Comparative Study on Curcumin Loaded in Golden Pompano (Trachinotus blochii) Head Phospholipid and Soybean Lecithin Liposomes: Preparation, Characteristics and Anti-Inflammatory Properties
Abstract
1. Introduction
2. Results
2.1. Characterization of Liposomes
2.1.1. Particle Size/ζ-Potential/Encapsulation Efficiency
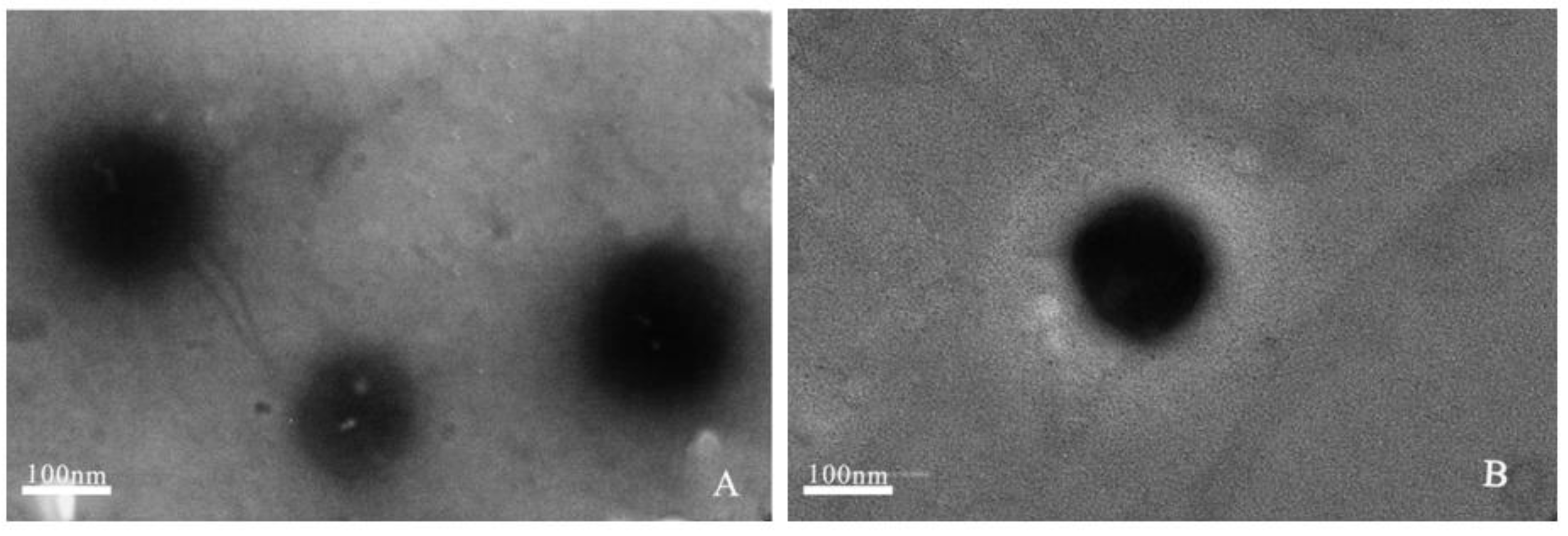
2.1.2. Transmission Electron Microscopy (TEM)
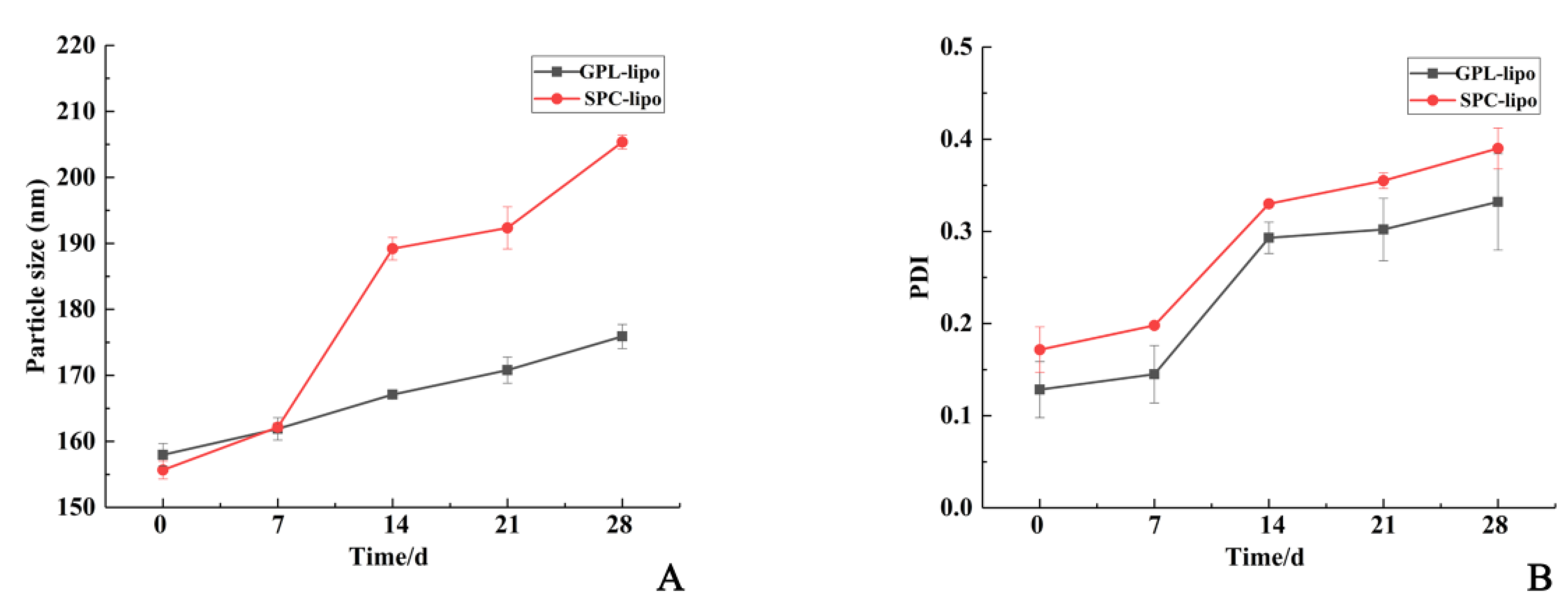
2.1.3. Storage Stability
2.2. In Vitro Evaluation of the Anti-Inflammatory Activity of Two Liposomes
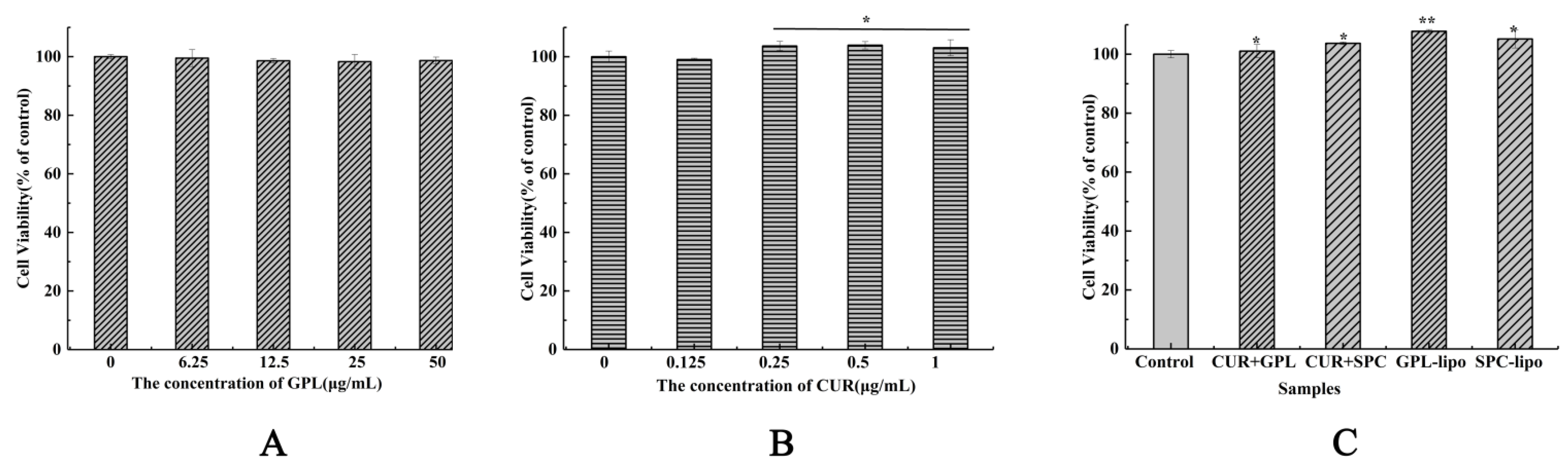
2.2.1. Cell Viability
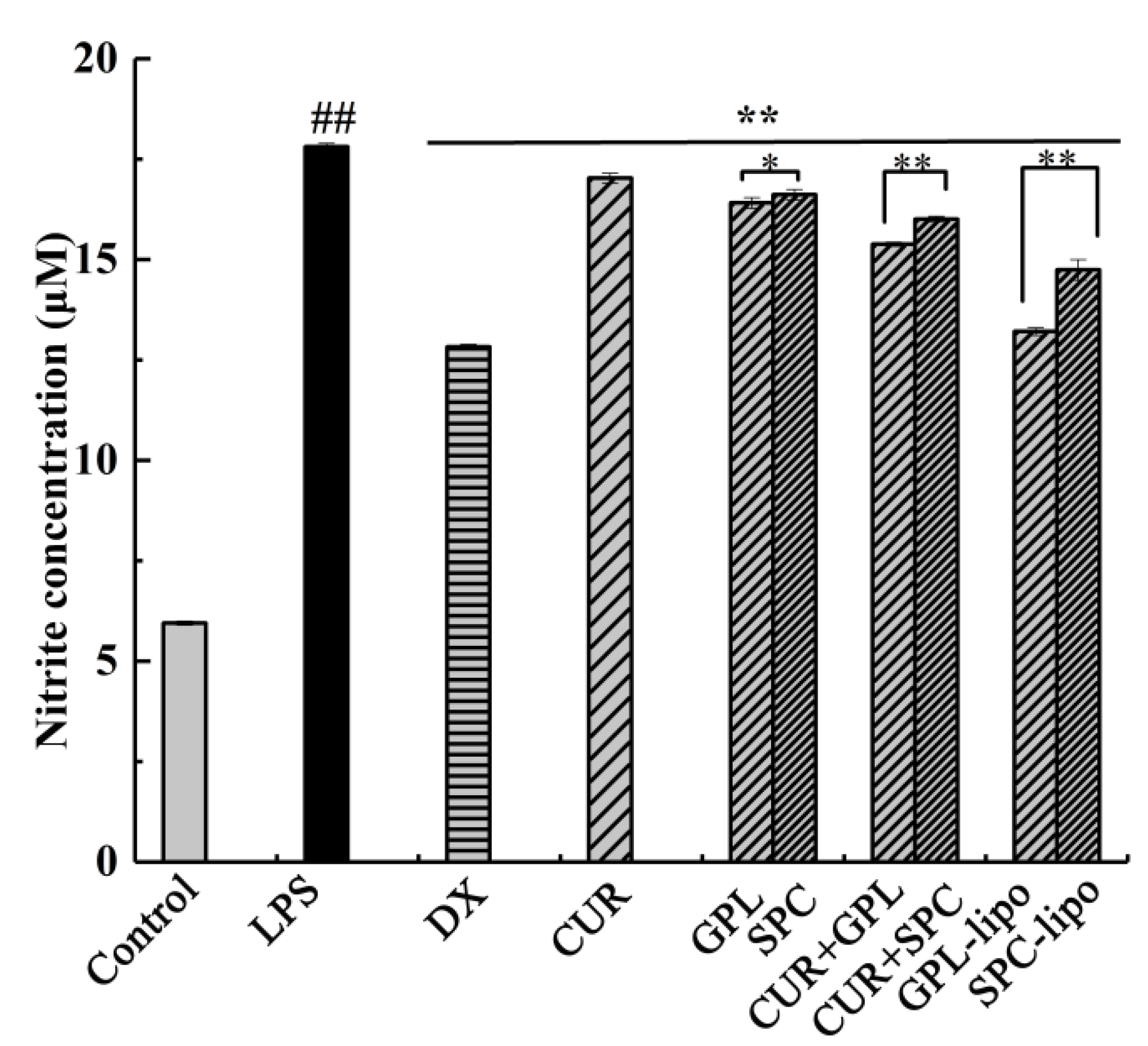
2.2.2. Nitric Oxide (NO) Production
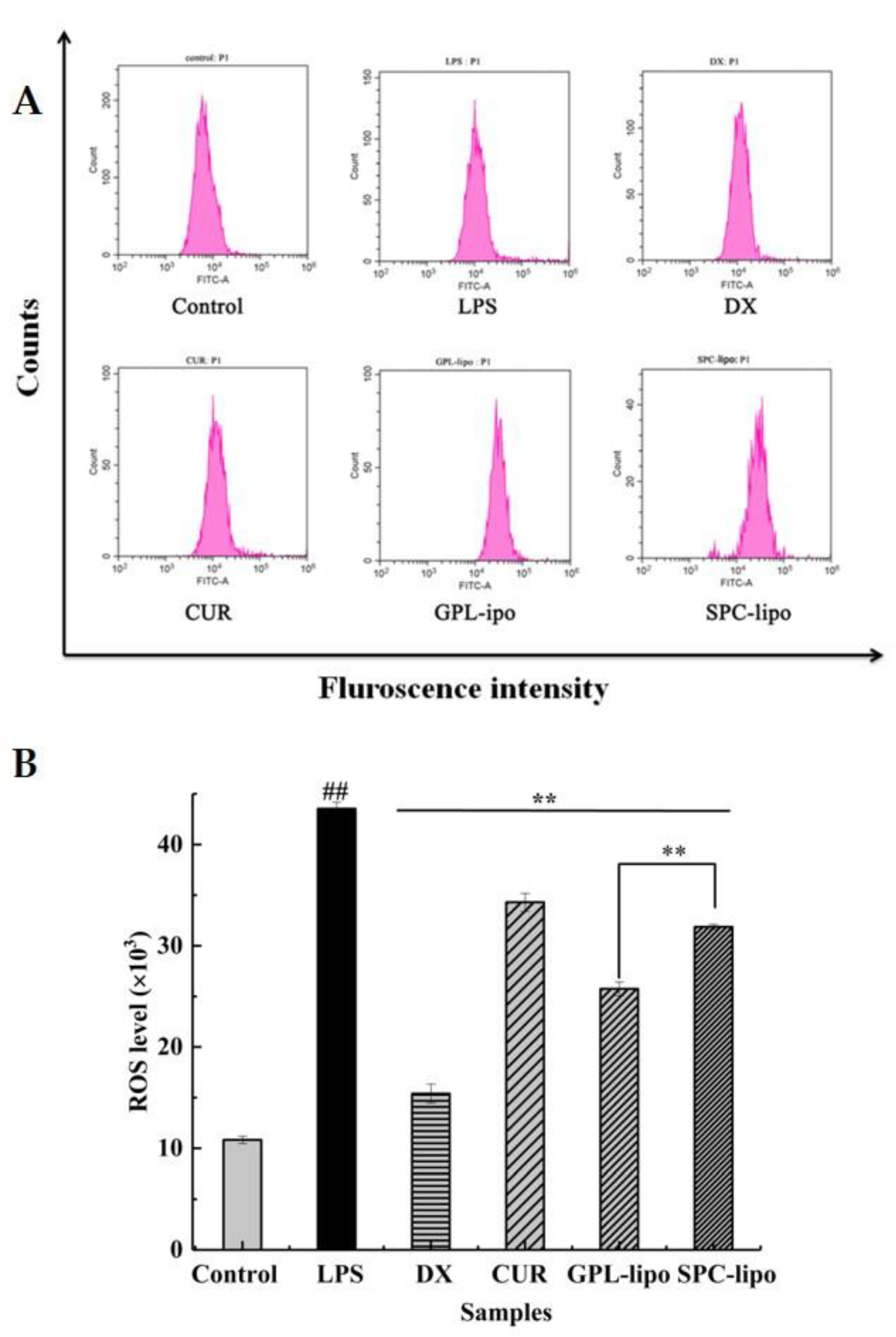
2.2.3. Reactive Oxygen Species (ROS) Expression
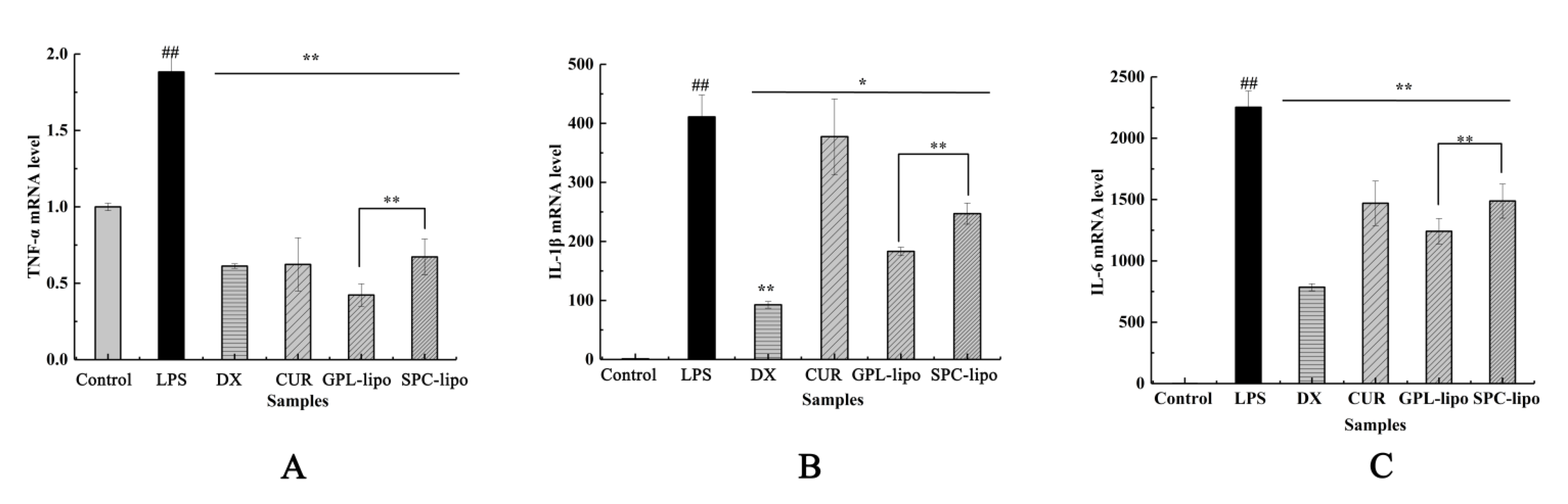
2.2.4. Quantitative Reverse Transcription-PCR (RT-qPCR)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction of GPL
4.3. Preparation of Liposomes
4.4. Measurement of Particle Size Distribution, PDI, ζ-Potential, and Encapsulation Efficiency
4.5. TEM
4.6. Storage Stability
4.7. Cell Viability Assay
4.8. NO Production Assays
4.9. ROS Production Assays
4.10. RT-qPCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ono, M. Molecular links between tumor angiogenesis and inflammation: Inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008, 99, 1501–1506. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, T.; Qiu, C.; Wu, B.; Zhang, Y.; Chen, L.; Xia, Q.; Li, C.; Zhou, B.; Liu, Z.; et al. Synthesis and optimization of novel allylated mono-carbonyl analogs of curcumin (MACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) in rats. Eur. J. Med. Chem. 2016, 121, 181–193. [Google Scholar] [CrossRef]
