Stability of Antimicrobial Drug Molecules in Different Gravitational and Radiation Conditions in View of Applications during Outer Space Missions
Abstract
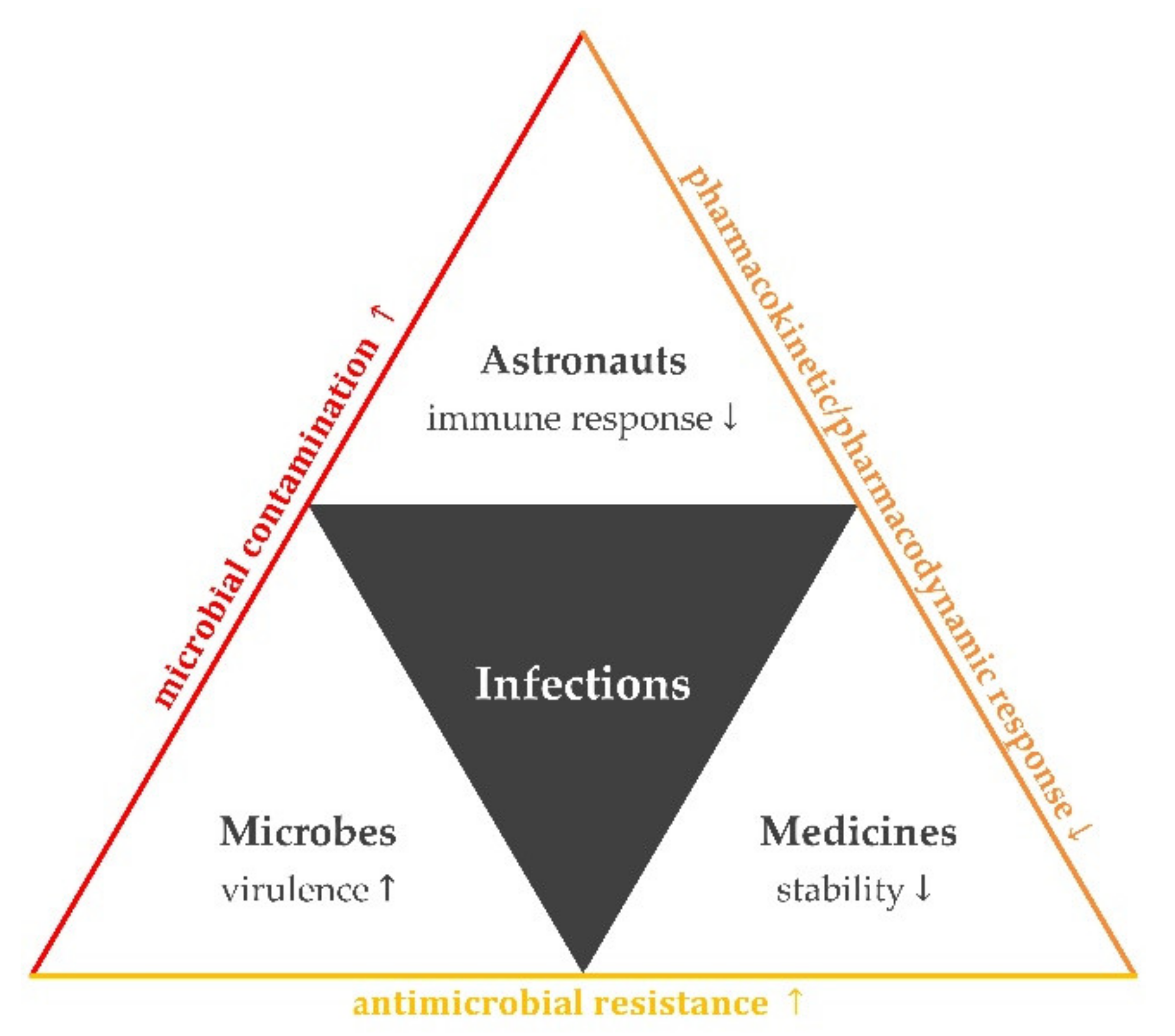
1. Introduction
- prolonged expedition duration (i.e., Martian expeditions lasting in the order of years);
- extended exposure to space environment;
- lack of restocking capability;
- absence of direct medical or pharmaceutical support from Earth and exclusive use of telemedicine; and
- limited or no capability for emergency evacuation of sick astronauts.
2. Impact of Spaceflight Environment on the Human Body
3. Terrestrial Microbial Concerns: Approaches for Multiple Drug Resistance
4. Spaceflight Microbial Concerns: From Bacterial Virulence and Antibiotic Susceptibility to Evidence for Microbial Contaminations
5. Impact of Spaceflight on Drug Stability: From Radiation to Gravitational Environment
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clément, G.; Bukley, A.P. Human Space Exploration–From Surviving to Performing. Acta Astronaut. 2014, 100, 101–106. [Google Scholar] [CrossRef]
- Thirsk, R.; Kuipers, A.; Mukai, C.; Williams, D. The Space-Flight Environment: The International Space Station and Beyond. CMAJ 2009, 180, 1216–1220. [Google Scholar] [CrossRef]
- International Space Exploration Coordination Group (ISECG). The Global Exploration Roadmap, 3rd ed.; NASA: Washington, DC, USA, 2018. Available online: https://www.globalspaceexploration.org/wordpress/wp-content/isecg/GER_2018_small_mobile.pdf (accessed on 5 March 2021).
