Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed
Abstract
1. Introduction
2. Results
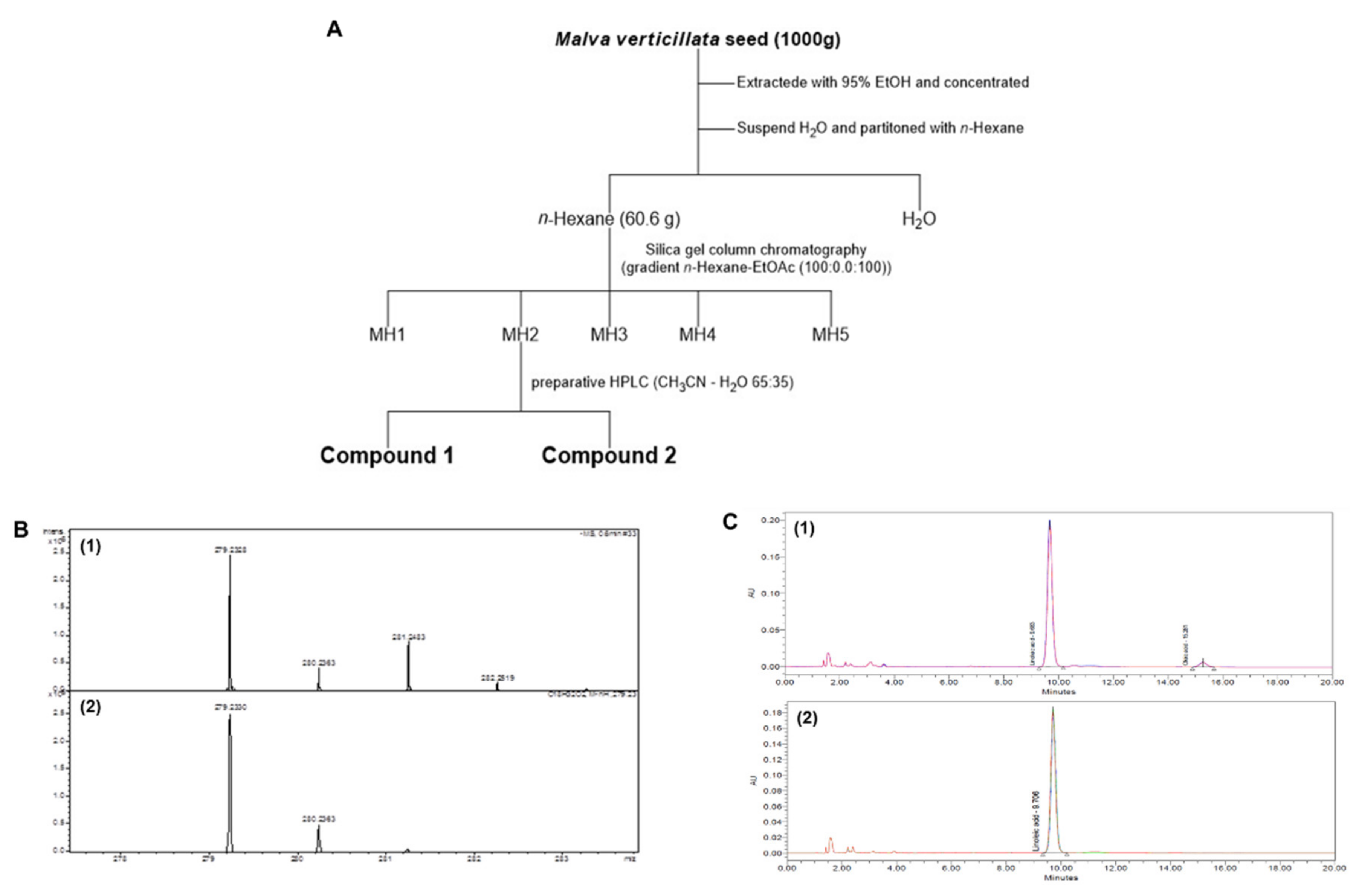
2.1. Malva verticillata Seed Extraction and Active Ingredient Separation
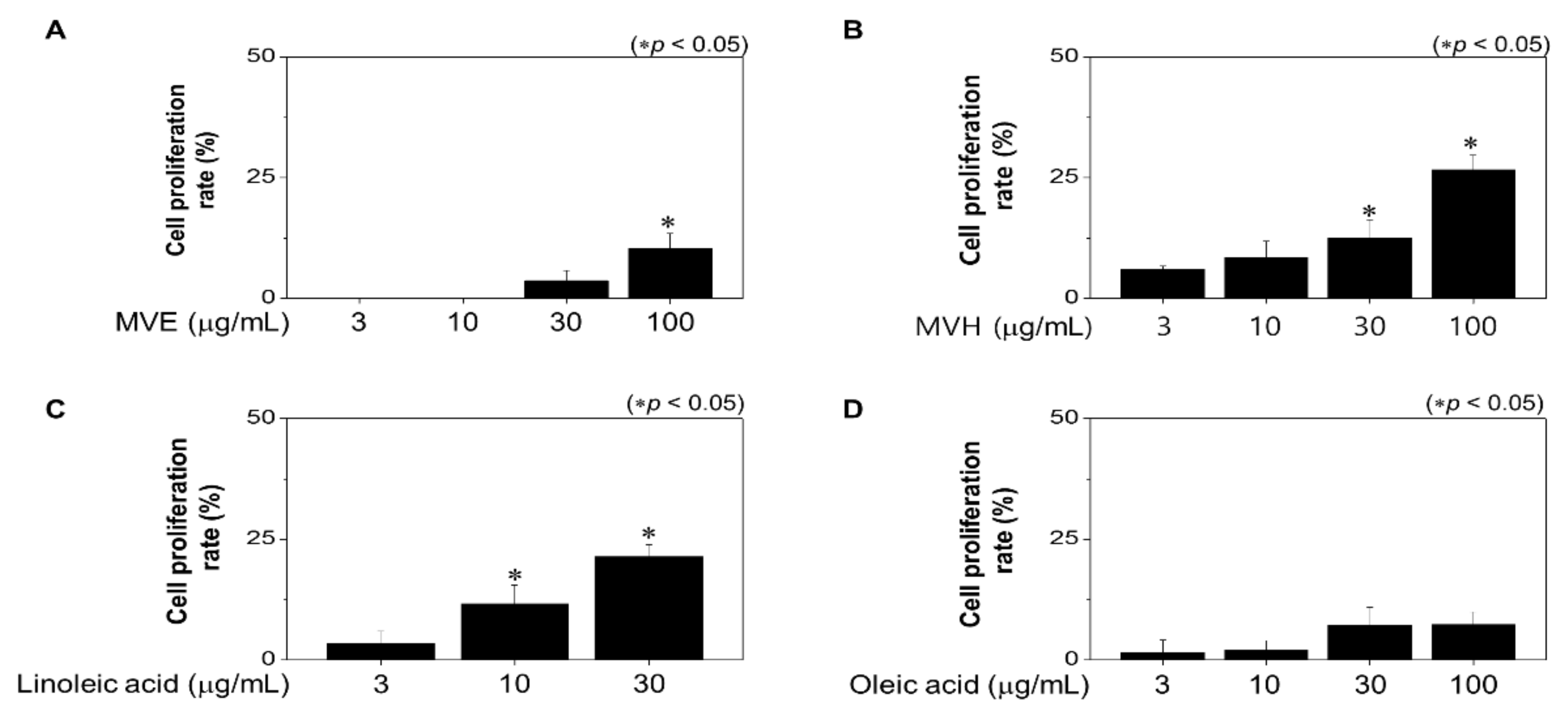
2.2. Effects of Malva verticillata Extract and LA Treatment on Hair DPC Proliferation
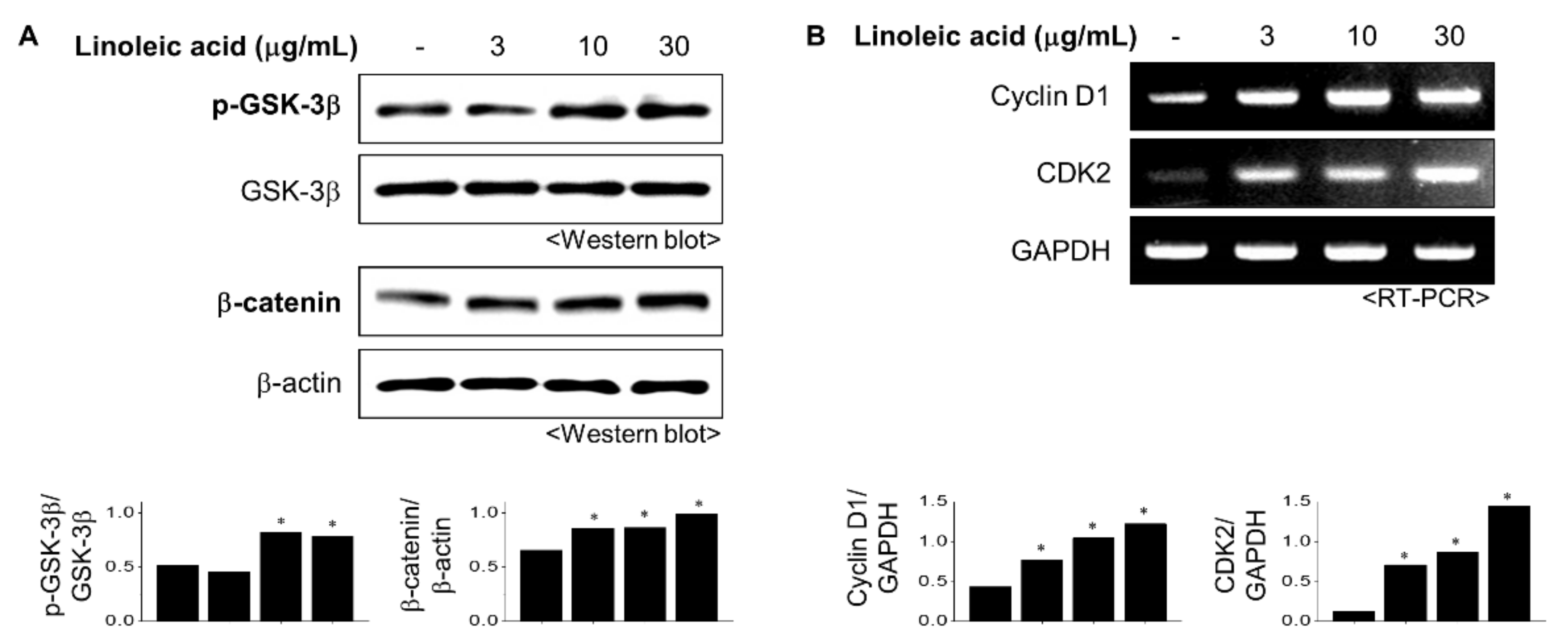
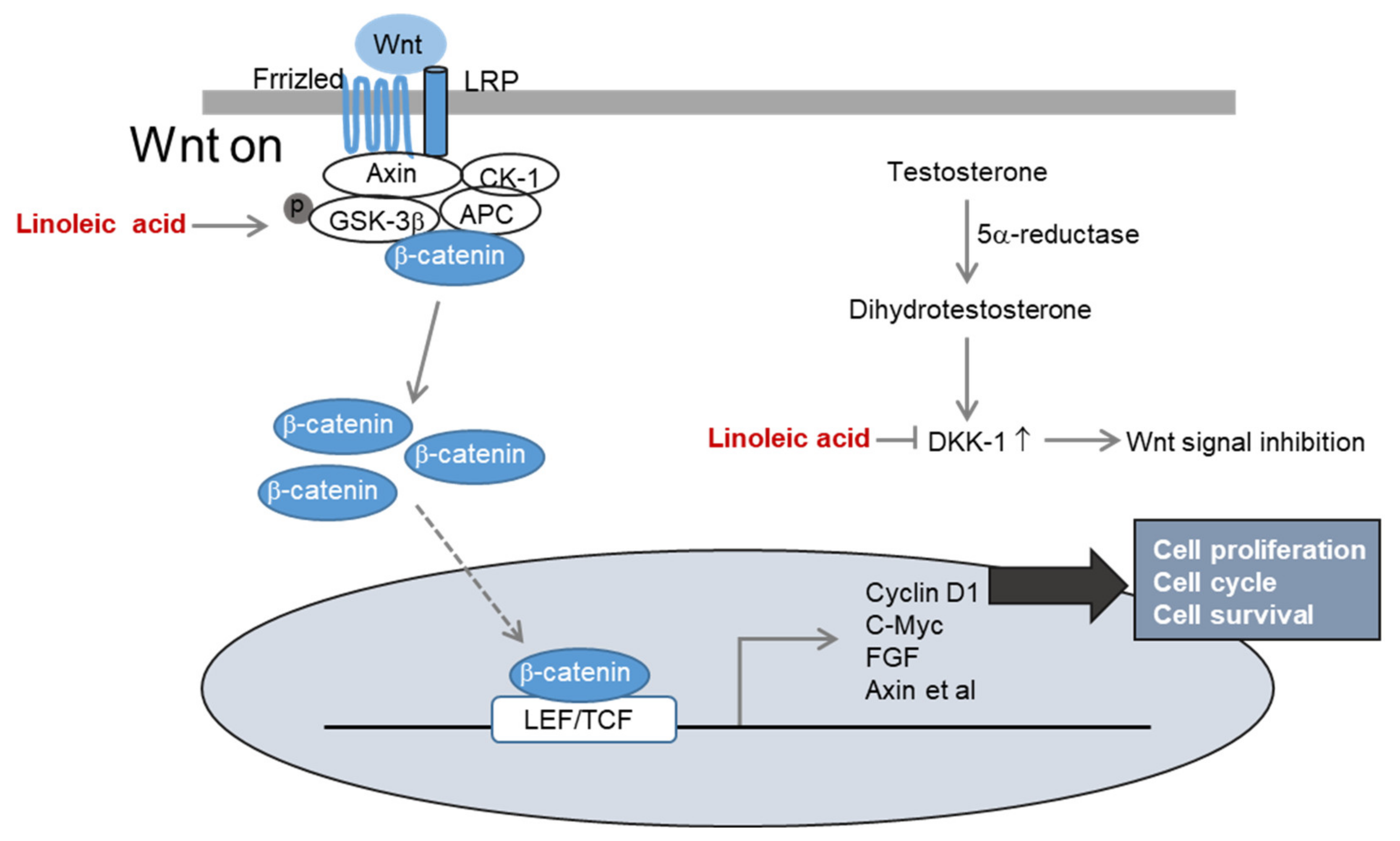
2.3. Effects of LA Treatment on the Wnt/β-Catenin Pathway
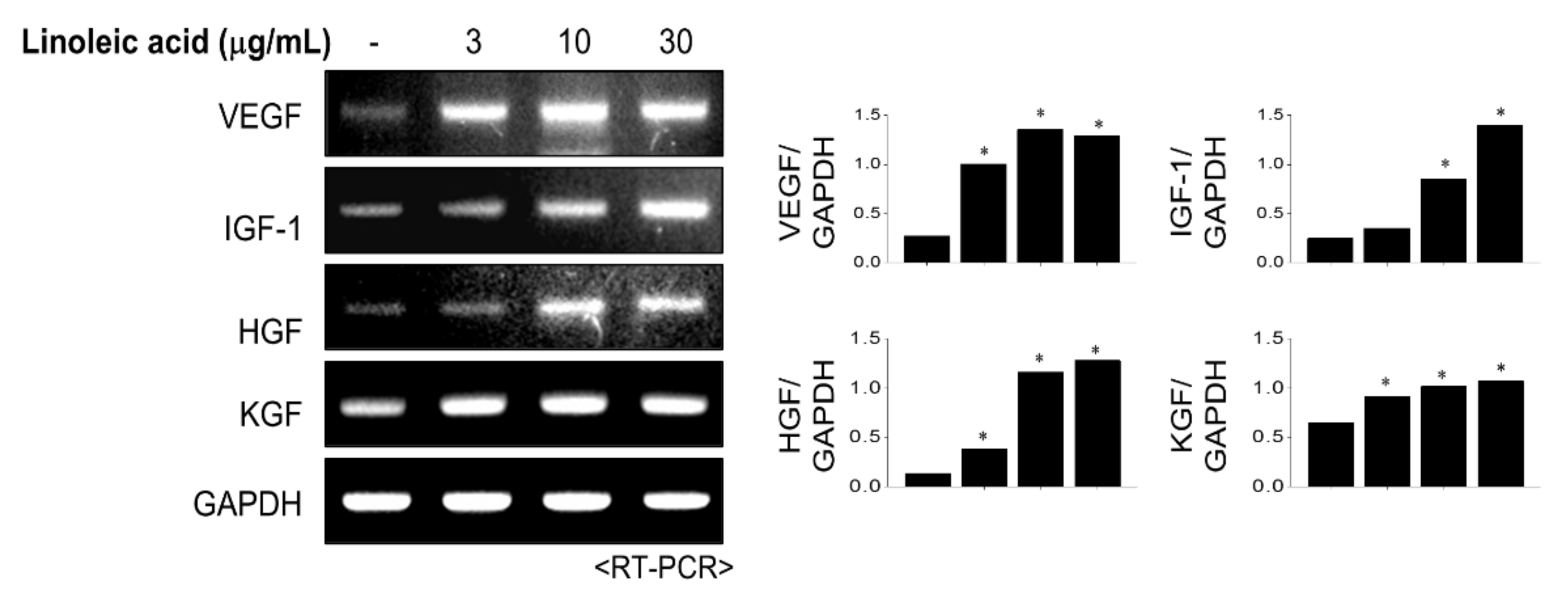
2.4. Effects of LA Treatment on Expression of Hair Growth Factors
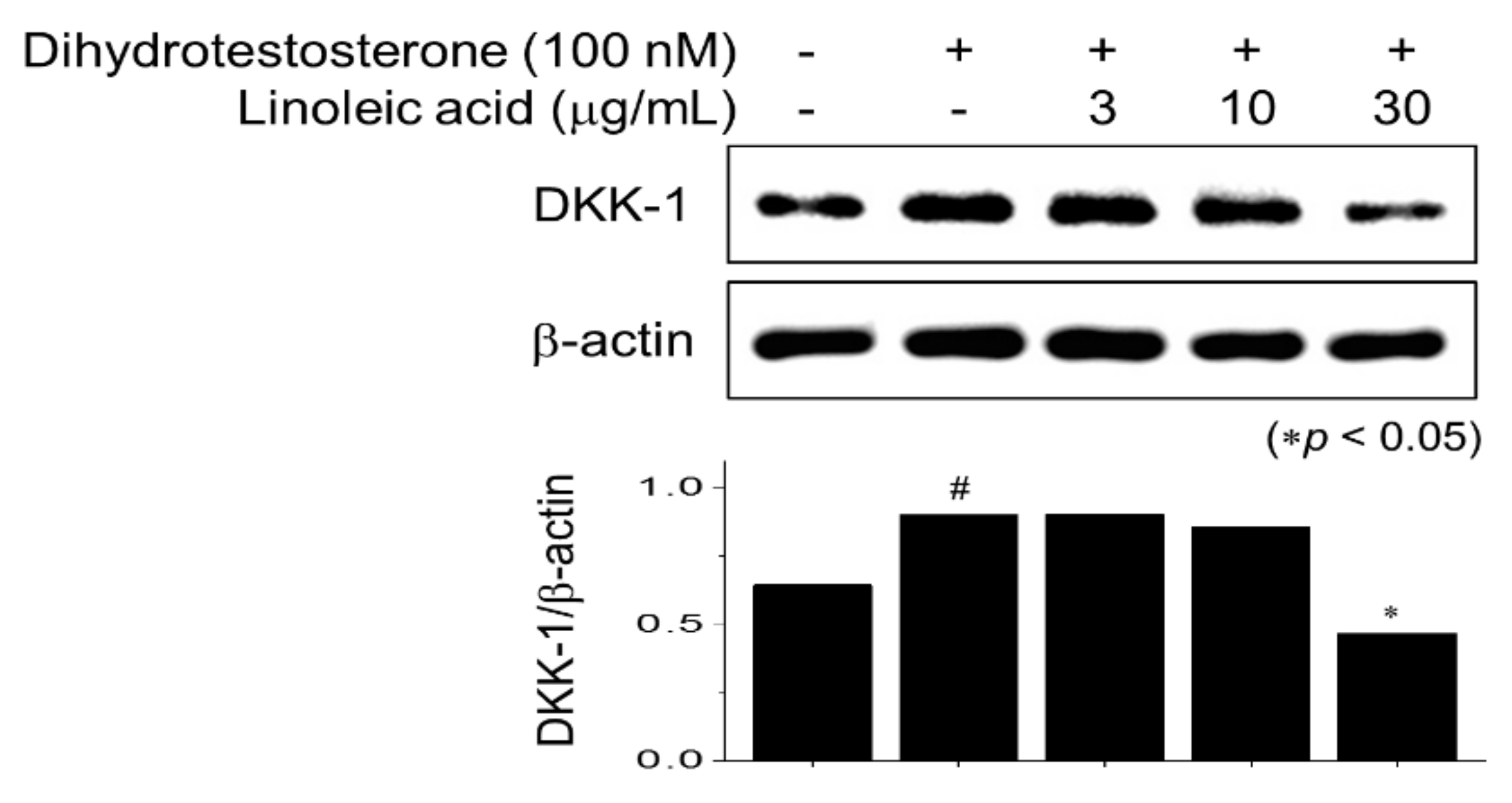
2.5. Inhibitory Effects of LA Treatment on Dihydrotestosterone-Induced DKK-1 Expression
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Malva verticillata Seed Extraction and Active Ingredient Separation
4.3. Cell Culture
4.4. Cell Proliferation Assay
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Western Blotting (WB)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| CDK | Cyclin-dependent kinase |
| DPC | Dermal papilla cells |
| LA | Linoleic acid |
