β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats
Abstract
1. Introduction
2. Results
2.1. SIT Reduces the Body Weight of HFD and Sucrose Fed Type 2 Diabetic Rats
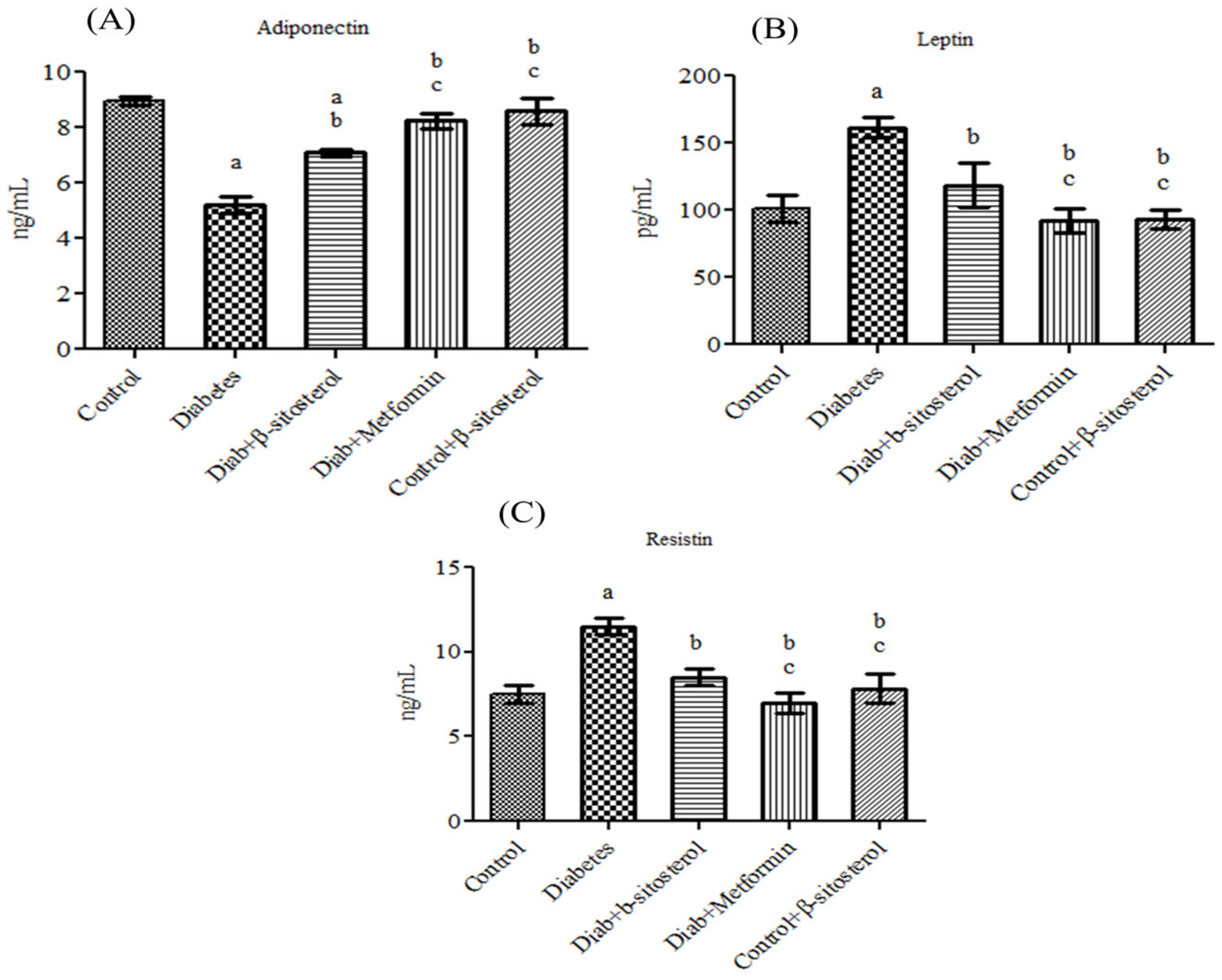
2.2. SIT Normalizes the Altered Levels of Serum