Therapeutic Effects of Decursin and Angelica gigas Nakai Root Extract in Gerbil Brain after Transient Ischemia via Protecting BBB Leakage and Astrocyte Endfeet Damage
Abstract
1. Introduction
2. Results
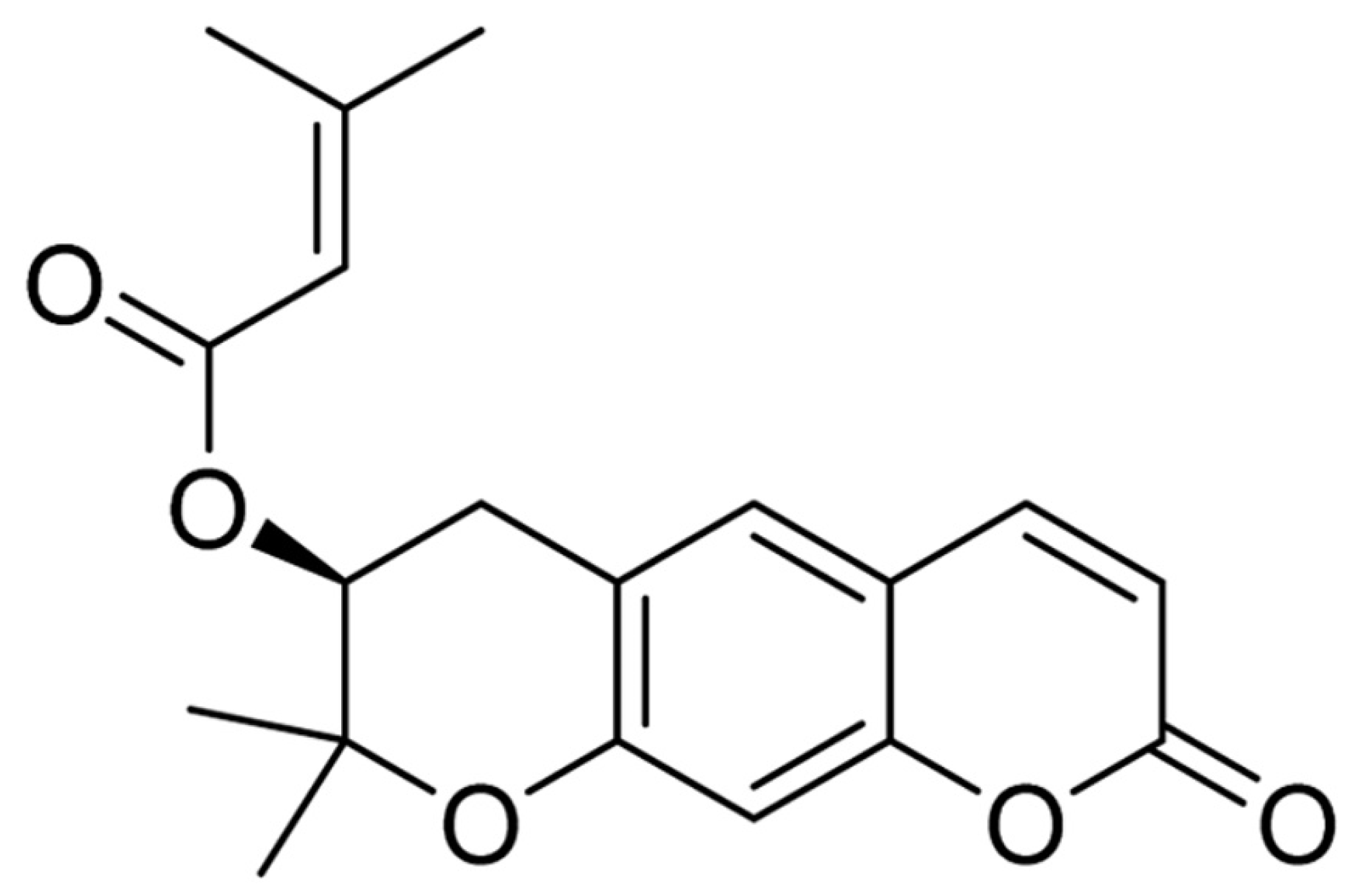
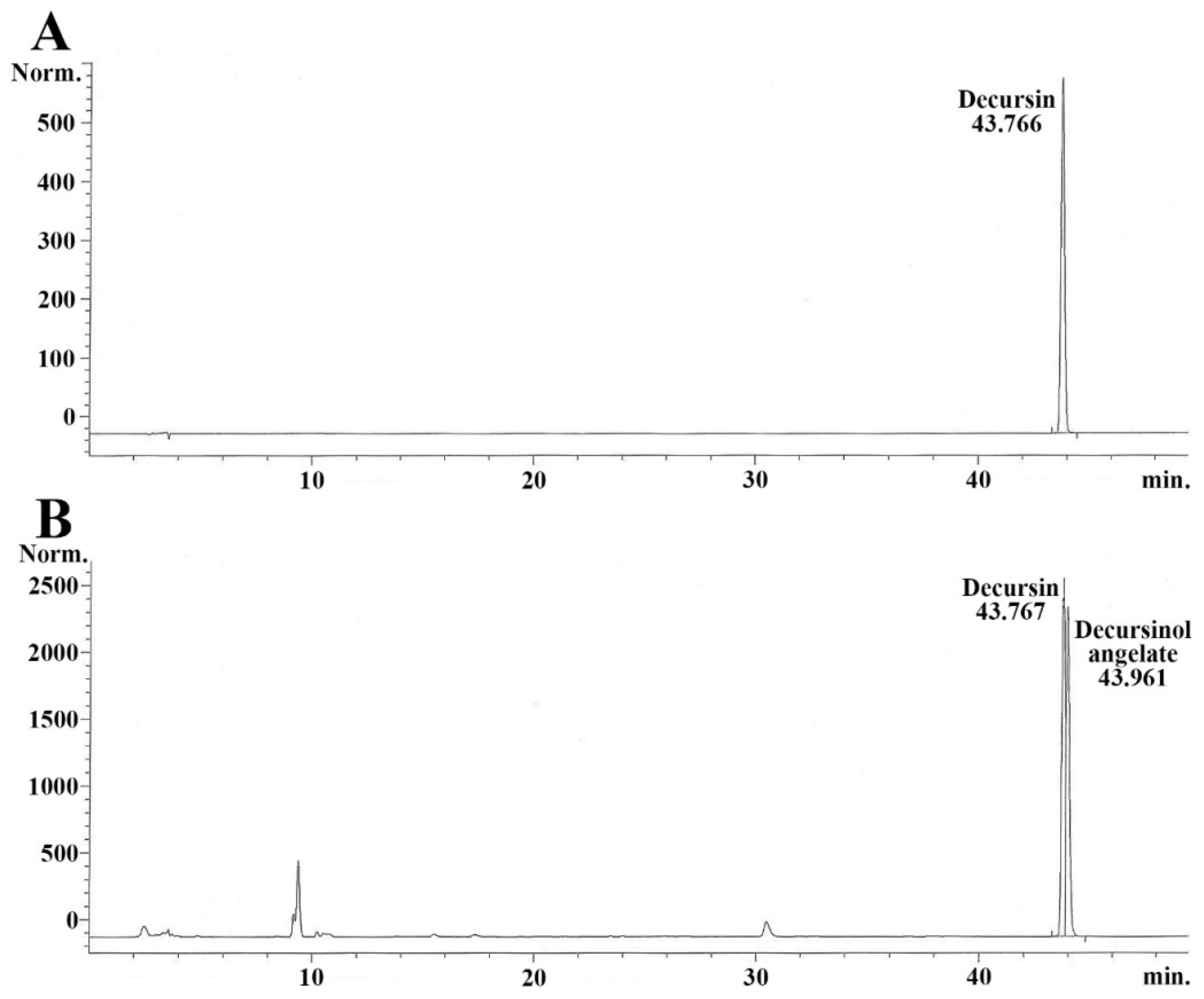
2.1. Quantity of Decursin in AGNE
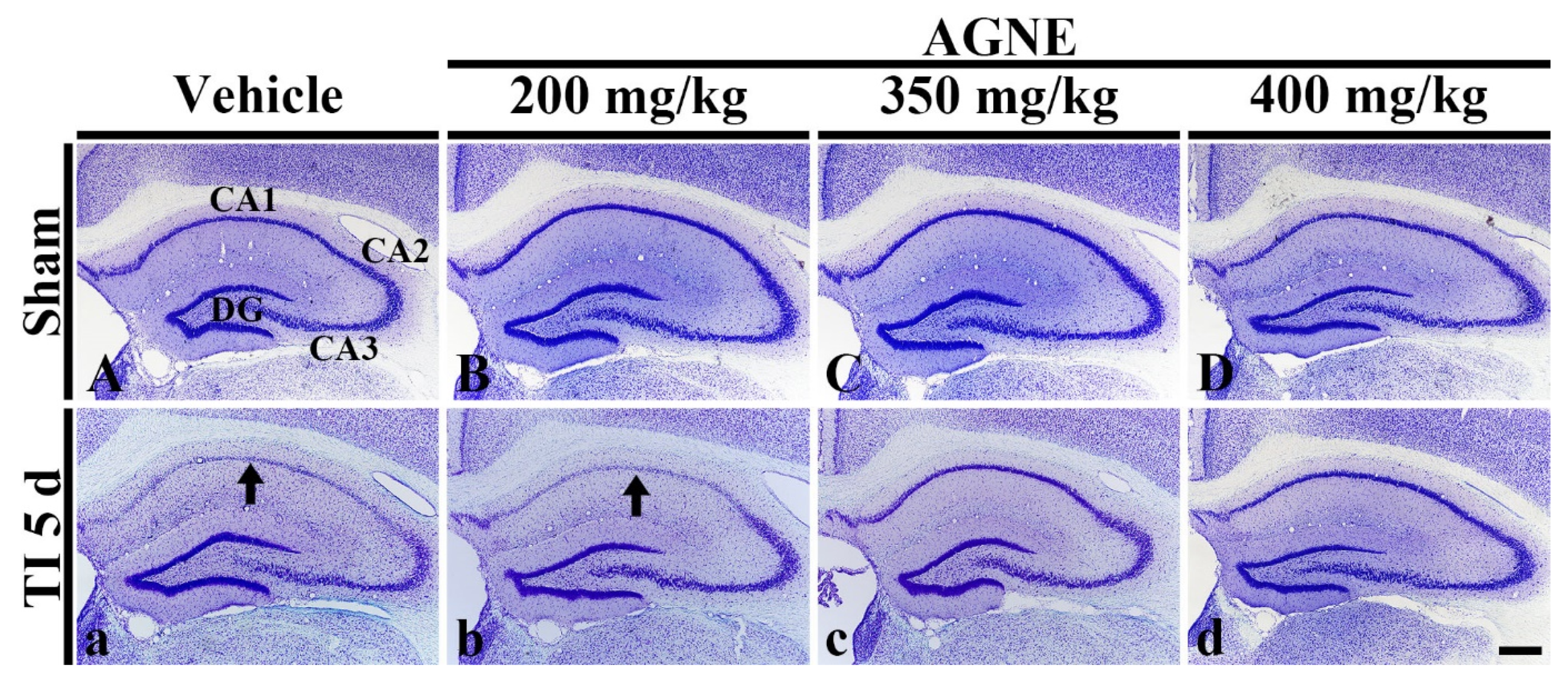
2.2. Dosage Determination of AGNE and Decursin for Neuroprotection against TI
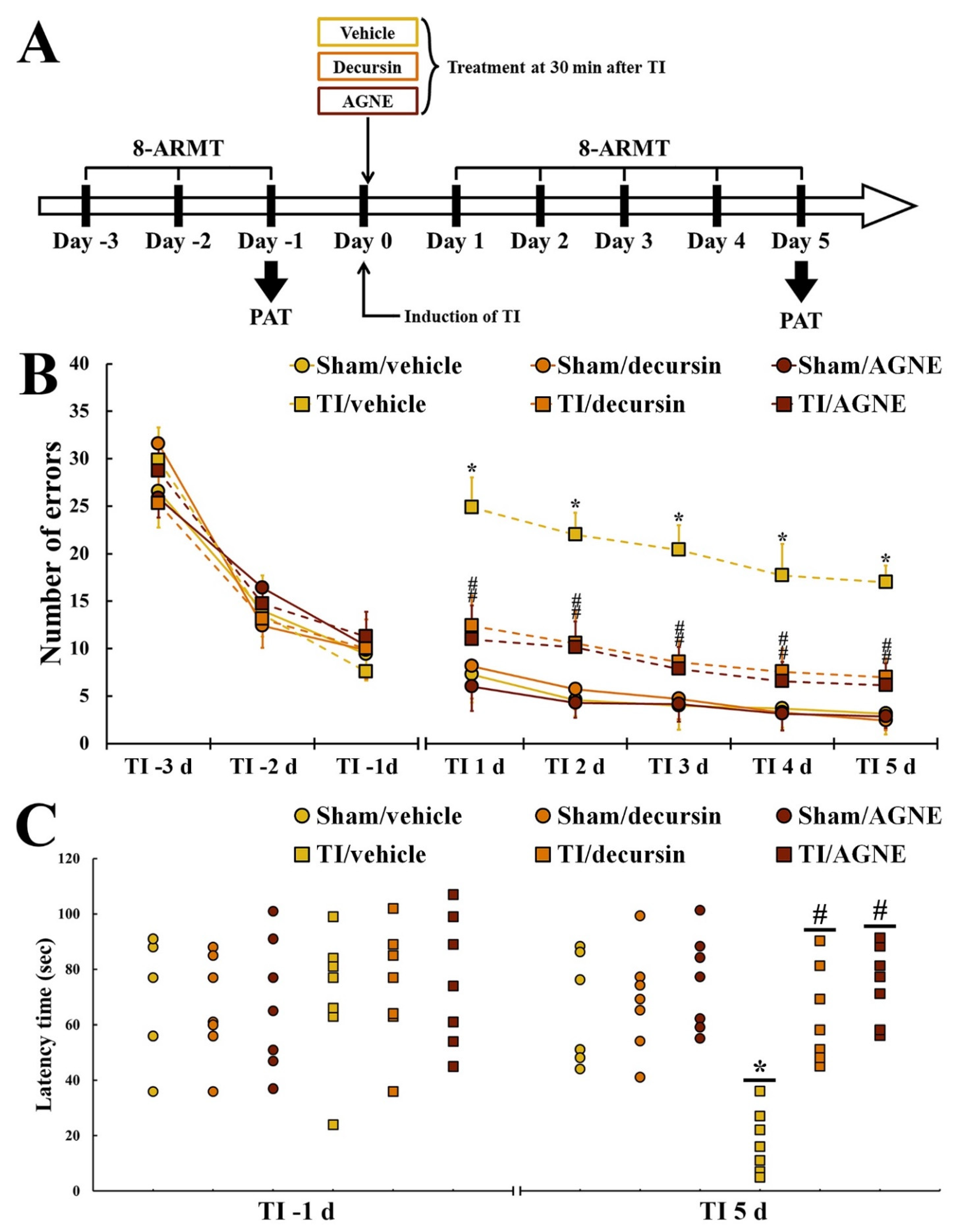
2.3. Attenuation of TI-Induced Cognitive Deficits by Decursin and AGNE
2.3.1. Spatial Memory
2.3.2. Learning Memory
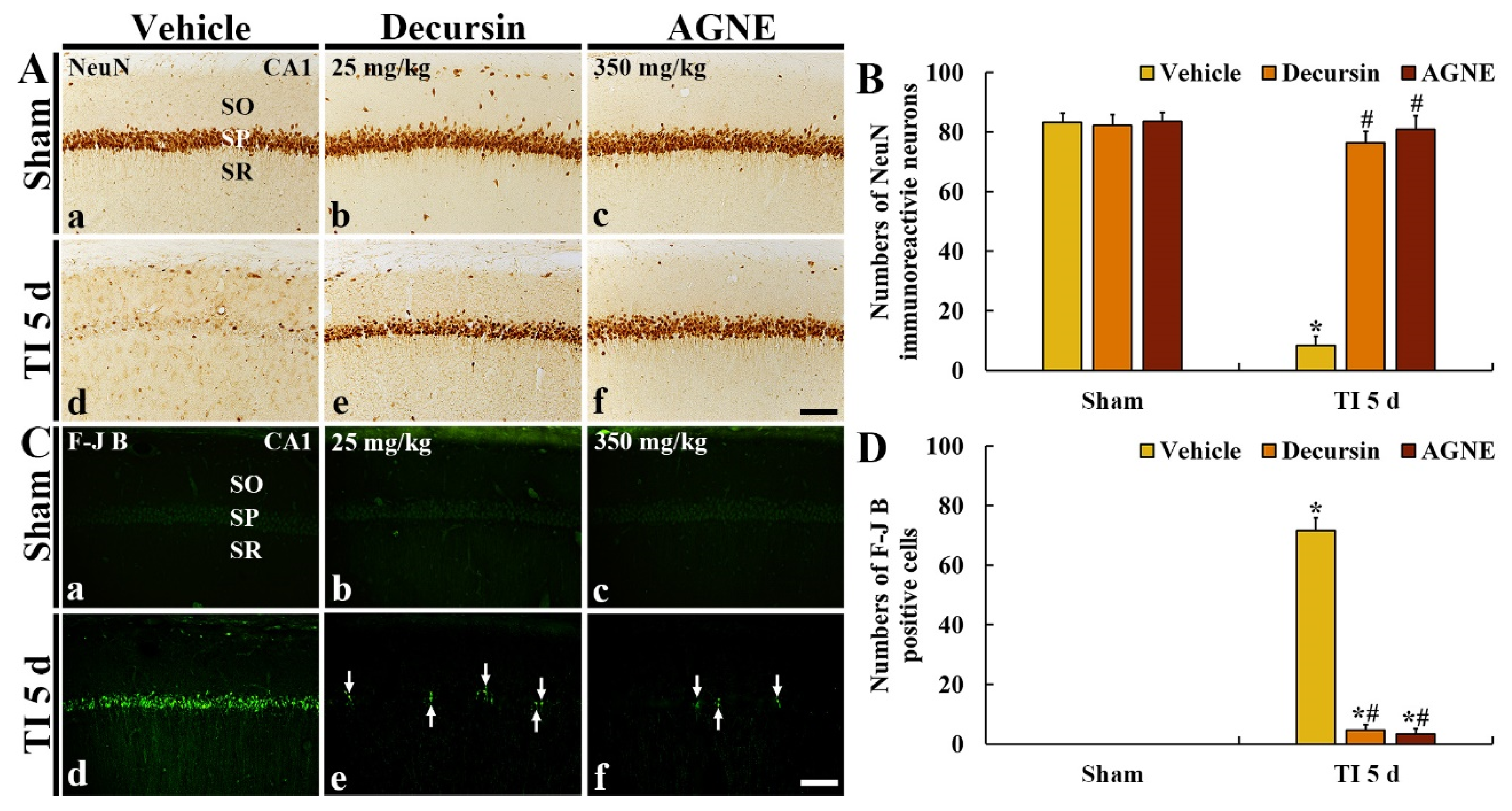
2.4. Neuroprotection by Decursin and AGNE
2.4.1. NeuN Immunoreactive Neurons
2.4.2. F-J B Positive Cells
2.5. Attenuation of TI-Induced BBB Leakage (IgG) by Decursin and AGNE
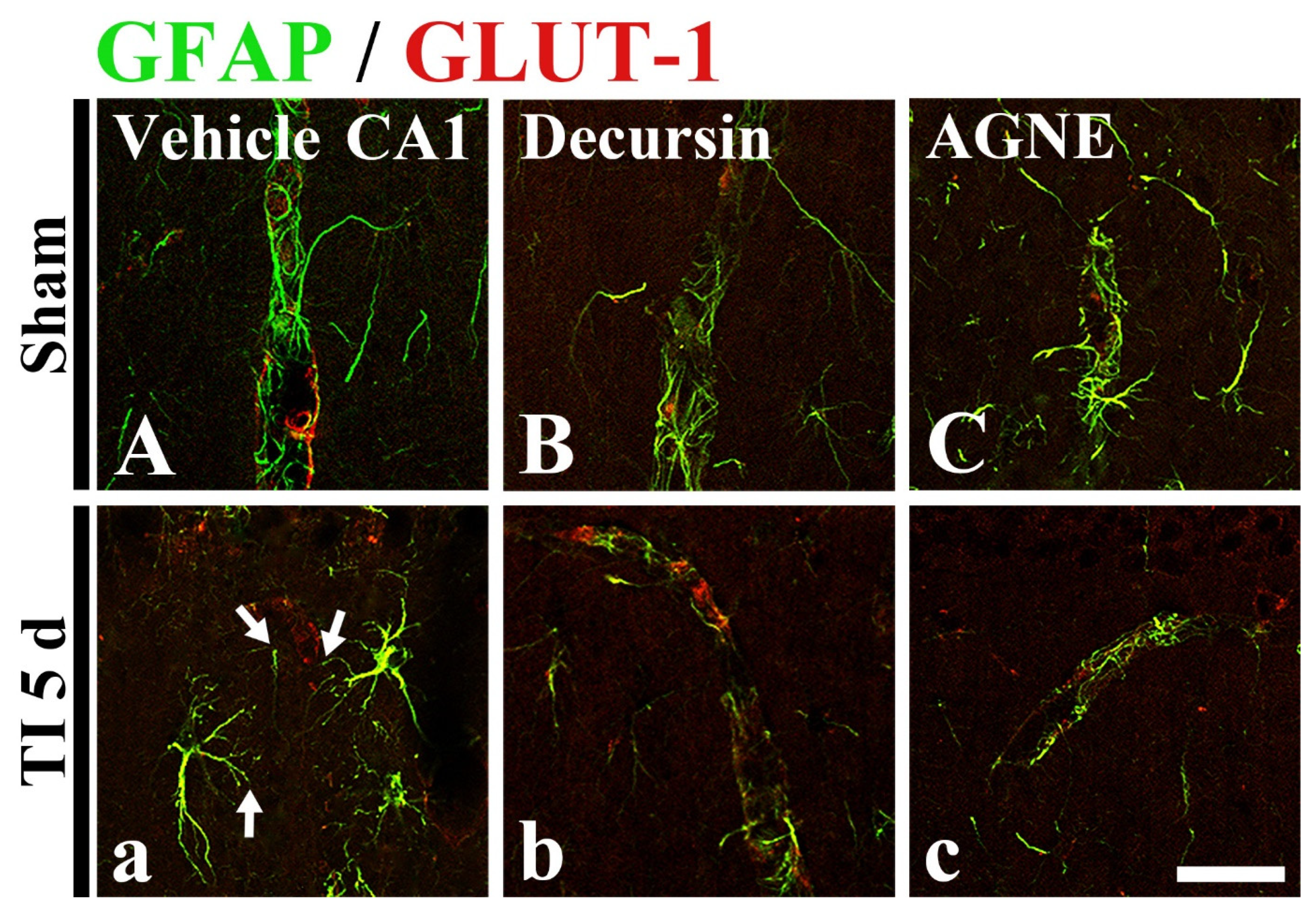
2.6. Attenuation of TI-Induced Astrocyte Endfeet (AEF) Damage by Decursin and AGNE
3. Discussion
4. Materials and Methods
4.1. Animals and Protocol Used in This Experiment
4.2. Experimental Groups
4.3. Decursin Preparation, and AGNE Extraction and Its Qualitative Analysis
4.4. Induction of TI
4.5. Post-Treatment with Decursin and AGNE
4.6. Tests for Cognitive Function
4.6.1. 8-ARMT
4.6.2. PAT
4.7. Preparation of Histological Sections
4.8. CV Histochemistry
4.9. F-J B Histofluorescence
4.10. Immunohistochemistry
4.11. Double Immunohistofluorescence
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 8-ARMT | 8-arm radial maze test |
| AEF | astrocyte endfeet |
| AGNE | Angelica gigas Nakai root extract |
| BBB | blood–brain barrier |
| CA | cornu ammonis |
| CCA | common carotid artery |
| CNS | central nervous system |
| CV | cresyl violet |
| DND | delayed neuronal death |
| F-J B | fluoro-Jade B |
| GFAP | glial fibrillary acidic protein |
| GLUT-1 | glucose transporter 1 |
| HPLC | high-performance liquid chromatography |
| IgG | immunoglobulin G |
| NeuN | neuronal nuclei-specific protein |
| PAT | passive avoidance test |
| ROS | reactive oxygen species |