| HFDPC | Human hair dermal papilla cells |
| DKK-1 | Dickkopf-related protein-1 |
References
- Son, K.H.; Suh, B.S.; Jeong, H.S.; Nam, M.W.; Kim, H.; Kim, H.C. Relationship between working hours and probability to take alopecia medicine among Korean male workers: A 4-year follow-up study. Ann. Occup. Environ. Med. 2019, 31. [Google Scholar] [CrossRef]
- Dinh, Q.Q.; Sinclair, R. Female pattern hair loss: Current treatment concepts. Clin. Interv. Aging. 2007, 2, 189–199. [Google Scholar]
- Alonso, L.; Fuchs, E. The hair cycle. J. Cell. Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef]
- Lai, J.J.; Chang, P.; Lai, K.P.; Chen, L.; Chang, C. The role of androgen and androgen receptor in skin-related disorders. Arch. Dermatol. Res. 2012, 304, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Milner, Y.; Sudnik, J.; Filippi, M.; Kizoulis, M.; Kashgarian, M.; Stenn, K. Exogen, shedding phase of the hair growth cycle: Characterization of a mouse model. J. Invest. Dermatol. 2002, 119, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A clinical and pathophysiological review. An. Bras. Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, L.Q.; Wang, Y.J.; Yang, W.; Geng, W.X.; Wei, J.; Li, L.W.; Chen, F.L. Treatment of alopecia by transplantation of hair follicle stem cells and dermal papilla cells encapsulated in alginate gels. Med. Hypotheses 2008, 70, 1014–1016. [Google Scholar] [CrossRef]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell. Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Song, Z.; Hao, F.; Yang, X. Identification of Wnt/beta-catenin signaling pathway in dermal papilla cells of human scalp hair follicles: TCF4 regulates the proliferation and secretory activity of dermal papilla cell. J. Dermatol. 2014, 41, 84–91. [Google Scholar] [CrossRef]
- Zakhem, G.A.; Goldberg, J.E.; Motosko, C.C.; Cohen, B.E.; Ho, R.S. Sexual dysfunction in men taking systemic dermatologic medication: A systematic review. J. Am. Acad. Dermatol. 2019, 81, 163–172. [Google Scholar] [CrossRef]
- Varothai, S.; Bergfeld, W.F. Androgenetic alopecia: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 217–230. [Google Scholar] [CrossRef]