- Singh, N.K.; Wood, J.M.; Karouia, F.; Venkateswaran, K. Succession and Persistence of Microbial Communities and Antimicrobial Resistance Genes Associated with International Space Station Environmental Surfaces. Microbiome 2018, 6, 204. [Google Scholar] [CrossRef]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.-P. Could Spaceflight-Associated Immune System Weakening Preclude the Expansion of Human Presence beyond Earth’s Orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef]
- Blue, R.S.; Bayuse, T.M.; Daniels, V.R.; Wotring, V.E.; Suresh, R.; Mulcahy, R.A.; Antonsen, E.L. Supplying a Pharmacy for NASA Exploration Spaceflight: Challenges and Current Understanding. Npj Microgravity 2019, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kohn, F.P.M.; Hauslage, J. The Gravity Dependence of Pharmacodynamics: The Integration of Lidocaine into Membranes in Microgravity. Npj Microgravity 2019, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Impact of Space Flight on Bacterial Virulence and Antibiotic Susceptibility. Infect. Drug Resist. 2015, 8, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Tangri, P.; Bisht, B. Who Role and Guidelines in Stability Study of Pharmaceuticals: A Regulatory Perspective. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 1380–1386. [Google Scholar]
- Huynh-Ba, K. Introduction. In Handbook of Stability Testing in Pharmaceutical Development: Regulations, Methodologies, and Best Practices; Huynh-Ba, K., Ed.; Springer: New York, NY, USA, 2009; pp. 1–6. [Google Scholar] [CrossRef]
- Lucas, T.I.; Bishara, R.H.; Seevers, R.H. A Stability Program for the Distribution of Drug Products. Pharm. Technol. 2004, 28, 68–73. [Google Scholar]
- Wotring, V. Space Pharmacology: How Space Affects Pharmacology. In Drug Discovery and Evaluation: Methods in Clinical Pharmacology; Hock, F.J., Gralinski, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–13. ISBN 978-3-319-56637-5. [Google Scholar]
- Norsk, P. Adaptation of the Cardiovascular System to Weightlessness: Surprises, Paradoxes and Implications for Deep Space Missions. Acta Physiol. 2020, 228, e13434. [Google Scholar] [CrossRef]
- Norsk, P.; Drummer, C.; Christensen, N.J.; Cirillo, M.; Heer, M.; Kramer, H.J.; Regnard, J.; Santo, N.G.D. Revised Hypothesis and Future Perspectives. Am. J. Kidney Dis. 2001, 38, 696–698. [Google Scholar] [CrossRef] [PubMed]
- Alfrey, C.P.; Udden, M.M.; Leach-Huntoon, C.; Driscoll, T.; Pickett, M.H. Control of Red Blood Cell Mass in Spaceflight. J. Appl. Physiol. 1996, 81, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Stenger, M.B.; Tarver, W.J.; Brunstetter, T.; Gibson, C.R.; Laurie, S.S.; Lee, S.M.C.; Macias, B.R.; Mader, T.H.; Otto, C.; Smith, S.M.; et al. Evidence Report: Risk of Spaceflight Associated Neuro-Ocular Syndrome (SANS); NASA: Washington, DC, USA, 2017. Available online: https://humanresearchroadmap.nasa.gov/Evidence/reports/SANS.pdf (accessed on 5 March 2021).
- Heer, M.; Paloski, W.H. Space Motion Sickness: Incidence, Etiology, and Countermeasures. Auton. Neurosci. 2006, 129, 77–79. [Google Scholar] [CrossRef]
- Levine, A.S. Psychological Effects of Long-Duration Space Missions and Stress Amelioration Techniques. In From Antarctica to Outer Space: Life in Isolation and Confinement; Harrison, A.A., Clearwater, Y.A., McKay, C.P., Eds.; Springer: New York, NY, USA, 1991; pp. 305–315. ISBN 978-0-387-97310-4. [Google Scholar]
- Williams, D.R. The Biomedical Challenges of Space Flight. Annu. Rev. Med. 2003, 54, 245–256. [Google Scholar] [CrossRef]
- Graebe, A.; Schuck, E.L.; Lensing, P.; Putcha, L.; Derendorf, H. Physiological, Pharmacokinetic, and Pharmacodynamic Changes in Space. J. Clin. Pharmacol. 2004, 44, 837–853. [Google Scholar] [CrossRef]
- Kast, J.; Yu, Y.; Seubert, C.N.; Wotring, V.E.; Derendorf, H. Drugs in Space: Pharmacokinetics and Pharmacodynamics in Astronauts. Eur. J. Pharm. Sci. 2017, 109, S2–S8. [Google Scholar] [CrossRef]
- Tietze, K.J.; Putcha, L. Factors Affecting Drug Bioavailability in Space. J. Clin. Pharm. 1994, 34, 671–676. [Google Scholar] [CrossRef]
- Derendorf, H. Pharmacokinetic/Pharmacodynamic Consequences of Space Flight. J. Clin. Pharm. 1994, 34, 684–691. [Google Scholar] [CrossRef]
- Geuna, S.; Brunelli, F.; Perino, M.A. Stressors, Stress and Stress Consequences during Long-Duration Manned Space Missions: A Descriptive Model. Acta Astronaut. 1995, 36, 347–356. [Google Scholar] [CrossRef]
- National Research Council; Space Studies Board. Behavioral Issues. In A Strategy for Research in Space Biology and Medicine in the New Century; National Academy Press: Washington, DC, USA, 1998; pp. 194–230. [Google Scholar]
- Palinkas, L.A. Psychosocial Issues in Long-Term Space Flight: Overview. Gravit. Space Biol. Bull. 2001, 14, 25–33. [Google Scholar] [PubMed]
- Vakoch, D.A. Psychology of Space Exploration: Contemporary Research in Historical Perspective; NASA: Washington, DC, USA, 2011; ISBN 978-1-62683-013-4.
- Stuster, J.W. Space Station Habitability Recommendations Based on a Systematic Comparative Analysis of Analogous Conditions; Contractor Report 3943; NASA: Washington, DC, USA, 1986; Public Domain.
- Harrison, A.A.; Clearwater, Y.A.; McKay, C.P. From Antarctica to Outer Space: Life in Isolation and Confinement, 1st ed.; Springer: New York, NY, USA, 1991; ISBN 978-0-387-97310-4. [Google Scholar]
- Kimzey, S.L.; Fischer, C.L.; Johnson, P.C.; Ritzmann, S.E.; Mengel, C.E. Hematology and Immunology Studies. In Biomedical Results of Apollo; NASA, SP-368; Johnston, R.S., Dietlein, L.F., Berry, C.A., Eds.; NASA: Washington, DC, USA, 1975; pp. 197–227. [Google Scholar]
- Kimzey, S.L. Hematology and Immunology Studies. In Biomedical Results from Skylab; NASA SP-377; Johnston, R.S., Dietlein, L.F., Eds.; NASA: Washington, DC, USA, 1977; pp. 249–282. [Google Scholar]
- Cogoli, A.; Tschopp, A.; Fuchs-Bislin, P. Cell Sensitivity to Gravity. Science 1984, 225, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Stowe, R.P.; Pierson, D.L.; Barrett, A.D. Elevated Stress Hormone Levels Relate to Epstein-Barr Virus Reactivation in Astronauts. Psychosom. Med. 2001, 63, 891–895. [Google Scholar] [CrossRef]
- Pierson, D.L.; Stowe, R.P.; Phillips, T.M.; Lugg, D.J.; Mehta, S.K. Epstein-Barr Virus Shedding by Astronauts during Space Flight. Brain Behav. Immun. 2005, 19, 235–242. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Mehta, S.K.; Schmid, D.S.; Gilden, D.H.; Pierson, D.L. Asymptomatic Reactivation and Shed of Infectious Varicella Zoster Virus in Astronauts. J. Med. Virol. 2008, 80, 1116–1122. [Google Scholar] [CrossRef]
