Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons
Abstract
1. Introduction
2. Preparation of Ga-Modified ZSM-5
2.1. Isomorphous Substitution
2.1.1. Hydrothermal Crystallization
2.1.2. Recrystallization
2.2. Ga-Modified Zeolites
2.2.1. Incipient Wetness Impregnation
2.2.2. Ion Exchange
2.2.3. Chemical Vapor Deposition (CVD)
2.2.4. Pre-Treatment
3. Reactive Species in Ga-Modified ZSM-5
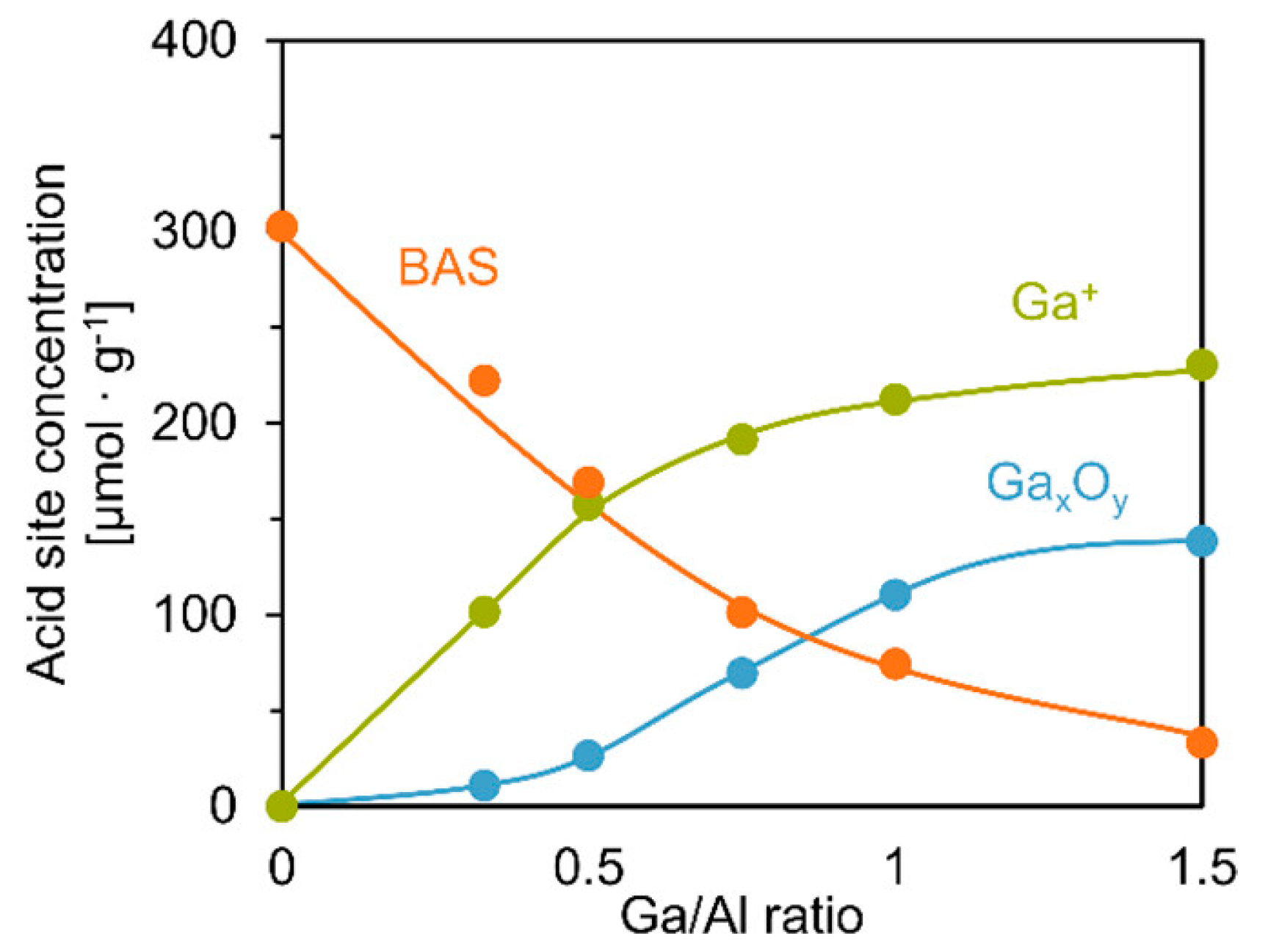
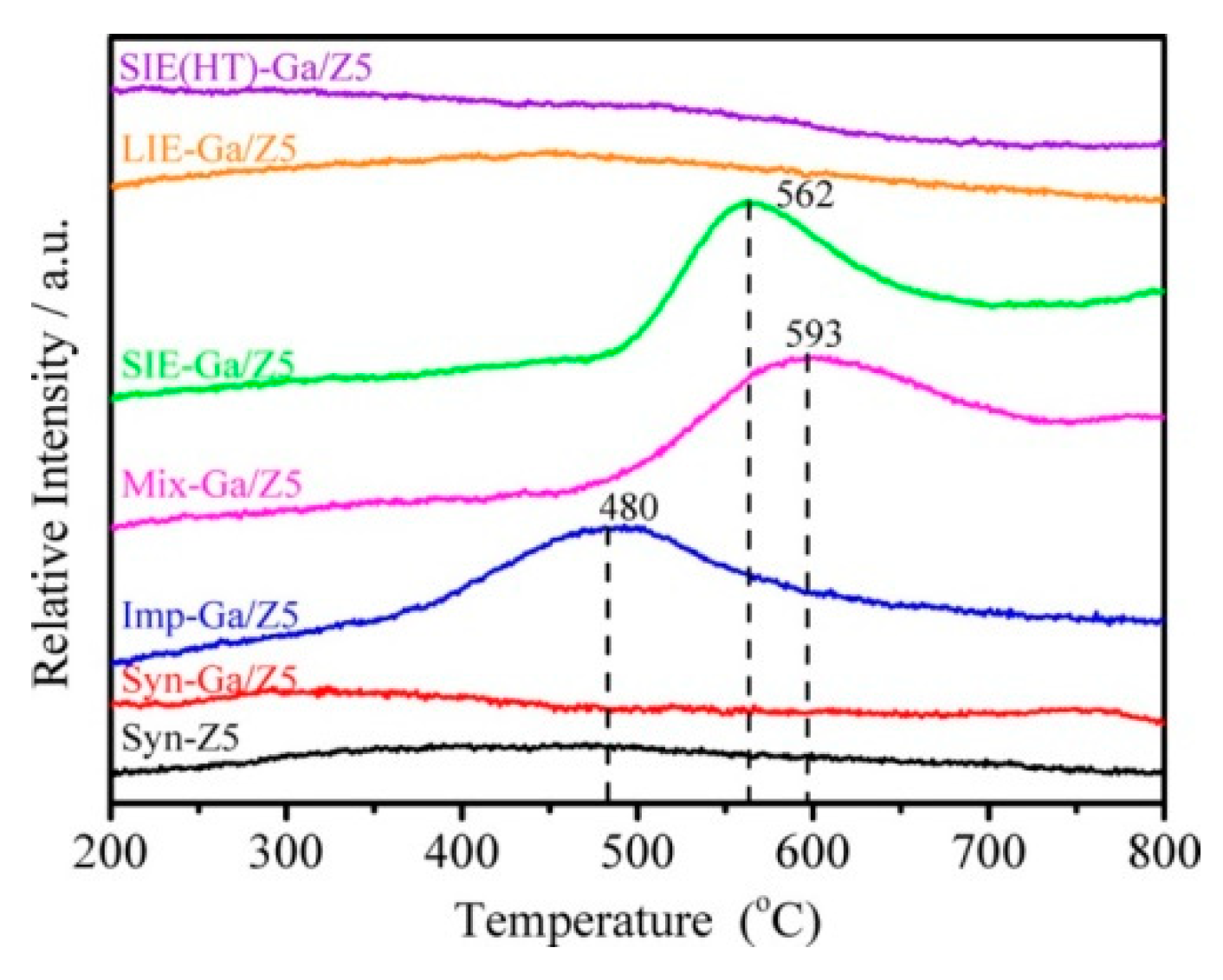
3.1. Oxidation State of Ga Species in Ga-Modified ZSM-5
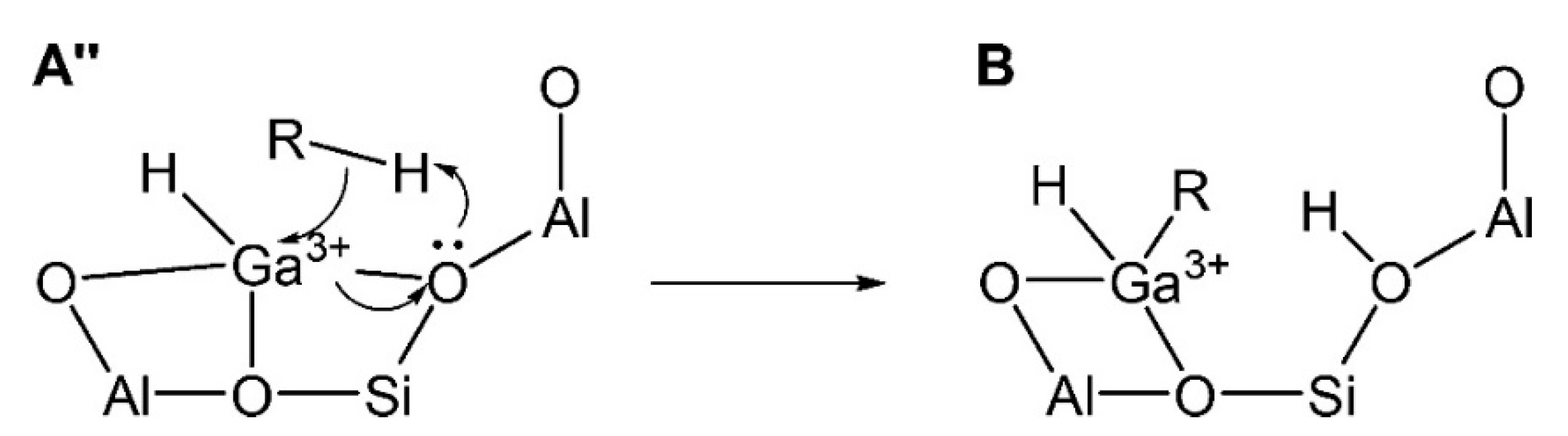
3.2. Structure of the Active Site
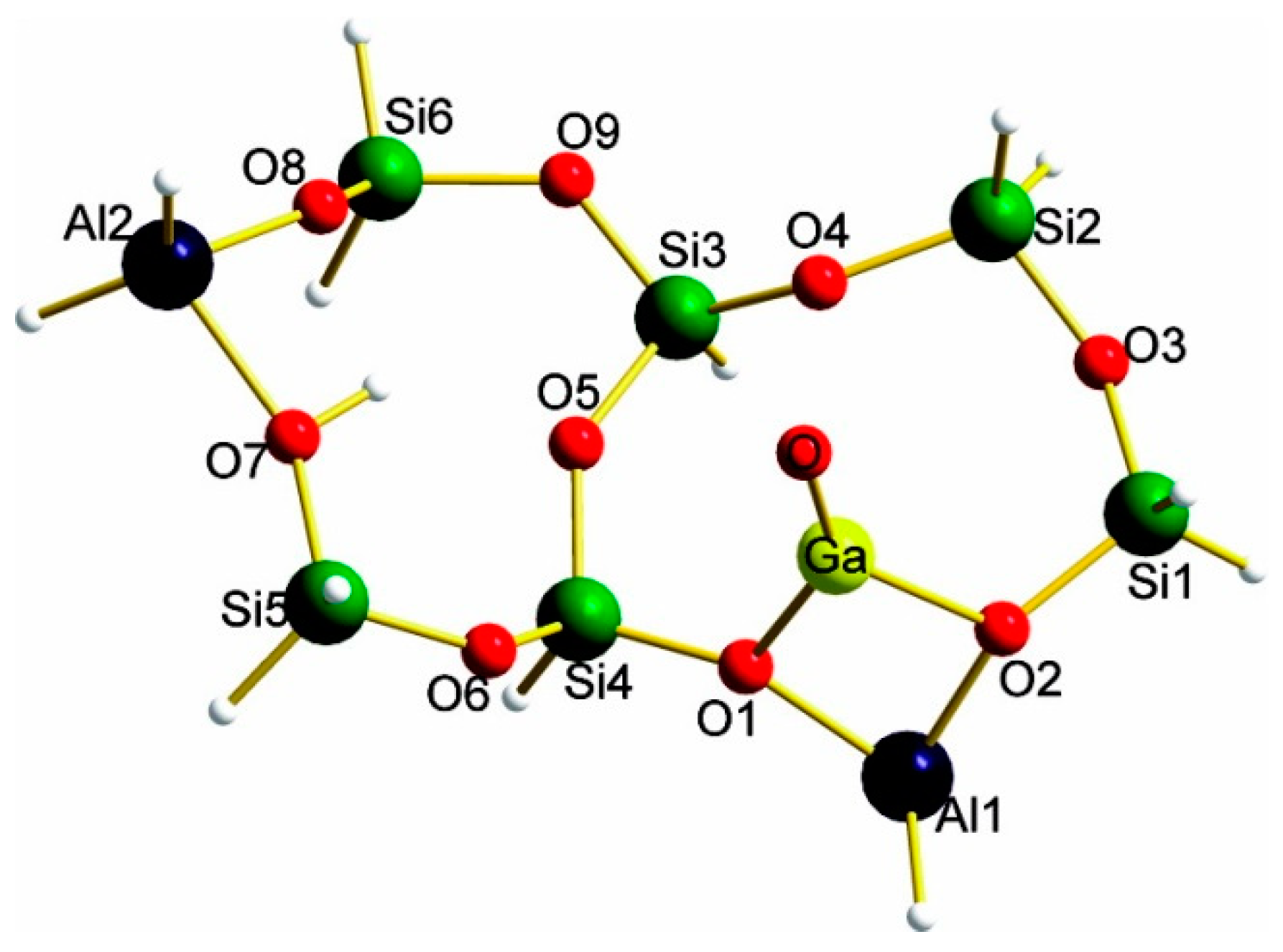
3.2.1. Framework Ga Species
3.2.2. Extra-Framework Ga Species
Gallium Oxide Particles
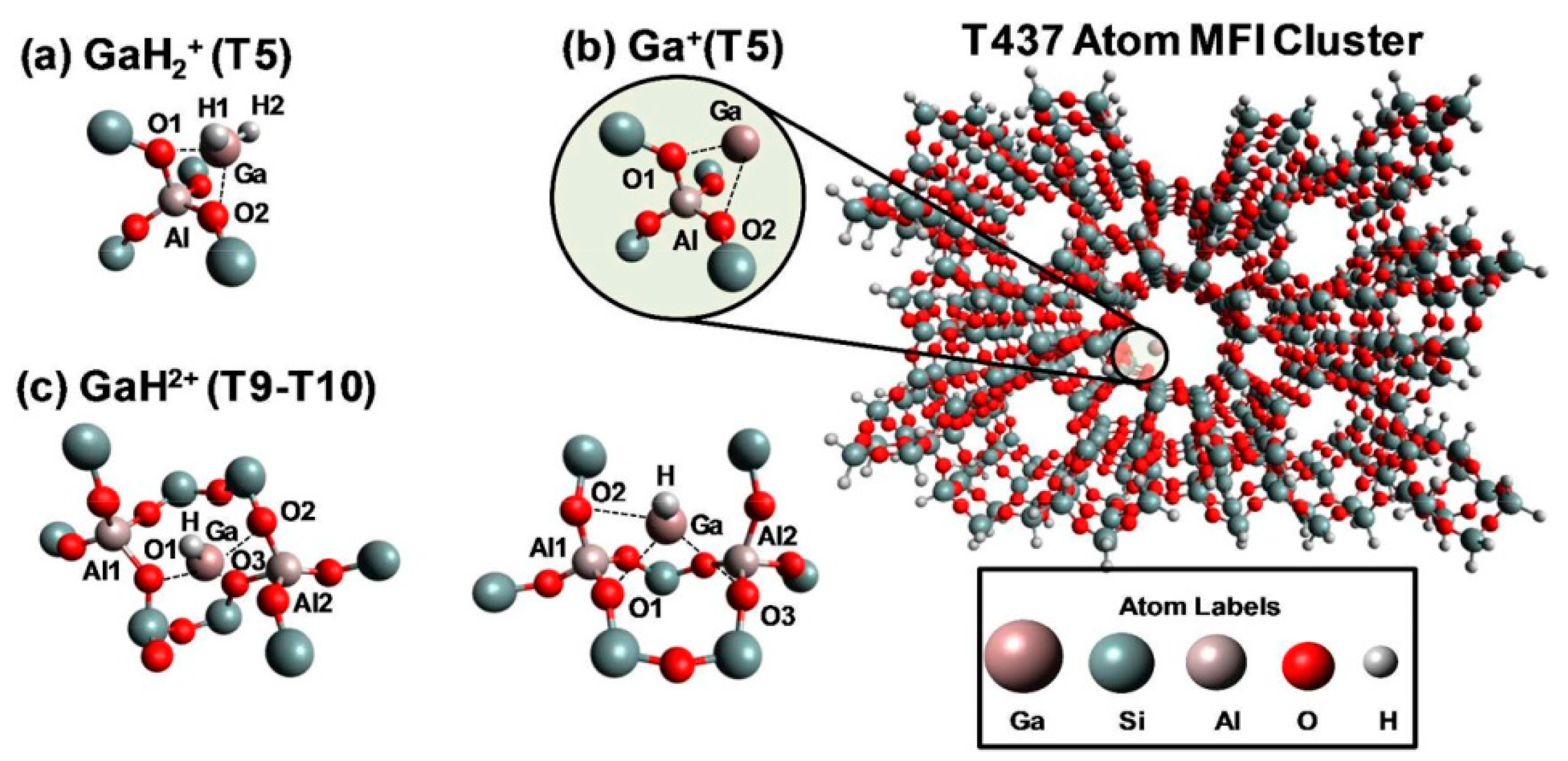
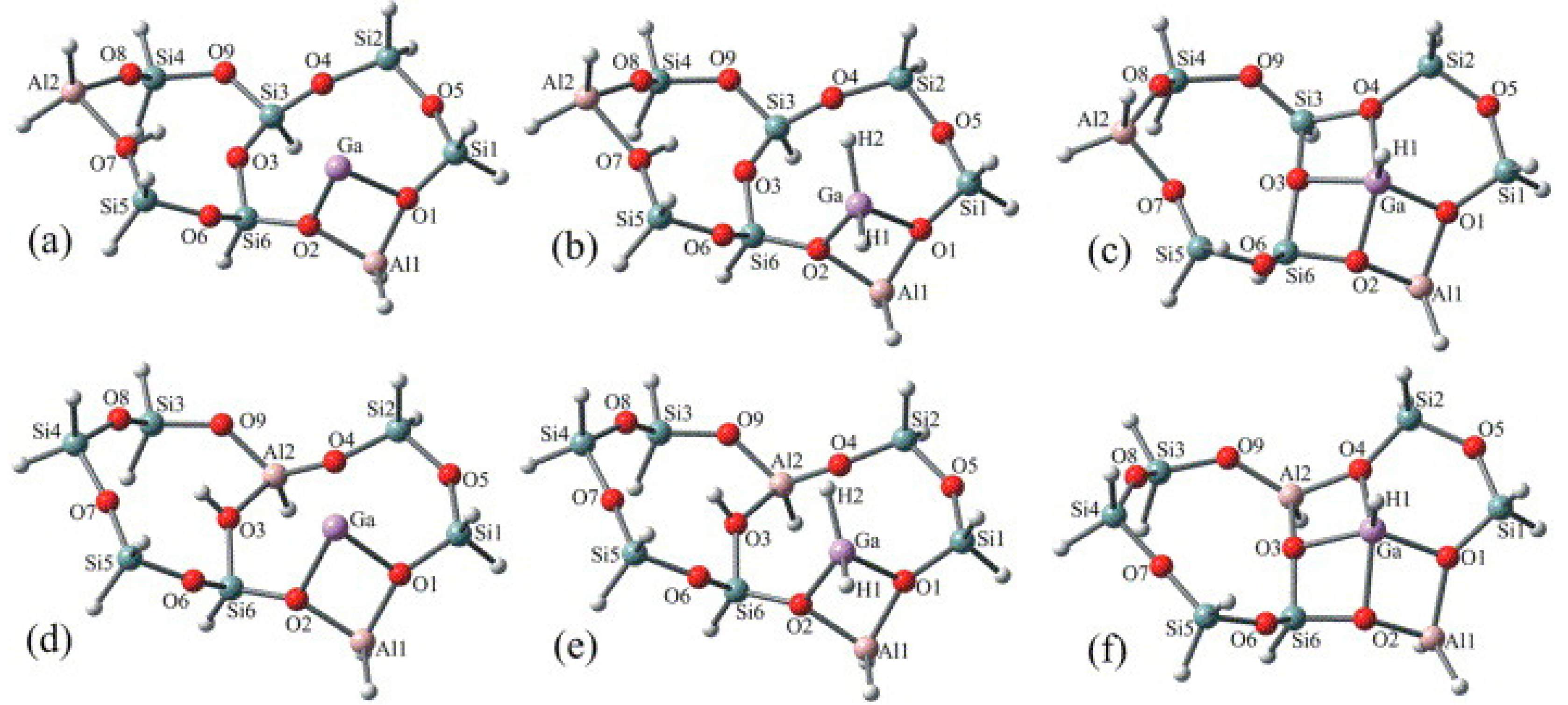
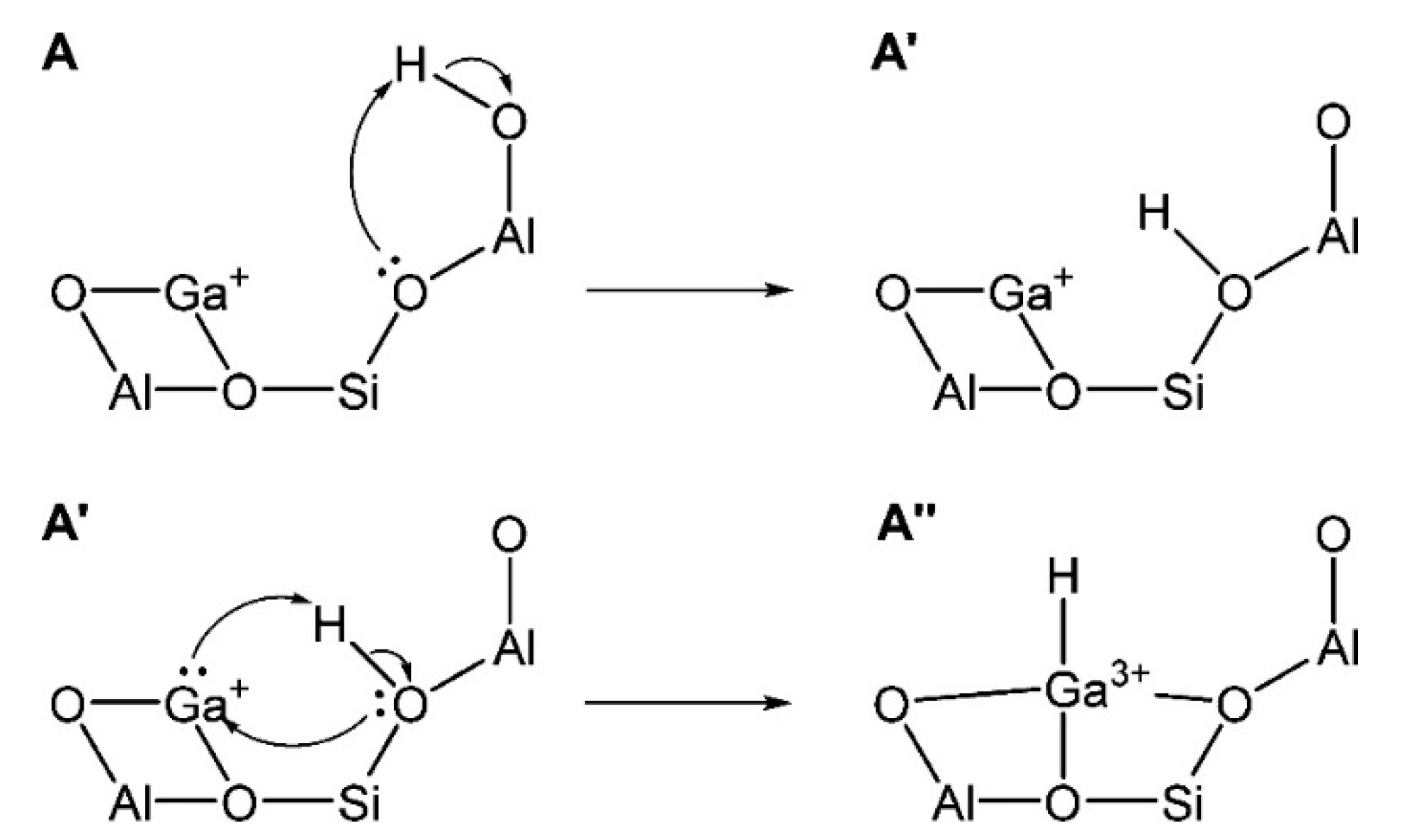
Cationic Ga Species
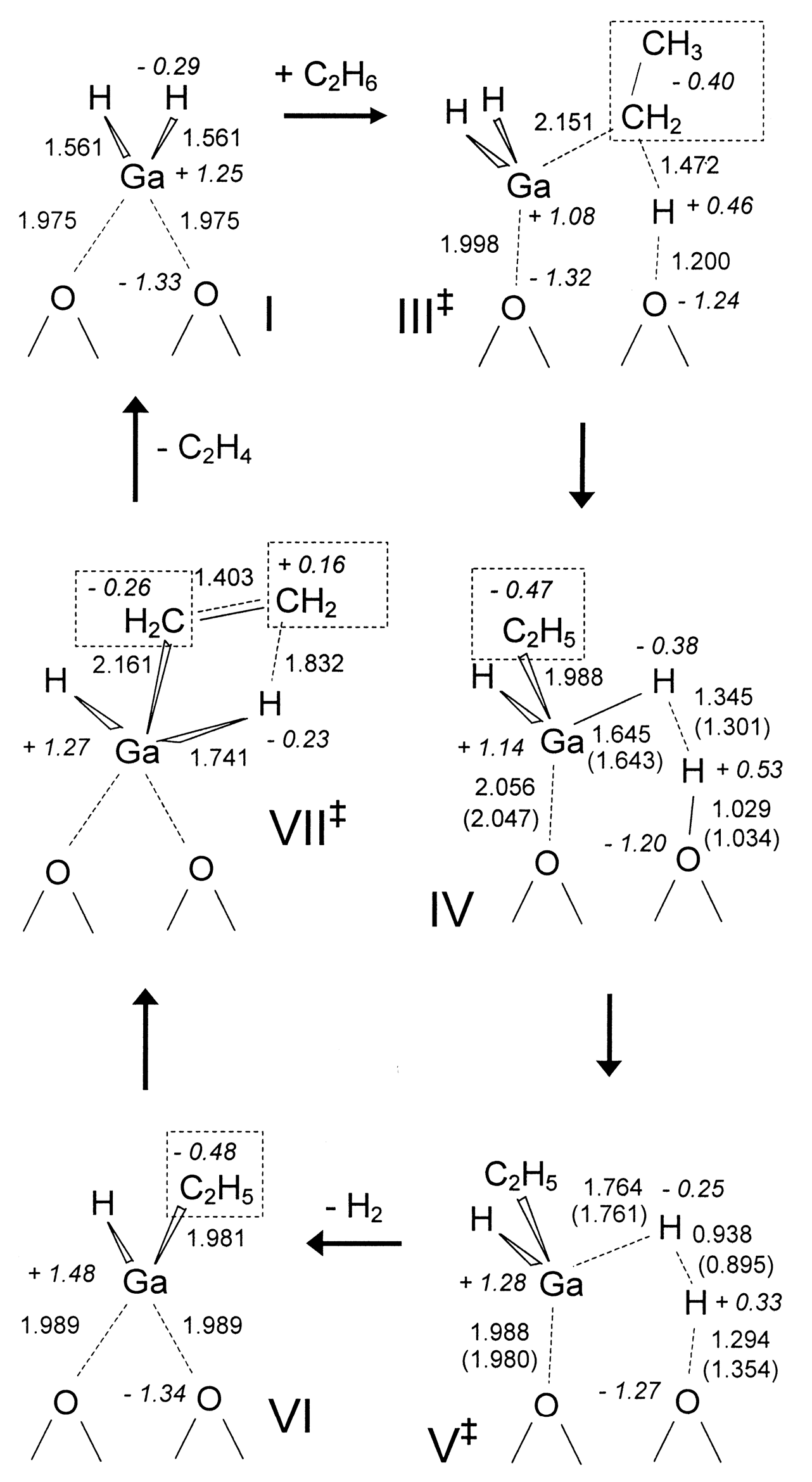
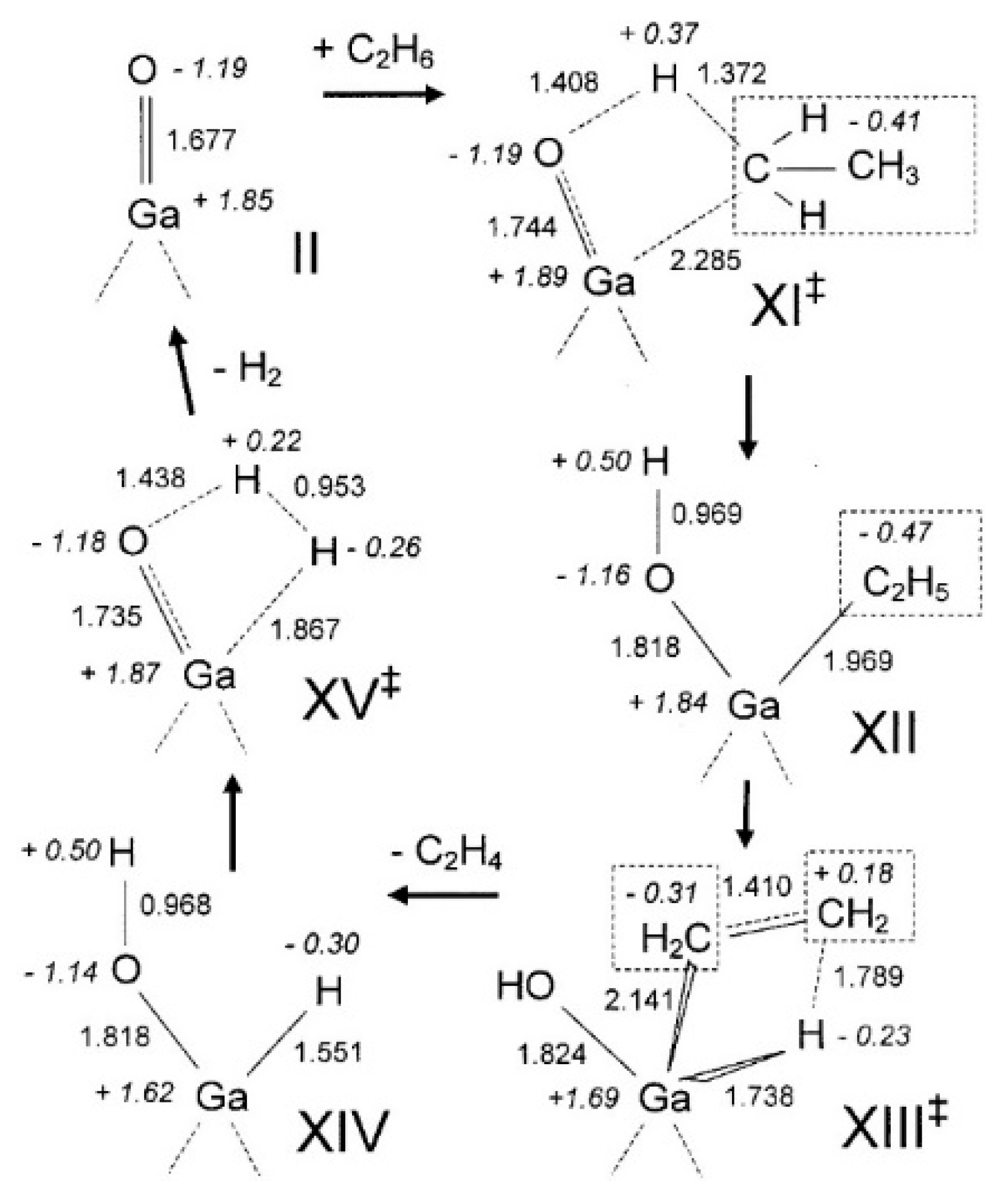
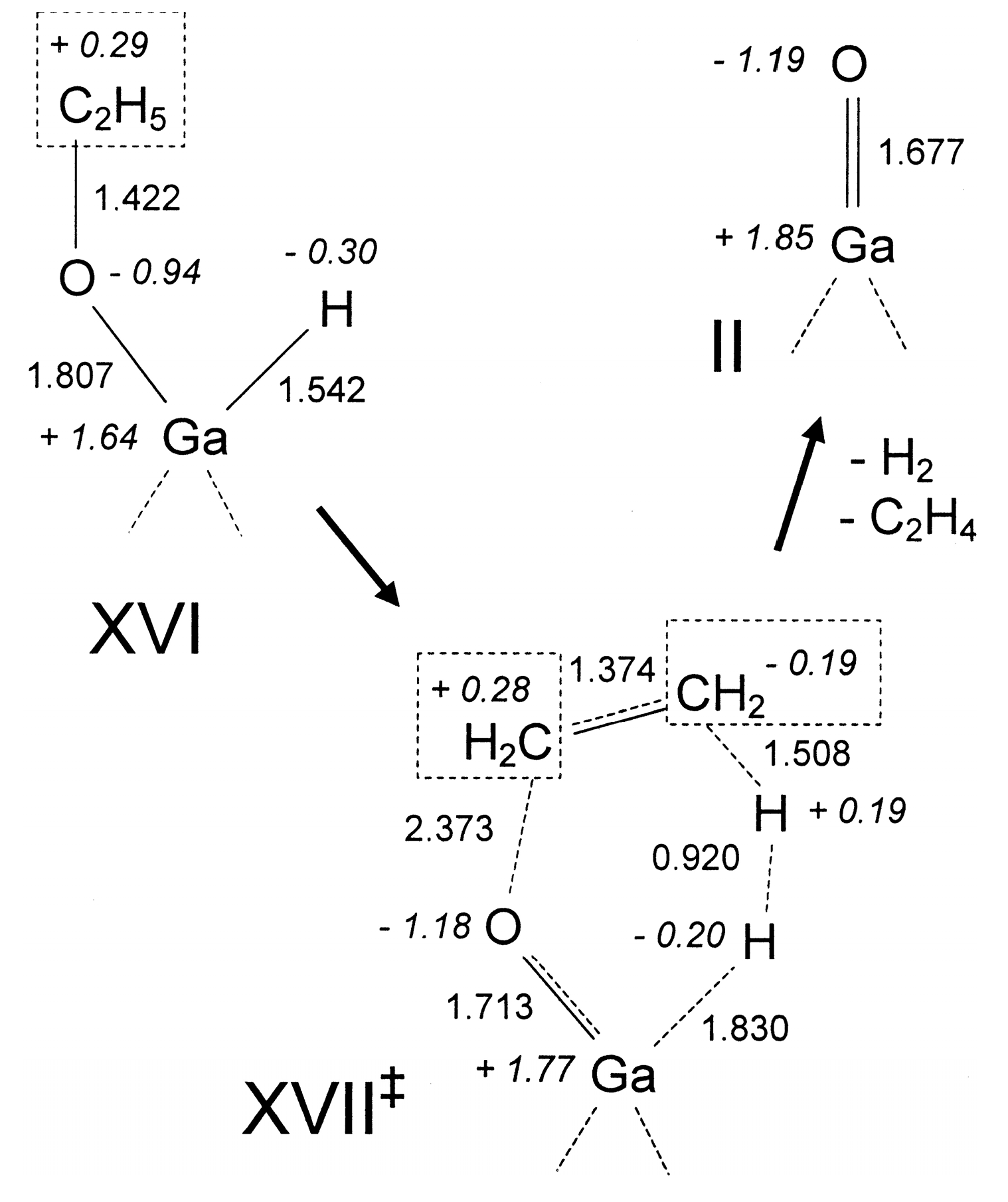
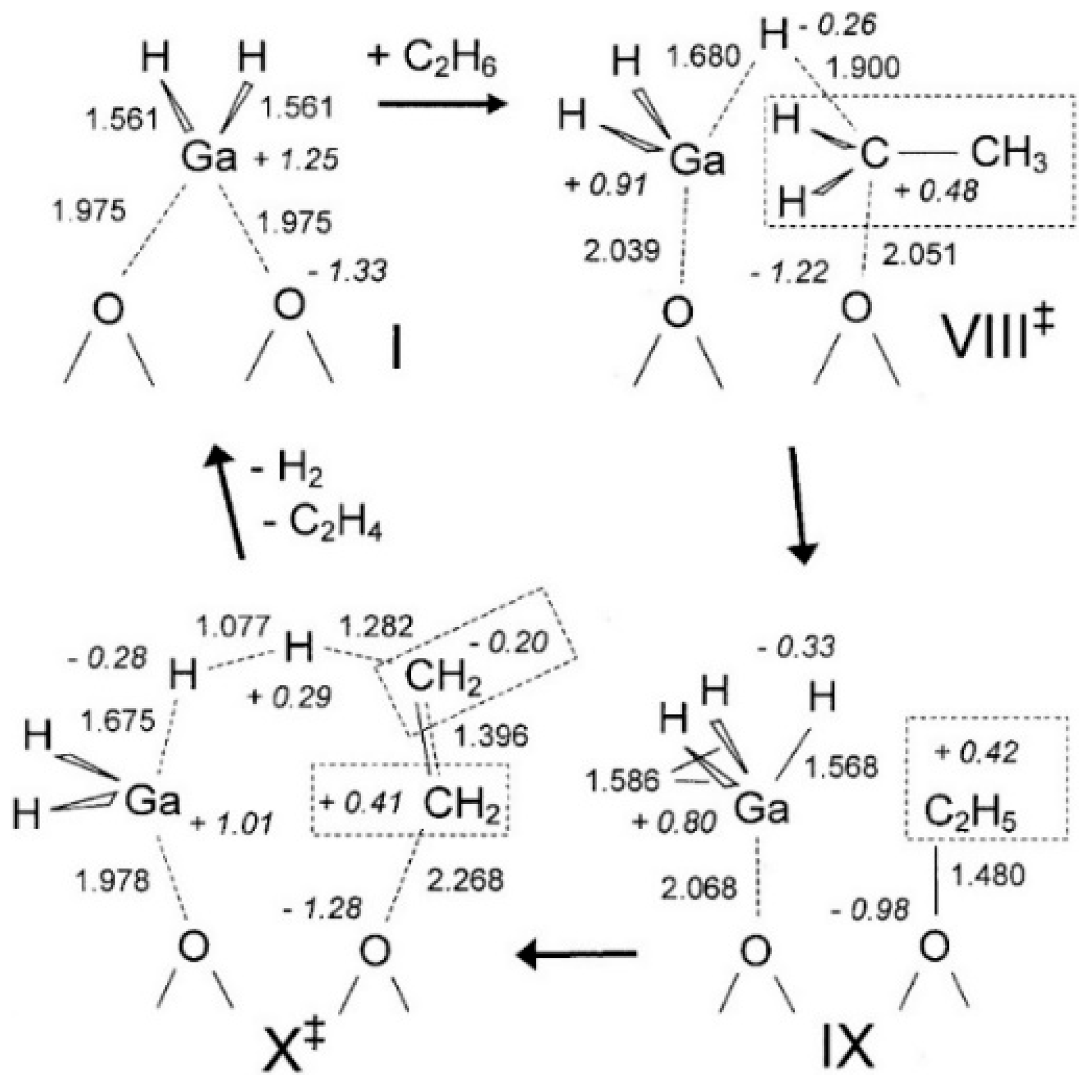
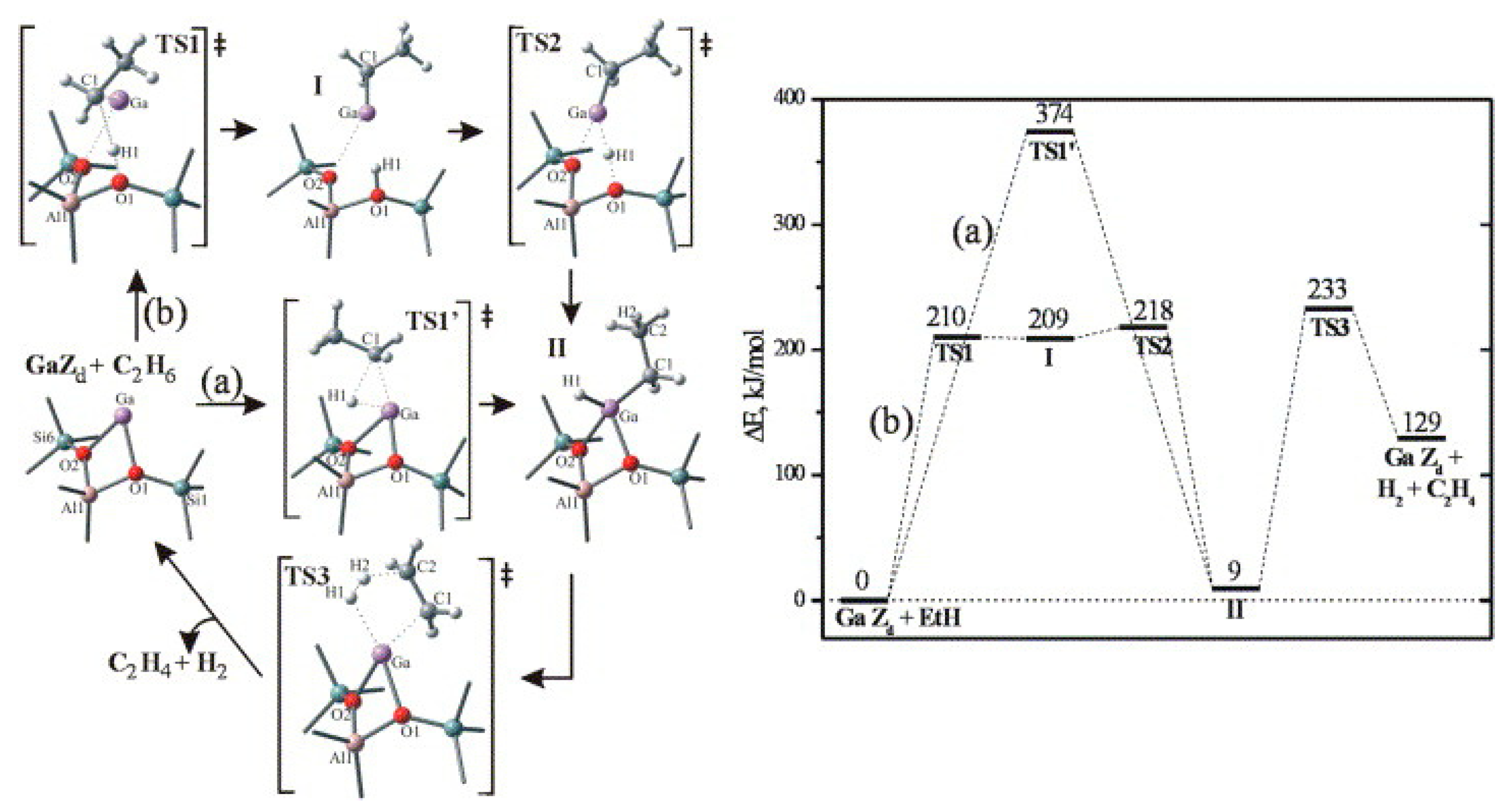
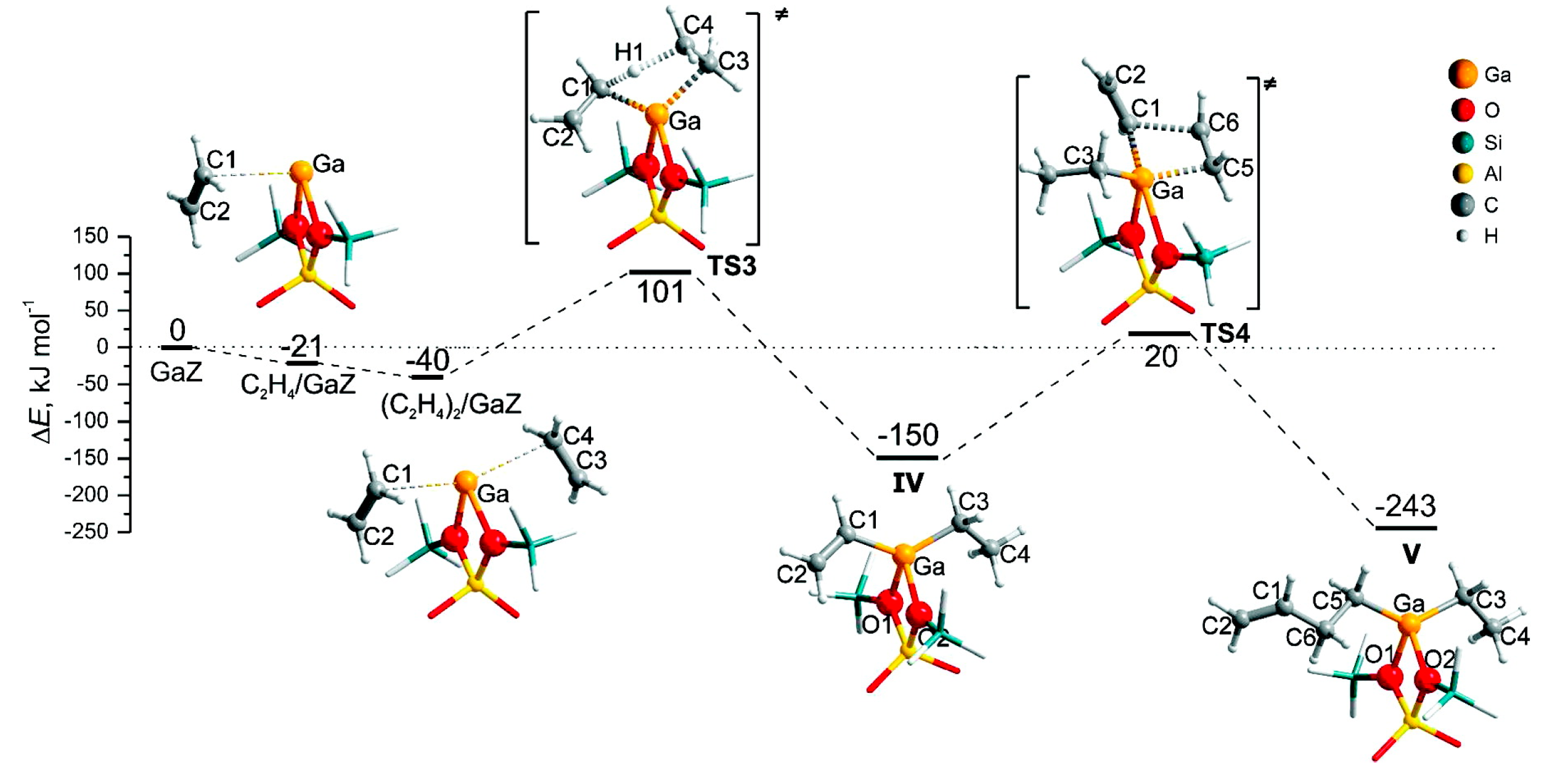
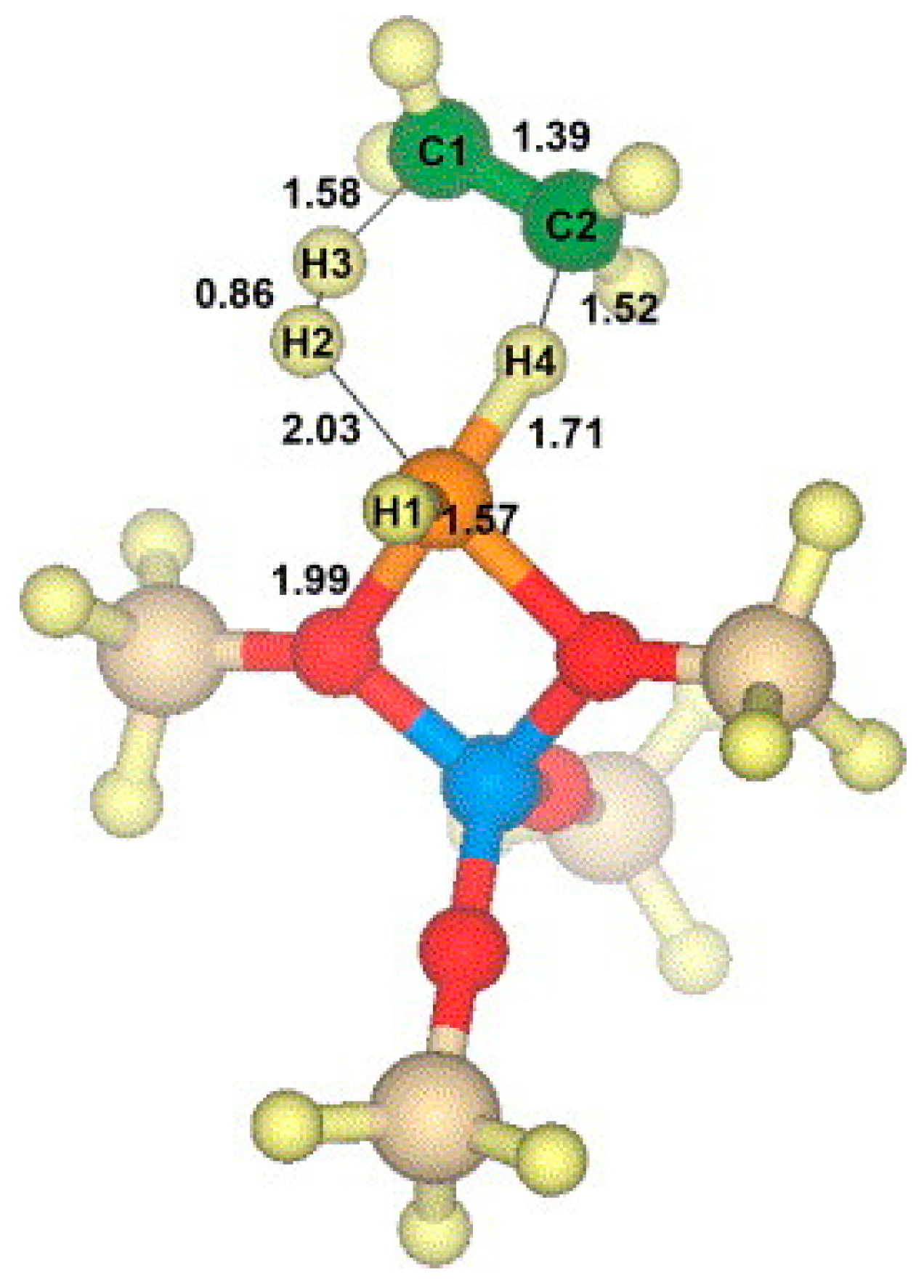
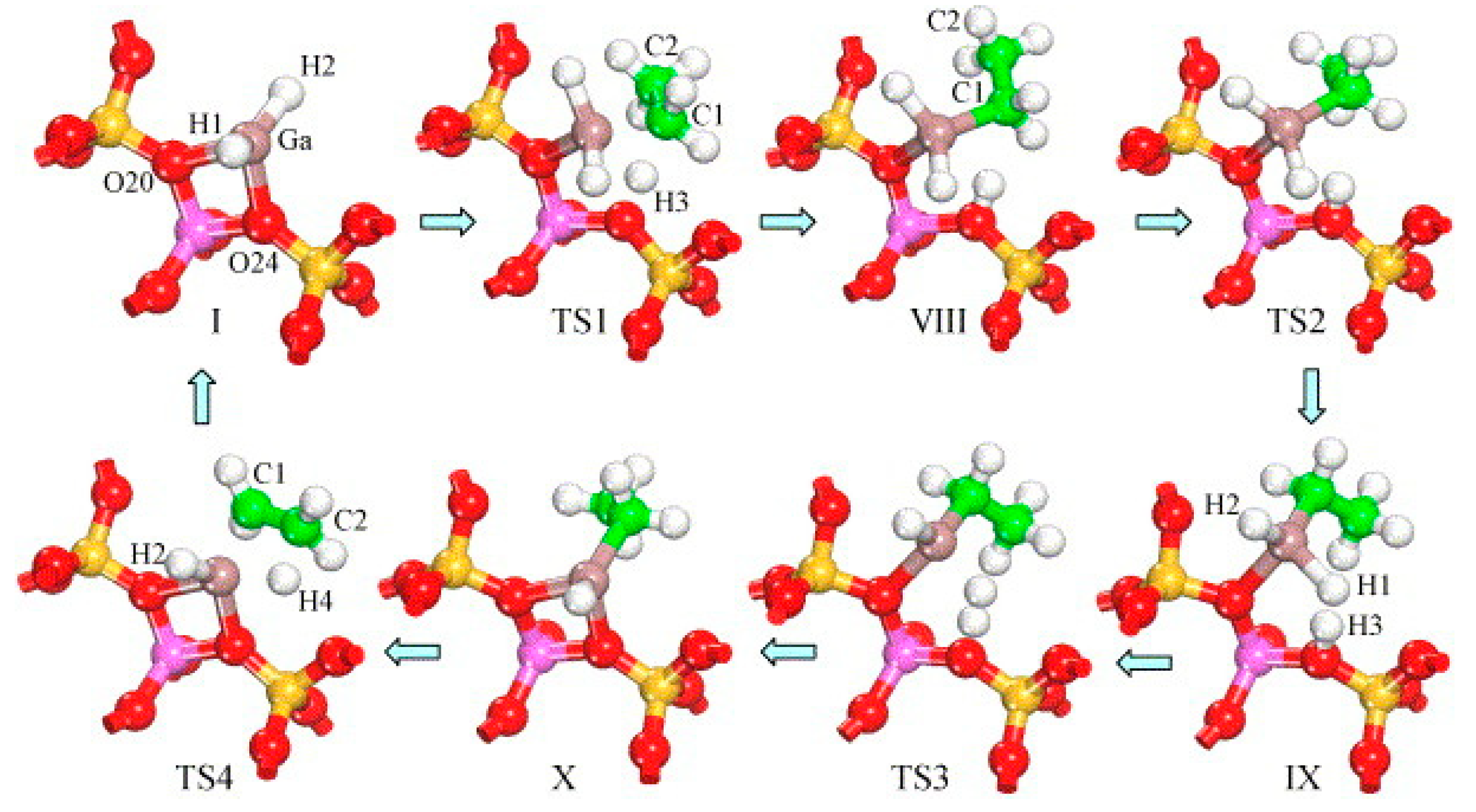
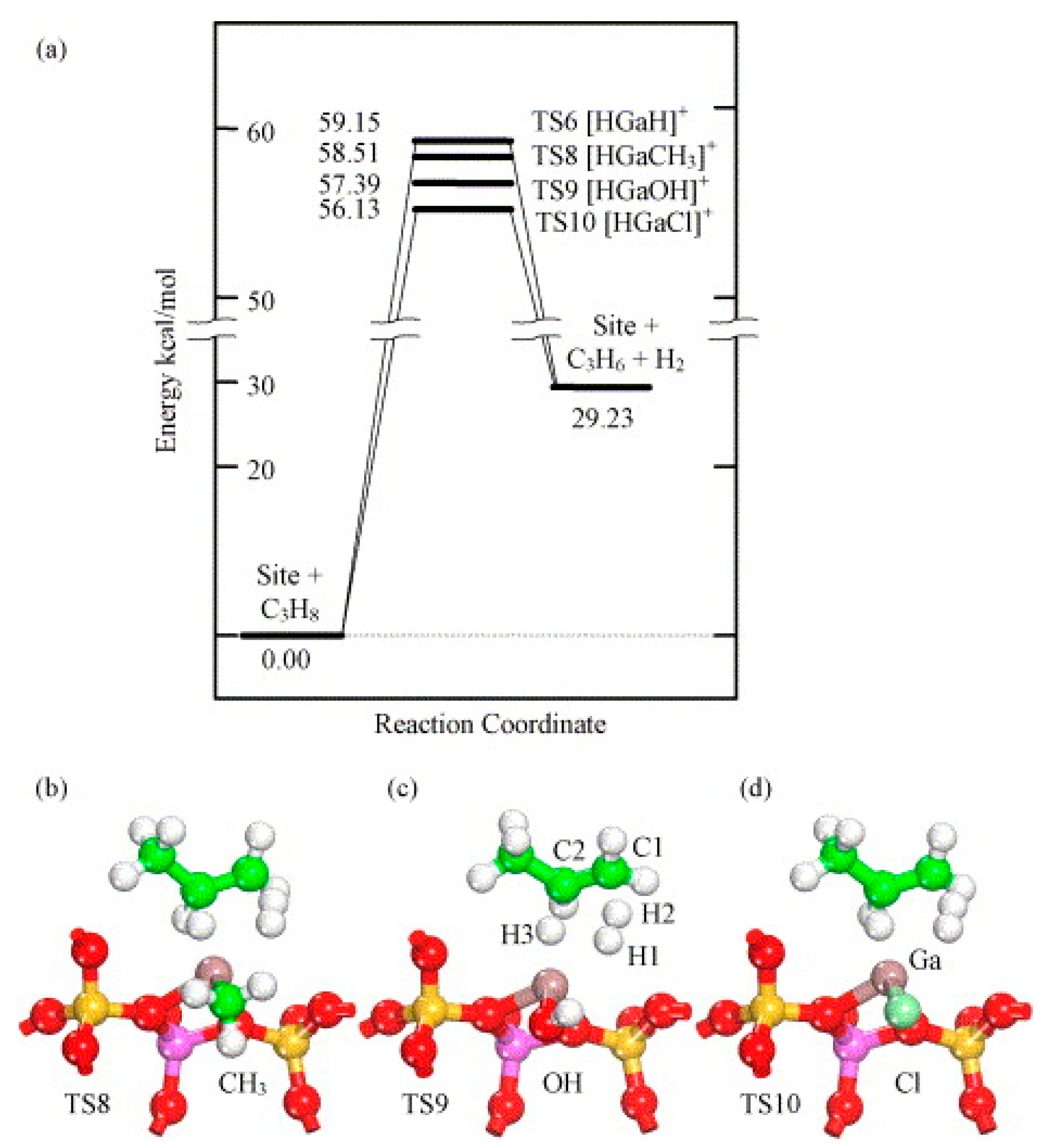
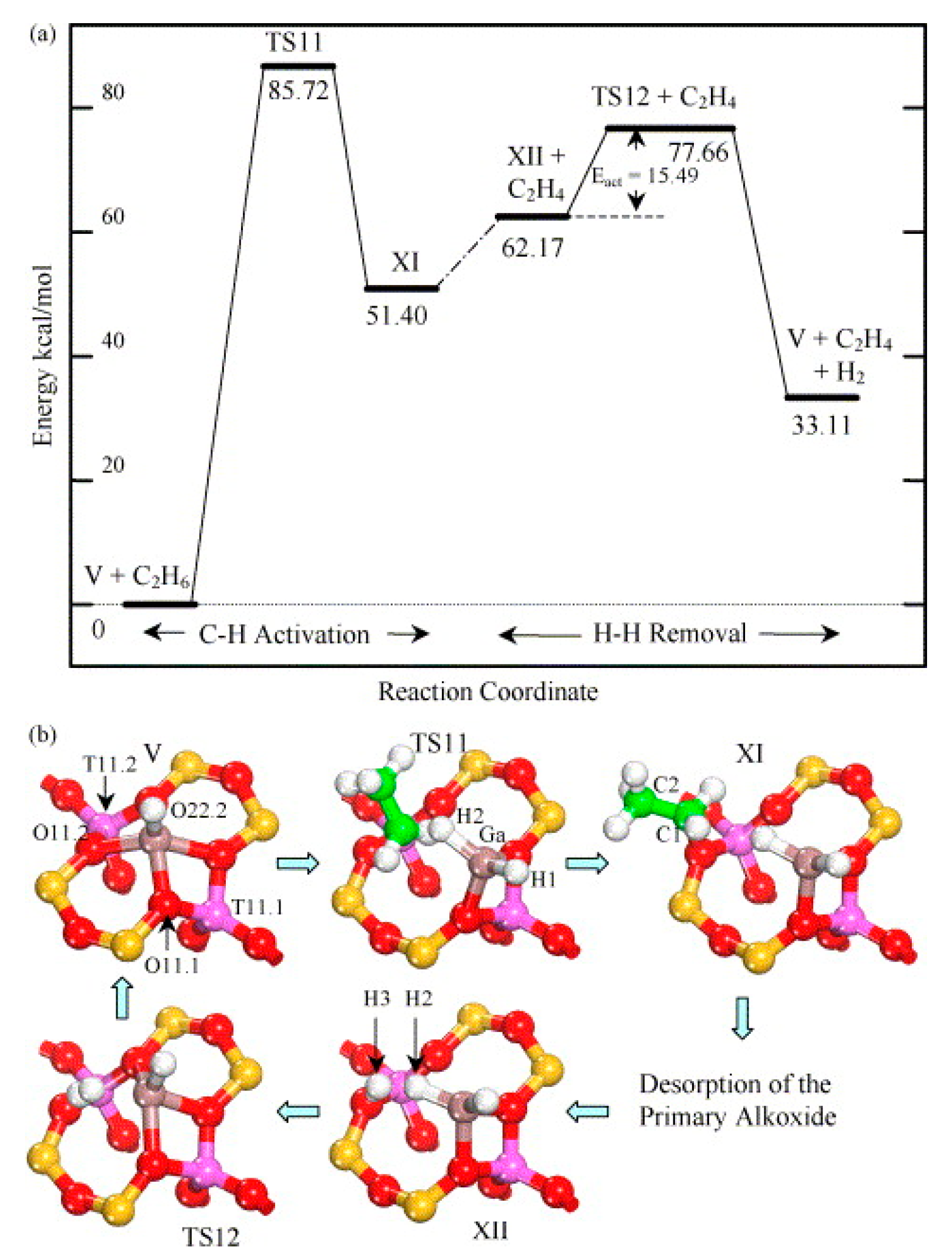
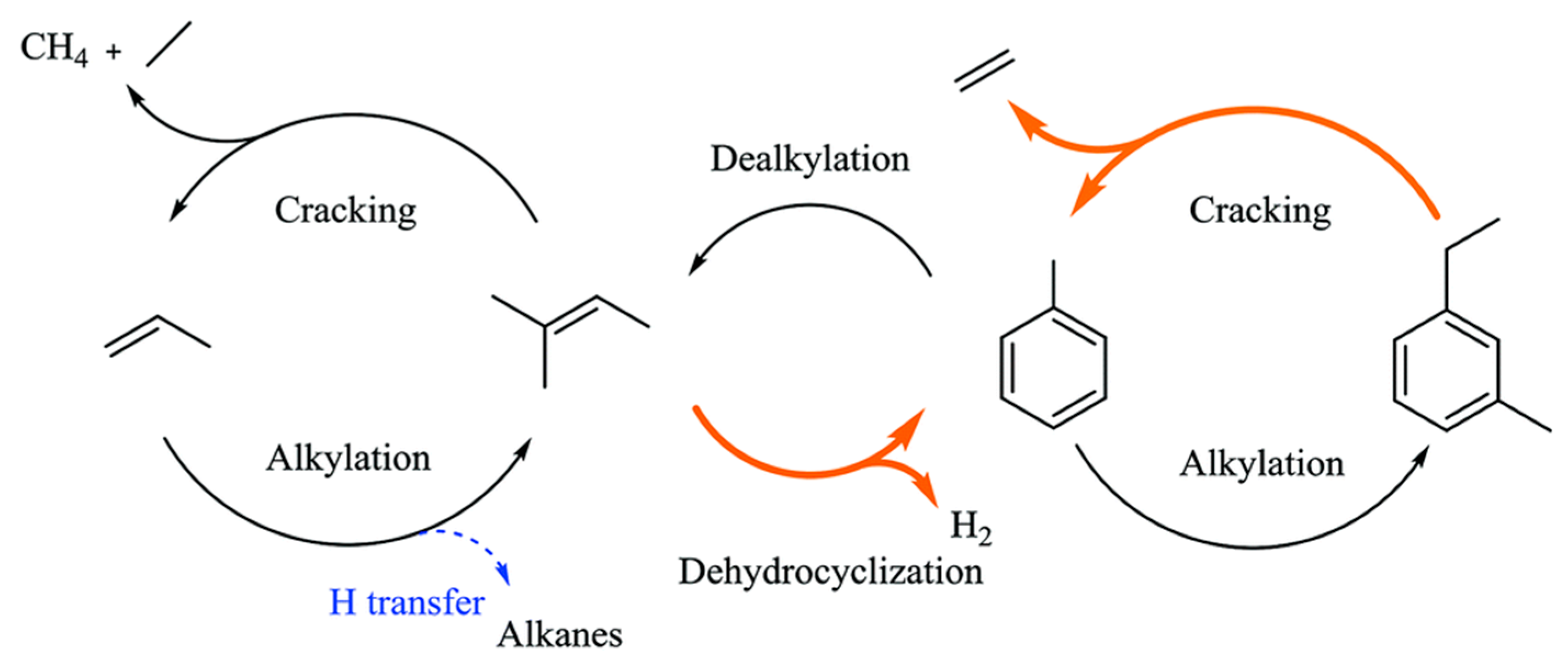
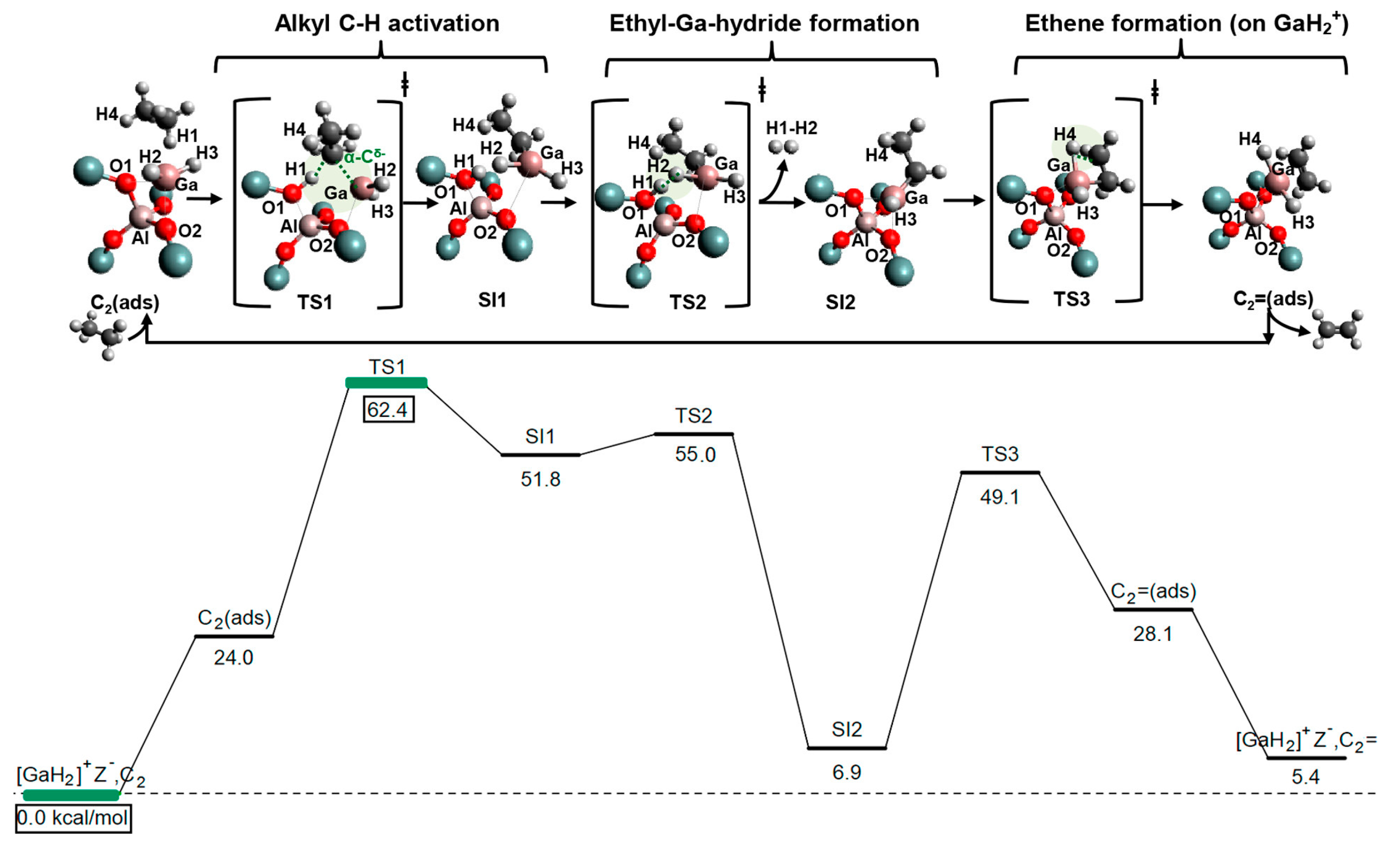
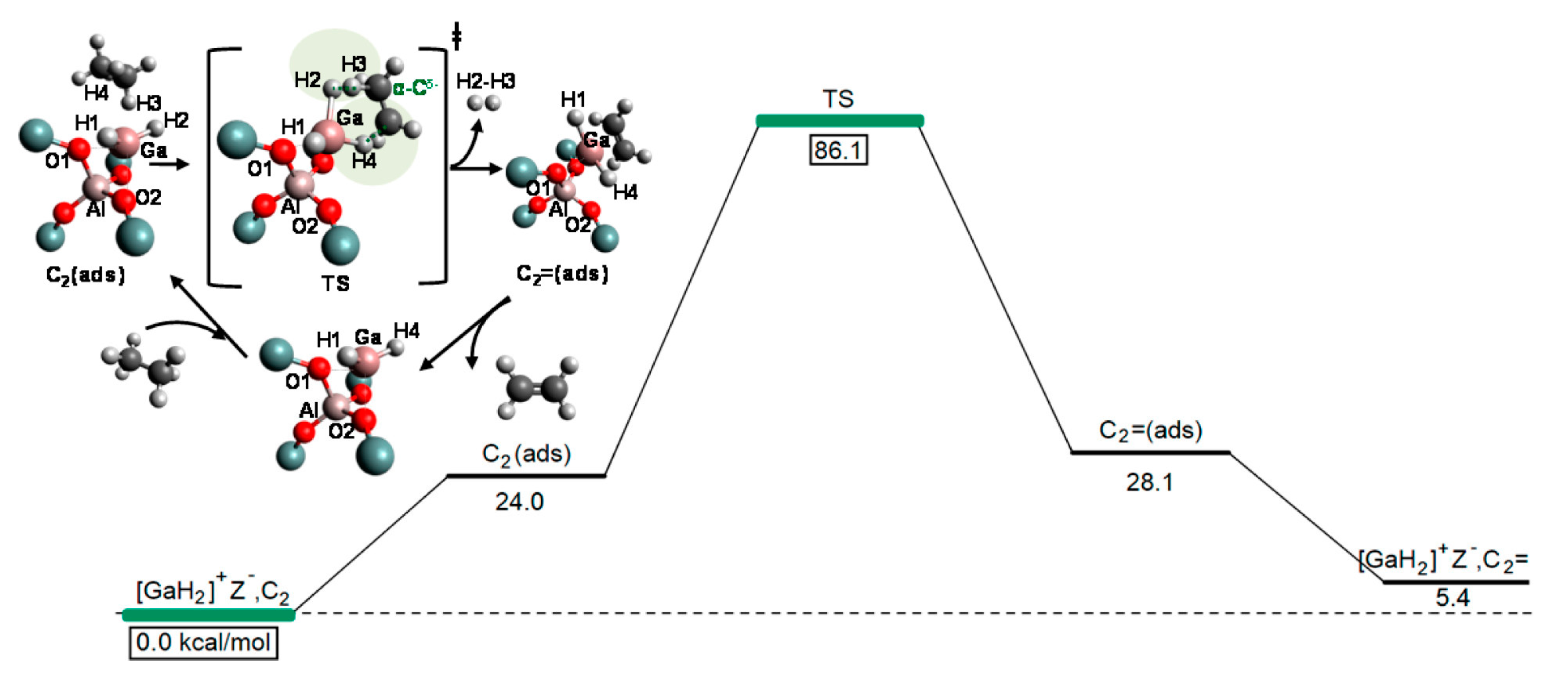
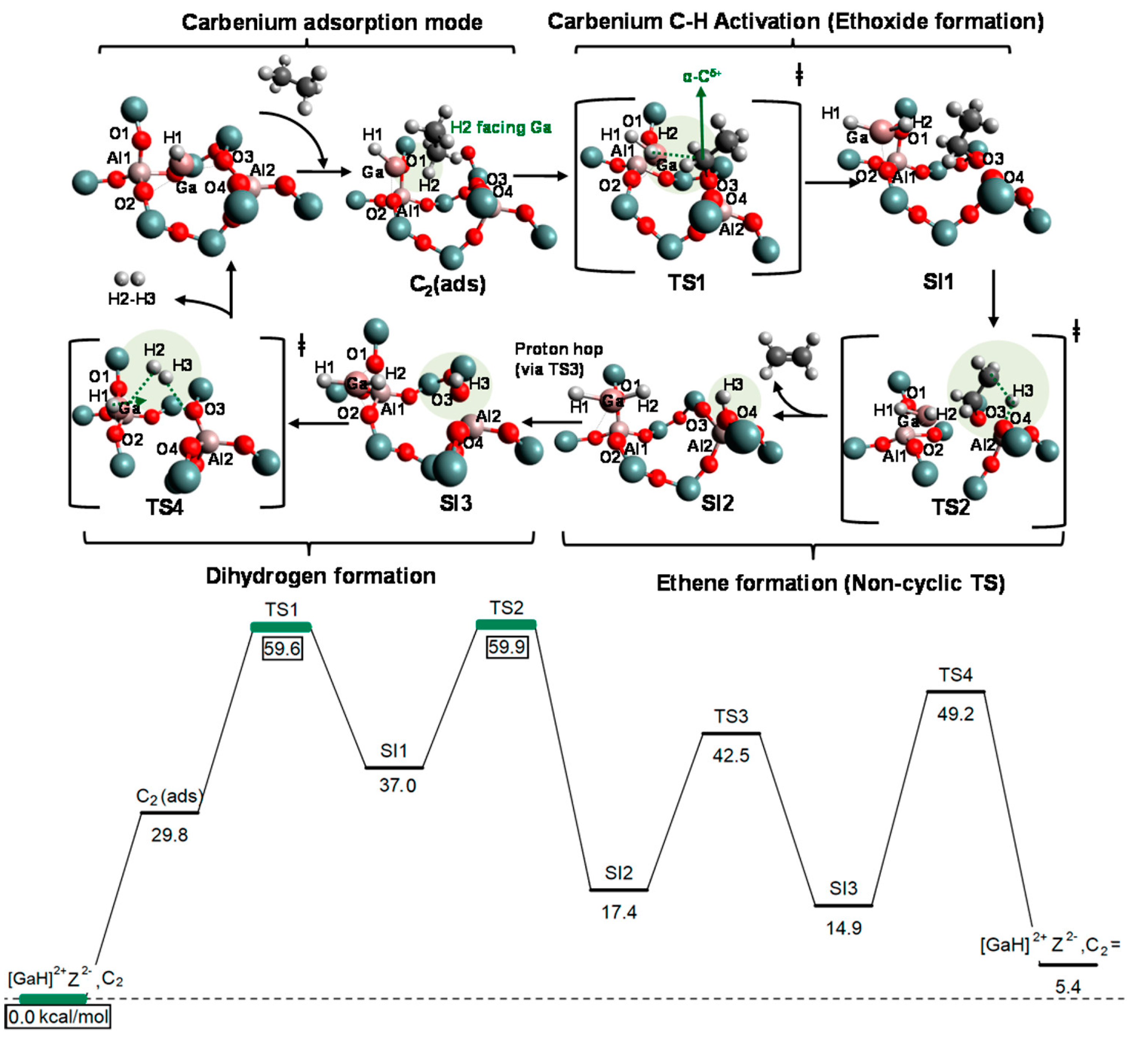
4. Mechanisms of Alkanes Conversion over Ga-Modified ZSM-5
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Breck, D.W. Crystalline molecular sieves. J. Chem. Educ. 1964, 41, 678. [Google Scholar] [CrossRef]
- Meier, W.M.; Olson, D.H.; Baerlocher, C. Atlas of zeolite structure types. Zeolites 1996, 17, 1–229. [Google Scholar]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Prieto, R.; Bilbao, J. Deactivation of a HZSM-5 zeolite catalyst in the transformation of the aqueous fraction of biomass pyrolysis oil into hydrocarbons. Energy Fuels 2004, 18, 1640–1647. [Google Scholar] [CrossRef]
- Taifan, W.; Baltrusaitis, J. CH4 conversion to value added products: Potential, limitations and extensions of a single step heterogeneous catalysis. Appl Catal. B Environ. 2016, 198, 525–547. [Google Scholar] [CrossRef]
- Lunsford, J.H. Catalytic conversion of methane to more useful chemicals and fuels: A challenge for the 21st century. Catal. Today 2000, 63, 165–174. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.W.; Im, S.W.; Lee, Y.H.; Suh, D.J.; Jun, K.W.; Lee, K.Y. Oxidative coupling of methane using non-stoichiometric lead hydroxyapatite catalyst mixtures. Fuel 2012, 94, 433–439. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S.; Alario, F. Aromatization of short chain alkanes on zeolite catalysts. Appl. Catal. A Gen. 1992, 89, 1–30. [Google Scholar] [CrossRef]
- Jin, M.H.; Lee, C.B.; Lee, D.W.; Lee, S.W.; Park, J.W.; Oh, D.; Hwang, K.R.; Lee, K.Y.; Park, J.S. Microchannel methane steam reformers with improved heat transfer efficiency and their long-term stability. Fuel 2016, 176, 86–92. [Google Scholar] [CrossRef]
- Gim, M.Y.; Song, C.; Kim, T.H.; Song, J.H.; Kim, D.H.; Lee, K.Y.; Song, I.K. BTX production by coaromatization of methane and propane over gallium oxide supported on mesoporous HZSM-5. Mol. Catal. 2017, 439, 134–142. [Google Scholar] [CrossRef]
- Qiu, B.; Jiang, F.; Lu, W.D.; Yan, B.; Li, W.C.; Zhao, Z.C.; Lu, A.H. Oxidative dehydrogenation of propane using layered borosilicate zeolite as the active and selective catalyst. J. Catal. 2020, 385, 176–182. [Google Scholar] [CrossRef]