| TI | transient ischemia |
References
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the american heart association/american stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; and the interdisciplinary council on peripheral vascular disease. The American academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009, 40, 2276–2293. [Google Scholar]
- Kirino, T.; Sano, K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984, 62, 201–208. [Google Scholar] [CrossRef]
- Lee, J.C.; Ahn, J.H.; Lee, D.H.; Yan, B.C.; Park, J.H.; Kim, I.H.; Cho, G.S.; Kim, Y.M.; Lee, B.; Park, C.W.; et al. Neuronal damage and gliosis in the somatosensory cortex induced by various durations of transient cerebral ischemia in gerbils. Brain Res. 2013, 1510, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kim, H.; Song, M.; Lee, J.C.; Park, J.H.; Ahn, J.H.; Yang, G.E.; Kim, H.; Ohk, T.G.; Shin, M.C.; et al. Time-course pattern of neuronal loss and gliosis in gerbil hippocampi following mild, severe, or lethal transient global cerebral ischemia. Neural Regen. Res. 2019, 14, 1394–1403. [Google Scholar]
- Park, J.H.; Cho, J.H.; Ahn, J.H.; Choi, S.Y.; Lee, T.K.; Lee, J.C.; Shin, B.N.; Hong, S.; Jeon, Y.H.; Kim, Y.M.; et al. Neuronal loss and gliosis in the rat striatum subjected to 15 and 30 minutes of middle cerebral artery occlusion. Metab. Brain Dis. 2018, 33, 775–784. [Google Scholar] [CrossRef]
- Kirino, T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982, 239, 57–69. [Google Scholar] [CrossRef]
- Ahn, J.H.; Park, J.H.; Park, J.; Shin, M.C.; Cho, J.H.; Kim, I.H.; Cho, J.H.; Lee, T.K.; Lee, J.C.; Shin, B.N.; et al. Long-term treadmill exercise improves memory impairment through restoration of decreased synaptic adhesion molecule 1/2/3 induced by transient cerebral ischemia in the aged gerbil hippocampus. Exp. Gerontol. 2018, 103, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Kang, I.J.; Kim, B.; Sim, H.J.; Kim, D.W.; Ahn, J.H.; Lee, J.C.; Ryoo, S.; Shin, M.C.; Cho, J.H.; et al. Experimental pretreatment with chlorogenic acid prevents transient ischemia-induced cognitive decline and neuronal damage in the hippocampus through anti-oxidative and anti-inflammatory effects. Molecules 2020, 25, 3578. [Google Scholar] [CrossRef]