- Chang, M.; Wu, M.; Li, H. Antitumor activities of novel glycyrrhetinic acid-modified curcumin-loaded cationic liposomes in vitro and in H22 tumor-bearing mice. Drug Deliv. 2018, 25, 1984–1995. [Google Scholar] [CrossRef]
- Li, J.; Hwang, I.-C.; Chen, X.; Park, H.J. Effects of chitosan coating on curcumin loaded nano-emulsion: Study on stability and in vitro digestibility. Food Hydrocoll. 2016, 60, 138–147. [Google Scholar] [CrossRef]
- Sinjari, B.; Pizzicannella, J.; D’Aurora, M.; Zappacosta, R.; Gatta, V.; Fontana, A.; Trubiani, O.; Diomede, F. Curcumin/Liposome Nanotechnology as Delivery Platform for Anti-inflammatory Activities via NFkB/ERK/pERK Pathway in Human Dental Pulp Treated With 2-HydroxyEthyl MethAcrylate (HEMA). Front. Physiol. 2019, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Crommelin, D.J.A.; van Hoogevest, P.; Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 2020, 318, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yu, L.; Zhao, X.; Ostrikov, K. Nanomaterials for oncotherapies targeting the hallmarks of cancer. Nanotechnology 2020, 31, 392001. [Google Scholar] [CrossRef] [PubMed]
- Cardia, M.C.; Caddeo, C.; Lai, F.; Fadda, A.M.; Sinico, C.; Luhmer, M. 1H NMR study of the interaction of trans-resveratrol with soybean phosphatidylcholine liposomes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.-H.; Xu, J.; Wang, Y.-M.; Xue, C.-H.; Kurihara, H.; Takahashi, K. Transport and uptake effects of marine complex lipid liposomes in small intestinal epithelial cell models. Food Funct. 2016, 7, 1904–1914. [Google Scholar] [CrossRef]