Adipokines, SREP-1c and PPAR-γ in Diabetic Rats
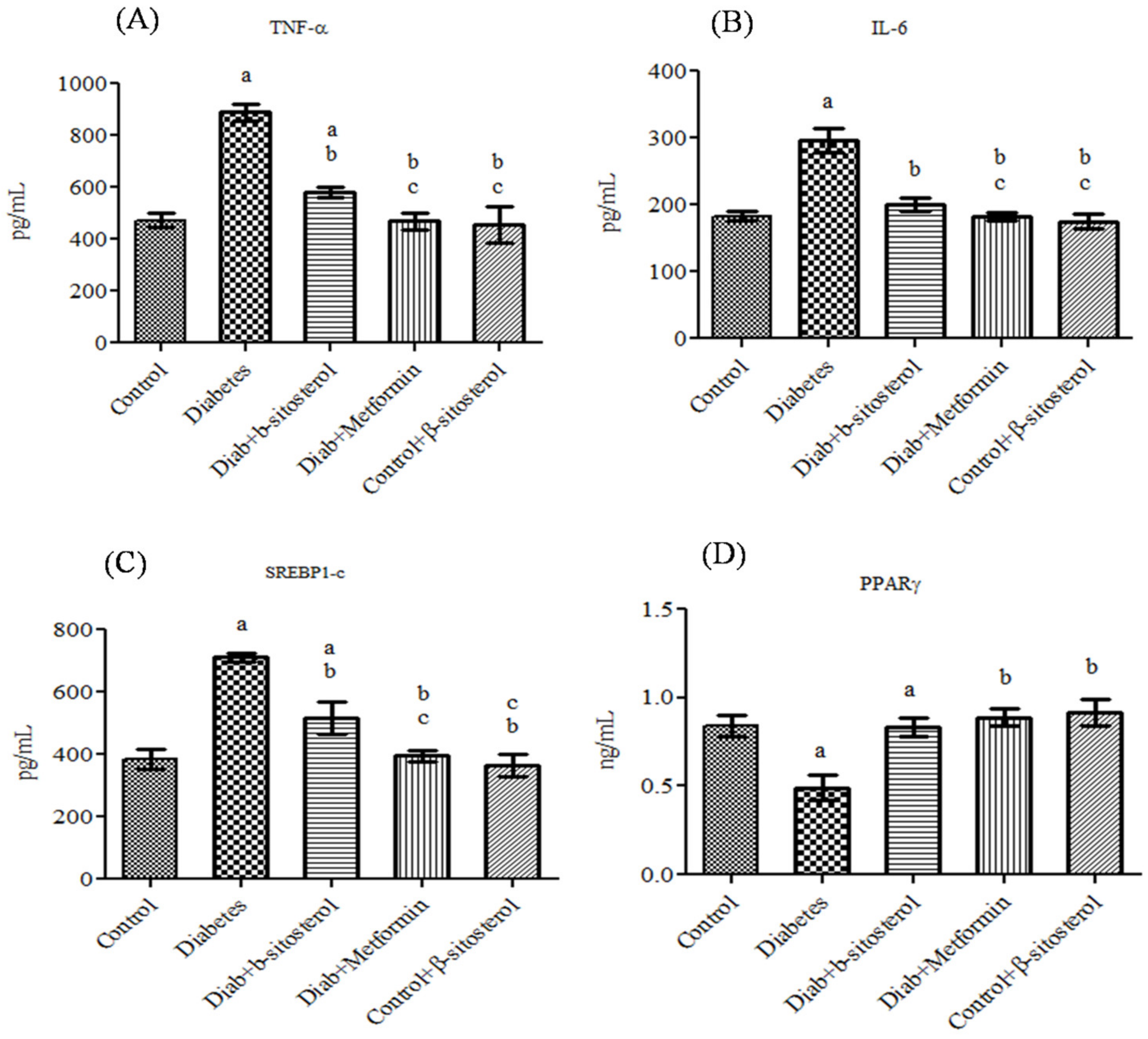
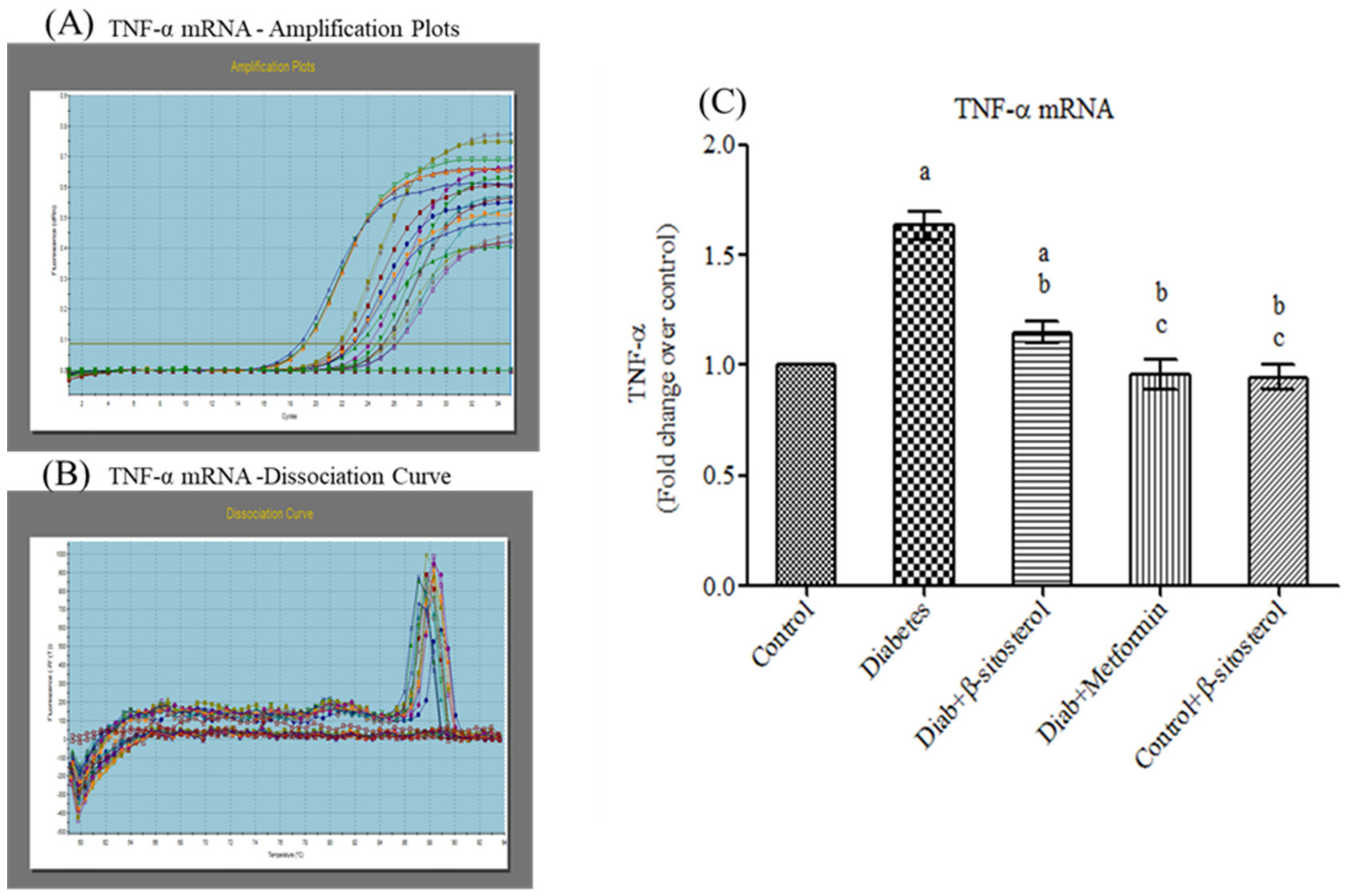