- Michorczyk, P.; Zenczak-Tomera, K.; Michorczyk, B.; Wegrzyniak, A.; Basta, M.; Millot, Y.; Valentin, L.; Dzwigaj, S. Effect of dealumination on the catalytic performance of Cr-containing Beta zeolite in carbon dioxide assisted propane dehydrogenation. J. Co2 Util. 2020, 36, 54–63. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, X.; Xie, Y.; Lin, R.B.; Krishna, R.; Cui, H.; Li, Z.; Shi, Y.; Wu, H.; Zhou, W.; et al. An Ultramicroporous Metal-Organic Framework for High Sieving Separation of Propylene from Propane. J. Am. Chem. Soc. 2020, 142, 17795–17801. [Google Scholar] [CrossRef] [PubMed]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Kosinov, N.; Hensen, E.J.M. Reactivity, Selectivity, and Stability of Zeolite-Based Catalysts for Methane Dehydroaromatization. Adv. Mater. 2020, 32, e2002565. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.-P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A Gen. 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujado, P.R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A Gen. 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Song, H.; Rioux, R.M.; Hoefelmeyer, J.D.; Komor, R.; Niesz, K.; Grass, M.; Yang, P.; Somorjai, G.A. Hydrothermal growth of mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: Synthesis, characterization, and catalytic properties. J. Am. Chem. Soc. 2006, 128, 3027–3037. [Google Scholar] [CrossRef]
- Hu, Z.P.; Wang, Z.; Yuan, Z.Y. Cr/Al2O3 catalysts with strong metal-support interactions for stable catalytic dehydrogenation of propane to propylene. Mol. Catal. 2020, 493, 111052. [Google Scholar] [CrossRef]
- Li, G.N.; Vollmer, I.; Liu, C.; Gascon, J.; Pidko, E.A. Structure and Reactivity of the Mo/ZSM-5 Dehydroaromatization Catalyst: An Operando Computational Study. ACS Catal. 2019, 9, 8731–8737. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Saito, H.; Sekine, Y.; Hensen, E.J.M.; Kosinov, N. Different mechanisms of ethane aromatization over Mo/ZSM-5 and Ga/ZSM-5 catalysts. Catal. Today 2020. [Google Scholar] [CrossRef]
- Ji, Z.H.; Lv, H.F.; Pan, X.L.; Bao, X.H. Enhanced ethylene selectivity and stability of Mo/ZSM5 upon modification with phosphorus in ethane dehydrogenation. J. Catal. 2018, 361, 94–104. [Google Scholar] [CrossRef]
- Hu, Z.P.; Chen, C.; Ren, J.T.; Yuan, Z.Y. Direct dehydrogenation of propane to propylene on surface-oxidized multiwall carbon nanotubes. Appl. Catal. A Gen. 2018, 559, 85–93. [Google Scholar] [CrossRef]
- Hu, Z.P.; Zhao, H.; Chen, C.; Yuan, Z.Y. Castanea mollissima shell-derived porous carbons as metal-free catalysts for highly efficient dehydrogenation of propane to propylene. Catal. Today 2018, 316, 214–222. [Google Scholar] [CrossRef]
- Shimada, H.; Akazawa, T.; Ikenaga, N.; Suzuki, T. Dehydrogenation of isobutane to isobutene with iron-loaded activated carbon catalyst. Appl. Catal. A Gen. 1998, 168, 243–250. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Lei, T.Q.; Miao, C.X.; Hua, W.M.; Yue, Y.H.; Gao, Z. Ga2O3/NaZSM-5 for C2H6 dehydrogenation in the presence of CO2: Conjugated effect of silanol. Microporous Mesoporous Mater. 2018, 268, 235–242. [Google Scholar] [CrossRef]
- Shen, Z.H.; Liu, J.; Xu, H.L.; Yue, Y.H.; Hua, W.M.; Shen, W. Dehydrogenation of ethane to ethylene over a highly efficient Ga2O3/HZSM-5 catalyst in the presence of CO2. Appl. Catal. A Gen. 2009, 356, 148–153. [Google Scholar] [CrossRef]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-surface-area catalyst design: Synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 2005, 109, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Moulijn, J.A.; van Diepen, A.E.; Kapteijn, F. Catalyst deactivation: Is it predictable? Appl. Catal. A Gen. 2001, 212, 3–16. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Schoonheydt, R.A. Alkane dehydrogenation over supported chromium oxide catalysts. Catal. Today 1999, 51, 223–232. [Google Scholar] [CrossRef]
- Wang, N.; Dong, X.; Liu, L.; Cai, D.; Cheng, Q.; Wang, J.; Hou, Y.; Emwas, A.-H.; Gascon, J.; Han, Y. Probing the Catalytic Active Sites of Mo/HZSM-5 and Their Deactivation during Methane Dehydroaromatization. Cell Rep. Phys. Sci. 2021, 2, 100309. [Google Scholar] [CrossRef]
- Mokrani, T.; Scurrell, M. Gas Conversion to Liquid Fuels and Chemicals: The Methanol Route-Catalysis and Processes Development. Catal. Rev. 2009, 51, 1–145. [Google Scholar] [CrossRef]
- Xie, S.B.; Chen, K.D.; Bell, A.T.; Iglesia, E. Structural characterization of molybdenum oxide supported on zirconia. J. Phys. Chem. B 2000, 104, 10059–10068. [Google Scholar] [CrossRef]
- Gnep, N.S.; Doyemet, J.Y.; Guisnet, M. Role Of Gallium Species on the Dehydrocyclodimerization Of Propane on Zsm5 Catalysts. J. Mol. Catal. 1988, 45, 281–284. [Google Scholar] [CrossRef]
