrossRef]
- Foley, R.C.; Gao, L.-L.; Spriggs, A.; Soo, L.Y.; Goggin, D.E.; Smith, P.M.; Atkins, C.A.; Singh, K.B. Identification and characterisation of seed storage protein transcripts from Lupinus angustifolius. BMC Plant Biol. 2011, 11, 59. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.M.; Beyer, K.; Vila, L.; Chatchatee, P.; Busse, P.J.; Sampson, H.A. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2002, 110, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, P.A.M.; McDaniel, M.R. Flavor characteristics of lactic, malic, citric, and acetic acids at various pH levels. J. Food Sci. 1995, 60, 384–388. [Google Scholar] [CrossRef]
- Mathare, S.; Bakal, S.; Dissanayake, T.; Jain, S. Effects of coagulation temperature on the texture and yield of soy paneer (tofu). J. Natl. Sci. Found. 2009, 37, 263–267. [Google Scholar] [CrossRef]
- Thomas, T.D.; Pritchard, G.G. Proteolytic enzymes of dairy starter cultures. FEMS Microbiol. Lett. 1987, 46, 245–268. [Google Scholar] [CrossRef]
- Obiegbuna James, E.; Morah Grace, N.; Ishiwu Charles, N. Comparison of yields and physicochemical properties of lime juice with acetic acid and calcium chloride coagulated soybean curds. J. Food Nutr. Sci. 2014, 2, 58–62. [Google Scholar]
- Zayas, J.F. Oil and fat binding properties of proteins. In Functionality of Proteins in Food; Springer: Berlin/Heidelberg, Germany, 1997; pp. 228–259. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting; Supplement; Association of Official Analytical Chemists: Arlington, VA, USA, 1991. [Google Scholar]
- Seleet, F.L.; Kassem, J.M.; Bayonmim, H.M.; Abd- Raabou, N.S.; Ahmed, N.S. Production of functional spreadable processed cheese analogue supplemented with chickpea. Int. J. Dairy Sci. 2014, 9, 1–14. [Google Scholar] [CrossRef]
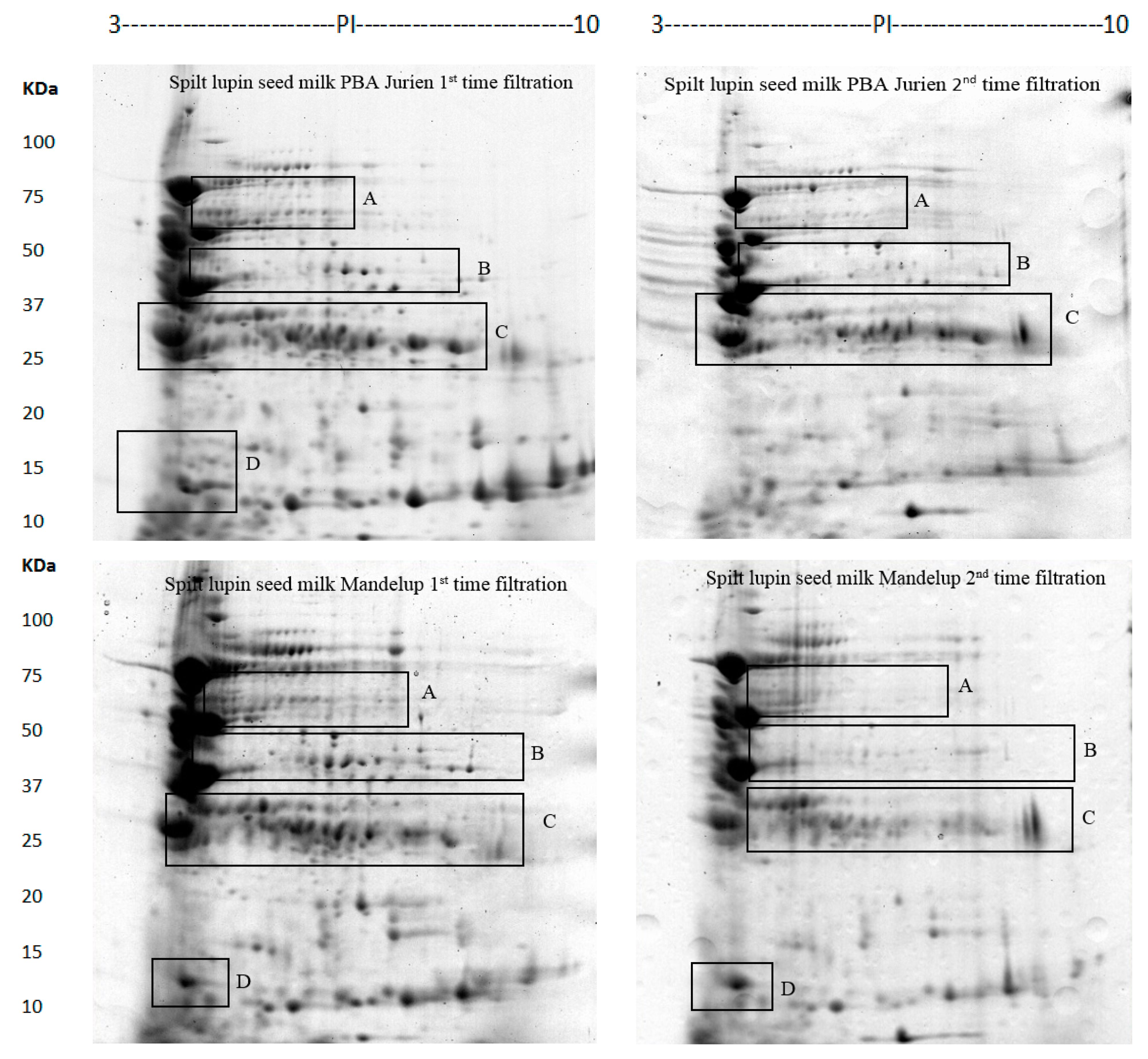


| Cultivars | Number of Filtrations | Spots Numbers Mean ± SD (n = 3) |
