Dragon Fruits as a Reservoir of Natural Polyphenolics with Chemopreventive Properties
Abstract
1. Introduction
2. Results and Discussion
2.1. Dragon Fruits Bioactive Compounds and Antioxidant Activities
2.2. Cytotoxic Activity
2.3. Nitric Oxide Inhibition
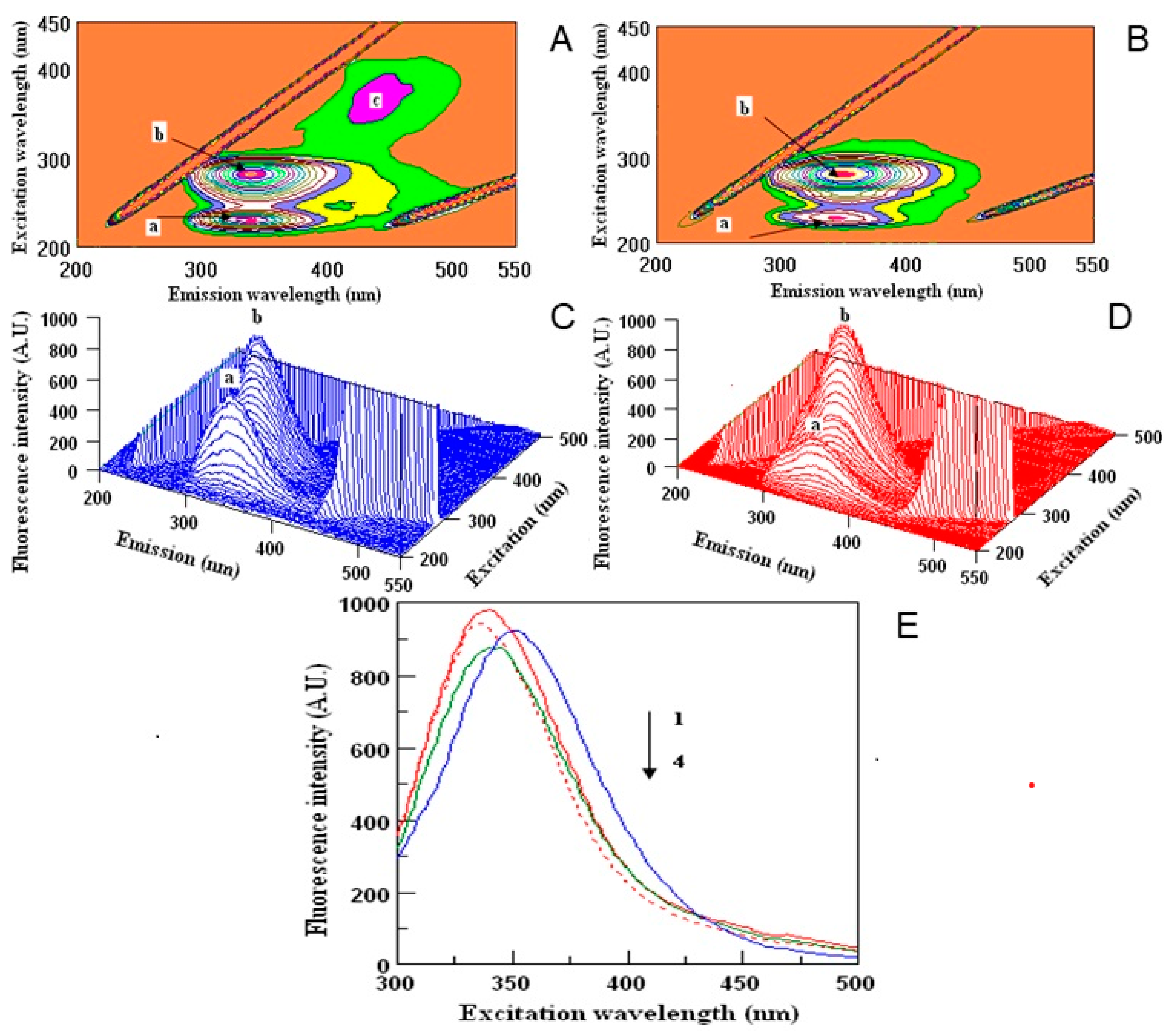
2.4. Fluorescence Properties of Interaction of Dragon Polyphenols with Human Serum Proteins (HSA)
3. Materials and Methods
3.1. Chemicals
3.2. Material
3.3. Methods
3.3.1. Determination of Polyphenols, Flavonoids, Flavanols, Tannins and Betacyanins
3.3.2. Determination of Flavonoids and Phenolic Acids
3.3.3. Determination of the Antioxidant Activity
- ABTS·+ was generated by the interaction of ABTS (7 mM) and K2S2O8 (2.45 mM). This solution was diluted with methanol until the absorbance reached 0.7 at 734 nm [41].