- Buang, Y.; Wang, Y.-M.; Cha, J.-Y.; Nagao, K.; Yanagita, T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition 2005, 21, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.T.; Zingg, J.-M.; Kwan, P.; Noble, T.; Smith, D.; Meydani, M. Curcumin modulation of high fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Atherosclerosis 2014, 232, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Li, P.; Xia, G.; Li, C.; Shen, X. Analysis and Identification of Golden pompano (Trachinotus blochii) Head Phospholipid Molecular Species by Liquid Chromatography-Mass Spectrometry. J. Oleo Sci. 2019, 68, 1187–1197. [Google Scholar] [CrossRef]
- Stulnig, T. Polyunsaturated fatty acids in the prevention of heart and vascular diseases. Ernahr. Umsch. 2015, 62, M596–M599. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Tai, K.; Rappolt, M.; Mao, L.; Gao, Y.; Li, X.; Yuan, F. The stabilization and release performances of curcumin-loaded liposomes coated by high and low molecular weight chitosan. Food Hydrocoll. 2020, 99, 105355. [Google Scholar] [CrossRef]
- Della Camera, G.; Lipsa, D.; Mehn, D.; Italiani, P.; Boraschi, D.; Gioria, S. A Step-by-Step Approach to Improve Clinical Translation of Liposome-Based Nanomaterials, a Focus on Innate Immune and Inflammatory Responses. Int. J. Mol. Sci. 2021, 22, 820. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Min, H.