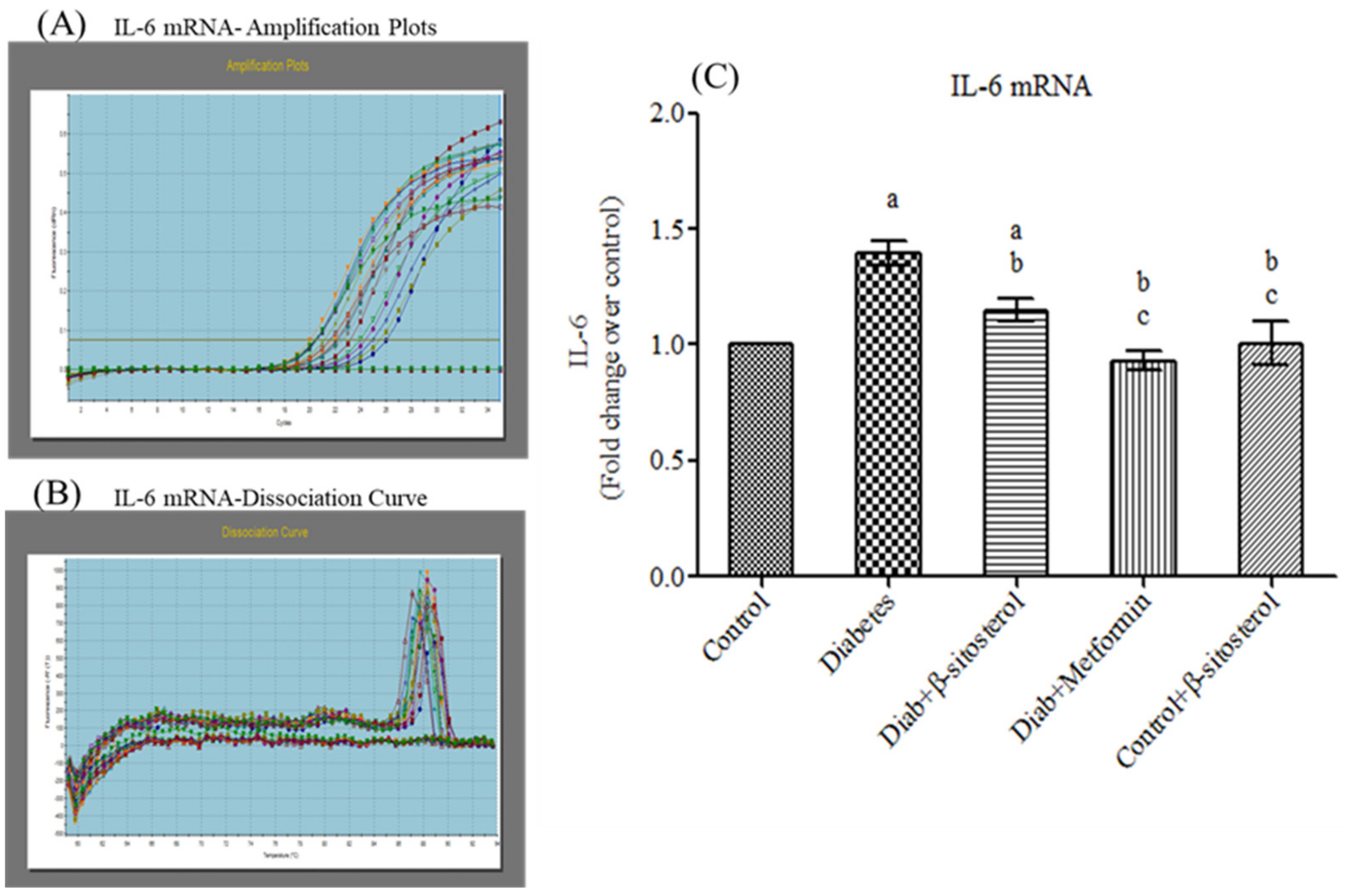
2.3. SIT Declines the Gene and Protein Expression of Proinflammatory Cytokines (TNF-α and IL- 6) in the Adipose Tissue