- CUPRAC is based on the use of the copper (II)–neocuproine [Cu (II)–Nc] reagent as the chromogenic oxidizing agent. The tested extracts or standard solution and H2O were added to the mixture of Cu (II), Nc, and NH4Ac buffer solution. The absorbance at 450 nm was measured against a reagent blank [42].
- DPPH solution (3.9 mL, 25 mg/L) in methanol was mixed with the tested extracts (0.1 mL). The reaction was monitored at 515 nm until the absorbance was stable [41].
- FRAP assay measures the ability to reduce ferric-tripyridyltriazine (Fe3+-TPTZ) to a ferrous form (Fe2+), which absorbs light at 593 nm [43].
3.3.4. Cytotoxic Activity
3.3.5. Inflammation In Vitro Model
3.3.6. Nitric Oxide Determination
3.3.7. Fluorometric Measurements
3.3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Le Bellec, F.; Vaillant, F.; Imbert, E. Pitahaya (Hylocereus spp.): A new fruit crop, a market with a future. Fruits 2006, 61, 237–250. [Google Scholar] [CrossRef]
- Mizrahi, Y. Do We Need New Crops for Arid Regions? A Review of Fruit Species Domestication in Israel. Agronomy 2020, 10, 1995. [Google Scholar] [CrossRef]
- Goenaga, R.; Marrero, A.; Pérez, D. Yield and Fruit Quality Traits of Dragon Fruit Cultivars Grown in Puerto Rico. HortTechnology 2020, 30, 803–808. [Google Scholar] [CrossRef]
- Sheng, K.; Wei, S.; Mei, J.; Xie, J. Chilling Injury, Physicochemical Properties, and Antioxidant Enzyme Activities of Red Pitahaya (Hylocereus polyrhizus) Fruits under Cold Storage Stress. Phyton 2021, 90, 291–305. [Google Scholar] [CrossRef]
- Jalgaonkar, K.; Mahawar, M.K.; Bibwe, B.; Kannaujia, P. Postharvest Profile, Processing and Waste Utilization of Dragon Fruit (Hylocereus Spp.): A Review. Food Rev. Int. 2020, 1–27. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Zagrodzki, P.; Ku, Y.G.; Luksirikul, P.; Weisz, M.; Gorinstein, S. Bioactivity and cytotoxicity of different species of pitaya fruits–A comparative study with advanced chemometric analysis. Food Biosci. 2021, 40, 2021100888. [Google Scholar] [CrossRef]
- Verona-Ruiz, A.; Urcia-Cerna, J.; Paucar-Menacho, L.M. Pitahaya (Hylocereus spp.): Culture, physicochemical characteristics, nutritional composition, and bioactive compounds. Sci. Agropecu. 2020, 11, 439–453. [Google Scholar] [CrossRef]
- Joshi, M.; Prabhakar, B. Phytoconstituents and pharmaco-therapeutic benefits of pitaya: A wonder fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef]