|---|---|---|
| PBA Jurien | First time | 231.33 ± 1.15 |
| Second time | 107.00 ± 1.00 | |
| Mandelup | First time | 204.00 ± 1.73 |
| Second time | 77.00 ± 1.00 |
| Split Lupin Milk PBA Jurien | Split Lupin Milk Mandelup | ||||
|---|---|---|---|---|---|
| First Filtration | Second Filtration | First Filtration | Second Filtration | ||
| Spot No | SSP | Mean ± SD (n = 3) | Mean ± SD (n = 3) | Mean ± SD (n = 3) | Mean ± SD (n = 3) |
| 1 | 6803 | 73.09 ± 0.57 | 8.07 ± 0.46 | 56.28 ± 0.83 | 0.52 ± 0.01 |
| 2 | 6806 | 82.91 ± 0.05 | 7.40 ± 0.53 | 63.40 ± 0.72 | 1.56 ± 0.25 |
| 3 | 7801 | 120.50 ± 0.73 | 5.58 ± 0.28 | 75.43 ± 0.49 | 1.81 ± 0.03 |
| 4 | 6501 | 30.41 ± 0.16 | 0.49 ± 0.01 | 25.13 ± 0.55 | 5.44 ± 0.41 |
| 5 | 7502 | 56.04 ± 0.78 | 3.25 ± 0.06 | 42.80 ± 0.64 | 6.91 ± 0.72 |
| 6 | 7204 | 30.93 ± 0.65 | 9.39 ± 0.58 | 25.13 ± 0.55 | 8.51 ± 0.40 |
| 7 | 7501 | 208.14 ± 0.34 | 0.48 ± 0.15 | 148.34 ± 0.79 | 2.53 ± 0.32 |
| 8 | 7503 | 170.08 ± 0.20 | 5.25 ± 0.68 | 101.25 ± 0.72 | 21.20 ± 0.58 |
| 9 | 8501 | 160.11 ± 0.68 | 5.72 ± 0.01 | 72.08 ± 0.26 | 1.66 ± 0.37 |
| 10 | 8503 | 61.84 ± 0.58 | 2.31 ± 0.01 | 37.62 ± 0.34 | 5.28 ± 0.18 |
| 11 | 4301 | 41.77 ± 0.86 | 18.56 ± 1.11 | 51.22 ± 0.75 | 15.30 ± 0.55 |
| 12 | 5304 | 108.78 ± 0.62 | 3.25 ± 0.35 | 16.10 ± 0.57 | ND |
| 13 | 6304 | 47.22 ± 0.66 | 1.86 ± 0.73 | 31.93 ± 0.60 | ND |
| 14 | 5402 | 215.24 ± 0.52 | 2.47 ± 0.61 | 101.35 ± 0.63 | ND |
| 15 | 2301 | 193.39 ± 0.49 | ND | 63.45 ± 0.27 | 30.63 ± 0.85 |
| 16 | 5303 | 233.49 ± 1.14 | ND | 19.31 ± 0.22 | 7.04 ± 0.42 |
| 17 | 6302 | 560.32 ± 0.95 | 30.92 ± 0.72 | 137.29 ± 0.84 | 20.94 ± 1.05 |
| 18 | 6203 | 274.02 ± 0.42 | ND | 129.94 ± 0.74 | 2.32 ± 0.50 |
| 19 | 6203 | 52.40 ± 0.53 | 15.51 ± 0.54 | 40.08 ± 0.25 | ND |
| 20 | 7306 | 622.24 ± 0.60 | 103.33 ± 3.51 | 436.37 ± 0.31 | 3.21 ± 0.30 |
| 21 | 8201 | 167.71 ± 0.56 | ND | 78.01 ± 0.66 | ND |