- Walker, E.J.; Rosenberg, G.A. Timp-3 and mmp-3 contribute to delayed inflammation and hippocampal neuronal death following global ischemia. Exp. Neurol. 2009, 216, 122–131. [Google Scholar] [CrossRef]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef]
- Lee, J.-C.; Won, M.-H. Neuroprotection of antioxidant enzymes against transient global cerebral ischemia in gerbils. Anat. Cell Biol. 2014, 47, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, Y.H.; Ahn, J.H.; Choi, S.Y.; Hong, S.; Kim, S.K.; Kang, I.J.; Kim, Y.-M.; Lee, T.-K.; Won, M.-H. Atomoxetine protects against nmda receptor-mediated hippocampal neuronal death following transient global cerebral ischemia. Curr. Neurovasc. Res. 2017, 14, 158–168. [Google Scholar] [CrossRef]
- Abdullahi, W.; Tripathi, D.; Ronaldson, P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Phys. Cell Physiol. 2018, 315, C343–C356. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Ok, S.; Oh, S.R.; Jung, T.S.; Jeon, S.O.; Jung, J.W.; Ryu, D.S. Effects of angelica gigas nakai as an anti-inflammatory agent in in vitro and in vivo atopic dermatitis models. Evid. Based Complement. Altern. Med. 2018, 2018, 2450712. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Jung, J.M.; Lee, H.E.; Lee, Y.W.; Kim, D.H.; Kim, J.M.; Hong, J.G.; Lee, C.H.; Jung, I.H.; Cho, Y.B.; et al. The memory ameliorating effects of inm-176, an ethanolic extract of angelica gigas, against scopolamine- or abeta(1-42)-induced cognitive dysfunction in mice. J. Ethnopharmacol. 2012, 143, 611–620. [Google Scholar] [CrossRef]
- Choi, Y.E.; Ahn, H.; Ryu, J.H. Polyacetylenes from angelica gigas and their inhibitory activity on nitric oxide synthesis in activated macrophages. Biol. Pharm. Bull. 2000, 23, 884–886. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Kim, S. Neuroprotective and cognitive enhancement potentials of angelica gigas nakai root: A review. Sci. Pharm. 2017, 85, 21. [Google Scholar] [CrossRef]