- Mehta, S.K.; Stowe, R.P.; Feiveson, A.H.; Tyring, S.K.; Pierson, D.L. Reactivation and Shedding of Cytomegalovirus in Astronauts during Spaceflight. J. Infect. Dis. 2000, 182, 1761–1764. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Sams, C.F.; Pierson, D.L. Multiple Latent Viruses Reactivate in Astronauts during Space Shuttle Missions. Brain Behav. Immun. 2014, 41, 210–217. [Google Scholar] [CrossRef]
- Crucian, B.E.; Stowe, R.P.; Pierson, D.L.; Sams, C.F. Immune System Dysregulation Following Short- vs Long-Duration Spaceflight. Aviat. Space Environ. Med. 2008, 79, 835–843. [Google Scholar] [CrossRef]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Mark Ott, C.; Pierson, D.L. Changes in Neutrophil Functions in Astronauts. Brain Behav. Immun. 2004, 18, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Simons, E.R.; Castro, V.A.; Ott, C.M.; Pierson, D.L. Changes in Monocyte Functions of Astronauts. Brain Behav. Immun. 2005, 19, 547–554. [Google Scholar] [CrossRef]
- Kaur, I.; Simons, E.R.; Kapadia, A.S.; Ott, C.M.; Pierson, D.L. Effect of Spaceflight on Ability of Monocytes to Respond to Endotoxins of Gram-Negative Bacteria. Clin. Vaccine Immunol. 2008, 15, 1523–1528. [Google Scholar] [CrossRef]
- Rykova, M.P.; Sonnenfeld, G.; Lesnyak, A.T.; Taylor, G.R.; Meshkov, D.O.; Mandel, A.D.; Medvedev, A.E.; Berry, W.D.; Fuchs, B.B.; Konstantinova, I.V. Effect of Spaceflight on Natural Killer Cell Activity. J. Appl. Physiol. 1992, 73, 196S–200S. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, I.V.; Rykova, M.; Meshkov, D.; Peres, C.; Husson, D.; Schmitt, D.A. Natural Killer Cells after ALTAIR Mission. Acta Astronaut. 1995, 36, 713–718. [Google Scholar] [CrossRef]
- Meshkov, D.; Rykova, M. The Natural Cytotoxicity in Cosmonauts on Board Space Stations. Acta Astronaut. 1995, 36, 719–726. [Google Scholar] [CrossRef]
- Tálas, M.; Bátkai, L.; Stöger, I.; Nagy, L.; Hiros, L.; Konstantinova, I.; Rykova, M.; Mozgovaya, I.; Guseva, O.; Kozharinov, V. Results of Space Experiment Program “Interferon”. I. Production of Interferon in Vitro by Human Lymphocytes Aboard Space Laboratory Solyut-6 (“Interferon I”) and Influence of Space Flight on Lymphocyte Functions of Cosmonauts (“Interferon III”). Acta Microbiol. Hung. 1983, 30, 53–61. [Google Scholar] [PubMed]
- Taylor, K.; Kleinhesselink, K.; George, M.D.; Morgan, R.; Smallwood, T.; Hammonds, A.S.; Fuller, P.M.; Saelao, P.; Alley, J.; Gibbs, A.G.; et al. Toll Mediated Infection Response Is Altered by Gravity and Spaceflight in Drosophila. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Cogoli, A.; Bechler, B.; Cogoli-Greuter, M.; Criswell, S.B.; Joller, H.; Joller, P.; Hunzinger, E.; Müller, O. Mitogenic Signal Transduction in T Lymphocytes in Microgravity. J. Leukoc. Biol. 1993, 53, 569–575. [Google Scholar] [CrossRef]
- Boxio, R.; Dournon, C.; Frippiat, J.-P. Effects of a Long-Term Spaceflight on Immunoglobulin Heavy Chains of the Urodele Amphibian Pleurodeles Waltl. J. Appl. Physiol. 2005, 98, 905–910. [Google Scholar] [CrossRef]
- Gridley, D.S.; Slater, J.M.; Luo-Owen, X.; Rizvi, A.; Chapes, S.K.; Stodieck, L.S.; Ferguson, V.L.; Pecaut, M.J. Spaceflight Effects on T Lymphocyte Distribution, Function and Gene Expression. J. Appl. Physiol. 2009, 106, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Courtade, M.; Caratero, A.; Jozan, S.; Pipy, B.; Caratero, M.C. Influence of Continuous, Very Low-Dose Gamma-Irradiation on the Mouse Immune System. Int. J. Radiat. Biol. 2001, 77, 587–592. [Google Scholar] [CrossRef]
- Taylor, G.R. Recovery of Medically Important Microorganisms from Apollo Astronauts. Aerosp. Med. 1974, 45, 824–828. [Google Scholar]
- Ferguson, J.K.; Taylor, G.R.; Mieszkuc, B.J. Microbiological Investigations. In Biomedical Results of Apollo; Johnston, R.S., Dietlein, L.F., Berry, C.A., Eds.; NASA: Washington, DC, USA, 1975; pp. 83–103. [Google Scholar]
- Ilyin, V.K. Microbiological Status of Cosmonauts during Orbital Spaceflights on Salyut and Mir Orbital Stations. Acta Astronaut. 2005, 56, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Novikova, N.; De Boever, P.; Poddubko, S.; Deshevaya, E.; Polikarpov, N.; Rakova, N.; Coninx, I.; Mergeay, M. Survey of Environmental Biocontamination on Board the International Space Station. Res. Microbiol. 2006, 157, 5–12. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London, UK, 2016. [Google Scholar]
- Younis, W.; Thangamani, S.; Seleem, M.N. Repurposing Non-Antimicrobial Drugs and Clinical Molecules to Treat Bacterial Infections. Curr. Pharm. Des. 2015, 21, 4106–4111. [Google Scholar] [CrossRef]
- Farrell, I.D.; Turner, P.J. Augmentin: Laboratory Studies. Scott. Med. J. 1982, 27, S21–S23. [Google Scholar] [CrossRef]
- Rolinson, G.N. The History and Background of Augmentin. S. Afr. Med. J. 1982, 62, 3A–4A. [Google Scholar] [PubMed]
- Van den Ende, J.; Gricius, M.E.; De Klerk, H.C.; Neiteler, B.F.; Forder, A.A.; Venter, L.; Hallett, A.F.; Koornhof, H.J.; Robinson, R.; Van Rensburg, A.J.; et al. The in Vitro Activity of Amoxycillin with Clavulanic Acid against Clinically Significant Bacteria. A Multicentre Study. S. Afr. Med. J. 1982, 62, 5A–8A. [Google Scholar]
- Rolinson, G.N. A Review of the Microbiology of Amoxycillin/Clavulanic Acid over the 15 Year Period 1978-1993. J. Chemother. 1994, 6, 283–318. [Google Scholar] [CrossRef]
- Lahiri, S.D.; Johnstone, M.R.; Ross, P.L.; McLaughlin, R.E.; Olivier, N.B.; Alm, R.A. Avibactam and Class C β-Lactamases: Mechanism of Inhibition, Conservation of the Binding Pocket, and Implications for Resistance. Antimicrob. Agents Chemother. 2014, 58, 5704–5713. [Google Scholar] [CrossRef]
- Mosley, J.F.; Smith, L.L.; Parke, C.K.; Brown, J.A.; Wilson, A.L.; Gibbs, L.V. Ceftazidime-Avibactam (Avycaz): For the Treatment of Complicated Intra-Abdominal and Urinary Tract Infections. Pharm. Ther. 2016, 41, 479–483. [Google Scholar]
- Lomovskaya, O.; Sun, D.; Rubio-Aparicio, D.; Nelson, K.; Tsivkovski, R.; Griffith, D.C.; Dudley, M.N. Vaborbactam: Spectrum of Beta-Lactamase Inhibition and Impact of Resistance Mechanisms on Activity in Enterobacteriaceae. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef]
- Vaborem. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/vaborem (accessed on 17 February 2021).