- Hagen, A.; Roessner, F. Ethane to aromatic hydrocarbons: Past, present, future. Catal. Rev. 2000, 42, 403–437. [Google Scholar] [CrossRef]
- Biscardi, J.A.; Iglesia, E. Structure and function of metal cations in light alkane reactions catalyzed by modified H-ZSM5. Catal. Today 1996, 31, 207–231. [Google Scholar] [CrossRef]
- Iglesia, E.; Baumgartner, J.E. Hydrogen-Transfer And Activation of Propane And Methane on Zsm5-Based Catalysts. Catal. Lett. 1993, 21, 55–70. [Google Scholar] [CrossRef]
- Dooley, K.M.; Guidry, T.F.; Price, G.L. Control of intrazeolitic gallium cation content and its effects on C2 dehydrogenation in Ga-MFI catalysts. J. Catal. 1995, 157, 66–75. [Google Scholar] [CrossRef]
- Price, G.L.; Kanazirev, V.; Dooley, K.M.; Hart, V.I. On the mechanism of propane dehydrocyclization over cation-containing, proton-poor MFI zeolite. J. Catal. 1998, 173, 17–27. [Google Scholar] [CrossRef]
- Kazansky, V.B.; Subbotina, I.R.; Rane, N.; van Santen, R.A.; Hensen, E.J. On two alternative mechanisms of ethane activation over ZSM-5 zeolite modified by Zn2+ and Ga1+ cations. Phys. Chem. Chem. Phys. PCCP 2005, 7, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Fanchiang, W.L.; Lin, Y.C. Catalytic fast pyrolysis of furfural over H-ZSM-5 and Zn/H-ZSM-5 catalysts. Appl. Catal. A Gen. 2012, 419, 102–110. [Google Scholar] [CrossRef]
- Bhan, A.; Delgass, W.N. Propane aromatization over HZSM-5 and Ga/HZSM-5 catalysts. Catal. Rev. 2008, 50, 19–151. [Google Scholar] [CrossRef]
- Krishnamurthy, G.; Bhan, A.; Delgass, W.N. Identity and chemical function of gallium species inferred from microkinetic modeling studies of propane aromatization over Ga/HZSM-5 catalysts. J. Catal. 2010, 271, 370–385. [Google Scholar] [CrossRef]
- Ono, Y.; Kitagawa, H.; Sendoda, Y. Transformation Of but-1-Ene into Aromatic-Hydrocarbons over ZSM-5 Zeolites. J. Chem. Soc. Faraday T 1 1987, 83, 2913–2923. [Google Scholar] [CrossRef]
- Davies, E.E.; Kolombos, A.J. Process for Converting C3-C12 Hydrocarbons to Aromatics over Gallia-Activated Zeolite. Google Patents US4180689A, 25 December 1979. [Google Scholar]
- Song, C.; Gim, M.Y.; Lim, Y.H.; Kim, D.H. Enhanced yield of benzene, toulene, and xylene from the co-aromatization of methane and propane over gallium supported on mesoporous ZSM-5 and ZSM-11. Fuel 2019, 251, 404–412. [Google Scholar] [CrossRef]
- Xin, M.D.; Xing, E.H.; Gao, X.Z.; Wang, Y.R.; Ouyang, Y.; Xu, G.T.; Luo, Y.B.; Shu, X.T. Ga Substitution during Modification of ZSM-5 and Its Influences on Catalytic Aromatization Performance. Ind. Eng. Chem. Res. 2019, 58, 6970–6981. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Chen, Y.Y.; Lin, Y.C. Ga-Substituted Nanoscale HZSM-5 in Methanol Aromatization: The Cooperative Action of the Bronsted Acid and the Extra-Framework Ga Species. Ind. Eng. Chem. Res. 2018, 57, 7742–7751. [Google Scholar] [CrossRef]
- Cybulskis, V.J.; Pradhan, S.U.; Lovon-Quintana, J.J.; Hock, A.S.; Hu, B.; Zhang, G.H.; Delgass, W.N.; Ribeiro, F.H.; Miller, J.T. The Nature of the Isolated Gallium Active Center for Propane Dehydrogenation on Ga/SiO2. Catal. Lett. 2017, 147, 1252–1262. [Google Scholar] [CrossRef]
- Li, Z.L.; Lepore, A.W.; Salazar, M.F.; Foo, G.S.; Davison, B.H.; Wu, Z.L.; Narula, C.K. Selective conversion of bio-derived ethanol to renewable BTX over Ga-ZSM-5. Green Chem. 2017, 19, 4344–4352. [Google Scholar] [CrossRef]
- Lai, P.C.; Chen, C.H.; Hsu, H.Y.; Lee, C.H.; Lin, Y.C. Methanol aromatization over Ga-doped desilicated HZSM-5. RSC Adv. 2016, 6, 67361–67371. [Google Scholar] [CrossRef]
- Rane, N.; Kersbulck, M.; van Santen, R.A.; Hensen, E.J.M. Cracking of n-heptane over Bronsted acid sites and Lewis acid Ga sites in ZSM-5 zeolite. Microporous Mesoporous Mater. 2008, 110, 279–291. [Google Scholar] [CrossRef]
- Phadke, N.M.; Van der Mynsbrugge, J.; Mansoor, E.; Getsoian, A.B.; Head-Gordon, M.; Bell, A.T. Characterization of Isolated Ga3+ Cations in Ga/H-MFI Prepared by Vapor-Phase Exchange of H-MFI Zeolite with GaCl3. ACS Catal. 2018, 8, 6106–6126. [Google Scholar] [CrossRef]