| 22 | 8202 | 77.22 ± 0.78 | 0.82 ± 0.04 | 63.13 ± 0.63 | ND |
| 23 | 8104 | 76.77 ± 0.11 | 0.33 ± 0.10 | 51.37 ± 0.98 | ND |
| 24 | 1101 | 439.11 ± 0.58 | ND | 234.32 ± 0.69 | 159.67 ± 0.58 |
| 25 | 1102 | 145.01 ± 0.74 | ND | 112.73 ± 1.43 | ND |
| 26 | 2101 | 275.02 ± 0.58 | ND | 121.38 ± 1.23 | ND |
| 27 | 8201 | ND | ND | 88.12 ± 0.67 | 3.49 ± 0.45 |
| Cultivar | Parameters | A Mean ± SD (n = 3) | B Mean ± SD (n = 3) | C Mean ± SD (n = 3) | D Mean ± SD (n = 3) | E Mean ± SD (n = 3) |
|---|---|---|---|---|---|---|
| PBA Jurien | Yield (%) | 17.63 ± 0.35 | 14.90 ± 0.14 | 12.70 ± 0.42 | 8.65 ± 0.49 | 10.25 ± 0.35 |
| Moisture (%) | 55.29 ± 0.55 | 61.78 ± 0.12 | 64.61 ± 0.31 | 71.42 ± 1.14 | 63.67 ± 0.15 | |
| Appearance | 4.37 ± 0.09 | 3.58 ± 0.09 | 3.00 ± 0.00 | 2.59 ± 0.09 | 2.93 ± 0.07 | |
| Color | 4.20 ± 0.61 | 3.58 ± 0.50 | 2.53 ± 0.51 | 2.52 ± 0.51 | 2.43 ± 0.50 | |
| Flavor | 4.20 ± 0.71 | 3.55 ± 0.51 | 2.80 ± 0.41 | 2.66 ± 0.48 | 2.63 ± 0.49 | |
| Texture | 4.20 ± 0.61 | 3.48 ± 0.51 | 2.90 ± 0.55 | 2.48 ± 0.51 | 2.60 ± 0.50 | |
| Overall acceptability | 4.73 ± 0.52 | 3.61 ± 0.50 | 2.87 ± 0.35 | 2.59 ± 0.50 | 3.47 ± 0.74 | |
| Mandelup | Yield (%) | 16.98 ± 0.28 | 14.25 ± 0.35 | 11.20 ± 0.28 | 7.15 ± 0.21 | 10.50 ± 0.71 |
| Moisture (%) | 55.39 ± 1.37 | 60.55 ± 0.14 | 66.27 ± 0.41 | 76.43 ± 0.15 | 62.55 ± 0.16 | |
| Appearance | 4.27 ± 0.12 | 3.50 ± 0.12 | 3.00 ± 0.11 | 2.83 ± 0.12 | 2.93 ± 0.10 | |
| Color | 4.43 ± 0.50 | 3.47 ± 0.51 | 2.90 ± 0.76 | 2.63 ± 0.62 | 2.57 ± 0.50 | |
| Flavor | 3.97 ± 0.72 | 3.37 ± 0.49 | 2.67 ± 0.55 | 2.50 ± 0.68 | 2.63 ± 0.56 | |
| Texture | 4.10 ± 0.48 | 3.57 ± 0.50 | 2.83 ± 0.59 | 2.30 ± 0.47 | 2.43 ± 0.50 | |
| Overall acceptability | 4.50 ± 0.63 | 3.57 ± 0.50 | 2.53 ± 0.51 | 2.67 ± 0.48 | 2.40 ± 0.50 | |
| Cow’s milk | Yield (%) | 17.60 ± 0.71 | 16.20 ± 0.28 | 14.65 ± 0.28 | 14.50 ± 0.20 | 15.20 ± 0.28 |
