Mono- and Binuclear Copper(II) and Nickel(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-Tetrazine Ligand
Abstract
1. Introduction
2. Results and Discussion
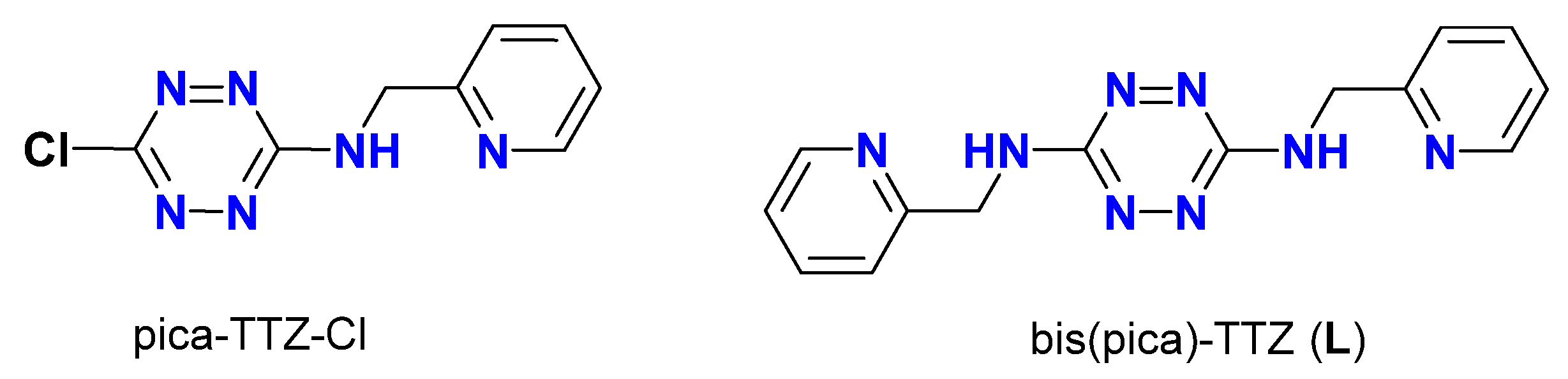
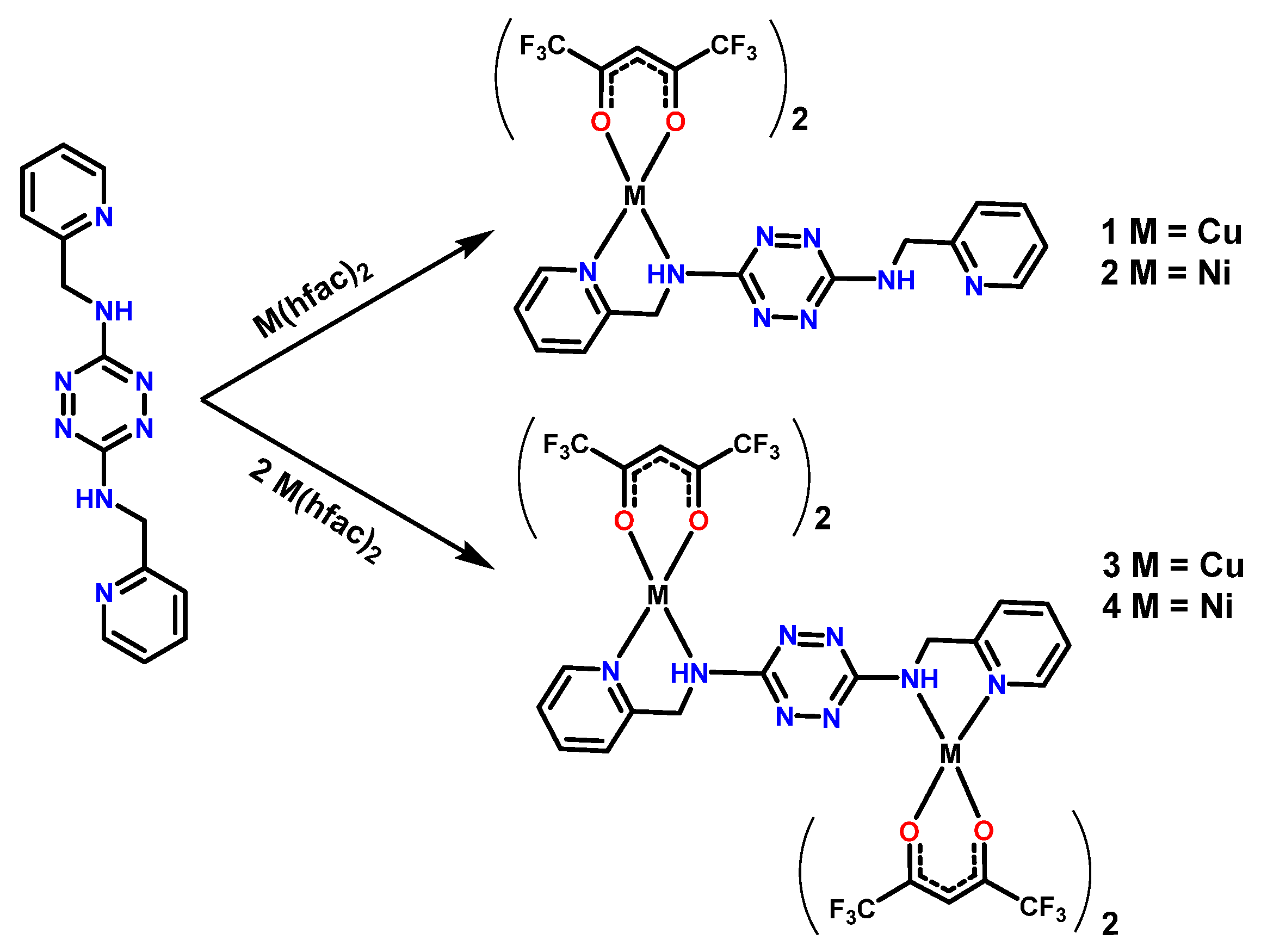
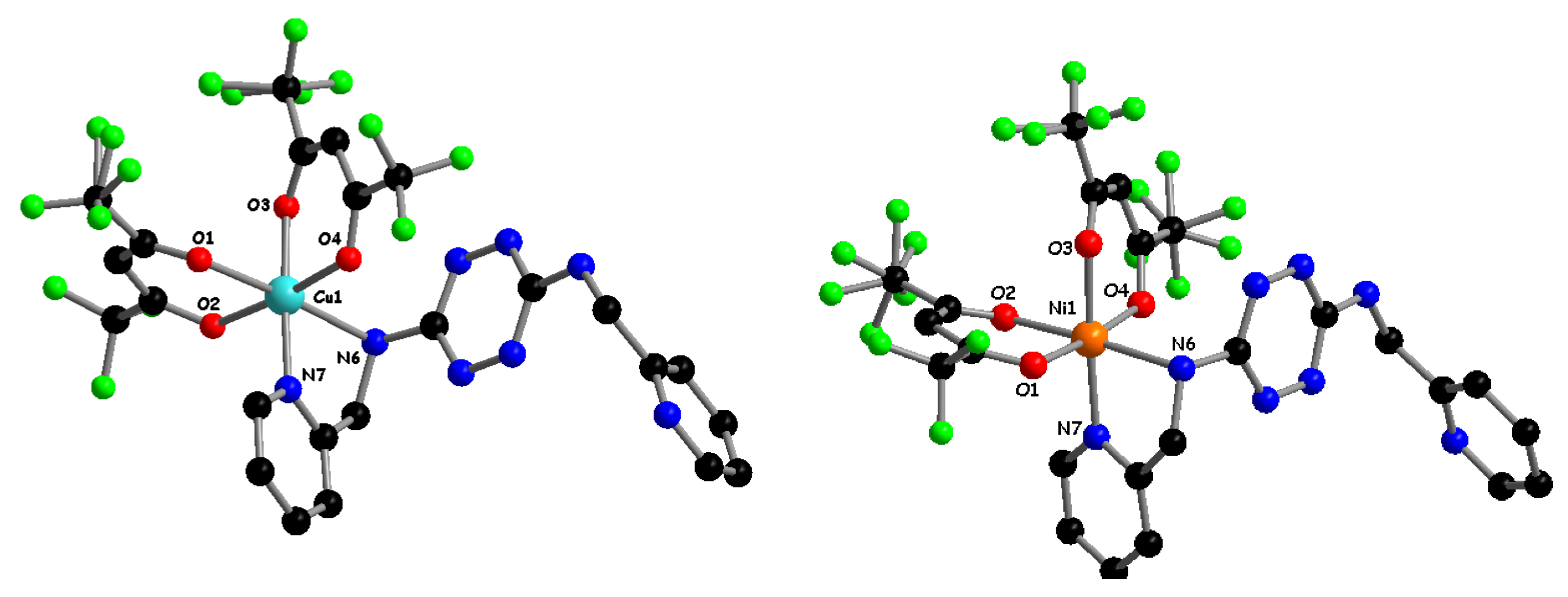
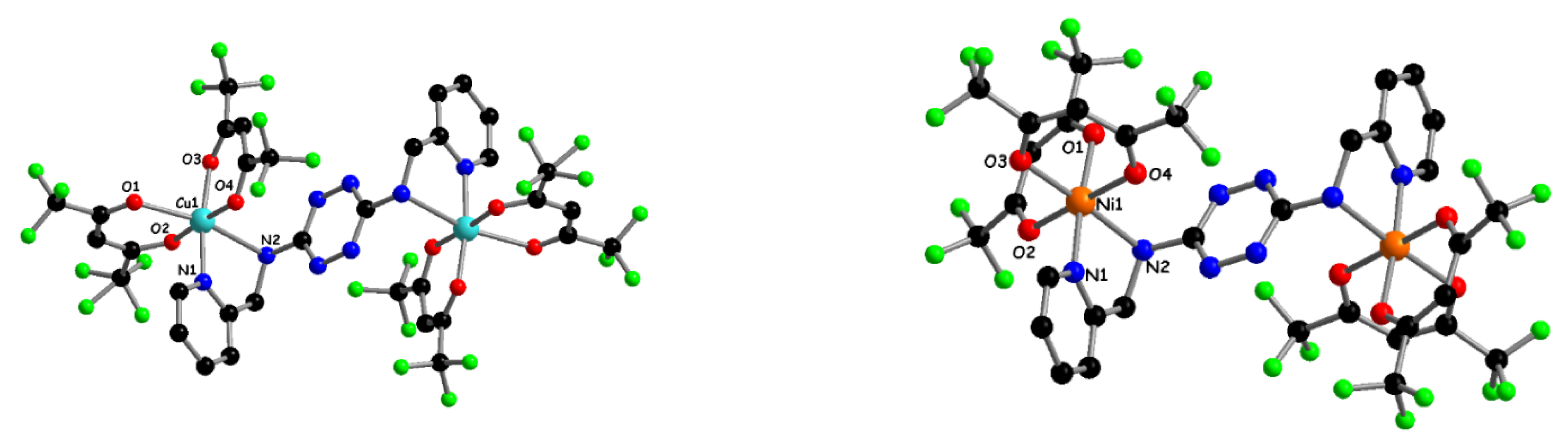
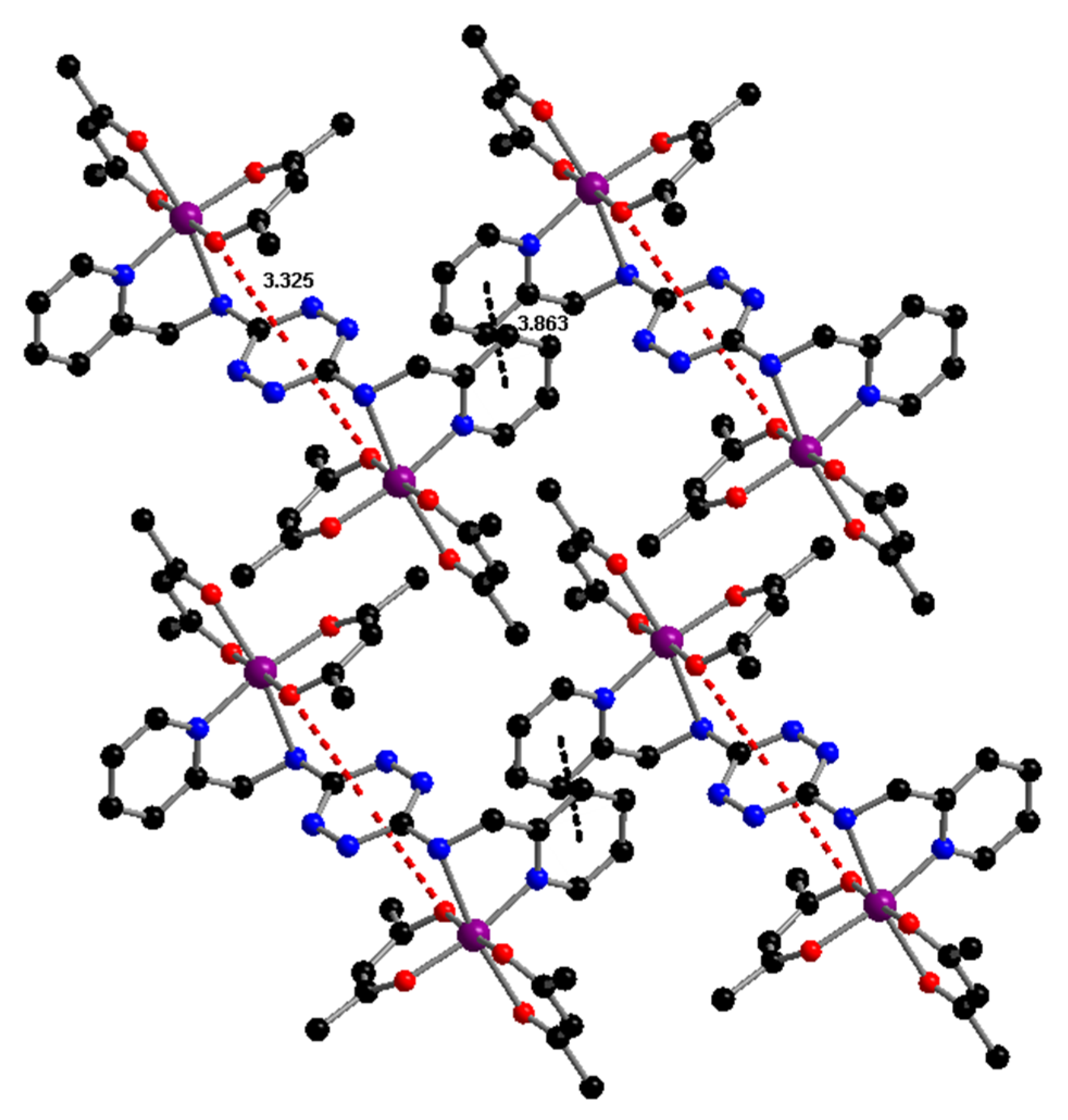
2.1. Synthesis and Characterization of TTZ-Based Complexes 1–4
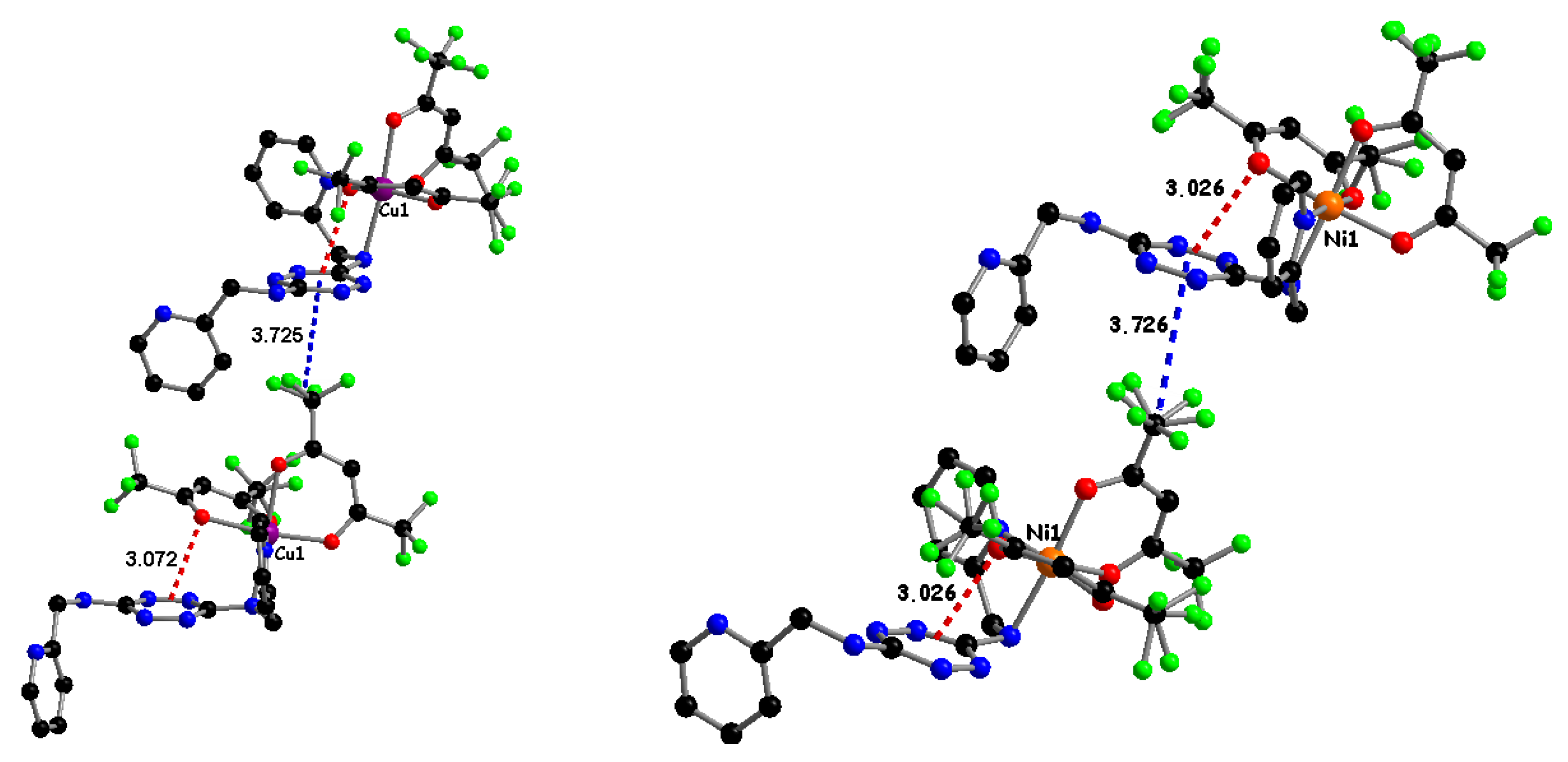
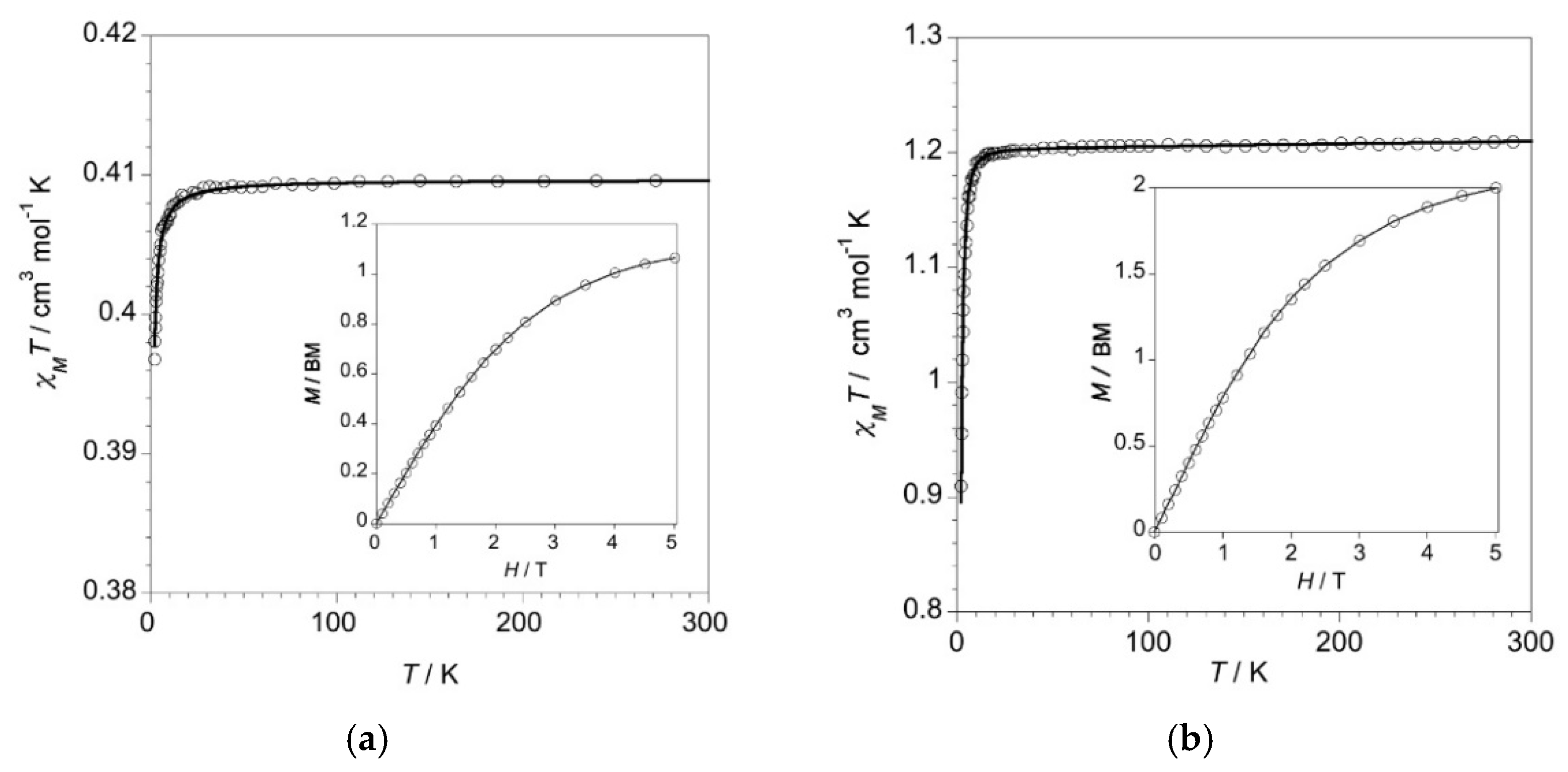
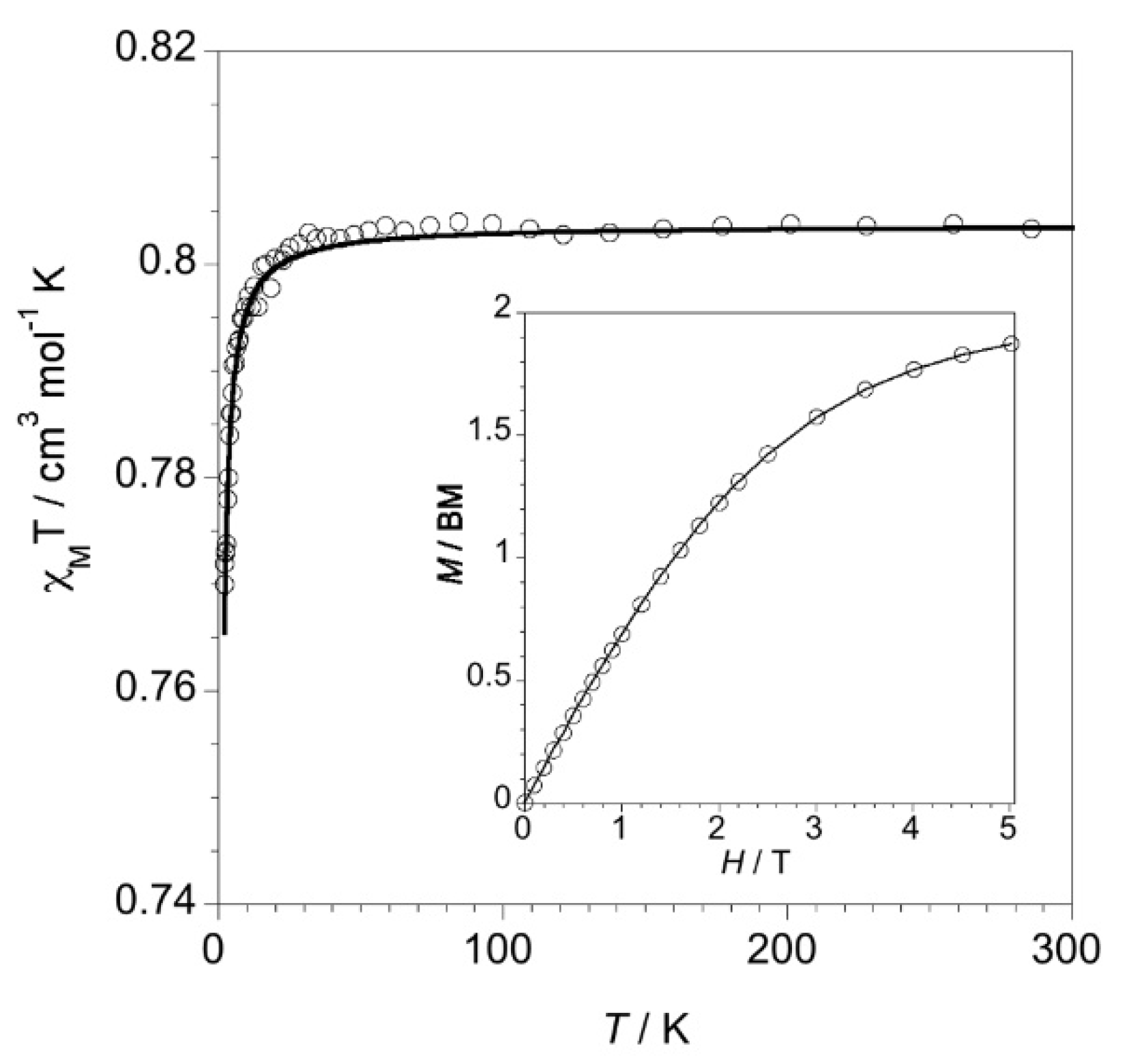
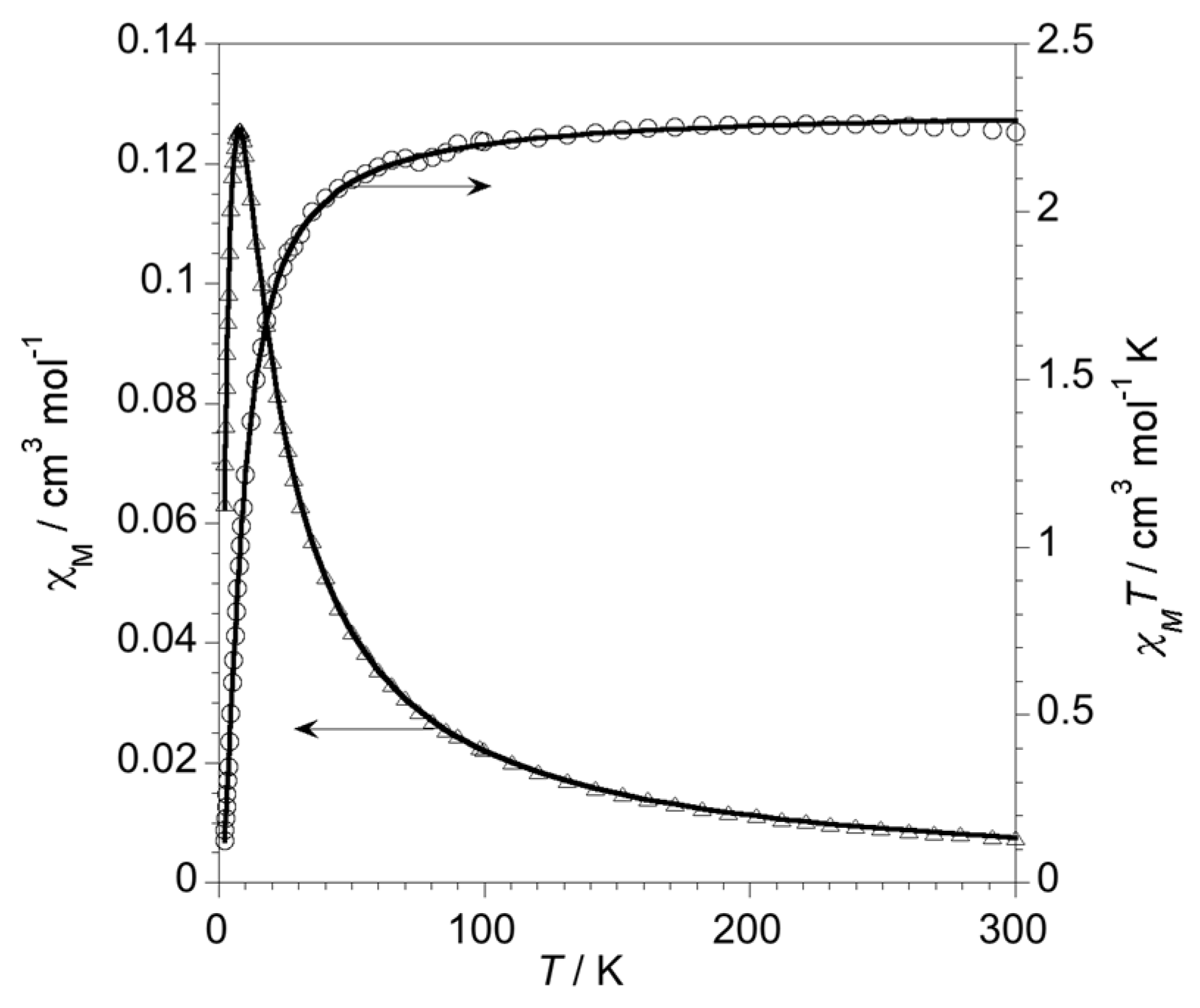
2.2. Magnetic Properties of Complexes 1–4
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Saracoglu, N. Recent advances and applications in 1,2,4,5-tetrazine chemistry. Tetrahedron 2007, 63, 4199–4236. [Google Scholar] [CrossRef]
- Clavier, G.; Audebert, P. s-Tetrazines as Building Blocks for New Functional Molecules and Molecular Materials. Chem. Rev. 2010, 110, 3299–3314. [Google Scholar] [PubMed]
- Audebert, P.; Miomandre, F.; Clavier, G.; Vernières, M.-C.; Badré, S.; Méallet-Renault, R. Synthesis and Properties of New Tetrazines Substituted by Heteroatoms: Towards the World’s Smallest Organic Fluorophores. Chem. Eur. J. 2005, 11, 5667–5673. [Google Scholar] [CrossRef]
- Gong, Y.-H.; Miomandre, F.; Méallet-Renault, R.; Badré, S.; Galmiche, L.; Tang, J.; Audebert, P.; Clavier, G. Synthesis and Physical Chemistry of s-Tetrazines: Which Ones are Fluorescent and Why? Eur. J. Org. Chem. 2009, 6121–6128. [Google Scholar] [CrossRef]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Clavier, G.; Miomandre, F.; Audebert, P. Design of New Tetrazine–Triphenylamine Bichromophores—Fluorescent Switching by Chemical Oxidation. Eur. J. Org. Chem. 2012, 2012, 1394–1403. [Google Scholar]
- Quinton, C.; Alain-Rizzo, V.; Dumas-Verdes, C.; Miomandre, F.; Audebert, P. Tetrazine–triphenylamine dyads: Influence of the nature of the linker on their properties. Electrochim. Acta 2013, 110, 693–701. [Google Scholar] [CrossRef]
- Jordan, B.J.; Pollier, M.A.; Miller, L.A.; Tiernan, C.; Clavier, G.; Audebert, P.; Rotello, V.M. Redox-Modulated Recognition of Tetrazines Using Thioureas. Org. Lett. 2007, 9, 2835–2838. [Google Scholar]