- Kim, S.; Oh, H.-K.; Kim, J.-Y.; Hong, J.-W.; Cho, S.-I. A review of pharmacological effects of angelica gigas, angelica sinensis, angelica acutiloba and their bioactive compounds. J. Korean Med. 2011, 32, 1–24. [Google Scholar]
- Lee, T.K.; Kim, B.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.C.; Yang, G.E.; Her, Y.; Park, J.H.; Kim, H.S.; et al. Effects of decursin and angelica gigas nakai root extract on hair growth in mouse dorsal skin via regulating inflammatory cytokines. Molecules 2020, 25, 3697. [Google Scholar] [CrossRef]
- Oh, S.T.; Lee, S.; Hua, C.; Koo, B.S.; Pak, S.C.; Kim, D.I.; Jeon, S.; Shin, B.A. Decursin induces apoptosis in glioblastoma cells, but not in glial cells via a mitochondria-related caspase pathway. Korean J. Physiol. Pharmacol. 2019, 23, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.W.; Park, K.H.; Jung, H.W.; Park, Y.K. Neuroprotective effect of the hairy root extract of angelica gigas nakai on transient focal cerebral ischemia in rats through the regulation of angiogenesis. BMC Complement. Altern. Med. 2015, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Du, J.; Zou, L.; Xia, H.; Wu, T.; Kim, Y.; Lee, Y. The neuroprotective effects of decursin isolated from angelica gigas nakai against amyloid beta-protein-induced apoptosis in pc 12 cells via a mitochondria-related caspase pathway. Neurochem. Res. 2015, 40, 1555–1562. [Google Scholar] [CrossRef]
- Zhang, H.; Park, J.H.; Maharjan, S.; Park, J.A.; Choi, K.S.; Park, H.; Jeong, Y.; Ahn, J.H.; Kim, I.H.; Lee, J.C.; et al. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J. Neuroinflamm. 2017, 14, 122. [Google Scholar] [CrossRef]
- Sapkota, A.; Gaire, B.P.; Cho, K.S.; Jeon, S.J.; Kwon, O.W.; Jang, D.S.; Kim, S.Y.; Ryu, J.H.; Choi, J.W. Eupatilin exerts neuroprotective effects in mice with transient focal cerebral ischemia by reducing microglial activation. PLoS ONE 2017, 12, e0171479. [Google Scholar] [CrossRef]