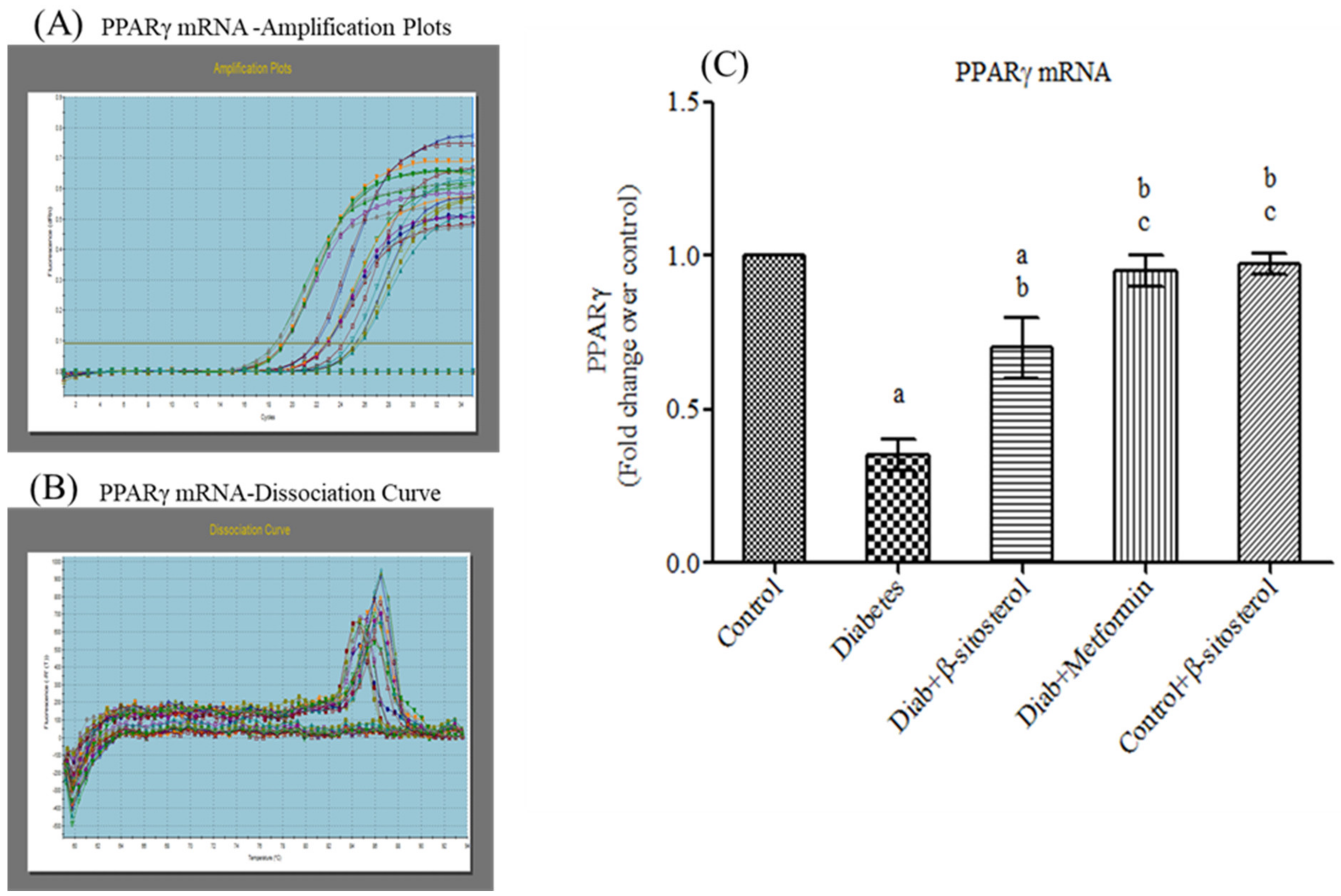
2.4. SIT Upregulates the Gene Expression of PPAR-γ in Adipose Tissue