-K.; Na, H.S.; Kwon, J.Y.; Ryu, J.; Cho, K.-H.; Choi, J.; Jung, K.; Lee, S.-Y.; Kim, S.J.; et al. Combinatmarion treatment with Lactobacillus acidophilus LA-1, vitamin B, and curcumin ameliorates the progression of osteoarthritis by inhibiting the pro-inflammatory mediators. Immunol. Lett. 2020, 228, 112–121. [Google Scholar] [CrossRef]
- Fallahi, F.; Borran, S.; Ashrafizadeh, M.; Zarrabi, A.; Pourhanifeh, M.H.; Mahabady, M.K.; Sahebkar, A.; Mirzaei, H. Curcumin and inflammatory bowel diseases: From in vitro studies to clinical trials. Mol. Immunol. 2021, 130, 20–30. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.-H.; Zhou, L.; Ding, X.-L.; Meng, Y.-C.; Han, K. Curcumin alleviates OGD/R-induced PC12 cell damage via repressing CCL3 and inactivating TLR4/MyD88/MAPK/NF-κB to suppress inflammation and apoptosis. J. Pharm. Pharmacol. 2020, 72, 1176–1185. [Google Scholar] [CrossRef]
- Shao, M.; Lou, D.; Yang, J.; Lin, M.; Deng, X.; Fan, Q. Curcumin and wikstroflavone B, a new biflavonoid isolated from Wikstroemia indica, synergistically suppress the proliferation and metastasis of nasopharyngeal carcinoma cells via blocking FAK/STAT3 signaling pathway. Phytomedicine 2020, 79, 153341. [Google Scholar] [CrossRef]
- Xu, D.; Xie, J.; Feng, X.; Zhang, X.; Ren, Z.; Zheng, Y.; Yang, J. Preparation and evaluation of a Rubropunctatin-loaded liposome anticancer drug carrier. RSC Adv. 2020, 10, 10352–10360. [Google Scholar] [CrossRef]
- Stuchlik, M.; Zák, S. Stuchl Lipid-based vehicle for oral drug delivey. Biomed. Pap. 2001, 145, 17–26. [Google Scholar] [CrossRef]