- Kårlund, A.; Moor, U.; Sandell, M.; Karjalainen, R.O. The impact of harvesting, storage and processing factors on health-promoting phytochemicals in berries and fruits. Processes 2014, 2, 596–624. [Google Scholar] [CrossRef]
- Paśko, P.; Tyszka-Czochara, M.; Trojan, S.; Bobis-Wozowicz, S.; Zagrodzki, P.; Namieśnik, J.; Haruenkit, R.; Poovarodom, S.; Pinsirodom, P.; Gorinstein, S. Glycolytic genes expression, proapoptotic potential in relation to the total content of bioactive compounds in durian fruits. Food Res. Int. 2019, 125, 108563. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Vearasilp, S.; Trakhtenberg, S.; Gorinstein, S. The multiple nutrition properties of some exotic fruits: Biological activity and active metabolites. Food Res. Int. 2011, 44, 1671–1701. [Google Scholar] [CrossRef]
- Pérez-Loredo, M.G.; García-Ochoa, F.; Barragán-Huerta, B.E. Comparative analysis of betalain content in Stenocereus stellatus fruits and other cactus fruits using principal component analysis. Int. J. Food Prop. 2016, 19, 326–338. [Google Scholar] [CrossRef]
- García-Cruz, L.; Valle-Guadarrama, S.; Salinas-Moreno, Y.; Joaquín-Cruz, E. Physical, chemical, and antioxidant activity characterization of pitaya (Stenocereus pruinosus) fruits. Plant Foods Hum. Nutr. 2013, 68, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Giangrieco, I.; Proietti, S.; Moscatello, S.; Tuppo, L.; Battistelli, A.; La Cara, F.; Tamburrini, M.; Famiani, F.; Ciardiello, M.A. Influence of geographical location of orchards on green kiwifruit bioactive components. J. Agric. Food Chem. 2016, 64, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cruz, L.; Valle-Guadarrama, S.; Salinas-Moreno, Y.; del Carmen Luna-Morales, C. Postharvest quality, soluble phenols, betalains content, and antioxidant activity of Stenocereus pruinosus and Stenocereus stellatus fruit. Postharvest Biol. Technol. 2016, 111, 69–76. [Google Scholar] [CrossRef]
- Jamilah, B.; Shu, C.E.; Kharidah, M.; Dzulkily, M.A.; Noranizan, A. Physico-chemical characteristics of red pitaya (Hylocereus polyrhizus) peel. Int. Food Res. J. 2011, 18, 279–285. [Google Scholar]
- Al-Mekhlafi, N.A.; Mediani, A.; Ismail, N.H.; Abas, F.; Dymerski, T.; Lubinska-Szczygeł, M.; Verasilp, S.; Gorinstein, S. Metabolomic and antioxidant properties of different varieties and origins of Dragon fruit. Microchem. J. 2021, 160, 105687. [Google Scholar] [CrossRef]
- Zain, N.M.; Nazeri, M.A.; Azman, N.A. Assessment on bioactive compounds and the effect of microwave on pitaya peel. J. Teknol. 2019, 81. [Google Scholar] [CrossRef]