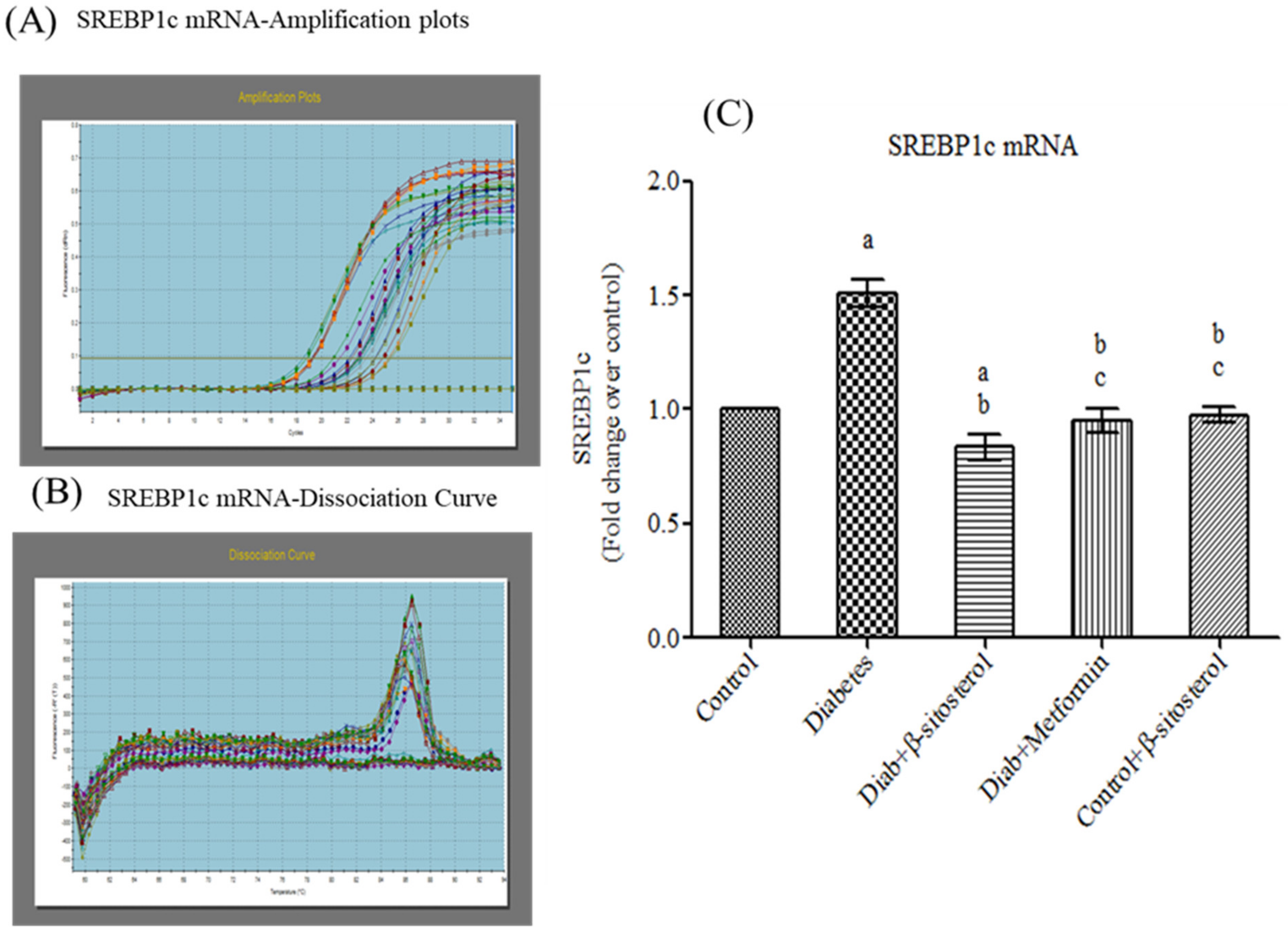
2.5. SIT Downregulates the Gene Expression of SREBP-1c in Adipose Tissue
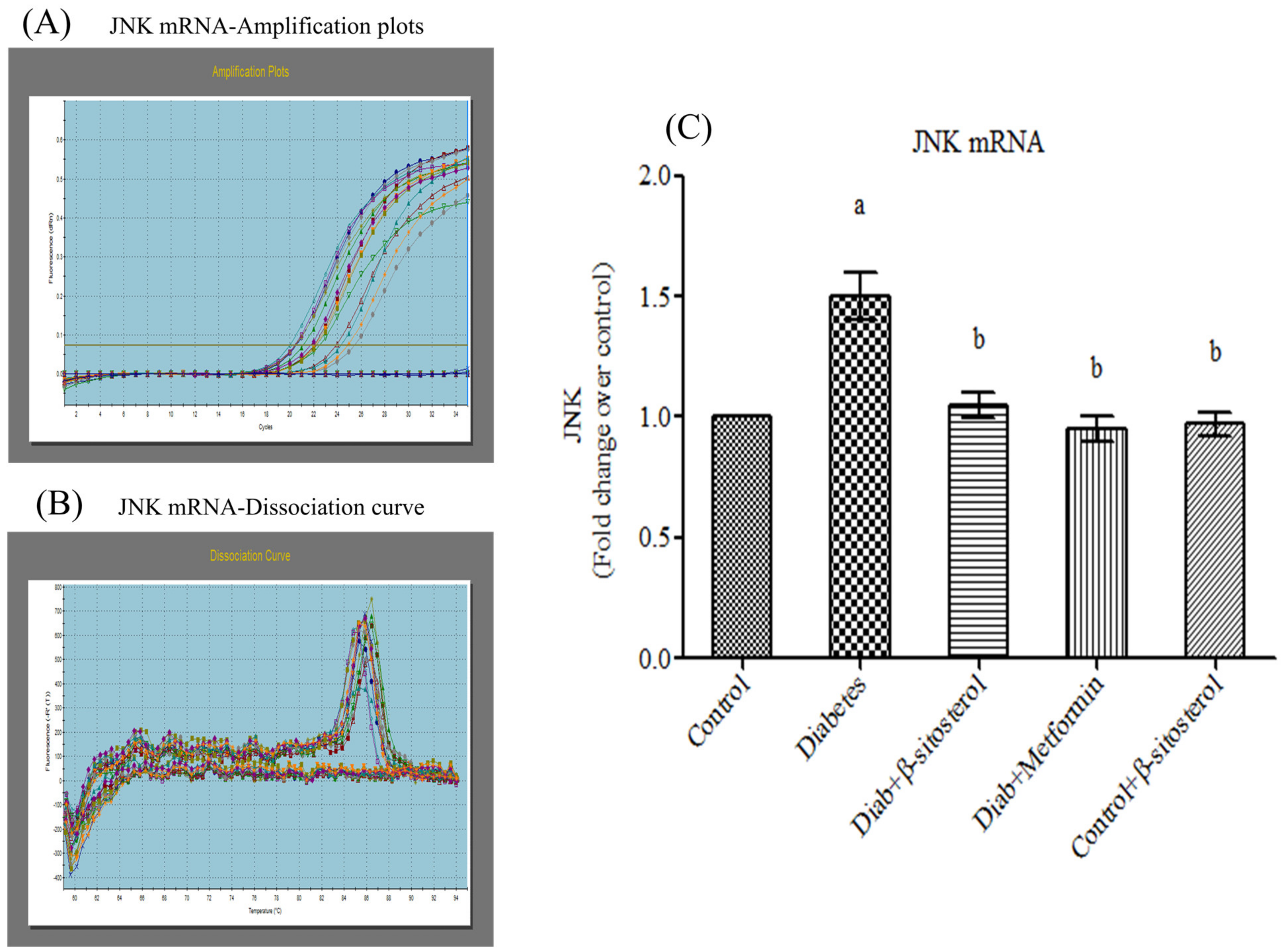