- Shao, C.T.; Lang, W.Z.; Yan, X.; Guo, Y.J. Catalytic performance of gallium oxide based-catalysts for the propane dehydrogenation reaction: Effects of support and loading amount. RSC Adv. 2017, 7, 4710–4723. [Google Scholar] [CrossRef]
- Han, Z.; Zhou, F.; Liu, Y.; Qiao, K.; Wu, G. Synthesis of gallium-containing ZSM-5 zeolites by the seed-induced method and catalytic performance of GaZSM-5 and AlZSM-5 during the conversion of methanol to olefins. J. Taiwan Inst. Chem. Eng. 2019, 103, 149–159. [Google Scholar] [CrossRef]
- Su, X.F.; Wang, G.L.; Bai, X.F.; Wu, W.; Xiao, L.F.; Fang, Y.J.; Zhang, J.W. Synthesis of nanosized HZSM-5 zeolites isomorphously substituted by gallium and their catalytic performance in the aromatization. Chem. Eng. J. 2016, 293, 365–375. [Google Scholar] [CrossRef]
- Li, J.; Yu, Y.Q.; Li, X.Y.; Wang, W.; Yu, G.; Deng, S.B.; Huang, J.; Wang, B.; Wang, Y.J. Maximizing carbon efficiency of petrochemical production from catalytic co-pyrolysis of biomass and plastics using gallium-containing MFI zeolites. Appl. Catal. B Environ. 2015, 172, 154–164. [Google Scholar] [CrossRef]
- Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Pinard, L. Propane aromatization on hierarchical Ga/HZSM-5 catalysts. J. Catal. 2018, 366, 223–236. [Google Scholar] [CrossRef]
- Wannapakdee, W.; Suttipat, D.; Dugkhuntod, P.; Yutthalekha, T.; Thivasasith, A.; Kidkhunthod, P.; Nokbin, S.; Pengpanich, S.; Limtrakul, J.; Wattanakit, C. Aromatization of C5 hydrocarbons over Ga-modified hierarchical HZSM-5 nanosheets. Fuel 2019, 236, 1243–1253. [Google Scholar] [CrossRef]
- Kosslick, H.; Richter, M.; Tuan, V.A.; Parlitz, B.; Szulzewsky, K.; Fricke, R. Genesis of gallosilicates with ZSM-5 structure. Insertion of Ga and zeolitic properties at various steps of crystallization. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 69, pp. 109–117. [Google Scholar]
- Nishi, K.; Komai, S.; Inagaki, K.; Satsuma, A.; Hattori, T. Structure and catalytic properties of Ga-MFI in propane aromatization. Appl. Catal. A Gen. 2002, 223, 187–193. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Banerjee, S.; Panjala, D. Product distribution in the aromatization of dilute ethene over H-GaAlMFI zeolite: Effect of space velocity. Microporous Mesoporous Mater. 2002, 51, 203–210. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Al-Khattaf, S. Physicochemical properties and catalytic performance of galloaluminosilicate in aromatization of lower alkanes: A comparative study with Ga/HZSM-5. J. Porous Mater. 2011, 19, 943–960. [Google Scholar] [CrossRef]
- Wannapakdee, W.; Wattanakit, C.; Paluka, V.; Yutthalekha, T.; Limtrakul, J. One-pot synthesis of novel hierarchical bifunctional Ga/HZSM-5 nanosheets for propane aromatization. RSC Adv. 2016, 6, 2875–2881. [Google Scholar] [CrossRef]
- Kim, W.G.; So, J.; Choi, S.W.; Liu, Y.J.; Dixit, R.S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W. Hierarchical Ga-MFI Catalysts for Propane Dehydrogenation. Chem. Mater. 2017, 29, 7213–7222. [Google Scholar] [CrossRef]
- Awate, S.V.; Joshi, P.N.; Shiralkar, V.P.; Kotasthane, A.N. Synthesis And Characterization Of Gallosilicate Pentasil (MFI) Framework Zeolites. J. Incl. Phenom. Mol. 1992, 13, 207–218. [Google Scholar] [CrossRef]
- Montes, A.; Giannetto, G. A new way to obtain acid or bifunctional catalysts V. Considerations on bifunctionality of the propane aromatization reaction over [Ga,Al]-ZSM-5 catalysts. Appl. Catal. A Gen. 2000, 197, 31–39. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Banerjee, S.; Panjala, D. Influence of Temperature on the Product Selectivity and Distribution of Aromatics and C8 Aromatic Isomers in the Conversion of Dilute Ethene over H-Galloaluminosilicate (ZSM-5 type) Zeolite. J. Catal. 2002, 205, 398–403. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Panjala, D.; Banerjee, S. Aromatization of propene and n-butene over H-galloaluminosilicate (ZSM-5 type) zeolite. Appl. Catal. A Gen. 2002, 231, 243–251. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Kinage, A.; Banerjee, S.; Choudhary, V.R. Propane Conversion to Aromatics on Highly Active H-GaAlMFI: Effect of Thermal Pretreatment. Energy Fuels 2006, 20, 919–922. [Google Scholar] [CrossRef]