- Azeredo, H.M. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Esquivel, P.; Stintzing, F.C.; Carle, R. Phenolic compound profiles and their corresponding antioxidant capacity of purple pitaya (Hylocereus sp.) genotypes. Z. Naturforsch. 2007, 62, 636–644. [Google Scholar] [CrossRef]
- Ramli, N.S.; Ismail, P.; Rahmat, A. Influence of conventional and ultrasonic-assisted extraction on phenolic contents, betacyanin contents, and antioxidant capacity of red dragon fruit (Hylocereus polyrhizus). Sci. World J. 2014, 964731. [Google Scholar] [CrossRef]
- Leong, H.Y.; Ooi, C.W.; Law, C.L.; Julkifle, A.L.; Ling, T.C.; Show, P.L. Application of liquid biphasic flotation for betacyanins extraction from peel and flesh of Hylocereus polyrhizus and antioxidant activity evaluation. Sep. Purif. Technol. 2018, 201, 156–166. [Google Scholar] [CrossRef]
- Gorinstein, S.; Poovarodom, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Vearasilp, S.; Haruenkit, R.; Ruamsuke, P.; Katrich, E.; Tashma, Z. Antioxidant properties and bioactive constituents of some rare exotic Thai fruits and comparison with conventional fruits: In vitro and in vivo studies. Food Res. Int. 2011, 44, 2222–2232. [Google Scholar] [CrossRef]
- Jayakumar, R.; Kanthimathi, M.S. Inhibitory effects of fruit extracts on nitric oxide-induced proliferation in MCF-7 cells. Food Chem. 2011, 126, 956–960. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Li, H.B.; Deng, G.F.; Ling, W.H.; Wu, S.; Xu, X.R.; Chen, F. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J. Funct. Foods 2013, 5, 1298–1309. [Google Scholar] [CrossRef]
- Luo, H.; Cai, Y.; Peng, Z.; Liu, T.; Yang, S. Chemical composition and in vitro evaluation of the cytotoxic and antioxidant activities of supercritical carbon dioxide extracts of pitaya (dragon fruit) peel. Chem. Cent. J. 2014, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Biedrawa, D.; Grabowska, K.; Galanty, A.; Sobolewska, D.; Żmudzki, P.; Podolak, I. Anti-melanoma potential of two benzoquinone homologues embelin and rapanone-a comparative in vitro study. Toxicol. In Vitro 2020, 65, 104826. [Google Scholar] [CrossRef] [PubMed]
- Macias-Ceja, D.C.; Cosín-Roger, J.; Ortiz-Masiá, D.; Salvador, P.; Hernández, C.; Calatayud, S.; Esplugues, J.V.; Barrachina, M.D. The flesh ethanolic extract of Hylocereus polyrhizus exerts anti-inflammatory effects and prevents murine colitis. Clin. Nutr. 2016, 35, 1333–1339. [Google Scholar] [CrossRef]