- Allijn, I.E.; Schiffelers, R.M.; Storm, G. Comparison of pharmaceutical nanoformulations for curcumin: Enhancement of aqueous solubility and carrier retention. Int. J. Pharm. 2016, 506, 407–413. [Google Scholar] [CrossRef]
- Jin, H.-H.; Lu, Q.; Jiang, J.-G. Curcumin liposomes prepared with milk fat globule membrane phospholipids and soybean lecithin. J. Dairy Sci. 2016, 99, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zou, L.; Liu, W.; Liu, C.; McClements, D.J. Fabrication and Characterization of Curcumin-Loaded Liposomes Formed from Sunflower Lecithin: Impact of Composition and Environmental Stress. J. Agric. Food Chem. 2018, 66, 12421–12430. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Antimisiaris, S.G. Effect of Amphiphilic Drugs on the Stability and Zeta-Potential of Their Liposome Formulations: A Study with Prednisolone, Diazepam, and Griseofulvin. J. Colloid Interface Sci. 2002, 251, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Laveti, D.; Kumar, M.; Hemalatha, R.; Sistla, R.; Naidu, V.G.M.; Talla, V.; Verma, V.; Kaur, N.; Nagpal, R. Anti-Inflammatory Treatments for Chronic Diseases: A Review. Inflamm. Allergy Drug Targets 2013, 12, 349–361. [Google Scholar] [CrossRef]
- Lu, G.M.; Zhang, R.; Geng, S.; Peng, L.; Jayaraman, P.; Chen, C.; Xu, F.; Yang, J.; Li, Q.; Zheng, H.; et al. Myeloid cell-derived inducible nitric oxide synthase suppresses M1 macrophage polarization. Nat. Commun. 2015, 6, 6676. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-J.; Liu, S.-C.; Chen, Y.-C.; Hsu, S.-H.; Chang, Y.-P.; Lin, J.-T. Three Pathways Assess Anti-Inflammatory Response of Epicatechin with Lipopolysaccharide-Mediated Macrophage RAW264.7 Cells. J. Food Biochem. 2015, 39, 334–343. [Google Scholar] [CrossRef]