- Zhou, Q.; Audebert, P.; Clavier, G.; Méallet-Renault, R.; Miomandre, F.; Shaukat, Z.; Vu, T.-T.; Tang, J. New Tetrazines Functionalized with Electrochemically and Optically Active Groups: Electrochemical and Photoluminescence Properties. J. Phys. Chem. C 2011, 115, 21899–21906. [Google Scholar] [CrossRef]
- Kurach, E.; Djurado, D.; Rimarčik, J.; Kornet, A.; Wlostowski, M.; Lukeš, V.; Pécaut, J.; Zagorska, M.; Pron, A. Effect of substituents on redox, spectroscopic and structural properties of conjugated diaryltetrazines—A combined experimental and theoretical study. Phys. Chem. Chem. Phys. 2011, 13, 2690–2700. [Google Scholar]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef]
- Frontera, A.; Gamez, P.; Mascal, M.; Mooibroek, T.J.; Reedijk, J. Putting Anion–π Interactions into Perspective. Angew. Chem. Int. Ed. 2011, 50, 9564–9583. [Google Scholar] [CrossRef]
- Savastano, M.; Bazzicalupi, C.; Giorgi, C.; García-Gallarín, C.; López de la Torre, M.D.; Pichierri, F.; Bianchi, A.; Melguizo, M. Anion Complexes with Tetrazine-Based Ligands: Formation of Strong Anion−π Interactions in Solution and in the Solid State. Inorg. Chem. 2016, 55, 8013–8024. [Google Scholar] [CrossRef]
- Savastano, M.; García-Gallarín, C.; López de la Torre, M.D.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Anion-π and lone pair-π interactions with s-tetrazine-based ligands. Coord. Chem. Rev. 2019, 397, 112–137. [Google Scholar] [CrossRef]
- Savastano, M.; García-Gallarín, C.; Giorgi, C.; Gratteri, P.; López de la Torre, M.D.; Bazzicalupi, C.; Bianchi, A.; Melguizo, M. Solid State and Solution Study on the Formation of Inorganic Anion Complexes with a Series of Tetrazine-Based Ligands. Molecules 2019, 24, 2247. [Google Scholar] [CrossRef]
- Kaim, W. The coordination chemistry of 1,2,4,5-tetrazines. Coord. Chem. Rev. 2002, 230, 127–139. [Google Scholar] [CrossRef]