- Yeh, W.J.; Tsai, C.C.; Ko, J.; Yang, H.Y. Hylocereus polyrhizus Peel Extract Retards Alcoholic Liver Disease Progression by Modulating Oxidative Stress and Inflammatory Responses in C57BL/6 Mice. Nutrients 2020, 12, 3884. [Google Scholar] [CrossRef]
- Bose, A. Interaction of tea polyphenols with serum albumins: A fluorescence spectroscopic analysis. J. Lumin. 2016, 169, 220–226. [Google Scholar] [CrossRef]
- López-Yerena, A.; Perez, M.; Vallverdú-Queralt, A.; Escribano-Ferrer, E. Insights into the Binding of Dietary Phenolic Compounds to Human Serum Albumin and Food-Drug Interactions. Pharmaceutics 2020, 12, 1123. [Google Scholar] [CrossRef] [PubMed]
- Gecibesler, I.H.; Aydin, M. Plasma Protein Binding of Herbal-Flavonoids to Human Serum Albumin and Their Anti-proliferative Activities. An. Acad. Bras. Cienc. 2020, 92. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Ulrih, N.P.; Sengupta, P.K.; Xiao, J. Plasma protein binding of dietary polyphenols to human serum albumin: A high performance affinity chromatography approach. Food Chem. 2019, 270, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, S.; Qin, Y.; Liu, J.; Liu, J.; Wang, Q.; Ren, F.; Zhang, H. Interaction of phenolic acids and their derivatives with human serum albumin: Structure—Affinity relationships and effects on antioxidant activity. Food Chem. 2018, 240, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Barasch, D.; Nemirovski, A.; Trakhtenberg, S.; Gorinstein, S. Bioactive compounds and the antioxidant capacity in new kiwi fruit cultivars. Food Chem. 2014, 165, 354–361. [Google Scholar] [CrossRef]
- Paśko, P.; Tyszka-Czochara, M.; Galanty, A.; Gdula-Argasińska, J.; Żmudzki, P.; Bartoń, H.; Zagrodzki, P.; Gorinstein, S. Comparative study of predominant phytochemical compounds and proapoptotic potential of broccoli sprouts and florets. Plant. Foods Hum. Nutr. 2018, 73, 95–100. [Google Scholar] [CrossRef]
- Haruenkit, R.; Poovarodom, S.; Vearasilp, S.; Namiesnik, J.; Sliwka-Kaszynska, M.; Park, Y.S.; Heo, B.G.; Cho, J.Y.; Jang, H.G.; Gorinstein, S. Comparison of bioactive compounds, antioxidant and antiproliferative activities of Mon Thong durian during ripening. Food Chem. 2010, 118, 540–547. [Google Scholar] [CrossRef]
- Cao, S.; Liu, T.; Jiang, Y.; He, S.; Harrison, D.K.; Joyce, D.C. The effects of host defence elicitors on betacyanin accumulation in Amaranthus mangostanus seedlings. Food Chem. 2012, 134, 1715–1718. [Google Scholar] [CrossRef]