- Raad, M.; Astafan, A.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Comparot, J.D.; Canaff, C.; Daou, T.J.; Patarin, J.; Pinard, L. Catalytic properties of Ga-containing MFI-type zeolite in cyclohexane dehydrogenation and propane aromatization. J. Catal. 2018, 365, 376–390. [Google Scholar] [CrossRef]
- Kosslick, H.; Tuan, V.A.; Parlitz, B.; Fricke, R.; Peuker, C.; Storek, W. Disruption Of the Mfi Framework by the Incorporation of Gallium. J. Chem. Soc. Faraday T 1993, 89, 1131–1138. [Google Scholar] [CrossRef]
- Kosslick, H.; Tuan, V.A.; Fricke, R. Ga–ZSM–5–Conditions of Synthesis. Cryst. Res. Technol. 1991, 26, K64–K67. [Google Scholar] [CrossRef]
- Schreiber, M.W.; Plaisance, C.P.; Baumgartl, M.; Reuter, K.; Jentys, A.; Bermejo-Deval, R.; Lercher, J.A. Lewis-Bronsted Acid Pairs in Ga/H-ZSM-5 To Catalyze Dehydrogenation of Light Alkanes. J. Am. Chem. Soc. 2018, 140, 4849–4859. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K.; Sivadinarayana, C. Influence of zeolite factors affecting zeolitic acidity on the propane aromatization activity and selectivity of Ga/H-ZSM-5. Microporous Mesoporous Mater. 2000, 37, 1–8. [Google Scholar] [CrossRef]
- Kazansky, V.; Subbotina, I.; Vansanten, R.; Hensen, E. DRIFTS study of the chemical state of modifying gallium ions in reduced Ga/ZSM-5 prepared by impregnationI. Observation of gallium hydrides and application of CO adsorption as a molecular probe for reduced gallium ions. J. Catal. 2004, 227, 263–269. [Google Scholar] [CrossRef]
- Kwak, B.S.; Sachtler, W.M.H. Effect Of Ga/Proton Balance In Ga/Hzsm-5 Catalysts on C-3 Conversion To Aromatics. J. Catal. 1994, 145, 456–463. [Google Scholar] [CrossRef]
- Nowak, I. Effect of H2–O2 pre-treatments on the state of gallium in Ga/H-ZSM-5 propane aromatisation catalysts. Appl. Catal. A Gen. 2003, 251, 107–120. [Google Scholar] [CrossRef]
- Kanazirev, V.; Price, G.L.; Dooley, K.M. Enhancement in Propane Aromatization with Ga2O3/HZSM-5 Catalysts. J. Chem. Soc. Chem. Comm. 1990, 9, 712–713. [Google Scholar] [CrossRef]
- Freeman, D.; Wells, R.P.; Hutchings, G.J. Methanol to hydrocarbons: Enhanced aromatic formation using a composite Ga2O3-H-ZSM-5 catalyst. Chem. Commun. 2001, 1754–1755. [Google Scholar] [CrossRef]
- Freeman, D.; Wells, R.P.K.; Hutchings, G.J. Conversion of methanol to hydrocarbons over Ga2O3/H-ZSM-5 and Ga2O3/WO3 catalysts. J. Catal. 2002, 205, 358–365. [Google Scholar] [CrossRef]
- Garciasanchez, M. Characterization of Ga/HZSM-5 and Ga/HMOR synthesized by chemical vapor deposition of trimethylgallium. J. Catal. 2003, 219, 352–361. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; García-Sánchez, M.; Rane, N.; Magusin, P.C.M.M.; Liu, P.-H.; Chao, K.-J.; van Santen, R.A. In situ Ga K edge XANES study of the activation of Ga/ZSM-5 prepared by chemical vapor deposition of trimethylgallium. Catal. Lett. 2005, 101, 79–85. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Bondil, M.; Head-Gordon, M.; Bell, A.T. Mechanism and Kinetics of Propane Dehydrogenation and Cracking over Ga/H-MFI Prepared via Vapor-Phase Exchange of H-MFI with GaCl3. J. Am. Chem. Soc. 2019, 141, 1614–1627. [Google Scholar] [CrossRef]
- El-Malki, E.M.; van Santen, R.A.; Sachtler, W.M.H. Introduction of Zn, Ga, and Fe into HZSM-5 cavities by sublimation: Identification of acid sites. J. Phys. Chem. B 1999, 103, 4611–4622. [Google Scholar] [CrossRef]
- Liu, R.-l.; Zhu, H.-q.; Wu, Z.-w.; Qin, Z.-f.; Fan, W.-b.; Wang, J.-g. Aromatization of propane over Ga-modified ZSM-5 catalysts. J. Fuel Chem. Technol. 2015, 43, 961–969. [Google Scholar] [CrossRef]
- Hamid, S.B.A.; Derouane, E.G.; Meriaudeau, P.; Naccache, C. Effect of reductive and oxidative atmospheres on the propane aromatisation activity and selectivity of Ga/H-ZSM-5 catalysts. Catal. Today 1996, 31, 327–334. [Google Scholar] [CrossRef]
- Rodrigues, V.D.; Faro, A.C. On catalyst activation and reaction mechanisms in propane aromatization on Ga/HZSM5 catalysts. Appl. Catal. A Gen. 2012, 435, 68–77. [Google Scholar] [CrossRef]