- Ai, J.; Wan, H.; Shu, M.; Zhou, H.; Zhao, T.; Fu, W.; He, Y. Guhong injection protects against focal cerebral ischemia-reperfusion injury via anti-inflammatory effects in rats. Arch. Pharmacal Res. 2017, 40, 610–622. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Yan, B.C.; Park, J.H.; Ahn, J.H.; Lee, D.H.; Kim, I.H.; Cho, J.H.; Chen, B.H.; Lee, J.C.; Cho, Y.S.; et al. Novel antiepileptic drug lacosamide exerts neuroprotective effects by decreasing glial activation in the hippocampus of a gerbil model of ischemic stroke. Exp. Ther. Med. 2015, 10, 2007–2014. [Google Scholar] [CrossRef]
- Lee, C.H.; Park, J.H.; Yoo, K.Y.; Choi, J.H.; Hwang, I.K.; Ryu, P.D.; Kim, D.H.; Kwon, Y.G.; Kim, Y.M.; Won, M.H. Pre- and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of bdnf expression and oxidative stress. Exp. Neurol. 2011, 229, 450–459. [Google Scholar] [CrossRef]
- Sweatt, J.D. Hippocampal function in cognition. Psychopharmacology 2004, 174, 99–110. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Preedy, V.R. Neuropathology of Drug Addictions and Substance Misuse Volume 1: Foundations of Understanding, Tobacco, Alcohol, Cannabinoids and Opioids; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Dhar, A.; Kaundal, R.K.; Sharma, S.S. Neuroprotective effects of fetmpyp: A peroxynitrite decomposition catalyst in global cerebral ischemia model in gerbils. Pharmacol. Res. 2006, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Yoshida, S.; Nagai, H.; Takeshita, A.; Mino, M.; Morioka, H.; Nakajima, T.; Kusakabe, K.T.; Okada, T. Transient forebrain ischemia induces impairment in cognitive performance prior to extensive neuronal cell death in mongolian gerbil (meriones unguiculatus). J. Vet. Sci. 2018, 19, 505–511. [Google Scholar] [CrossRef]
- Kim, B.; Lee, T.K.; Park, C.W.; Kim, D.W.; Ahn, J.H.; Sim, H.; Lee, J.C.; Yang, G.E.; Kim, J.D.; Shin, M.C.; et al. Pycnogenol((r)) supplementation attenuates memory deficits and protects hippocampal ca1 pyramidal neurons via antioxidative role in a gerbil model of transient forebrain ischemia. Nutrients 2020, 12, 2477. [Google Scholar] [CrossRef]