- Stetsiuk, O.; Abhervé, A.; Avarvari, N. 1,2,4,5-Tetrazine based ligands and complexes. Dalton Trans. 2020, 49, 5759–5777. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, N.; Kitos, A.A.; Brusso, J.L.; Murugesu, M. Enhancing Magnetic Communication between Metal Centres: The Role of s-Tetrazine Based Radicals as Ligands. Chem. Eur. J. 2021, 27, 5091–5106. [Google Scholar] [CrossRef] [PubMed]
- Schottel, B.L.; Chifotides, H.T.; Shatruk, M.; Chouai, A.; Pérez, L.M.; Bacsa, J.; Dunbar, K.R. Anion−π Interactions as Controlling Elements in Self-Assembly Reactions of Ag(I) Complexes with π-Acidic Aromatic Rings. J. Am. Chem. Soc. 2006, 128, 5895–5912. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulos, D.I.; Dolinar, B.S.; Vignesh, K.R.; Dunbar, K.R. Putting a New Spin on Supramolecular Metallacycles: Co3 Triangle and Co4 Square Bearing Tetrazine-Based Radicals as Bridges. J. Am. Chem. Soc. 2017, 139, 11040–11043. [Google Scholar] [CrossRef]
- Giles, I.D.; Chifotides, H.T.; Shatruck, M.; Dunbar, K.R. Anion-templated self-assembly of highly stable Fe(II) pentagonal metallacycles with short anion–π contacts. Chem. Commun. 2011, 47, 12604–12606. [Google Scholar] [CrossRef] [PubMed]
- Glöckle, M.; Hubler, K.; Kümmerer, H.-J.; Denninger, G.; Kaim, W. Dicopper(I) Complexes with Reduced States of 3,6-Bis(2‘-pyrimidyl)-1,2,4,5-tetrazine: Crystal Structures and Spectroscopic Properties of the Free Ligand, a Radical Species, and a Complex of the 1,4-Dihydro Form. Inorg. Chem. 2001, 40, 2263–2269. [Google Scholar] [CrossRef]
- Woods, T.J.; Ballesteros-Rivas, M.F.; Ostrovsky, S.M.; Palii, A.V.; Reu, O.S.; Klokishiner, S.I.; Dunbar, K.R. Strong Direct Magnetic Coupling in a Dinuclear CoII Tetrazine Radical Single-Molecule Magnet. Chem. Eur. J. 2015, 21, 10302–10305. [Google Scholar] [CrossRef]
- Woods, T.J.; Stout, H.D.; Dolinar, B.S.; Vignesh, K.R.; Ballesteros-Rivas, M.F.; Achim, C.; Dunbar, K.R. Strong Ferromagnetic Exchange Coupling Mediated by a Bridging Tetrazine Radical in a Dinuclear Nickel Complex. Inorg. Chem. 2017, 56, 12094–12097. [Google Scholar] [CrossRef]