- Arnaldo da Costa Faro Júniora, A.; de Oliveira Rodriguesa, V. Pulse reaction studies of gallium modified H-ZSM5 catalysts with propane. Adv. Pharmacol. 2008, 1155–1158. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T. Tunable activity of [Ga]HZSM-5 with H2 treatment: Ethane dehydrogenation. Catal. Commun. 2014, 45, 63–68. [Google Scholar] [CrossRef]
- Fricke, R.; Kosslick, H.; Lischke, G.; Richter, M. Incorporation of gallium into zeolites: Syntheses, properties and catalytic application. Chem. Rev. 2000, 100, 2303–2406. [Google Scholar] [CrossRef]
- Su, X.; Fang, Y.; Gao, P.; Liu, Y.; Hou, G.; Bai, X.; Wu, W. In-situ microwave synthesis of nano-GaZSM-5 bifunctional catalysts with controllable location of active GaO+ species for olefins aromatization. Microporous Mesoporous Mater. 2020, 306, 110388. [Google Scholar] [CrossRef]
- Thivasasith, A.; Maihom, T.; Pengpanich, S.; Limtrakul, J.; Wattanakit, C. Insights into the reaction mechanism of n-hexane dehydroaromatization to benzene over gallium embedded HZSM-5: Effect of H2 incorporated on active sites. Phys. Chem. Chem. Phys. PCCP 2019, 21, 5359–5367. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; van Santen, R.A. Self-organization of extraframework cations in zeolites. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2070–2086. [Google Scholar] [CrossRef]
- Mansoor, E.; Head-Gordon, M.; Bell, A.T. Computational Modeling of the Nature and Role of Ga Species for Light Alkane Dehydrogenation Catalyzed by Ga/H-MFI. ACS Catal. 2018, 8, 6146–6162. [Google Scholar] [CrossRef]
- Kuo, Y.-T.; Almansa, G.A.; Vreugdenhil, B.J. Catalytic aromatization of ethylene in syngas from biomass to enhance economic sustainability of gas production. Appl. Energy 2018, 215, 21–30. [Google Scholar] [CrossRef]
- Meitzner, G.D.; Iglesia, E.; Baumgartner, J.E.; Huang, E.S. The Chemical State of Gallium in Working Alkane Dehydrocyclodimerization Catalysts. In situ Gallium K-Edge X-Ray Absorption Spectroscopy. J. Catal. 1993, 140, 209–225. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Conte, M.; Landon, P.; Zhou, W.; Bartley, J.K.; Taylor, S.H.; Carley, A.F.; Kiely, C.J.; Khalid, K.; Hutchings, G.J. Reactivity of Ga2O3 Clusters on Zeolite ZSM-5 for the Conversion of Methanol to Aromatics. Catal. Lett. 2012, 142, 1049–1056. [Google Scholar] [CrossRef]
- Sirotin, S.V.; Moskovskaya, I.F.; Romanovsky, B.V. Synthetic strategy for Fe-MCM-41 catalyst: A key factor for homogeneous or heterogeneous phenol oxidation. Catal. Sci. Technol. 2011, 1, 971–980. [Google Scholar] [CrossRef]
- Michorczyk, P. Dehydrogenation of propane to propene over gallium oxide in the presence of CO2. Appl. Catal. A Gen. 2003, 251, 425–433. [Google Scholar] [CrossRef]
- Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of propane to propene over different polymorphs of gallium oxide. J. Catal. 2005, 232, 143–151. [Google Scholar] [CrossRef]
- Michorczyk, P.; Kuśtrowski, P.; Kolak, A.; Zimowska, M. Ordered mesoporous Ga2O3 and Ga2O3–Al2O3 prepared by nanocasting as effective catalysts for propane dehydrogenation in the presence of CO2. Catal. Commun. 2013, 35, 95–100. [Google Scholar] [CrossRef]
- Xu, B.; Li, T.; Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Enhanced Stability of HZSM-5 Supported Ga2O3 Catalyst in Propane Dehydrogenation by Dealumination. Catal. Lett. 2007, 119, 283–288. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.F.; Wang, P.; Zhang, Z.Z.; Zhang, Q.D.; Xie, H.J.; Yang, G.H.; Han, Y.Z.; Tan, Y.S. Mechanistic insight to acidity effects of Ga/HZSM-5 on its activity for propane aromatization. RSC Adv. 2015, 5, 92222–92233. [Google Scholar] [CrossRef]
- Al-Yassir, N.; Akhtar, M.N.; Ogunronbi, K.; Al-Khattaf, S. Synthesis of stable H-galloaluminosilicate MFI with hierarchical pore architecture by surfactant-mediated base hydrolysis, and their application in propane aromatization. J. Mol. Catal. A Chem. 2012, 360, 1–15. [Google Scholar] [CrossRef]
- Rodrigues, V.d.O.; Vasconcellos, F.J.; Faro Júnior, A.d.C. Mechanistic studies through H–D exchange reactions: Propane aromatization in HZSM5 and Ga/HZSM5 catalysts. J. Catal. 2016, 344, 252–262. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, J.; Wang, X.; Zhang, Q.; Xie, H.; Han, Y.; Tan, Y. A highly efficient Ga/ZSM-5 catalyst prepared by formic acid impregnation and in situ treatment for propane aromatization. Catal. Sci. Technol. 2015, 5, 4081–4090. [Google Scholar] [CrossRef]