- Dhillon, S. Meropenem/Vaborbactam: A Review in Complicated Urinary Tract Infections. Drugs 2018, 78, 1259–1270. [Google Scholar] [CrossRef]
- Chevalier, J.; Mahamoud, A.; Baitiche, M.; Adam, E.; Viveiros, M.; Smarandache, A.; Militaru, A.; Pascu, M.L.; Amaral, L.; Pagès, J.-M. Quinazoline Derivatives Are Efficient Chemosensitizers of Antibiotic Activity in Enterobacter Aerogenes, Klebsiella Pneumoniae and Pseudomonas Aeruginosa Resistant Strains. Int. J. Antimicrob. Agents 2010, 36, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Militaru, A.; Smarandache, A.; Mahamoud, A.; Alibert, S.; Pages, J.-M.; Pascu, M.-L. Time Stability Studies of Quinazoline Derivative Designed to Fight Drug Resistance Acquired by Bacteria. Lett. Drug Des. Discov. 2011, 8, 124–129. [Google Scholar] [CrossRef]
- Matys, A.; Podlewska, S.; Witek, K.; Witek, J.; Bojarski, A.J.; Schabikowski, J.; Otrębska-Machaj, E.; Latacz, G.; Szymańska, E.; Kieć-Kononowicz, K.; et al. Imidazolidine-4-One Derivatives in the Search for Novel Chemosensitizers of Staphylococcus Aureus MRSA: Synthesis, Biological Evaluation and Molecular Modeling Studies. Eur. J. Med. Chem. 2015, 101, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Smarandache, A.; Boni, M.; Andrei, I.R.; Handzlik, J.; Kiec-Kononowicz, K.; Staicu, A.; Pascu, M.-L. Spectroscopic Investigations of Novel Pharmaceuticals: Stability and Resonant Interaction with Laser Beam. Appl. Surf. Sci. 2017, 417, 143–148. [Google Scholar] [CrossRef]
- Machado, L.; Spengler, G.; Evaristo, M.; Handzlik, J.; Molnár, J.; Viveiros, M.; Kiec-Kononowicz, K.; Amaral, L. Biological Activity of Twenty-Three Hydantoin Derivatives on Intrinsic Efflux Pump System of Salmonella Enterica Serovar Enteritidis NCTC 13349. In Vivo 2011, 25, 769–772. [Google Scholar]
- Nozawa, R.; Yokota, T.; Fujimoto, T. Susceptibility of Methicillin-Resistant Staphylococcus Aureus to the Selenium-Containing Compound 2-Phenyl-1,2-Benzoisoselenazol-3(2H)-One (PZ51). Antimicrob. Agents Chemother. 1989, 33, 1388–1390. [Google Scholar] [CrossRef]
- Favrot, L.; Grzegorzewicz, A.E.; Lajiness, D.H.; Marvin, R.K.; Boucau, J.; Isailovic, D.; Jackson, M.; Ronning, D.R. Mechanism of Inhibition of Mycobacterium Tuberculosis Antigen 85 by Ebselen. Nat. Commun. 2013, 4, 2748. [Google Scholar] [CrossRef]
- Thangamani, S.; Younis, W.; Seleem, M.N. Repurposing Ebselen for Treatment of Multidrug-Resistant Staphylococcal Infections. Sci. Rep. 2015, 5, 11596. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgren, A.; Zou, L. Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus Aureus LT-1 Targeting Thioredoxin Reductase. Front. Microbiol. 2019, 10, 3016. [Google Scholar] [CrossRef]
- Rooney, J.; Luz, A.; González-Hunt, C.; Bodhicharla, R.; Ryde, I.; Anbalagan, C.; Meyer, J. Effects of 5’-Fluoro-2-Deoxyuridine on Mitochondrial Biology in Caenorhabditis Elegans. Exp. Gerontol. 2014, 56, 69–76. [Google Scholar] [CrossRef]
- Ejim, L.; Farha, M.A.; Falconer, S.B.; Wildenhain, J.; Coombes, B.K.; Tyers, M.; Brown, E.D.; Wright, G.D. Combinations of Antibiotics and Nonantibiotic Drugs Enhance Antimicrobial Efficacy. Nat. Chem. Biol. 2011, 7, 348–350. [Google Scholar] [CrossRef]
- Grimsey, E.M.; Piddock, L.J.V. Do Phenothiazines Possess Antimicrobial and Efflux Inhibitory Properties? FEMS Microbiol. Rev. 2019, 43, 577–590. [Google Scholar] [CrossRef]
- Viola, G.; Latterini, L.; Vedaldi, D.; Aloisi, G.G.; Dall’acqua, F.; Gabellini, N.; Elisei, F.; Barbafina, A. Photosensitization of DNA Strand Breaks by Three Phenothiazine Derivatives. Chem. Res. Toxicol. 2003. [Google Scholar] [CrossRef] [PubMed]
- Buckner, M.M.C.; Ciusa, M.L.; Piddock, L.J.V. Strategies to Combat Antimicrobial Resistance: Anti-Plasmid and Plasmid Curing. FEMS Microbiol. Rev. 2018, 42, 781–804. [Google Scholar] [CrossRef]
- Zilberstein, D.; Liveanu, V.; Gepstein, A. Tricyclic Drugs Reduce Proton Motive Force in Leishmania Donovani Promastigotes. Biochem. Pharm. 1990, 39, 935–940. [Google Scholar] [CrossRef]
- Plenge-Tellechea, F.; Domínguez-Solís, C.A.; Díaz-Sánchez, Á.G.; Meléndez-Martínez, D.; Vargas-Medrano, J.; Sierra-Fonseca, J.A. Chlorpromazine and Dimethyl Sulfoxide Modulate the Catalytic Activity of the Plasma Membrane Ca2+-ATPase from Human Erythrocyte. J. Bioenerg. Biomembr. 2018, 50, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Baugh, S.; Phillips, C.R.; Ekanayaka, A.S.; Piddock, L.J.V.; Webber, M.A. Inhibition of Multidrug Efflux as a Strategy to Prevent Biofilm Formation. J. Antimicrob. Chemother. 2014, 69, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Opperman, T.J.; Nguyen, S.T. Recent Advances toward a Molecular Mechanism of Efflux Pump Inhibition. Front. Microbiol. 2015, 6, 421. [Google Scholar] [CrossRef]
- Amaral, L.; Cerca, P.; Spengler, G.; Machado, L.; Martins, A.; Couto, I.; Viveiros, M.; Fanning, S.; Pagès, J.-M. Ethidium Bromide Efflux by Salmonella: Modulation by Metabolic Energy, PH, Ions and Phenothiazines. Int. J. Antimicrob. Agents 2011, 38, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Takács, D.; Cerca, P.; Martins, A.; Riedl, Z.; Hajós, G.; Molnár, J.; Viveiros, M.; Couto, I.; Amaral, L. Evaluation of Forty New Phenothiazine Derivatives for Activity Against Intrinsic Efflux Pump Systems of Reference Escherichia Coli, Salmonella Enteritidis, Enterococcus Faecalis and Staphylococcus Aureus Strains. In Vivo 2011, 25, 719–724. [Google Scholar] [PubMed]