- Tyszka-Czochara, M.; Pasko, P.; Zagrodzki, P.; Gajdzik, E.; Wietecha-Posluszny, R.; Gorinstein, S. Selenium supplementation of amaranth sprouts influences betacyanin content and improves anti-inflammatory properties via NFκB in murine RAW 264.7 macrophages. Biol. Trace Elem. Res. 2016, 169, 320–330. [Google Scholar] [CrossRef]
- Paśko, P.; Galanty, A.; Żmudzki, P.; Gdula-Argasińska, J.; Zagrodzki, P. Influence of different light conditions and time of sprouting on harmful and beneficial aspects of rutabaga sprouts in comparison to their roots and seeds. J. Sci. Food Agric. 2019, 99, 302–308. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z.; Scheerens, J.C.; Miller, A.R. Modified 2, 2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2 ‘-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, Y.W.; Park, Y.; Lee, H.J.; Kim, K.W. Anti-inflammatory effects of chlorogenic acid in lipopolysaccharide-stimulated RAW 264.7 cells. Inflamm. Res. 2014, 63, 81–90. [Google Scholar] [CrossRef] [PubMed]
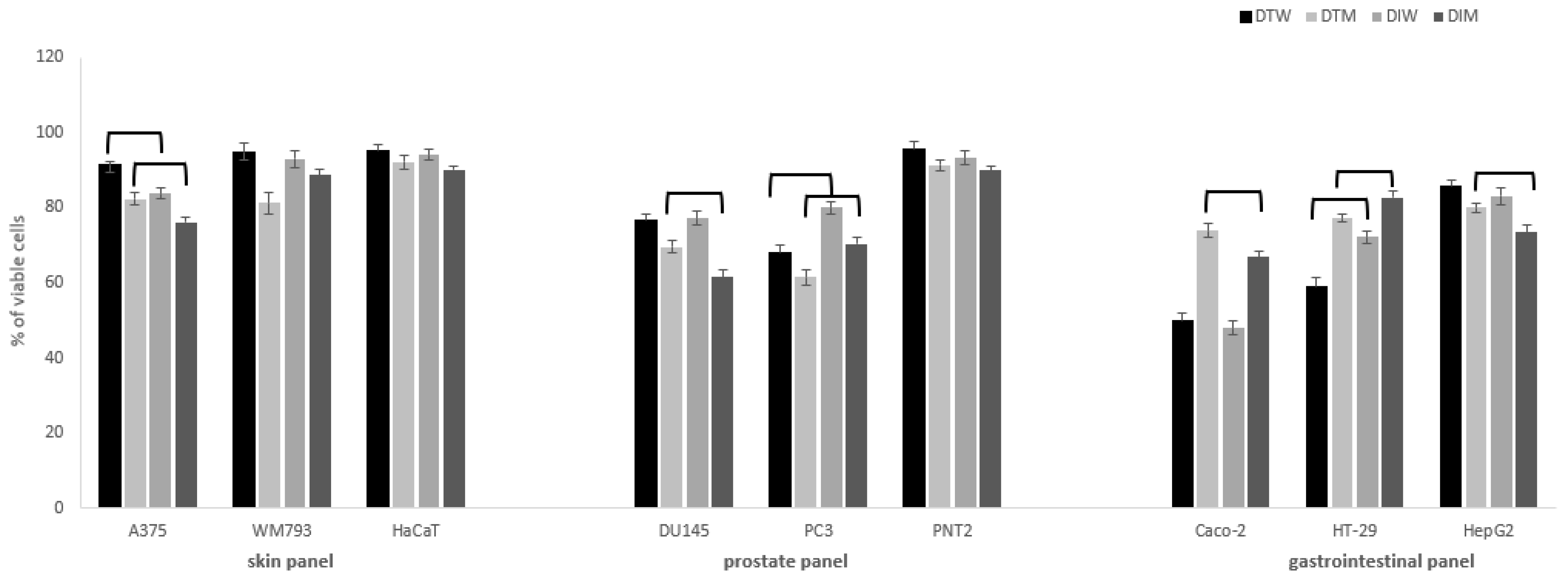
| Parameter | Dragon Fruits from Israel (DI) | Dragon Fruits from Thailand (DT) | ||
|---|---|---|---|---|
| Methanol (DIM) | Water (DIW) | Methanol (DTM) | Water (DTW) | |
| Total Bioactive Compounds | ||||
| Total Polyphenols [mg GAE/g DW] | 7.78 ± 0.24 a1c1 | 9.11 ± 0.35 a1d1 | 2.51 ± 0.10 b1c1 | 4.52 ± 0.19 b1d1 |
| Flavanols [µg CE/g DW] | 60.40 ± 4.42 a1c3 | 111.75 ± 4.35 a1d1 | 46.94 ± 5.00 b1c3 | 81.23 ± 4.43 b1d1 |
| Flavonoids [mg CE/g DW] | 1.57 ± 0.10 | − | 0.71 ± 0.14 | − |
| Tannins [mg CE/g DW] | 3.07 ± 0.12 | 2.42 ± 0.26 | 2.29 ± 0.22 | 1.37 ± 0.25 |
| Total Betacyanins [mg/100 g DW] | 9.30 ± 1.53 a1b2 | 2.56 ± 0.25 a1c3 | 5.33 ± 0.12 b2 | 4.99 ± 0.21 c3 |
| Selected Flavonoids [mg/100 g DW] | ||||
| Myricetin | 8.71 ± 0.79 a1 | Nd | 17.95 ± 0.32 a1 | Nd |
| Rutin | 3.67 ± 0.28 a1 | Nd | 1.46 ± 0.13 a1 | Nd |
| Quercetin | Nd | Nd | 6.37 ± 0.14 | Nd |
| Selected Phenolic Acids [mg/100 g DW] | ||||
| Gallic acid | 34.26 ± 1.75 a1c1 | 1.92 ± 0.59 a1 | 23.86 ± 0.70 b1c1 | 1.32 ± 0.26 b1 |
| Caffeic acid | Nd | Nd | Nd | 1.25 ± 0.27 |
| Protocatechuic acid | Nd | Nd | Nd | 0.57 ± 0.16 |
| Parameter | Dragon Fruits from Israel (DI) | Dragon Fruits from Thailand (DT) | ||
|---|---|---|---|---|
| Methanol (DIM) | Water (DIW) | Methanol (DTM) | Water (DTW) | |
| ABTS [µM Trolox/gDW] | 21.85 ± 0.73 a2b1 | 24.49 ± 0.94 a2c1 | 15.82 ± 0.42 b1 | 17.53 ± 0.58 c1 |