- Traish, A.M. Post-finasteride syndrome: A surmountable challenge for clinicians. Fertil. Steril. 2020, 113, 21–50. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Marks, J.G., Jr. Pseudoacromegaly induced by the long-term use of minoxidil. J. Am. Acad. Dermatol. 2003, 48, 962–965. [Google Scholar] [CrossRef]
- Dawber, R.P.; Rundegren, J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 271–275. [Google Scholar] [CrossRef]
- Rogers, N.E.; Avram, M.R. Medical treatments for male and female pattern hair loss. J. Am. Acad. Dermatol. 2008, 59, 547–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Choi, E.J.; Kim, J.A.; Hwang, Y.L.; Kim, C.D.; Lee, M.H.; Roh, S.S.; Kim, Y.H.; Han, I.; Kang, S. Malva verticillata seed extracts upregulate the Wnt pathway in human dermal papilla cells. Int. J. Cosmet. Sci. 2016, 38, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gonda, R.; Tomoda, M.; Shimizu, N.; Kanari, M. Characterization of an acidic polysaccharide from the seeds of Malva verticillata stimulating the phagocytic activity of cells of the RES. Planta Med. 1990, 56, 73–76. [Google Scholar] [CrossRef]
- Fritsche, K.L. Linoleic acid, vegetable oils & inflammation. Mo. Med. 2014, 111, 41–43. [Google Scholar] [PubMed]
- Whelan, J.; Fritsche, K. Linoleic acid. Adv. Nutr. 2013, 4, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, S.Y. Anti-cancer activities of omega-6 polyunsaturated fatty acids. Biomed. J. 2014, 37, 112–119. [Google Scholar]
- Lu, X.; Yu, H.; Ma, Q.; Shen, S.; Das, U.N. Linoleic acid suppresses colorectal cancer cell growth by inducing oxidant stress and mitochondrial dysfunction. Lipids Health Dis. 2010, 9, 106–116. [Google Scholar] [CrossRef]
- Maggiora, M.; Bologna, M.; Ceru, M.P.; Possati, L.; Angelucci, A.; Cimini, A.; Miglietta, A.; Bozzo, F.; Margiotta, C.; Muzio, G.; et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int. J. Cancer 2004, 112, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, F.; Agostoni, C.; Borghi, C.; Catapano, A.L.; Cena, H.; Ghiselli, A.; La Vecchia, C.; Lercker, G.; Manzato, E.; Pirillo, A.; et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 2020, 292, 90–98. [Google Scholar] [CrossRef]