- Lemes, M.A.; Brunet, G.; Pialat, A.; Ungur, L.; Korobkov, I.; Murugesu, M. Strong ferromagnetic exchange coupling in a {NiII4} cluster mediated through an air-stable tetrazine-based radical anion. Chem. Commun. 2017, 53, 8660–8663. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, S.K.; van der Meer, M.; Sahoo, A.; Laha, P.; Dehury, N.; Plebst, S.; Sarkar, B.; Samanta, K.; Patra, S. A dinuclear [{(p-cym)RuIICl}2(μ-bpytz•−)]+ complex bridged by a radical anion: Synthesis, spectroelectrochemical, EPR and theoretical investigation (bpytz = 3,6-bis(3,5-dimethylpyrazolyl)1,2,4,5-tetrazine; p-cym = p-cymene). Dalton Trans. 2016, 45, 12532–12538. [Google Scholar] [CrossRef] [PubMed]
- Lemes, M.A.; Stein, H.N.; Gabidullin, B.; Robeyns, K.; Clérac, R.; Murugesu, M. Probing Magnetic-Exchange Coupling in Supramolecular Squares Based on Reducible Tetrazine-Derived Ligands. Chem. Eur. J. 2018, 24, 4259–4263. [Google Scholar] [CrossRef] [PubMed]
- Nazarenko, I.; Pop, F.; Sun, Q.; Hauser, A.; Lloret, F.; Julve, M.; El-Ghayoury, A.; Avarvari, N. Structural, photophysical and magnetic properties of transition metal complexes based on the dipicolylamino-chloro-1,2,4,5-tetrazine ligand. Dalton Trans. 2015, 44, 8855–8866. [Google Scholar] [CrossRef]
- Pop, F.; Ding, J.; Lawson Daku, L.M.; Hauser, A.; Avarvari, N. Tetrathiafulvalene-s-tetrazine: Versatile platform for donor–acceptor systems and multifunctional ligands. RSC Adv. 2013, 3, 3218–3221. [Google Scholar] [CrossRef]
- Stetsiuk, O.; El-Ghayoury, A.; Hauser, A.; Avarvari, N. Dipicolylamino-methoxy-1,2,4,5-tetrazine ligand and its metal complexes: Structural and photophysical studies. Polyhedron 2019, 170, 232–238. [Google Scholar] [CrossRef]
- Stetsiuk, O.; Petrusenko, S.R.; Sorace, L.; Lupan, A.; Attia, A.A.A.; Kokozay, V.N.; El-Ghayoury, A.; Avarvari, N. Versatile coordination behaviour of the chloro-tetrazine-picolylamine ligand: Mixed-valence binuclear Cu(I)/Cu(II) complexes. Dalton Trans 2019, 48, 11966–11977. [Google Scholar] [CrossRef]
- Stetsiuk, O.; El-Ghayoury, A.; Lloret, F.; Julve, M.; Avarvari, N. Mononuclear and One-Dimensional Cobalt(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-tetrazine Ligand. Eur. J. Inorg. Chem. 2018, 449–457. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinheim, Germany, 1993; p. 18. [Google Scholar]