| CUPRAC [µM Trolox/gDW] | 22.28 ± 0.70 a1c1 | 26.57 ± 1.15 a1d1 | 16.14 ± 0.61 b2c1 | 18.98 ± 0.50 b2d1 |
| DPPH [µM Trolox/gDW] | 13.65 ± 0.37 a3b1 | 14.69 ± 0.46 a3c1 | 5.11 ± 0.28 b1 | 5.04 ± 0.18 c1 |
| FRAP [µM Trolox/gDW] | 9.06 ± 0.57 a3b2 | 10.76 ± 0.76 a3c1 | 6.77 ± 0.13 b2 | 7.86 ± 0.35 c1 |
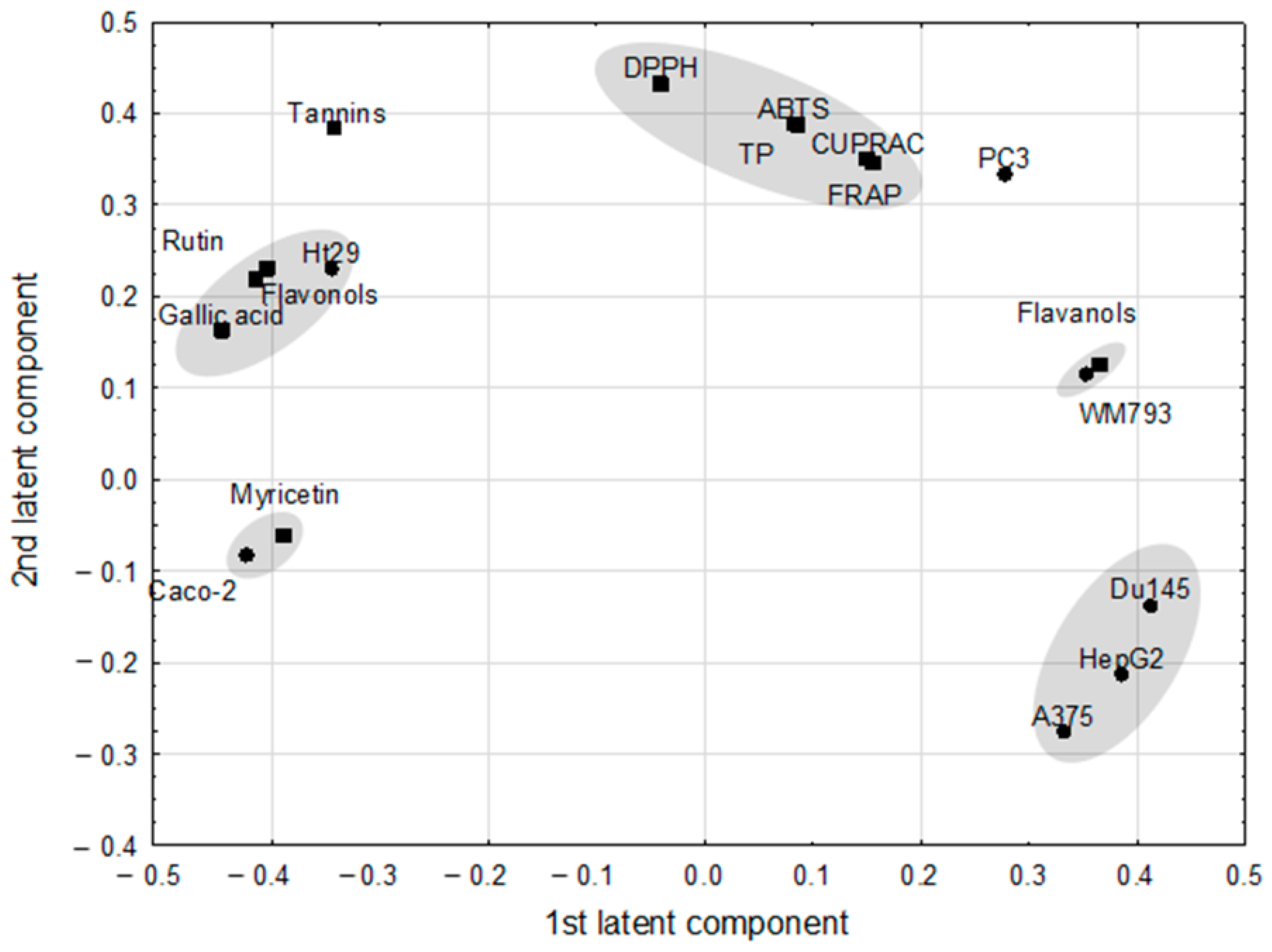
| Pairs of Correlated Parameters | Correlation Weights | |
|---|---|---|
| Gallic acid | Flavonoids | 0.184 |
| Gallic acid | Rutin | 0.178 |
| Rutin | Flavonoids | 0.168 |
| DPPH | ABTS | 0.167 |
| DPPH | TP | 0.167 |
| Myricetin | Caco-2 | 0.165 |
| Gallic acid | Caco-2 | 0.162 |
| HepG2 | DU145 | 0.157 |
| Myricetin | Gallic acid | 0.152 |
| TP | ABTS | 0.151 |
| Gallic acid | HT-29 | 0.150 |
| Flavanols | Flavonoids | –0.150 |
| HepG2 | Caco-2 | –0.156 |
| Gallic acid | WM793 | –0.158 |
| Myricetin | DU145 | –0.159 |
| Gallic acid | Flavanols | –0.163 |
| DU145 | Caco-2 | –0.173 |
| Sample | Peak a | Peak b | Peak c | |||
|---|---|---|---|---|---|---|
| λex/λem (nm/nm) | Int F0 | λex/λem (nm/nm) | Int F0 | λex/λem (nm/nm) | Int F0 | |
| HSA + Water | 228/347 | 744.10 | 280/350 | 853.41 | – | – |
| HSA + DT | 230/345 | 634.15 | 281/341 | 850.53 | 366/439 | 132.45 |
| HSA + DI | 228/345 | 335.22 | 280/349 | 871.70 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paśko, P.; Galanty, A.; Zagrodzki, P.; Luksirikul, P.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Dragon Fruits as a Reservoir of Natural Polyphenolics with Chemopreventive Properties. Molecules 2021, 26, 2158. https://doi.org/10.3390/molecules26082158
Paśko P, Galanty A, Zagrodzki P, Luksirikul P, Barasch D, Nemirovski A, Gorinstein S. Dragon Fruits as a Reservoir of Natural Polyphenolics with Chemopreventive Properties. Molecules. 2021; 26(8):2158. https://doi.org/10.3390/molecules26082158
Chicago/Turabian StylePaśko, Paweł, Agnieszka Galanty, Paweł Zagrodzki, Patraporn Luksirikul, Dinorah Barasch, Alina Nemirovski, and Shela Gorinstein. 2021. "Dragon Fruits as a Reservoir of Natural Polyphenolics with Chemopreventive Properties" Molecules 26, no. 8: 2158. https://doi.org/10.3390/molecules26082158
APA StylePaśko, P., Galanty, A., Zagrodzki, P., Luksirikul, P., Barasch, D., Nemirovski, A., & Gorinstein, S. (2021). Dragon Fruits as a Reservoir of Natural Polyphenolics with Chemopreventive Properties. Molecules, 26(8), 2158. https://doi.org/10.3390/molecules26082158







