The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines
Abstract
1. Introduction
2. Results
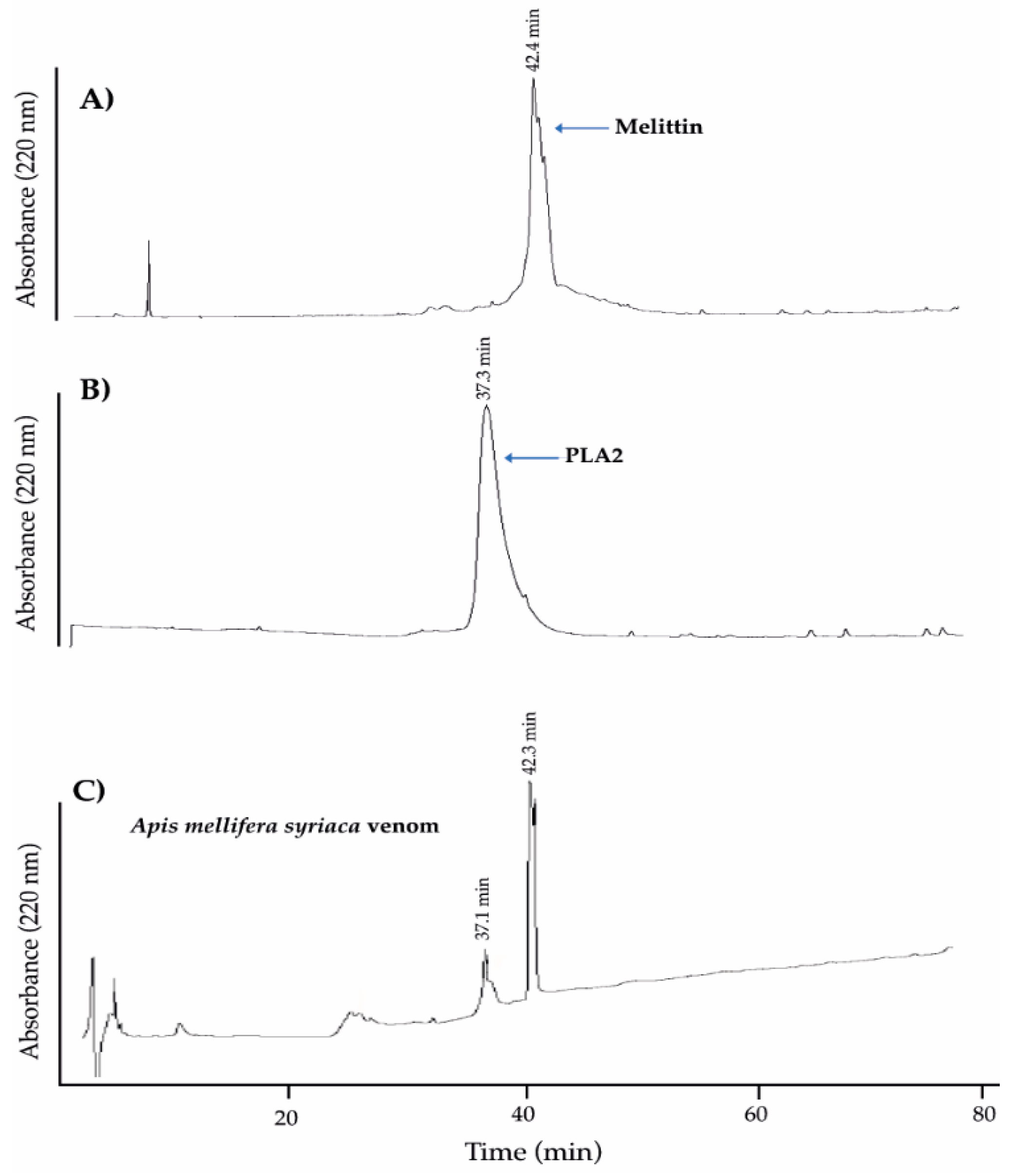
2.1. Analysis of A. mellifera syriaca Venom (Used in Our Experiments) and Its Two Main Components, MEL and PLA2, by HPLC
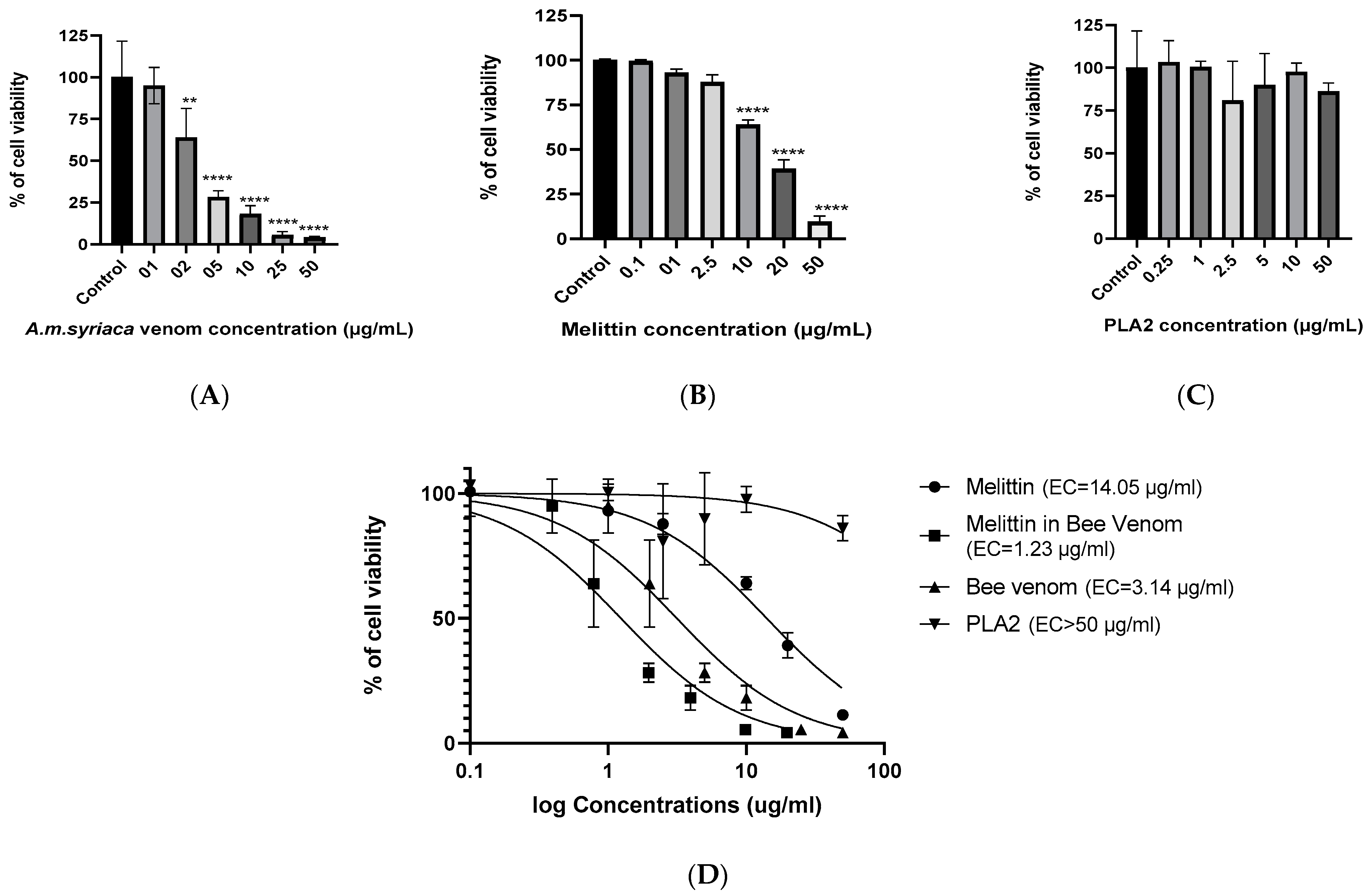
2.2. Dose-Dependent Effect of A. mellifera Venom on Cell Viability of Colon Cancer HCT116 Cells
2.3. Dose-Dependent Effect of MEL on Cell Viability of Colon Cancer HCT116 Cells
2.4. Effect of PLA2 on Cell Viability of Colon Cancer HCT116 Cells
2.5. Effect of MEL in A. mellifera Venom on Cell Viability of Colon Cancer HCT116 Cells
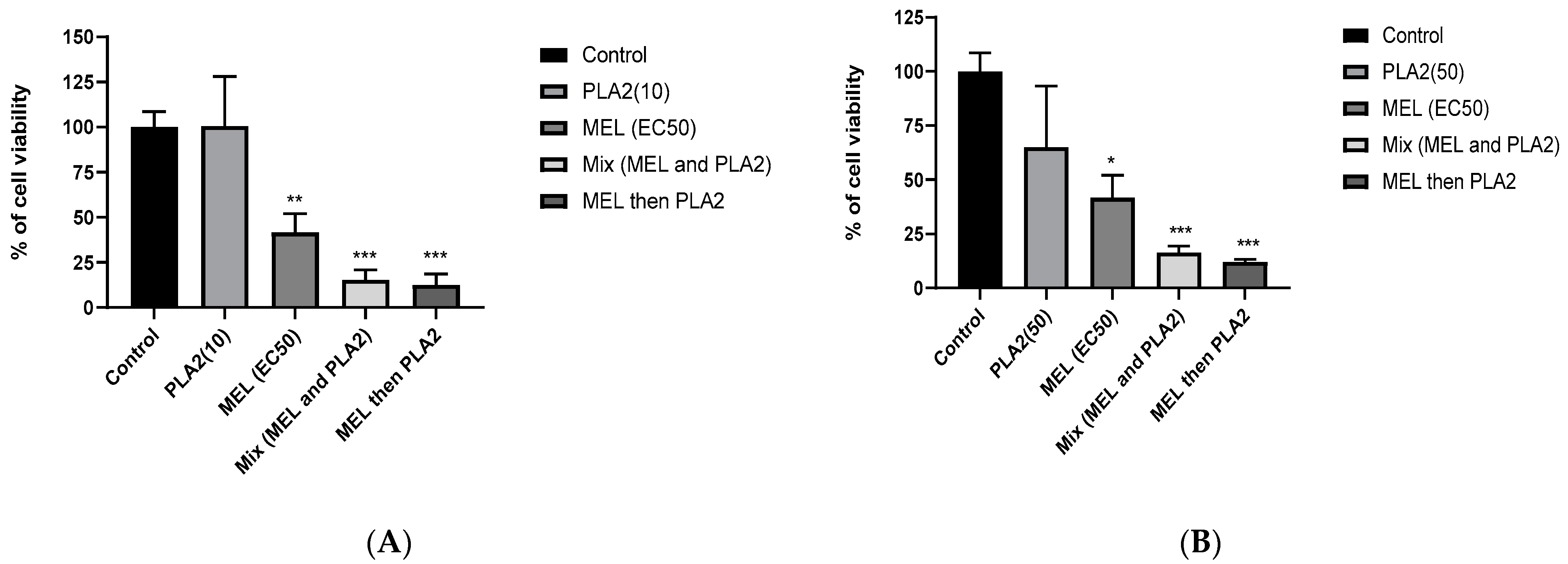
2.6. Synergistic Effects between MEL and PLA2
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Analysis of A. mellifera syriaca Venom Components, MEL, and PLA2 by High-Pressure Liquid Chromatography (HPLC) Technique
4.3. Cell Culture
4.4. Cellular Viability Assay
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Khachfe, H.H.; Rahal, Z.; Sammouri, J.; Kheil, M.; Baydoun, H.; Chatila, D. Cancer in Lebanon: A review of incidence rates from 2008 to 2015 and projections till 2025. S. Asian J. Cancer 2019, 60, 61. [Google Scholar]
- Peluso, G.; Incollingo, P.; Calogero, A.; Tammaro, V.; Rupealta, N.; Chiacchio, G. Current tissue molecular markers in colorectal cancer: A literature review. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Hao, D.-J.; Zhang, Q.; An, J.; Zhao, J.-J.; Chen, B. Application of bee venom and its main constituent melittin for cancer treatment. Cancer Chemother. Pharmacol. 2016, 78, 1113–1130. [Google Scholar] [CrossRef]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-B.; Lee, J.-D.; Lee, H.-J.; Han, H.-J.; Mar, W.-C.; Kang, S.-K. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain 2001, 90, 271–280. [Google Scholar] [CrossRef]