- De Faria, P.A.; Bettanin, F.; Cunha, R.L.O.R.; Paredes-Gamero, E.J.; Homem-de-Mello, P.; Nantes, I.L.; Rodrigues, T. Cytotoxicity of Phenothiazine Derivatives Associated with Mitochondrial Dysfunction: A Structure-Activity Investigation. Toxicology 2015, 330, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Smarandache, A.; Kristiansen, J.B.; Christensen, J.; Pascu, M.-L. Optical Studies of the Spectral Properties of Phenothiazines. Lett. Drug Des. Discov. 2012, 9, 352–360. [Google Scholar] [CrossRef]
- Smarandache, A.; Simon, A.; Tozar, T.; Nastasa, V.; Pascu, M.L. Stability Studies on Promethazine Unexposed and Exposed to UV Laser Radiation. In Physical Chemistry of Interfaces and Nanomaterials XIV, Proceedings of SPIE Optics + Photonics, San Diego, CA, USA, 9–13 August 2015; Hayes, S.C., Bittner, E.R., Eds.; SPIE: Bellingham, WA, USA, 2015; p. 954916. [Google Scholar] [CrossRef]
- Farha, M.A.; Brown, E.D. Drug Repurposing for Antimicrobial Discovery. Nat. Microbiol. 2019, 4, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Gajdács, M.; Spengler, G. The Role of Drug Repurposing in the Development of Novel Antimicrobial Drugs: Non-Antibiotic Pharmacological Agents as Quorum Sensing-Inhibitors. Antibiotics 2019, 8, 270. [Google Scholar] [CrossRef]
- Kesselheim, A.S.; Tan, Y.T.; Avorn, J. The Roles of Academia, Rare Diseases, And Repurposing In The Development Of The Most Transformative Drugs. Health Aff. 2015, 34, 286–293. [Google Scholar] [CrossRef]
- Pascu, M.L. Laser Optofluidics in Fighting Multiple Drug Resistance; Bentham Science Publishers: Sharjah, United Arab Emirates, 2017; ISBN 978-1-68108-498-5. [Google Scholar]
- Tozar, T.; Costa, S.S.; Udrea, A.-M.; Nastasa, V.; Couto, I.; Viveiros, M.; Pascu, M.L.; Romanitan, M.O. Anti-Staphylococcal Activity and Mode of Action of Thioridazine Photoproducts. Sci. Rep. 2020, 10, 18043. [Google Scholar] [CrossRef] [PubMed]
- Tozar, T.; Pascu, M.L. Time Stability of Laser Exposed Phenothiazines Aqueous Solutions in View of Antimicrobial Research. Proc. Rom. Acad. Ser. A Math. Phys. 2018, 19, 537–544. [Google Scholar]
- Morán, M.C.; Tozar, T.; Simon, A.; Dinache, A.; Smarandache, A.; Andrei, I.R.; Boni, M.; Pascu, M.L.; Cirisano, F.; Ferrari, M. Toxicity Study in Blood and Tumor Cells of Laser Produced Medicines for Application in Fabrics. Colloids Surf. B Biointerfaces 2016, 137, 91–103. [Google Scholar] [CrossRef]
- Andrei, I.R.; Tozar, T.; Dinache, A.; Boni, M.; Nastasa, V.; Pascu, M.L. Chlorpromazine Transformation by Exposure to Ultraviolet Laser Beams in Droplet and Bulk. Eur. J. Pharm. Sci. 2016, 81, 27–35. [Google Scholar] [CrossRef]
- Pascu, M.L.; Danko, B.; Martins, A.; Jedlinszki, N.; Alexandru, T.; Nastasa, V.; Boni, M.; Militaru, A.; Andrei, I.R.; Staicu, A.; et al. Exposure of Chlorpromazine to 266 Nm Laser Beam Generates New Species with Antibacterial Properties: Contributions to Development of a New Process for Drug Discovery. PLoS ONE 2013, 8, e55767. [Google Scholar] [CrossRef]
- Tozar, T.; Nastasa, V.; Stoicu, A.; Chifiriuc, M.C.; Popa, M.; Kamerzan, C.; Pascu, M.L. In Vitro Antimicrobial Efficacy of Laser Exposed Chlorpromazine against Gram-Positive Bacteria in Planktonic and Biofilm Growth State. Microb. Pathog. 2019, 129, 250–256. [Google Scholar] [CrossRef]
- Nistorescu, S.; Pircalabioru, G.G.; Udrea, A.-M.; Simon, A.; Pascu, M.L.; Chifiriuc, M.-C. Laser-Irradiated Chlorpromazine as a Potent Anti-Biofilm Agent for Coating of Biomedical Devices. Coatings 2020, 10, 1230. [Google Scholar] [CrossRef]
- Velema, W.A.; van der Berg, J.P.; Hansen, M.J.; Szymanski, W.; Driessen, A.J.M.; Feringa, B.L. Optical Control of Antibacterial Activity. Nat. Chem. 2013, 5, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Babalhavaeji, A.; Samanta, S.; Beharry, A.A.; Woolley, G.A. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc. Chem. Res. 2015, 48, 2662–2670. [Google Scholar] [CrossRef] [PubMed]
- Mayer, G.; Heckel, A. Biologically Active Molecules with a “Light Switch”. Angew. Chem. Int. Ed. Engl. 2006, 45, 4900–4921. [Google Scholar] [CrossRef]
- Weinstain, R.; Slanina, T.; Kand, D.; Klán, P. Visible-to-NIR-Light Activated Release: From Small Molecules to Nanomaterials. Chem. Rev. 2020, 120, 13135–13272. [Google Scholar] [CrossRef] [PubMed]
- Chaguza, C. Bacterial Survival: Evolve and Adapt or Perish. Nat. Rev. Microbiol. 2020, 18, 5. [Google Scholar] [CrossRef] [PubMed]
- Zhukov-Verezhnikov, N.N.; Mayskiy, I.N.; Yazdovskii, V.I.; Pekhov, A.P.; Gyurdzhian, A.A.; Nefed’eva, N.P.; Kapichnikov, M.M.; Podoplelov, I.I.; Rybakov, N.I.; Klemparskaya, N.N.; et al. Results of First Microbiological and Cytological Experiments on Earth Satellites. Artif. Earth Satell. 1962, 11, 47–71. [Google Scholar]
- Saunders, J.F. The Experiments of Biosatellite II. NASA SP-204; NASA: Washington, DC, USA, 1971.