| Moisture (%) | 54.66 ± 0.19 | 55.94 ± 0.29 | 69.33 ± 1.40 | 65.21 ± 0.91 | 63.35 ± 1.06 | |
| Appearance | 3.97 ± 0.12 | 3.60 ± 0.10 | 3.77 ± 0.13 | 3.90 ± 0.12 | 3.52 ± 0.11 | |
| Color | 4.07 ± 0.14 | 3.90 ± 0.16 | 3.87 ± 0.12 | 3.83 ± 0.14 | 3.83 ± 0.13 | |
| Flavor | 3.60 ± 0.10 | 3.97 ± 0.12 | 3.90 ± 0.14 | 4.13 ± 0.12 | 3.66 ± 0.11 | |
| Texture | 3.80 ± 0.14 | 3.89 ± 0.06 | 3.93 ± 0.13 | 4.20 ± 0.12 | 4.07 ± 0.12 | |
| Overall acceptability | 4.00 ± 0.14 | 3.70 ± 0.11 | 3.77 ± 0.16 | 3.67 ± 0.12 | 3.93 ± 0.14 |
| Temperature | Parameters | A Mean ± SD (n = 3) | B Mean ± SD (n = 3) | C Mean ± SD (n = 3) | D Mean ± SD (n = 3) | E Mean ± SD (n = 3) |
|---|---|---|---|---|---|---|
| 45 °C | Yield% | 17.75 ± 0.35 | 15.25 ± 0.35 | 12.67 ± 0.35 | 8.70 ± 0.42 | 10.75 ± 0.35 |
| Moisture (%) | 54.43 ± 0.15 | 62.82 ± 0.63 | 63.00 ± 0.50 | 72.67 ± 0.31 | 63.26 ± 0.39 | |
| Appearance | 4.33 ± 0.48 | 3.67 ± 0.61 | 2.83 ± 0.46 | 2.87 ± 0.57 | 2.63 ± 0.56 | |
| Color | 4.47 ± 0.51 | 3.50 ± 0.51 | 3.00 ± 0.70 | 2.80 ± 0.61 | 2.60 ± 0.50 | |
| Flavor | 4.27 ± 0.74 | 3.43 ± 0.50 | 2.63 ± 0.49 | 2.60 ± 0.56 | 2.63 ± 0.50 | |
| Texture | 4.27 ± 0.58 | 3.30 ± 0.54 | 2.97 ± 0.51 | 2.60 ± 0.56 | 2.63 ± 0.49 | |
| Overall acceptability | 4.63 ± 0.49 | 3.60 ± 0.50 | 3.80 ± 0.41 | 2.80 ± 0.41 | 2.43 ± 0.50 | |
| 90 °C | Yield% | 8.45 ± 0.07 | 7.75 ± 0.35 | 6.75 ± 0.35 | 6.95 ± 0.78 | 6.50 ± 0.70 |
| Moisture (%) | 61.28 ± 0.70 | 65.50 ± 0.77 | 66.78 ± 0.15 | 72.38 ± 0.72 | 67.87 ± 0.28 | |
| Appearance | 4.20 ± 0.66 | 3.57 ± 0.68 | 3.03 ± 0.56 | 2.80 ± 0.66 | 2.93 ± 0.53 | |
| Color | 4.23 ± 0.68 | 3.47 ± 0.51 | 2.87 ± 0.78 | 2.67 ± 0.61 | 2.59 ± 0.50 | |
| Flavor | 3.90 ± 0.80 | 3.40 ± 0.50 | 2.67 ± 0.55 | 2.53 ± 0.68 | 2.62 ± 0.56 | |
| Texture | 2.70 ± 0.52 | 2.90 ± 0.49 | 2.80 ± 0.61 | 2.30 ± 0.47 | 2.45 ± 0.51 | |
| Overall acceptability | 3.40 ± 0.77 | 3.20 ± 0.56 | 2.57 ± 0.50 | 2.32 ± 0.48 | 2.38 ± 0.49 |