- Noh, Y.; Ahn, J.H.; Lee, J.W.; Hong, J.; Lee, T.K.; Kim, B.; Kim, S.S.; Won, M.H. Brain factor-7(r) improves learning and memory deficits and attenuates ischemic brain damage by reduction of ros generation in stroke in vivo and in vitro. Lab. Anim. Res. 2020, 36, 24. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Ahn, J.H.; Chen, B.H.; Park, J.H.; Shin, B.N.; Lee, T.K.; Cho, J.H.; Lee, J.C.; Park, J.R.; Yang, S.R.; Ryoo, S.; et al. Early iv-injected human dermis-derived mesenchymal stem cells after transient global cerebral ischemia do not pass through damaged blood-brain barrier. J. Tissue Eng. Regen. Med. 2018, 12, 1646–1657. [Google Scholar] [CrossRef]
- Park, J.H.; Park, J.A.; Ahn, J.H.; Kim, Y.H.; Kang, I.J.; Won, M.H.; Lee, C.H. Transient cerebral ischemia induces albumin expression in microglia only in the ca1 region of the gerbil hippocampus. Mol. Med. Rep. 2017, 16, 661–665. [Google Scholar] [CrossRef]
- Lee, T.K.; Park, Y.; Kim, B.; Lee, J.C.; Shin, M.C.; Ohk, T.G.; Park, C.W.; Cho, J.H.; Park, J.H.; Lee, C.H.; et al. Long-term alternating fasting increases interleukin-13 in the gerbil hippocampus, but does not protect bbb and pyramidal neurons from ischemia-reperfusion injury. Neurochem. Res. 2020, 45, 2352–2363. [Google Scholar] [CrossRef]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Ahn, M.J.; Lee, M.K.; Kim, Y.C.; Sung, S.H. The simultaneous determination of coumarins in angelica gigas root by high performance liquid chromatography-diode array detector coupled with electrospray ionization/mass spectrometry. J. Pharm. Biomed. Anal. 2008, 46, 258–266. [Google Scholar] [CrossRef]
- Carpenter, J.; Marion, C. Exotic Animal Formulary; Elsevier: St. Louis, MO, USA, 2013. [Google Scholar]
- Choi, Y.S.; Horning, P.; Aten, S.; Karelina, K.; Alzate-Correa, D.; Arthur, J.S.C.; Hoyt, K.R.; Obrietan, K. Mitogen- and stress-activated protein kinase 1 regulates status epilepticus-evoked cell death in the hippocampus. ASN Neuro 2017, 9, 1759091417726607. [Google Scholar] [CrossRef]