- Bruen, R.; Fitzsimons, S.; Belton, O. Atheroprotective effects of conjugated linoleic acid. Br. J. Clin. Pharmacol. 2017, 83, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Thom, E.; Wadstein, J.; Gudmundsen, O. Conjugated linoleic acid reduces body fat in healthy exercising humans. J. Int. Med. Res. 2001, 29, 392–396. [Google Scholar] [CrossRef]
- den Hartigh, L.J. Conjugated Linoleic Acid Effects on Cancer, Obesity, and Atherosclerosis: A Review of Pre-Clinical and Human Trials with Current Perspectives. Nutrients 2019, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Lee, H.K.; Kim, J.S.; Kim, Y.G.; Pyo, M.; Yun, J.; Hwang, B.Y.; Hong, J.T.; Kim, Y.; Han, S.B. Saucerneol D inhibits dendritic cell activation by inducing heme oxygenase-1, but not by directly inhibiting toll-like receptor 4 signaling. J. Ethnopharmacol. 2015, 166, 92–101. [Google Scholar] [CrossRef]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid. Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Barison, A.; da Silva, C.W.; Campos, F.R.; Simonelli, F.; Lenz, C.A.; Ferreira, A.G. A simple methodology for the determination of fatty acid composition in edible oils through 1H NMR spectroscopy. Magn. Reason. Chem. 2010, 48, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Niehrs, C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 2006, 25, 7469–7481. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.K.; Shao, C.; Wang, J.; Wei, Q.; Wang, X.; Collier, Z.; Tang, S.; Liu, H.; Zhang, F.; Huang, J.; et al. Wnt/ β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis, and cancer chemoresistance. Genes Dis. 2016, 3, 11–40. [Google Scholar] [CrossRef]
- Rastegar, H.; Ashtiani, H.A.; Aghaei, M.; Barikbin, B.; Ehsani, A. Herbal Extracts Induce Dermal Papilla Cell Proliferation of Human Hair Follicles. Ann. Dermatol. 2015, 27, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Invest. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
| Name | Primer | Gene Bank Accession | |
|---|---|---|---|
| Forward | Reverse | ||
| Cyclin D1 | CGTGGCCTCTAAGATGAAGG | TGCGGATGATCTGTTTGTTC | CR542099.1 |
| CDK2 | AAAGCCAGAAACAAGTTGACG | GAGATCTCTCGGATGGCAGT | X62071.1 |
| VEGF | AAGGAGGAGGGCAGAATCAT | TTTCTTGCGCTTTCGTTTTT | NM001025368 |