- Ma, F.; Liu, F.; Ding, L.; You, M.; Yue, H.; Zhou, Y.; Hou, Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef]
- Charman, W.N. Lipids, Lipophilic Drugs, and Oral Drug Delivery—Some Emerging Concepts. J. Pharm. Sci. 2000, 89, 967–978. [Google Scholar] [CrossRef]
- Tőkés, T.; Erős, G.; Várszegi, S.; Hartmann, P.; Bebes, A.; Kaszaki, J.; Boros, M. Protective effects of phosphatidylcholine pretreatment in endotoxin-induced systemic inflammation in the rat hippocampus. Acta Physiol. Hung. 2010, 97, 483. [Google Scholar]
- Wang, G.; Wang, J.; Wu, W.; To, S.S.T.; Zhao, H.; Wang, J. Advances in lipid-based drug delivery: Enhancing efficiency for hydrophobic drugs. Expert Opin. Drug Deliv. 2015, 12, 1475–1499. [Google Scholar] [CrossRef]
- Żelechowska, P.; Różalska, S.; Wiktorska, M.; Brzezińska-Błaszczyk, E.; Agier, J. Curdlan stimulates tissue mast cells to synthesize pro-inflammatory mediators, generate ROS, and migrate via Dectin-1 receptor. Cell. Immunol. 2020, 351, 104079. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Yu, H.; Zhang, X.; Cui, G.; Wang, K.; Mao, S.; Pan, Y. Typhonium giganteum Lectin Exerts A Pro-Inflammatory Effect on RAW 264.7 via ROS and the NF-κB Signaling Pathway. Toxins 2017, 9, 275. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Chen, L.; Shen, X.; Chen, G.; Cao, X.; Yang, J. Effect of Three-spot Seahorse Petroleum Ether Extract on Lipopolysaccharide Induced Macrophage RAW264.7 Inflammatory Cytokine Nitric Oxide and Composition Analysis. J. Oleo Sci. 2015, 64, 933–942. [Google Scholar] [CrossRef]