- Jang, M.-H.; Shin, M.-C.; Lim, S.; Han, S.-M.; Park, H.-J.; Shin, I. Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J. Pharmacol. Sci. 2003, 91, 95–104. [Google Scholar] [CrossRef]
- Jung, G.B.; Huh, J.-E.; Lee, H.-J.; Kim, D.; Lee, G.-J.; Park, H.-K. Anti-cancer effect of bee venom on human MDA-MB-231 breast cancer cells using Raman spectroscopy. Biomed. Opt. Express 2018, 9, 5703–5718. [Google Scholar] [CrossRef]
- Lee, M.-T.; Sun, T.-L.; Hung, W.-C.; Huang, H.W. Process of inducing pores in membranes by melittin. Proc. Natl. Acad. Sci. USA 2013, 110, 14243–14248. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Abd El-Wahed, A.A.; Khalifa, S.A.; Sheikh, B.Y.; Farag, M.A.; Saeed, A.; Larik, F.A. Bee venom composition: From chemistry to biological activity. Stud. Nat. Prod. Chem. 2019, 60, 459–484. [Google Scholar]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Bitar, L.; Jundi, D.; Rima, M.; Al-Alam, J.; Sabatier, J.-M.; Fajloun, Z. Bee Venom PLA2 Versus Snake Venom PLA2: Evaluation of Structural and Functional Properties. Venoms Toxins 2021, 1. [Google Scholar]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef]
- Gajski, G.; Garaj-Vrhovac, V. Melittin: A lytic peptide with anticancer properties. Environ. Toxicol. Pharmacol. 2013, 36, 697–705. [Google Scholar] [CrossRef]
- Marcussi, S.; Sant’Ana, C.D.; Oliveira, C.Z.; Rueda, A.Q.; Menaldo, D.L.; Beleboni, R.O. Snake venom phospholipase A2 inhibitors: Medicinal chemistry and therapeutic potential. Curr. Top. Med. Chem. 2007, 7, 743–756. [Google Scholar] [CrossRef]