- Lee, B.J.; Hur, Y.G.; Kim, D.H.; Lee, S.H.; Lee, K.-Y. Non-oxidative aromatization and ethylene formation over Ga/HZSM-5 catalysts using a mixed feed of methane and ethane. Fuel 2019, 253, 449–459. [Google Scholar] [CrossRef]
- Ogunronbi, K.E.; Al-Yassir, N.; Al-Khattaf, S. New insights into hierarchical metal-containing zeolites; synthesis and kinetic modelling of mesoporous gallium-containing ZSM-5 for propane aromatization. J. Mol. Catal. A Chem. 2015, 406, 1–18. [Google Scholar] [CrossRef]
- Kazansky, V.; Subbotina, I.; Vansanten, R.; Hensen, E. DRIFTS study of the nature and chemical reactivity of gallium ions in Ga/ZSM-5II. Oxidation of reduced Ga species in ZSM-5 by nitrous oxide or water. J. Catal. 2005, 233, 351–358. [Google Scholar] [CrossRef]
- Faro Júnior, A.d.C.; Oliveira Rodrigues, V.d. Pulse reaction studies of gallium modified H-ZSM5 catalysts with propane. In Studies in Surface Science and Catalysis; Gédéon, A., Massiani, P., Babonneau, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 174, pp. 1155–1158. [Google Scholar]
- Serykh, A.I.; Kolesnikov, S.P. On the nature of gallium species in gallium-modified mordenite and MFI zeolites. A comparative DRIFT study of carbon monoxide adsorption and hydrogen dissociation. Phys. Chem. Chem. Phys. 2011, 13, 6892–6900. [Google Scholar] [CrossRef] [PubMed]
- Rane, N.; Overweg, A.R.; Kazansky, V.B.; Santen, R.A.V.; Hensen, E.J.M. Characterization and reactivity of Ga+ and GaO+ cations in zeolite ZSM-5. J. Catal. 2006, 239, 478–485. [Google Scholar] [CrossRef]
- Pidko, E.A.; Kazansky, V.B.; Hensen, E.J.M.; van Santen, R.A. A comprehensive density functional theory study of ethane dehydrogenation over reduced extra-framework gallium species in ZSM-5 zeolite. J. Catal. 2006, 240, 73–84. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; van Santen, R.A. Anionic Oligomerization of Ethylene over Ga/ZSM-5 Zeolite: A Theoretical Study. J. Phys. Chem. C 2008, 112, 19604–19611. [Google Scholar] [CrossRef]
- Rodrigues, V.D.; Eon, J.G.; Faro, A.C. Correlations between Dispersion, Acidity, Reducibility, and Propane Aromatization Activity of Gallium Species Supported on HZSM5 Zeolites. J. Phys. Chem. C 2010, 114, 4557–4567. [Google Scholar] [CrossRef]
- Pidko, E.A.; Hensen, E.J.M.; van Santen, R.A. Dehydrogenation of light alkanes over isolated gallyl ions in Ga/ZSM-5 zeolites. J. Phys. Chem. C 2007, 111, 13068–13075. [Google Scholar] [CrossRef]
- Frash, M.V.; Van Santen, R.A. Activation of small alkanes on Ga-exchanged zeolites: A quantum-chemical study of ethane dehydrogenation. J. Phys. Chem. A 2000, 104, 2468–2475. [Google Scholar] [CrossRef]
- Wongnongwa, Y.; Kidkhunthod, P.; Sukkha, U.; Pengpanich, S.; Thavornprasert, K.-a.; Phupanit, J.; Kungwan, N.; Feng, G.; Keawin, T.; Jungsuttiwong, S. Local structure elucidation and reaction mechanism of light naphtha aromatization over Ga embedded H-ZSM-5 zeolite: Combined DFT and experimental study. Microporous Mesoporous Mater. 2020, 306, 110414. [Google Scholar] [CrossRef]
- Joshi, Y.V.; Thomson, K.T. The roles of gallium hydride and Bronsted acidity in light alkane dehydrogenation mechanisms using Ga-exchanged HZSM-5 catalysts: A DFT pathway analysis. Catal. Today 2005, 105, 106–121. [Google Scholar] [CrossRef]
- Pereira, M.S.; Chaer Nascimento, M.A. Theoretical study on the dehydrogenation reaction of alkanes catalyzed by zeolites containing nonframework gallium species. J. Phys. Chem. B 2006, 110, 3231–3238. [Google Scholar] [CrossRef]
- Chao, K.-J.; Liu, P.-H. Gallium-containing zeolites: Characterization of catalytic role of gallium species in converting light paraffins to aromatics. Catal. Surv. Asia 2005, 9, 11–15. [Google Scholar] [CrossRef]
- Gao, P.; Xu, J.; Qi, G.; Wang, C.; Wang, Q.; Zhao, Y.; Zhang, Y.; Feng, N.; Zhao, X.; Li, J.; et al. A Mechanistic Study of Methanol-to-Aromatics Reaction over Ga-Modified ZSM-5 Zeolites: Understanding the Dehydrogenation Process. ACS Catal. 2018, 8, 9809–9820. [Google Scholar] [CrossRef]