- Coker, R.K.; Laurent, G.J. Pulmonary fibrosis: Cytokines in the balance. Eur. Respir. J 1998, 11, 1218–1221. [Google Scholar] [CrossRef]
- Mei, F.; Liu, J.; Wu, J.; Duan, Z.; Chen, M.; Meng, K.; Chen, S.; Shen, X.; Xia, G.; Zhao, M. Collagen Peptides Isolated from Salmo salar and Tilapia nilotica Skin Accelerate Wound Healing by Altering Cutaneous Microbiome Colonization via Upregulated NOD2 and BD14. J. Agric. Food Chem. 2020, 68, 1621–1633. [Google Scholar] [CrossRef]
- Mei, F.; Meng, K.; Gu, Z.; Yun, Y.; Zhang, W.; Zhang, C.; Zhong, Q.; Pan, F.; Shen, X.; Xia, G.; et al. Arecanut (Areca catechu L.) Seed Polyphenol-Ameliorated Osteoporosis by Altering Gut Microbiome via LYZ and the Immune System in Estrogen-Deficient Rats. J. Agric. Food Chem. 2021, 69, 246–258. [Google Scholar] [CrossRef]
| Liposomes Formulations | Particle Size (nm) | ζ-Potential (mV) | Encapsulation Efficiency (%) | |
|---|---|---|---|---|
| Pre-Extrusion | Pro-Extrusion | |||
| Empty GPL liposome | 834.07 ± 25.72 | 170.60 ± 1.69 | −28.23 ± 1.46 | - |
| Empty SPC liposome | 788.87 ± 11.52 | 169.83 ± 0.6 | −22.33 ± 0.86 | - |
| GPL-lipo | 596.4 ± 26.63 | 155.66 ± 1.10 | −35.47 ± 0.87 | 86.72 ± 2.74 |
| SPC-lipo | 787.83 ± 7.38 | 157.97 ± 1.41 | −25.30 ± 0.56 | 89.25 ± 3.20 |
| Genes | Forward | Reverse |
|---|---|---|
| GAPDH | CATGTTCCAGTATGACTCCACTC | GGCCTCACCCCATTTGATGT |