- Boça, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Girerd, J.J.; Charlot, M.F.; Kahn, O. Orbital interaction in one-dimensional magnetic compounds. Mol. Phys. 1977, 34, 1063–1076. [Google Scholar] [CrossRef]
- Kahn, O.; Charlot, M.F. Overlap density in binuclear complexes: A topological approach of the exchange interaction. Nouv. J. Chim. 1980, 4, 567–576. [Google Scholar]
- De Munno, G.; Julve, M.; Lloret, F.; Derory, A. 2,2′-Bipyrimidine (bpym)-bridged Dinuclear Complexes-Part 1. Preparation, Crystal Structure, and Magnetic Properties of [Ni2(H2O)8(bpym)](NO3)4 and [Ni2(H2O)8(bpym)](SO4)2·2H2O. J. Chem. Soc. Dalton Trans. 1993, 1179–1184. [Google Scholar] [CrossRef]
- McConnell, H.M. Ferromagnetism in Solid Free Radicals. J. Chem. Phys. 1963, 39, 1910. [Google Scholar] [CrossRef]
- Izuoka, A.; Murata, S.; Sugawara, T.; Iwamura, H. Molecular Design and Model Experiments of Ferromagnetic Intermolecular Interaction in the Assembly of High-spin Organic Molecules. Generation and Characterization of the Spin States of Isomeric Bis(phenylmethylenyl)[2.2]paracyclophanes. J. Am. Chem. Soc. 1987, 109, 2631–2639. [Google Scholar] [CrossRef]
- Oshio, H. Control of the Intermolecular Magnetic interaction by the Spin Polarization of dπ-Electrons: Ferromagnetic Interaction between Iron Centres through an Organic Bridging Ligand. J. Chem. Soc. Chem. Commun. 1991, 240–241. [Google Scholar] [CrossRef]
- Kollmar, C.; Couty, M.; Kahn, O. A Mechanism for the Ferromagnetic Coupling in Decamethylferrocenium Tetracyanoethenide. J. Am. Chem. Soc. 1991, 113, 7994–8005. [Google Scholar] [CrossRef]
- Kollmar, C.; Kahn, O. Spin Polarization and Ferromagnetic coupling in metallocenium charge transfer complexes. J. Chem. Phys. 1992, 96, 2988–2997. [Google Scholar] [CrossRef]
- Cargill Thompson, A.M.W.; Gatteschi, D.; McCleverty, J.A.; Navas, J.A.; Rentschler, E.; Ward, M.D. Effects of Systematic Variation in Bridging Ligand Structure on the Electrochemical and Magnetic Properties of a Series of Dinuclear Molybdenum Complexes. Inorg. Chem. 1996, 35, 2701–2703. [Google Scholar] [CrossRef]