- Mahar, I.; Albuquerque, M.S.; Mondragon-Rodriguez, S.; Cavanagh, C.; Davoli, M.A.; Chabot, J.G.; Williams, S.; Mechawar, N.; Quirion, R.; Krantic, S. Phenotypic alterations in hippocampal npy- and pv-expressing interneurons in a presymptomatic transgenic mouse model of alzheimer’s disease. Front. Aging Neurosci. 2016, 8, 327. [Google Scholar] [CrossRef]
- Radtke-Schuller, S.; Schuller, G.; Angenstein, F.; Grosser, O.S.; Goldschmidt, J.; Budinger, E. Brain atlas of the mongolian gerbil (meriones unguiculatus) in ct/mri-aided stereotaxic coordinates. Brain Struct. Funct. 2016, 221 (Suppl. 1), 1–272. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, F.; Zou, X.; Torbey, M. Comparison of unbiased estimation of neuronal number in the rat hippocampus with different staining methods. J. Neurosci. Methods 2015, 254, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Schmued, L.C.; Hopkins, K.J. Fluoro-jade b: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Anderson, K.J.; Miller, K.M.; Fugaccia, I.; Scheff, S.W. Regional distribution of fluoro-jade b staining in the hippocampus following traumatic brain injury. Exp. Neurol. 2005, 193, 125–130. [Google Scholar] [CrossRef]
- Meurer, R.T.; Martins, D.T.; Hilbig, A.; Ribeiro Mde, C.; Roehe, A.V.; Barbosa-Coutinho, L.M.; Fernandes Mda, C. Immunohistochemical expression of markers ki-67, neun, synaptophysin, p53 and her2 in medulloblastoma and its correlation with clinicopathological parameters. Arq. Neuro Psiquiatr. 2008, 66, 385–390. [Google Scholar] [CrossRef]
- Paizs, M.; Engelhardt, J.I.; Siklos, L. Quantitative assessment of relative changes of immunohistochemical staining by light microscopy in specified anatomical regions. J. Microsc. 2009, 234, 103–112. [Google Scholar] [CrossRef]

| Type | Ingredients | |
|---|---|---|
| Coumarin derivatives | Major ingredients | Decursin |
| Decursinol angelate | ||
| Decursinol | ||