- Taylor, G.R.; Graves, R.C.; Kelton Ferguson, J.; Brockett, R.M.; Mieszkuc, B.J. SKYLAB Environmental and Crew Microbiology Studies. Biomed. Results Skylab 1977, 377, 53. [Google Scholar]
- Planel, H.; Tixador, R.; Nefedov, I.G.; Gretchko, G.; Richoilley, G. Preliminary Results of Cytos Experiment Flown in Salyut VI: Investigations on Paramecium Aurelia. Life Sci. Space Res. 1979, 17, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Planel, H.; Tixador, R.; Nefedov, Y.; Gretchko, G.; Richoilley, G. Effects of Space Flight Factors at the Cellular Level: Results of the Cytos Experiment. Aviat. Space Environ. Med. 1982, 53, 370–374. [Google Scholar] [PubMed]
- Benoit, M.R.; Li, W.; Stodieck, L.S.; Lam, K.S.; Winther, C.L.; Roane, T.M.; Klaus, D.M. Microbial Antibiotic Production Aboard the International Space Station. Appl. Microbiol. Biotechnol. 2006, 70, 403–411. [Google Scholar] [CrossRef]
- Pollard, E.C. Theoretical Studies on Living Systems in the Absence of Mechanical Stress. J. Theor. Biol. 1965, 8, 113–123. [Google Scholar] [CrossRef]
- Benoit, M.R.; Klaus, D.M. Microgravity, Bacteria, and the Influence of Motility. Adv. Space Res. 2007, 39, 1225–1232. [Google Scholar] [CrossRef]
- Su, L.; Chang, D.; Liu, C. The Development of Space Microbiology in the Future: The Value and Significance of Space Microbiology Research. Future Microbiol. 2013, 8, 5–8. [Google Scholar] [CrossRef]
- Lam, K.S.; Mamber, S.W.; Pack, E.J.; Forenza, S.; Fernandes, P.B.; Klaus, D.M. The Effects of Space Flight on the Production of Monorden by Humicola Fuscoatra WC5157 in Solid-State Fermentation. Appl. Microbiol. Biotechnol. 1998, 49, 579–583. [Google Scholar] [CrossRef]
- Lam, K.S.; Gustavson, D.R.; Pirnik, D.L.; Pack, E.; Bulanhagui, C.; Mamber, S.W.; Forenza, S.; Stodieck, L.S.; Klaus, D.M. The Effect of Space Flight on the Production of Actinomycin D by Streptomyces Plicatus. J. Ind. Microbiol. Biotechnol. 2002, 29, 299–302. [Google Scholar] [CrossRef]
- Zea, L.; Stodieck, L.; Klaus, D.M. The First Fifty Years of Bacterial Growth and Antibiotic Effectiveness Research in Space. Poster Presentation, American Society for Gravitational and Space Research (ASGSR) Conference, Pasadena, CA, USA, 22–26 October 2014. [Google Scholar]
- Tixador, R.; Richoilley, G.; Gasset, G.; Planel, H.; Moatti, N.; Lapchine, L.; Enjalbert, L.; Raffin, J.; Bost, R.; Zaloguev, S.N.; et al. Preliminary Results of Cytos 2 Experiment. Acta Astronaut. 1985, 12, 131–134. [Google Scholar] [CrossRef]
- Moatti, N.; Lapchine, L.; Gasset, G.; Richoilley, G.; Templier, J.; Tixador, R. Preliminary Results of the “Antibio” Experiment. Naturwissenschaften 1986, 73, 413–414. [Google Scholar] [CrossRef]
- Tixador, R.; Gasset, G.; Eche, B.; Moatti, N.; Lapchine, L.; Woldringh, C.; Toorop, P.; Moatti, J.P.; Delmotte, F.; Tap, G. Behavior of Bacteria and Antibiotics under Space Conditions. Aviat. Space Environ. Med. 1994, 65, 551–556. [Google Scholar]
- Lapchine, L.; Moatti, N.; Gasset, G.; Richoilley, G.; Templier, J.; Tixador, R. Antibiotic Activity in Space. Drugs Exp. Clin. Res. 1986, 12, 933–938. [Google Scholar]
- Aunins, T.R.; Erickson, K.E.; Prasad, N.; Levy, S.E.; Jones, A.; Shrestha, S.; Mastracchio, R.; Stodieck, L.; Klaus, D.; Zea, L.; et al. Spaceflight Modifies Escherichia Coli Gene Expression in Response to Antibiotic Exposure and Reveals Role of Oxidative Stress Response. Front. Microbiol. 2018, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Tixador, R.; Richoilley, G.; Gasset, G.; Templier, J.; Bes, J.C.; Moatti, N.; Lapchine, L. Study of Minimal Inhibitory Concentration of Antibiotics on Bacteria Cultivated in Vitro in Space (Cytos 2 Experiment). Aviat. Space Environ. Med. 1985, 56, 748–751. [Google Scholar] [PubMed]
- Sieradzki, K.; Tomasz, A. Alterations of Cell Wall Structure and Metabolism Accompany Reduced Susceptibility to Vancomycin in an Isogenic Series of Clinical Isolates of Staphylococcus Aureus. J. Bacteriol. 2003, 185, 7103–7110. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.W.; Ott, C.M.; zu Bentrup, K.H.; Ramamurthy, R.; Quick, L.; Porwollik, S.; Cheng, P.; McClelland, M.; Tsaprailis, G.; Radabaugh, T.; et al. Space Flight Alters Bacterial Gene Expression and Virulence and Reveals a Role for Global Regulator Hfq. Proc. Natl. Acad. Sci. USA 2007, 104, 16299–16304. [Google Scholar] [CrossRef] [PubMed]
- Crabbé, A.; Schurr, M.J.; Monsieurs, P.; Morici, L.; Schurr, J.; Wilson, J.W.; Ott, C.M.; Tsaprailis, G.; Pierson, D.L.; Stefanyshyn-Piper, H.; et al. Transcriptional and Proteomic Responses of Pseudomonas Aeruginosa PAO1 to Spaceflight Conditions Involve Hfq Regulation and Reveal a Role for Oxygen. Appl. Environ. Microbiol. 2011, 77, 1221–1230. [Google Scholar] [CrossRef]
- Zea, L.; Prasad, N.; Levy, S.E.; Stodieck, L.; Jones, A.; Shrestha, S.; Klaus, D. A Molecular Genetic Basis Explaining Altered Bacterial Behavior in Space. PLoS ONE 2016, 11, e0164359. [Google Scholar] [CrossRef]
- Zea, L.; Larsen, M.; Estante, F.; Qvortrup, K.; Moeller, R.; Dias de Oliveira, S.; Stodieck, L.; Klaus, D. Phenotypic Changes Exhibited by E. Coli Cultured in Space. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Deguchi, S.; Shimoshige, H.; Tsudome, M.; Mukai, S.; Corkery, R.W.; Ito, S.; Horikoshi, K. Microbial Growth at Hyperaccelerations up to 403,627 x g. Proc. Natl. Acad. Sci. USA 2011, 108, 7997–8002. [Google Scholar] [CrossRef] [PubMed]