- Damianoglou, A.; Rodger, A.; Pridmore, C.; Dafforn, T.R.; Mosely, J.A.; Sanderson, J.M. The synergistic action of melittin and phospholipase A2 with lipid membranes: Development of linear dichroism for membrane-insertion kinetics. Protein Pept. Lett. 2010, 17, 1351–1362. [Google Scholar] [CrossRef]
- Nehme, H.; Ayde, H.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Potential Inhibitory Effect of Apis mellifera’s Venom and of Its Two Main Components—Melittin and PLA2—on Escherichia coli F1F0-ATPase. Antibiotics 2020, 9, 824. [Google Scholar] [CrossRef]
- Cajal, Y.; Jain, M.K. Synergism between mellitin and phospholipase A2 from bee venom: Apparent activation by intervesicle exchange of phospholipids. Biochemistry 1997, 36, 3882–3893. [Google Scholar] [CrossRef]
- Chen, J.; Guan, S.-M.; Sun, W.; Fu, H. Melittin, the major pain-producing substance of bee venom. Neurosci. Bull. 2016, 32, 265–272. [Google Scholar] [CrossRef]
- Samanci, T.; Kekeçoglu, M. Comparison of commercial and antolian bee venom in terms of chemical composition. Uludağ Arıcılık Derg. 2019, 19, 61–68. [Google Scholar] [CrossRef]
- Hong, S.-J.; Rim, G.S.; Yang, H.I.; Yin, C.S.; Koh, H.G.; Jang, M.-H. Bee venom induces apoptosis through caspase-3 activation in synovial fibroblasts of patients with rheumatoid arthritis. Toxicon 2005, 46, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.-W.; Wei, H.-C.; Lin, J.-P.; Kuo, H.-M.; Liu, K.-C.; Hsu, S.-C. Bee venom induced cell cycle arrest and apoptosis in human cervical epidermoid carcinoma Ca Ski cells. Anticancer Res. 2008, 28, 833–842. [Google Scholar] [PubMed]
- Duffy, C.; Sorolla, A.; Wang, E.; Golden, E.; Woodward, E.; Davern, K. Honeybee venom and melittin suppress growth factor receptor activation in HER2-enriched and triple-negative breast cancer. NPJ Precis. Oncol. 