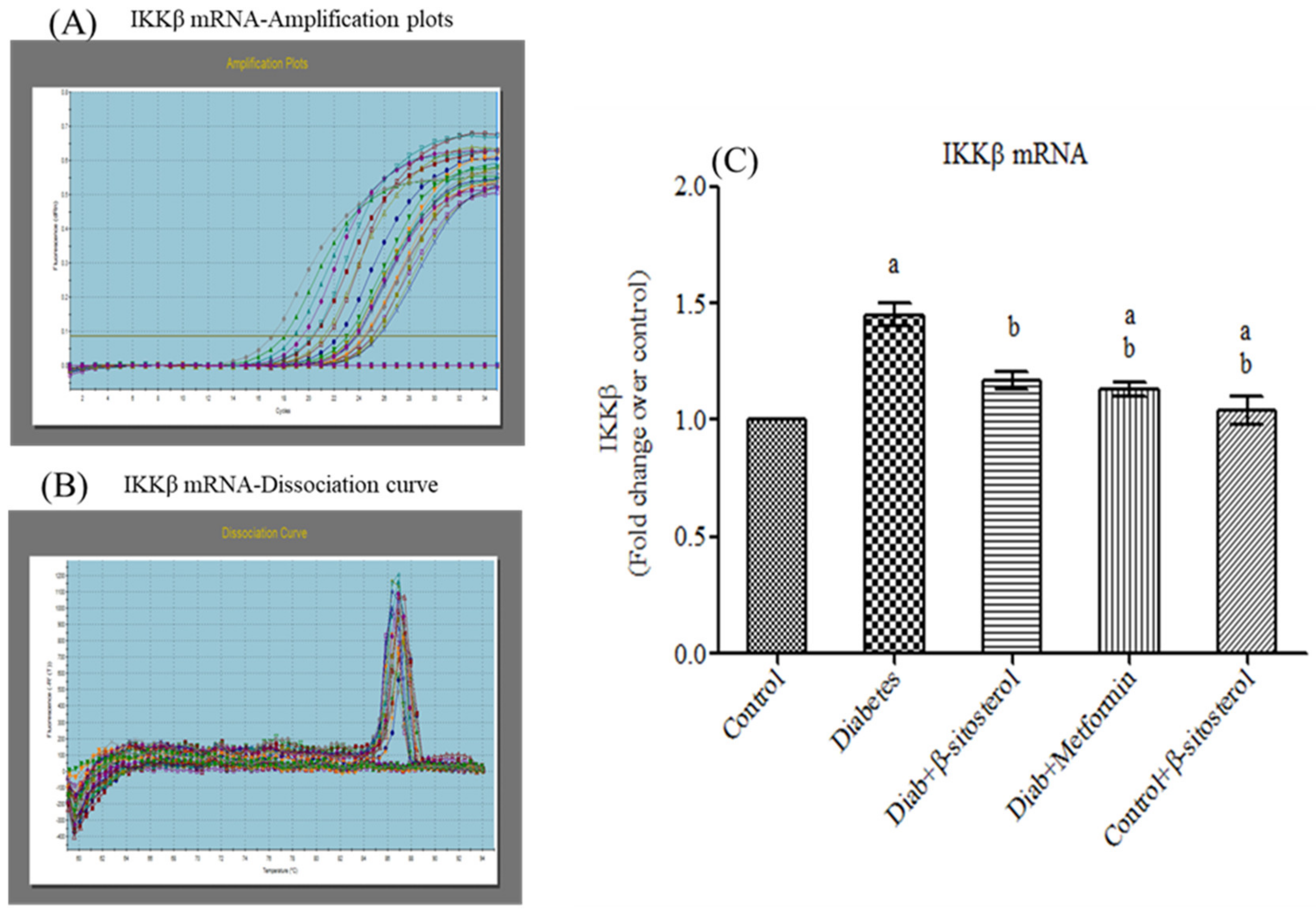
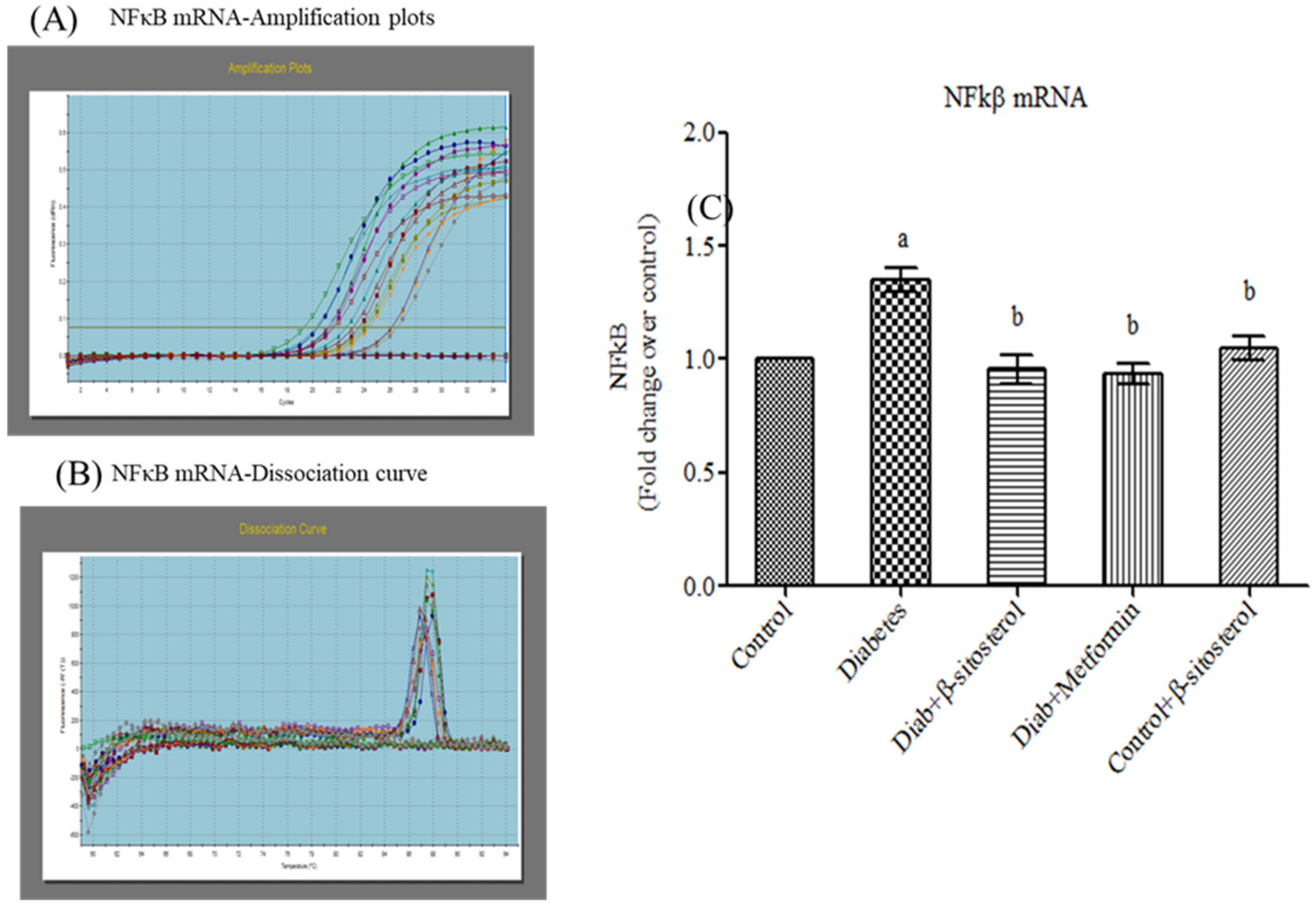
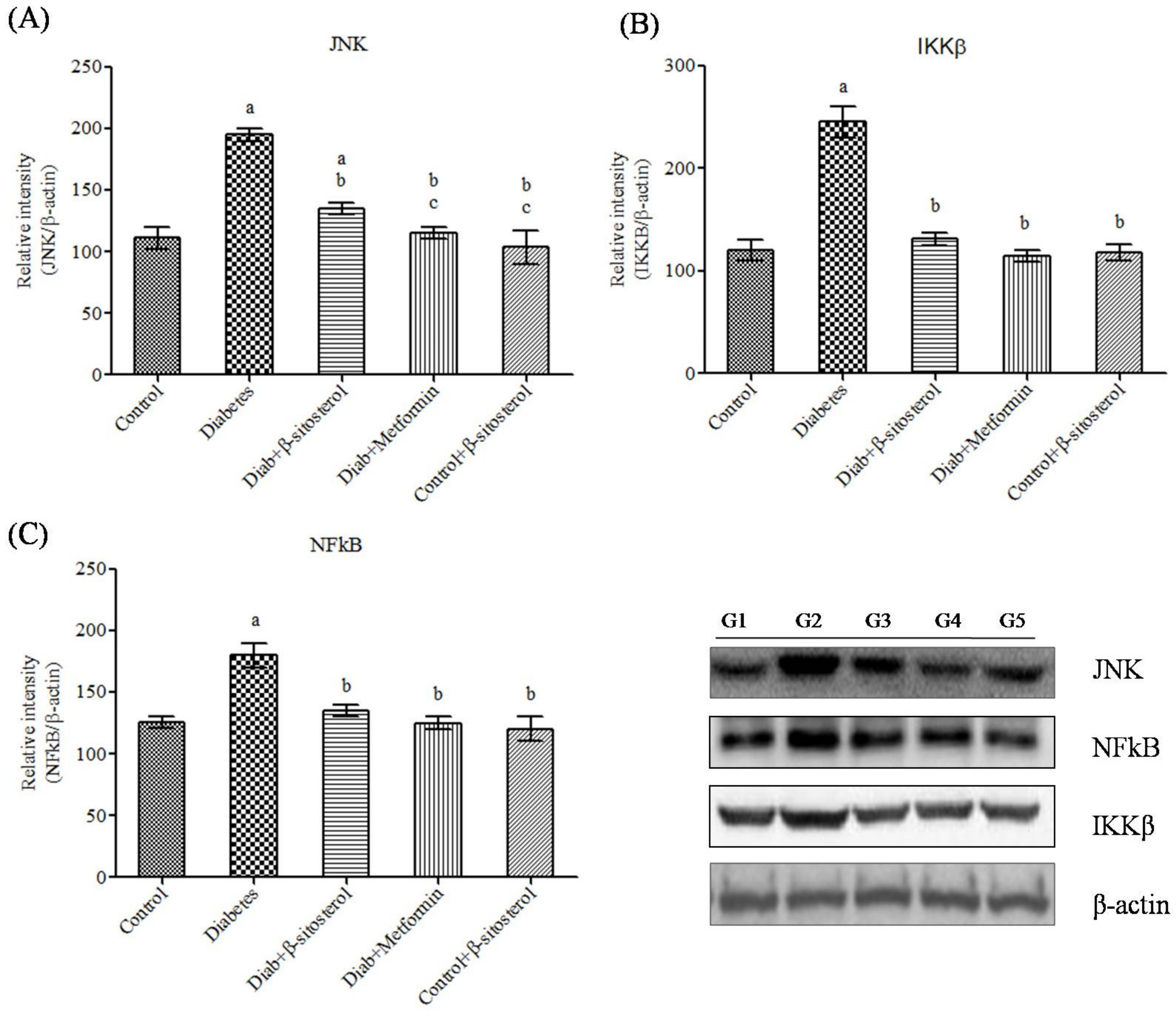