- Mastrapa, R.M.E.; Glanzberg, H.; Head, J.N.; Melosh, H.J.; Nicholson, W.L. Survival of Bacteria Exposed to Extreme Acceleration: Implications for Panspermia. Earth Planet. Sci. Lett. 2001, 189, 1–8. [Google Scholar] [CrossRef]
- Benardini, J.N.; Sawyer, J.; Venkateswaran, K.; Nicholson, W.L. Spore UV and Acceleration Resistance of Endolithic Bacillus Pumilus and Bacillus Subtilis Isolates Obtained from Sonoran Desert Basalt: Implications for Lithopanspermia. Astrobiology 2003, 3, 709–717. [Google Scholar] [CrossRef]
- Yoshida, N.; Minamimura, T.; Yoshida, T.; Ogawa, K. Effect of Hypergravitational Stress on Microbial Cell Viability. J. Biosci. Bioeng. 1999, 88, 342–344. [Google Scholar] [CrossRef]
- Zea, L.; Nisar, Z.; Rubin, P.; Cortesão, M.; Luo, J.; McBride, S.A.; Moeller, R.; Klaus, D.; Müller, D.; Varanasi, K.K.; et al. Design of a Spaceflight Biofilm Experiment. Acta Astronaut. 2018, 148, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Pyle, B.H.; McFeters, G.A.; Broadaway, S.C.; Johnsrud, C.K.; Storfa, R.T.; Borkowski, J. Bacterial Growth on Surfaces and in Suspensions. In Biorack on Spacehab. Biological Experiments on Three Shuttle-to-Mir Missions; SP-1222; European Space Agency: Paris, France, 1999; pp. 95–102. [Google Scholar]
- McLean, R.J.C.; Cassanto, J.M.; Barnes, M.B.; Koo, J.H. Bacterial Biofilm Formation under Microgravity Conditions. FEMS Microbiol. Lett. 2001, 195, 115–119. [Google Scholar] [CrossRef]
- Kim, W.; Tengra, F.K.; Young, Z.; Shong, J.; Marchand, N.; Chan, H.K.; Pangule, R.C.; Parra, M.; Dordick, J.S.; Plawsky, J.L.; et al. Spaceflight Promotes Biofilm Formation by Pseudomonas Aeruginosa. PLoS ONE 2013, 8, e62437. [Google Scholar] [CrossRef] [PubMed]
- Vaishampayan, A.; Grohmann, E. Multi-Resistant Biofilm-Forming Pathogens on the International Space Station. J. Biosci. 2019, 44, 125. [Google Scholar] [CrossRef] [PubMed]
- Zea, L.; McLean, R.J.C.; Rook, T.A.; Angle, G.; Carter, D.L.; Delegard, A.; Denvir, A.; Gerlach, R.; Gorti, S.; McIlwaine, D.; et al. Potential Biofilm Control Strategies for Extended Spaceflight Missions. Biofilm 2020, 2, 100026. [Google Scholar] [CrossRef]
- Novikova, N.D. Review of the Knowledge of Microbial Contamination of the Russian Manned Spacecraft. Microb. Ecol. 2004, 47, 127–132. [Google Scholar] [CrossRef]
- Song, B.; Leff, L.G. Identification and Characterization of Bacterial Isolates from the Mir Space Station. Microbiol. Res. 2005, 160, 111–117. [Google Scholar] [CrossRef]
- Senatore, G.; Mastroleo, F.; Leys, N.; Mauriello, G. Effect of Microgravity & Space Radiation on Microbes. Future Microbiol. 2018, 13, 831–847. [Google Scholar] [CrossRef]
- Fukuda, T.; Fukuda, K.; Takahashi, A.; Ohnishi, T.; Nakano, T.; Sato, M.; Gunge, N. Analysis of Deletion Mutations of the RpsL Gene in the Yeast Saccharomyces Cerevisiae Detected after Long-Term Flight on the Russian Space Station Mir. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2000, 470, 125–132. [Google Scholar] [CrossRef]
- Horneck, G.; Klaus, D.M.; Mancinelli, R.L. Space Microbiology. Microbiol. Mol. Biol. Rev. 2010, 74, 121–156. [Google Scholar] [CrossRef] [PubMed]
- Cucinotta, F.A.; Durante, M. Cancer Risk from Exposure to Galactic Cosmic Rays: Implications for Space Exploration by Human Beings. Lancet Oncol. 2006, 7, 431–435. [Google Scholar] [CrossRef]
- Mehta, P.; Bhayani, D. Impact of Space Environment on Stability of Medicines: Challenges and Prospects. J. Pharm. Biomed. Anal. 2017, 136, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Chancellor, J.C.; Blue, R.S.; Cengel, K.A.; Auñón-Chancellor, S.M.; Rubins, K.H.; Katzgraber, H.G.; Kennedy, A.R. Limitations in Predicting the Space Radiation Health Risk for Exploration Astronauts. Npj Microgravity 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Blue, R.S.; Chancellor, J.C.; Antonsen, E.L.; Bayuse, T.M.; Daniels, V.R.; Wotring, V.E. Limitations in Predicting Radiation-Induced Pharmaceutical Instability during Long-Duration Spaceflight. Npj Microgravity 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Norbury, J.W.; Slaba, T.C.; Aghara, S.; Badavi, F.F.; Blattnig, S.R.; Clowdsley, M.S.; Heilbronn, L.H.; Lee, K.; Maung, K.M.; Mertens, C.J.; et al. Advances in Space Radiation Physics and Transport at NASA. Life Sci. Space Res. 2019, 22, 98–124. [Google Scholar] [CrossRef]
- National Council on Radiation Protection and Measurements (NCRP). Guidance on Radiation Received in Space Activities; NCRP: Bethesda, MD, USA, 1989; Technical Report 98. [Google Scholar]
- National Council on Radiation Protection and Measurements (NCRP). Radiation Protection for Space Activities: Supplement to Previous Recommendations; Technical Report Commentary 23; NCRP: Bethesda, MD, USA, 2014. [Google Scholar]
- Abuhanoğlu, G.; Özer, A.Y. Radiation Sterilization of New Drug Delivery Systems. Interv. Med. Appl. Sci. 2014, 6, 51–60. [Google Scholar] [CrossRef]
- Domańska, I.M.; Oledzka, E.; Sobczak, M. Sterilization Process of Polyester Based Anticancer-Drug Delivery Systems. Int. J. Pharm. 2020, 587, 119663. [Google Scholar] [CrossRef] [PubMed]
- Smarandache, A.; Moeller, R.; Pascu, M.L. UV-Vis and FTIR Spectroscopic Investigations of Gamma-Ray Irradiated Antibiotics. Rom. Rep. Phys. 2018, 70, 602. [Google Scholar]
- Kim, M.-H.; Plante, I. An Assessment of How Radiation Incurred During a Mars Mission Could Affect Food and Pharmaceuticals; Wyle Science, Technology, and Engineering Group—NASA: Houston, TX, USA, 2015. [Google Scholar]