- Gao, P.; Wang, Q.; Xu, J.; Qi, G.; Wang, C.; Zhou, X.; Zhao, X.; Feng, N.; Liu, X.; Deng, F. Brønsted/Lewis Acid Synergy in Methanol-to-Aromatics Conversion on Ga-Modified ZSM-5 Zeolites, As Studied by Solid-State NMR Spectroscopy. ACS Catal. 2018, 8, 69–74. [Google Scholar] [CrossRef]
- Hensen, E.J.; Pidko, E.A.; Rane, N.; van Santen, R.A. Water-promoted hydrocarbon activation catalyzed by binuclear gallium sites in ZSM-5 zeolite. Angew. Chem. 2007, 46, 7273–7276. [Google Scholar] [CrossRef]
- Jr, A.C.F.; Rodrigues, V.D.O.; Eon, J.G. In Situ X-ray Absorption Study of the Genesis and Nature of the Reduced Gallium Species in Ga/HZSM5 Catalysts. J. Phys. Chem. C 2011, 115, 4749–4756. [Google Scholar]
- Fang, Y.J.; Su, X.F.; Bai, X.F.; Wu, W.; Wang, G.L.; Xiao, L.F.; Yu, A.R. Aromatization over nanosized Ga-containing ZSM-5 zeolites prepared by different methods: Effect of acidity of active Ga species on the catalytic performance. J. Energy Chem. 2017, 26, 768–775. [Google Scholar] [CrossRef]
- Uslamin, E.A.; Luna-Murillo, B.; Kosinov, N.; Bruijnincx, P.C.A.; Pidko, E.A.; Weckhuysen, B.M.; Hensen, E.J.M. Gallium-promoted HZSM-5 zeolites as efficient catalysts for the aromatization of biomass-derived furans. Chem. Eng. Sci. 2019, 198, 305–316. [Google Scholar] [CrossRef]
- Broclawik, E.; Himei, H.; Yamadaya, M.; Kubo, M.; Miyamoto, A.; Vetrivel, R. Density-functional theory calculations of the reaction pathway for methane activation on a gallium site in metal exchanged ZSM-5. J. Chem. Phys. 1995, 103, 2102–2108. [Google Scholar] [CrossRef]
- Gomez, E.; Yan, B.; Kattel, S.; Chen, J.G. Carbon dioxide reduction in tandem with light-alkane dehydrogenation. Nat. Rev. Chem. 2019, 3, 638–649. [Google Scholar] [CrossRef]
- Guisnet, M.; Gnep, N.S. Aromatization of propane over GaHMFI catalysts. Reaction scheme, nature of the dehydrogenating species and mode of coke formation. Catal. Today 1996, 31, 275–292. [Google Scholar] [CrossRef]
- Bandiera, J.; Ben Taarit, Y. Ethane conversion: Kinetic evidence for the competition of consecutive steps for the same active centre. Appl. Catal. A Gen. 1997, 152, 43–51. [Google Scholar] [CrossRef]
- Pereira, M.S.; Nascimento, M.A.C. Theoretical study of the dehydrogenation reaction of ethane catalyzed by zeolites containing non-framework gallium species: The 3-step mechanism×the 1-step concerted mechanism. Chem. Phys. Lett. 2005, 406, 446–451. [Google Scholar] [CrossRef]
- Pereira, M.S.; da Silva, A.M.; Chaer Nascimento, M.A. Effect of the Zeolite Cavity on the Mechanism of Dehydrogenation of Light Alkanes over Gallium-Containing Zeolites. J. Phys. Chem. C 2011, 115, 10104–10113. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The Strength of Brønsted Acid Sites in Microporous Aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Conte, M.; Lopez-Sanchez, J.A.; He, Q.; Morgan, D.J.; Ryabenkova, Y.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Kiely, C.J.; Khalid, K.; et al. Modified zeolite ZSM-5 for the methanol to aromatics reaction. Catal. Sci. Technol. 2012, 2, 105–112. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Devadas, P.; Banerjee, S.; Kinage, A.K. Aromatization of dilute ethylene over Ga-modified ZSM-5 type zeolite catalysts. Microporous Mesoporous Mater. 2001, 47, 253–267. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Z.; Liu, X.; Wang, Y.; Meng, C. Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons. Molecules 2021, 26, 2234. https://doi.org/10.3390/molecules26082234
Feng Z, Liu X, Wang Y, Meng C. Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons. Molecules. 2021; 26(8):2234. https://doi.org/10.3390/molecules26082234
Chicago/Turabian StyleFeng, Zhe, Xin Liu, Yu Wang, and Changgong Meng. 2021. "Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons" Molecules 26, no. 8: 2234. https://doi.org/10.3390/molecules26082234
APA StyleFeng, Z., Liu, X., Wang, Y., & Meng, C. (2021). Recent Advances on Gallium-Modified ZSM-5 for Conversion of Light Hydrocarbons. Molecules, 26(8), 2234. https://doi.org/10.3390/molecules26082234