| IGF-1 | TCAACAAGCCCACAGGGTAT | CGTGCAGAGCAAAGGAT | A29119.1 |
| HGF | GCCTGAAAGATATCCCGACA | TTCCATGTTCTTGTCCCACA | NM001010932.3 |
| KGF | GACATGGATCCTGCCAACTT | AATTCCAACTGCCACTGTCC | S81661.1 |
| GAPDH | TCCATGACAACTTTGGTATC | TGTAGCCAAATTCGTTGTCA | NM001289745.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.-N.; Park, S.-M.; Lee, Y.-M. Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules 2021, 26, 2117. https://doi.org/10.3390/molecules26082117
Ryu HS, Jeong J, Lee CM, Lee KS, Lee J-N, Park S-M, Lee Y-M. Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules. 2021; 26(8):2117. https://doi.org/10.3390/molecules26082117
Chicago/Turabian StyleRyu, Hwa Sun, JiYeon Jeong, Chun Mong Lee, Kwang Sik Lee, Jung-No Lee, Sung-Min Park, and Yong-Moon Lee. 2021. "Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed" Molecules 26, no. 8: 2117. https://doi.org/10.3390/molecules26082117
APA StyleRyu, H. S., Jeong, J., Lee, C. M., Lee, K. S., Lee, J.-N., Park, S.-M., & Lee, Y.-M. (2021). Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules, 26(8), 2117. https://doi.org/10.3390/molecules26082117