- Putcha, L.; Berens, K.L.; Marshburn, T.H.; Ortega, H.J.; Billica, R.D. Pharmaceutical Use by U.S. Astronauts on Space Shuttle Missions. Aviat. Space Environ. Med. 1999, 70, 705–708. [Google Scholar]
- Wotring, V.E. Medication Use by U.S. Crewmembers on the International Space Station. FASEB J. 2015, 29, 4417–4423. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Daniels, V.R.; Vaksman, Z.; Boyd, J.L.; Crady, C.; Putcha, L. Evaluation of Physical and Chemical Changes in Pharmaceuticals Flown on Space Missions. AAPS J. 2011, 13, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Wotring, V.E. Space Pharmacology; Springer Briefs in Space Development; Springer: New York, NY, USA, 2012; ISBN 978-1-4614-3395-8. [Google Scholar]
- Marciniec, B.; Stawny, M.; Olszewski, K.; Kozak, M.; Naskrent, M. Analytical Study on Irradiated Methylxanthine Derivatives. J. Anal. Calorim. 2013, 111, 2165–2170. [Google Scholar] [CrossRef][Green Version]
- Maquille, A.; Jiwan, J.-L.H.; Tilquin, B. Radiosterilization of Drugs in Aqueous Solutions May Be Achieved by the Use of Radioprotective Excipients. Int. J. Pharm. 2008, 349, 74–82. [Google Scholar] [CrossRef]
- Marciniec, B.; Ogrodowczyk, M.; Czajka, B.; Hofman, M. The Influence of Radiation Sterilisation on Some β-Blockers in the Solid State. Thermochim. Acta 2011, 514, 10–15. [Google Scholar] [CrossRef]
- Hasanain, F.; Guenther, K.; Mullett, W.M.; Craven, E. Gamma Sterilization of Pharmaceuticals—A Review of the Irradiation of Excipients, Active Pharmaceutical Ingredients, and Final Drug Product Formulations. PDA J. Pharm. Sci. Technol. 2014, 68, 113–137. [Google Scholar] [CrossRef]
- Wotring, V.E. Chemical Potency and Degradation Products of Medications Stored Over 550 Earth Days at the International Space Station. AAPS J. 2016, 18, 210–216. [Google Scholar] [CrossRef]
- Chuong, M.C.; Prasad, D.; Leduc, B.; Du, B.; Putcha, L. Stability of Vitamin B Complex in Multivitamin and Multimineral Supplement Tablets after Space Flight. J. Pharm. Biomed. Anal. 2011, 55, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Zwart, S.R.; Kloeris, V.L.; Perchonok, M.H.; Braby, L.; Smith, S.M. Assessment of Nutrient Stability in Foods from the Space Food System After Long-Duration Spaceflight on the ISS. J. Food Sci. 2009, 74, H209–H217. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, J.J.W.A.; Krause, J.; Cunha, H.; Goncalves, J.; Almeida, H.; Schiller, P. The Large Diameter Centrifuge, LDC, for Life and Physical Sciences and Technology. In Proceedings of the Life in Space for Life on Earth Symposium, Angers, France, 22–27 June 2008; ESA: Paris, France, 2008. ESA SP-663. [Google Scholar]
- Simon, Á.; Smarandache, A.; Tozar, T.; Andrei, I.R.; Stoicu, A.; van Loon, J.J.W.A.; Dowson, A.; Pascu, M.L. Photoactive Chlorpromazine and Promazine Drugs Exposed to Hypergravity Conditions after Interaction with UV Laser Radiation. Sent Publ. (Under Rev.) 2021. [Google Scholar]
| Drug | Formulation | Spaceflight Duration | Stability Criterion | Study |
|---|---|---|---|---|
| acetaminophen *,** aspirin ibuprofen loperamide *** loratadine *,**,*** melatonin * modafinil *** pseudoephedrine zolpidem *** | solid | 550 days | API content degradation products | Wotring [163] |
| acyclovir * amoxicillin/clavulanate * atorvastatin azithromycin cefadroxil ciprofloxacin * clotrimazole * cobalamine dextroamphetamine * epinephrine * fluconazole * furosemide * ibuprofen imipenem/cilastatin * levofloxacin * levothyroxine * lidocaine * metoprolol succinate * metronidazole mupirocin * phenytoin * progestin/estrogen * promethazine * risedronate * sertraline * silver sulfadiazine * sulfamethoxazole/trimethoprim * temazepam * triamcinolone * | solid semi-solid liquid | 14 days 353 days 596 days 880 days | API content physical propertieschemical properties | Du et al. [157] |
| Centrum Silver® multivitamin Once A Day® women’s multivitamin * | solid | 14–20 days 12–19 months | API content (B complex only) | Chuong et al. [164] |
| Centrum Silver® multivitamin **** Nature’s Way® vitamin D supplement **** | solid | 13 days 353 days 596 days 880 days | API content | Zwart et al. [165] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, Á.; Smarandache, A.; Iancu, V.; Pascu, M.L. Stability of Antimicrobial Drug Molecules in Different Gravitational and Radiation Conditions in View of Applications during Outer Space Missions. Molecules 2021, 26, 2221. https://doi.org/10.3390/molecules26082221
Simon Á, Smarandache A, Iancu V, Pascu ML. Stability of Antimicrobial Drug Molecules in Different Gravitational and Radiation Conditions in View of Applications during Outer Space Missions. Molecules. 2021; 26(8):2221. https://doi.org/10.3390/molecules26082221
Chicago/Turabian StyleSimon, Ágota, Adriana Smarandache, Vicentiu Iancu, and Mihail Lucian Pascu. 2021. "Stability of Antimicrobial Drug Molecules in Different Gravitational and Radiation Conditions in View of Applications during Outer Space Missions" Molecules 26, no. 8: 2221. https://doi.org/10.3390/molecules26082221
APA StyleSimon, Á., Smarandache, A., Iancu, V., & Pascu, M. L. (2021). Stability of Antimicrobial Drug Molecules in Different Gravitational and Radiation Conditions in View of Applications during Outer Space Missions. Molecules, 26(8), 2221. https://doi.org/10.3390/molecules26082221