| TNF-α | TATGGCTCAGGGTCCAACTC | GGAAAGCCCATTTGAGTCCT |
| IL-1β | GTTGACGGACCCCAAAAGAT | CCTCATCCTGGAAGGTCCAC |
| IL-6 | CACGGCCTTCCCTACTTCAC | TGCAAGTGCATCATCGTTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Yi, X.; Liu, Z.; Dong, X.; Xia, G.; Zhang, X.; Shen, X. Comparative Study on Curcumin Loaded in Golden Pompano (Trachinotus blochii) Head Phospholipid and Soybean Lecithin Liposomes: Preparation, Characteristics and Anti-Inflammatory Properties. Molecules 2021, 26, 2328. https://doi.org/10.3390/molecules26082328
Gao X, Yi X, Liu Z, Dong X, Xia G, Zhang X, Shen X. Comparative Study on Curcumin Loaded in Golden Pompano (Trachinotus blochii) Head Phospholipid and Soybean Lecithin Liposomes: Preparation, Characteristics and Anti-Inflammatory Properties. Molecules. 2021; 26(8):2328. https://doi.org/10.3390/molecules26082328
Chicago/Turabian StyleGao, Xia, Xiangzhou Yi, Zhongyuan Liu, Xiuping Dong, Guanghua Xia, Xueying Zhang, and Xuanri Shen. 2021. "Comparative Study on Curcumin Loaded in Golden Pompano (Trachinotus blochii) Head Phospholipid and Soybean Lecithin Liposomes: Preparation, Characteristics and Anti-Inflammatory Properties" Molecules 26, no. 8: 2328. https://doi.org/10.3390/molecules26082328
APA StyleGao, X., Yi, X., Liu, Z., Dong, X., Xia, G., Zhang, X., & Shen, X. (2021). Comparative Study on Curcumin Loaded in Golden Pompano (Trachinotus blochii) Head Phospholipid and Soybean Lecithin Liposomes: Preparation, Characteristics and Anti-Inflammatory Properties. Molecules, 26(8), 2328. https://doi.org/10.3390/molecules26082328