2020, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.E.; Hwang, C.J.; Gu, S.M.; Park, M.H.; Kim, J.H.; Park, J.H. Cancer cell growth inhibitory effect of bee venom via increase of death receptor 3 expression and inactivation of NF-kappa B in NSCLC cells. Toxins 2014, 6, 2210–2228. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.; Chen, Y.; Li, H.; Xu, S.; Li, Y.; Sun, J. Melittin inhibits tumor angiogenesis modulated by endothelial progenitor cells associated with the SDF-1α/CXCR4 signaling pathway in a UMR-106 osteosarcoma xenograft mouse model. Mol. Med. Rep. 2016, 14, 57–68. [Google Scholar] [CrossRef]
- Liu, S.; Yu, M.; He, Y.; Xiao, L.; Wang, F.; Song, C. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology 2008, 47, 1964–1973. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaacoub, C.; Rifi, M.; El-Obeid, D.; Mawlawi, H.; Sabatier, J.-M.; Coutard, B.; Fajloun, Z. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines. Molecules 2021, 26, 2264. https://doi.org/10.3390/molecules26082264
Yaacoub C, Rifi M, El-Obeid D, Mawlawi H, Sabatier J-M, Coutard B, Fajloun Z. The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines. Molecules. 2021; 26(8):2264. https://doi.org/10.3390/molecules26082264
Chicago/Turabian StyleYaacoub, Carole, Mariam Rifi, Dany El-Obeid, Hiba Mawlawi, Jean-Marc Sabatier, Bruno Coutard, and Ziad Fajloun. 2021. "The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines" Molecules 26, no. 8: 2264. https://doi.org/10.3390/molecules26082264
APA StyleYaacoub, C., Rifi, M., El-Obeid, D., Mawlawi, H., Sabatier, J.-M., Coutard, B., & Fajloun, Z. (2021). The Cytotoxic Effect of Apis mellifera Venom with a Synergistic Potential of Its Two Main Components—Melittin and PLA2—On Colon Cancer HCT116 Cell Lines. Molecules, 26(8), 2264. https://doi.org/10.3390/molecules26082264