- Hernández-Molina, R.; Mederos, A.; Gili, P.; Domínguez, S.; Lloret, F.; Cano, J.; Julve, M.; Ruiz-Pérez, C.; Solans, X. Dimer species in dimethyl sulfoxide-water (80:20 w/w) solution of N,N’-bis(salicylidene)-m-phenylenediamine (H2sal-m-phen) and similar Schiff bases with CuII, NiII, CoII and ZnII. Crystal structure of [Co2(sal-m-phen)2] · CHCl3. J. Chem. Soc. Dalton Trans. 1997, 4327–4334. [Google Scholar] [CrossRef]
- Lloret, F.; De Munno, G.; Julve, M.; Cano, J.; Ruiz, R.; Caneschi, A. Spin Polarization and Ferromagnetism in Two-Dimensional Sheetlike Cobalt(II) Polymers: [Co(L)2(NCS)2] (L = Pyrimidine or Pyrazine). Angew. Chem. Int. Ed. 1998, 37, 135–138. [Google Scholar] [CrossRef]
- Fernández, I.; Ruiz, R.; Faus, J.; Julve, M.; Lloret, F.; Cano, J.; Ottewaelder, X.; Journaux, Y.; Muñoz, M.C. Ferromagnetic Coupling through Spin Polarization in a Dinuclear Copper(II) Metallacyclophane. Angew. Chem. Int. Ed. 2001, 40, 3039–3042. [Google Scholar] [CrossRef]
- Pardo, E.; Morales-Osorio, I.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Pasán, J.; Ruiz-Pérez, C.; Ottewaelder, X.; Journaux, Y. Magnetic Anisotropy of a High-Spin Octanuclear Nickel(II) Complex with a meso-Helicate Core. Inorg. Chem. 2004, 43, 7594–7596. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Bernot, K.; Julve, M.; Lloret, F.; Cano, J.; Ruiz-García, R.; Delgado, F.S.; Ruiz-Pérez, C.; Ottenwaelder, X.; Journaux, Y. Spin Control in Ladderlike Hexanuclear Copper(II) Complexes with Metallacyclophane Cores. Inorg. Chem. 2004, 43, 2768–2770. [Google Scholar] [CrossRef] [PubMed]
- Foxon, S.P.; Torres, G.R.; Walter, O.; Pedersen, J.Z.; Toflund, H.; Hüber, M.; Falk, K.; Haase, W.; Cano, J.; Lloret, F.; et al. Syntheses, Structures, and Magnetic Properties of Copper(II) Complexes with 1,3-[Bis(2-pyridylmethyl)amino]benzene (1,3-tpbd) as Ligand. Eur. J. Inorg. Chem. 2004, 335–343. [Google Scholar] [CrossRef]
- Pascu, M.; Lloret, F.; Avarvari, N.; Julve, M.; Andruh, M. Ferromagnetic Coupling through Spin Polarization in the Hexanuclear [MnII3CuII3] Complex. Inorg. Chem. 2004, 43, 5189–5191. [Google Scholar] [CrossRef] [PubMed]