| Component (g/100 g) | PBA Jurien 1st Mean ± SD (n = 3) | PBA Jurien. 2nd Mean ± SD (n = 3) | PBA Jurien 3rd Mean ± SD (n = 3) | Mandelup 1st Mean ± SD (n = 3) | Mandelup 2nd Mean ± SD (n = 3) | Mandelup 3rd Mean ± SD (n = 3) | |
|---|---|---|---|---|---|---|---|
| Yield(w/v) | 17.69 ± 0.21 | 12.25 ± 0.35 | 9.50 ± 0.14 | 16.86 ± 0.14 | 11.10 ± 0.14 | 8.70 ± 0.42 | |
| Protein | 27.33 ± 0.57 | 6.10 ± 0.10 | 4.10 ± 0.10 | 20.60 ± 0.43 | 9.1 ± 0.10 | 5.33 ± 0.11 | |
| Fat | 9.90 ± 0.10 | 1.00 ± 0.10 | 0.99 ± 0.05 | 6.23 ± 0.57 | 2.26 ± 0.57 | 0.99 ± 0.05 | |
| Moisture | 53.67 ± 1.52 | 85.00 ± 1.52 | 87.24 ± 1.01 | 64.45 ± 1.52 | 79.67 ± 1.52 | 81.29 ± 1.11 | |
| Ash | 4.60 ± 0.26 | 3.50 ± 0.02 | 3.15 ± 0.15 | 4.20 ± 0.20 | 3.70 ± 0.03 | 3.63 ± 0.41 | |
| Carbohydrates | 3.96 ± 0.57 | 4.60 ± 0.25 | 4.90 ± 0.10 | 5.33 ± 0.152 | 4.56 ± 0.15 | 7.23 ± 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saedi, N.; Agarwal, M.; Islam, S.; Ren, Y.-L. Study on the Correlation between the Protein Profile of Lupin Milk and Its Cheese Production Compared with Cow’s Milk. Molecules 2021, 26, 2395. https://doi.org/10.3390/molecules26082395
Al-Saedi N, Agarwal M, Islam S, Ren Y-L. Study on the Correlation between the Protein Profile of Lupin Milk and Its Cheese Production Compared with Cow’s Milk. Molecules. 2021; 26(8):2395. https://doi.org/10.3390/molecules26082395
Chicago/Turabian StyleAl-Saedi, Nadia, Manjree Agarwal, Shahidul Islam, and Yong-Lin Ren. 2021. "Study on the Correlation between the Protein Profile of Lupin Milk and Its Cheese Production Compared with Cow’s Milk" Molecules 26, no. 8: 2395. https://doi.org/10.3390/molecules26082395
APA StyleAl-Saedi, N., Agarwal, M., Islam, S., & Ren, Y.-L. (2021). Study on the Correlation between the Protein Profile of Lupin Milk and Its Cheese Production Compared with Cow’s Milk. Molecules, 26(8), 2395. https://doi.org/10.3390/molecules26082395