| Other ingredients | (2′′R,3′′R)-epoxyangeloyldecursinol, (2′′S,3′′S)-epoxyangeloyldecursinol, 4′′-Hydroxytigloyldecursinol, 4′′-hydroxydecursin, columbianetin-O-β-D-glucopyranoside, Marmesinin, Nodakenin, etc. | |
| Saccharides | Polysaccharide | Angelan (peptic polysaccharide) |
| Monosaccharides | Arabinose, Galactose, Galacturonic acid (sugar acid), etc. | |
| Polyacetylene | 18-acetoxy-octadeca-1,9-dien-4,6-diyn-3,8-diol, Octadeca-1,9-dien-4,6-diyn-3,8,18-triol, etc. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.-K.; Kang, I.-J.; Sim, H.; Lee, J.-C.; Ahn, J.-H.; Kim, D.-W.; Park, J.-H.; Lee, C.-H.; Kim, J.-D.; Won, M.-H.; et al. Therapeutic Effects of Decursin and Angelica gigas Nakai Root Extract in Gerbil Brain after Transient Ischemia via Protecting BBB Leakage and Astrocyte Endfeet Damage. Molecules 2021, 26, 2161. https://doi.org/10.3390/molecules26082161
Lee T-K, Kang I-J, Sim H, Lee J-C, Ahn J-H, Kim D-W, Park J-H, Lee C-H, Kim J-D, Won M-H, et al. Therapeutic Effects of Decursin and Angelica gigas Nakai Root Extract in Gerbil Brain after Transient Ischemia via Protecting BBB Leakage and Astrocyte Endfeet Damage. Molecules. 2021; 26(8):2161. https://doi.org/10.3390/molecules26082161
Chicago/Turabian StyleLee, Tae-Kyeong, II-Jun Kang, Hyejin Sim, Jae-Chul Lee, Ji-Hyeon Ahn, Dae-Won Kim, Joon-Ha Park, Choong-Hyun Lee, Jong-Dai Kim, Moo-Ho Won, and et al. 2021. "Therapeutic Effects of Decursin and Angelica gigas Nakai Root Extract in Gerbil Brain after Transient Ischemia via Protecting BBB Leakage and Astrocyte Endfeet Damage" Molecules 26, no. 8: 2161. https://doi.org/10.3390/molecules26082161
APA StyleLee, T.-K., Kang, I.-J., Sim, H., Lee, J.-C., Ahn, J.-H., Kim, D.-W., Park, J.-H., Lee, C.-H., Kim, J.-D., Won, M.-H., & Choi, S.-Y. (2021). Therapeutic Effects of Decursin and Angelica gigas Nakai Root Extract in Gerbil Brain after Transient Ischemia via Protecting BBB Leakage and Astrocyte Endfeet Damage. Molecules, 26(8), 2161. https://doi.org/10.3390/molecules26082161