- Foxon, S.P.; Walter, O.; Koch, R.; Rupp, H.; Müller, P.; Schindler, S. Tuning the Magnetic Properties of a Dinuclear Copper Complex: From Ferromagnetic to Antiferromagnetic Coupling. Eur. J. Inorg. Chem. 2004, 344–348. [Google Scholar] [CrossRef]
- Paital, A.R.; Mitra, T.; Ray, D.; Wong, W.T.; Ribas-Ariño, J.; Novoa, J.J.; Ribas, J.; Aromí, G. Substituted m-phenylene bridges as strong ferromagnetic couplers for CuII-bridge-CuII magnetic interactions: New perspectives. Chem Commun. 2005, 5172–5174. [Google Scholar] [CrossRef]
- Cangussu, D.; Pardo, E.; Dul, M.-C.; Lescouëzec, R.; Herson, P.; Journaux, Y.; Pedroso, E.F.; Pereira, C.L.M.; Stumpf, H.O.; Muñoz, M.C.; et al. Rational design of a new class of heterobimetallic molecule-based magnets. Synthesis, crystal structures, and magnetic properties of oxamato-bridged M’3M2 (M’ = LiI and MnII·; M = NiII and CoII) open frameworks with a three-dimensional honeycomb architecture. Inorg. Chim. Acta 2008, 361, 3304–3402. [Google Scholar]
- Turba, S.; Walter, O.; Schindler, S.; Nielsen, L.P.; Hazell, A.; McKenzie, C.J.; Lloret, F.; Cano, J.; Julve, M. Syntheses, Structures, and Properties of Copper(II) Complexes of Bis(2-pyridylmethyl) Derivatives of o-, m-, and p-Phenylenediamine and Aniline. Inorg. Chem. 2008, 47, 9612–9623. [Google Scholar] [CrossRef]
- Palacios, M.A.; Rodríguez-Diéguez, A.; Sironi, A.; Herrera, J.M.; Mota, A.; Cano, J.; Colacio, E. Enhanced ferromagnetic interaction in metallacyclic complexes incorporating m-phenylenediamidate bridges. Dalton Trans. 2009, 8538–8547. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Fabelo, O.; Castellano, M.; Cano, J.; Fordham, S.; Zhou, H.-C. Multielectron oxidation in a ferromagnetically coupled nickel(II) triple mesocate. Chem. Commun. 2015, 51, 13381–13384. [Google Scholar] [CrossRef]
- Fernandes, T.S.; Vilela, R.S.; Valdo, A.K.; Martins, F.T.; García-España, E.; Inclán, M.; Cano, J.; Lloret, F.; Julve, M.; Stumpf, H.O.; et al. Dicopper(II) Metallacyclophanes with N,N′-2,6-Pyridinebis(oxamate): Solution Study, Synthesis, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2016, 55, 2390–2401. [Google Scholar] [CrossRef] [PubMed]
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Formula | C24H16F12N8O4Cu | C24H16F12N8O4Ni | C36H26F24N8O10Cu2 | C38H24F24N10O8Ni2 |
| M [g∙mol−1] | 771.99 | 767.16 | 1313.75 | 1322.05 |
| T [K] | 89.9(3) | 150.00(10) | 150.00(10) | 149.56(10) |
| Crystal system | monoclinic | monoclinic | triclinic | triclinic |
| Space group | P21/n | P21/n | P-1 | P-1 |
| a [Å] | 9.479(3) | 9.4370(10) | 9.901(7) | 9.2160(2) |
| b [Å] | 14.191(5) | 14.299(2) | 11.416(7) | 10.5636(3) |
| c [Å] | 22.002(6) | 22.162(4) | 11.823(3) | 13.4199(3) |
| α [°] | 90 | 90 | 91.277(4) | 89.369(2) |
| β [°] | 98.581(2) | 99.569(15) | 96.619(5) | 77.005(2) |
| γ [°] | 90 | 90 | 100.342(5) | 73.297(2) |
| V [Å3] | 2926.5(16) | 2948.9(7) | 1304.6(13) | 1217.30(5) |
| Z | 4 | 4 | 1 | 1 |
| ρcalcd [g cm−3] | 1.752 | 1.728 | 1.672 | 1.803 |
| μ [mm−1] | 2.216 | 2.103 | 2.343 | 2.388 |
| Goodness-of-fit on F2 | 1.027 | 1.041 | 1.080 | 1.055 |
| Final R1 a/wR2 b [I > 2σ(I)] | 0.0632/0.1576 | 0.0555/0.1457 | 0.0881/0.2453 | 0.0573/0.1524 |
| R1a/wR2b (all data) | 0.0698/0.1635 | 0.0600/0.1498 | 0.0931/0.2525 | 0.0625/0.1589 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stetsiuk, O.; El-Ghayoury, A.; Lloret, F.; Julve, M.; Avarvari, N. Mono- and Binuclear Copper(II) and Nickel(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-Tetrazine Ligand. Molecules 2021, 26, 2122. https://doi.org/10.3390/molecules26082122
Stetsiuk O, El-Ghayoury A, Lloret F, Julve M, Avarvari N. Mono- and Binuclear Copper(II) and Nickel(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-Tetrazine Ligand. Molecules. 2021; 26(8):2122. https://doi.org/10.3390/molecules26082122
Chicago/Turabian StyleStetsiuk, Oleh, Abdelkrim El-Ghayoury, Francesc Lloret, Miguel Julve, and Narcis Avarvari. 2021. "Mono- and Binuclear Copper(II) and Nickel(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-Tetrazine Ligand" Molecules 26, no. 8: 2122. https://doi.org/10.3390/molecules26082122
APA StyleStetsiuk, O., El-Ghayoury, A., Lloret, F., Julve, M., & Avarvari, N. (2021). Mono- and Binuclear Copper(II) and Nickel(II) Complexes with the 3,6-Bis(picolylamino)-1,2,4,5-Tetrazine Ligand. Molecules, 26(8), 2122. https://doi.org/10.3390/molecules26082122