2.6. SIT Declines the Gene and Protein Expression of JNK, IKKβ and NF-κB in Adipose Tissue
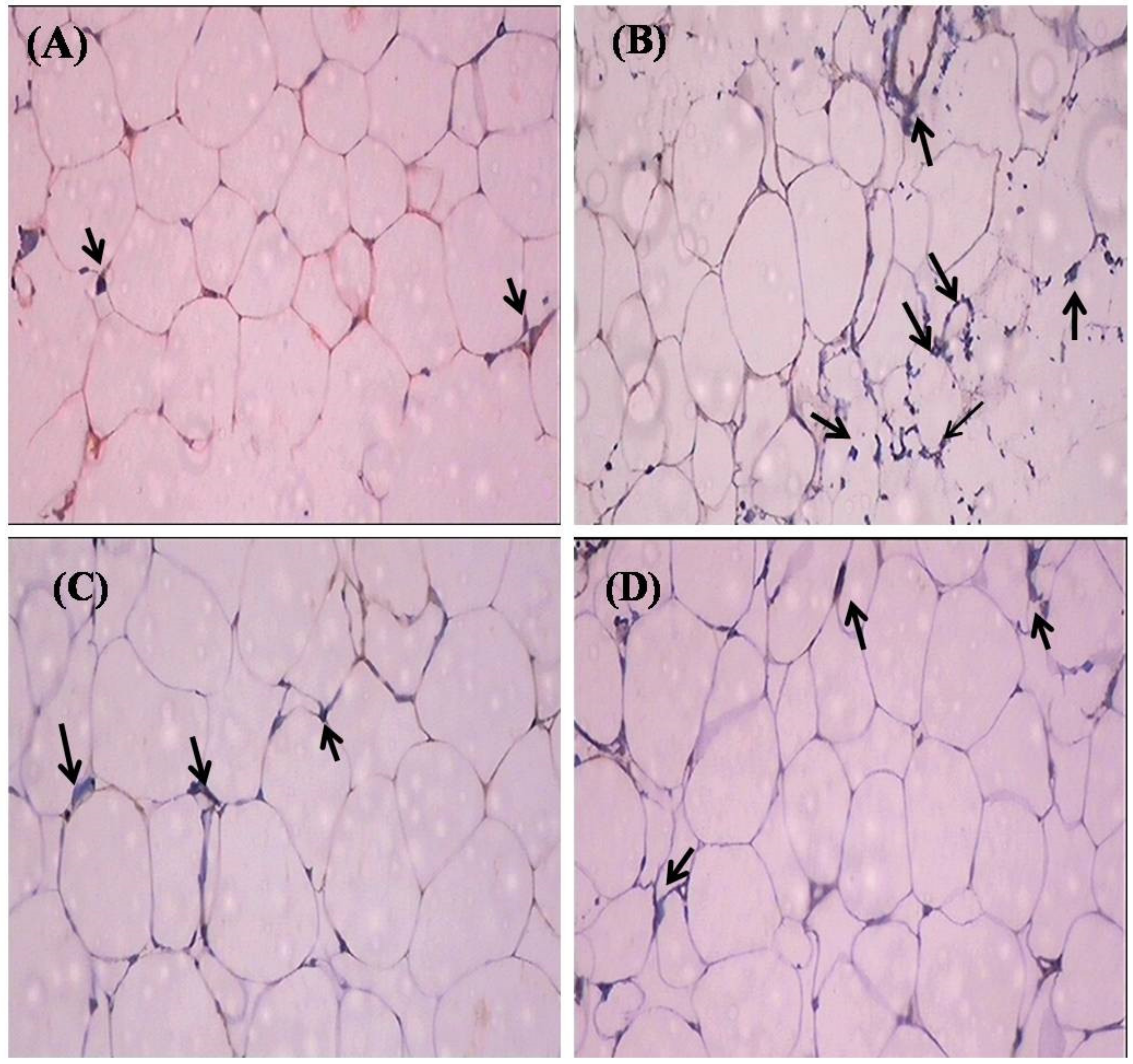
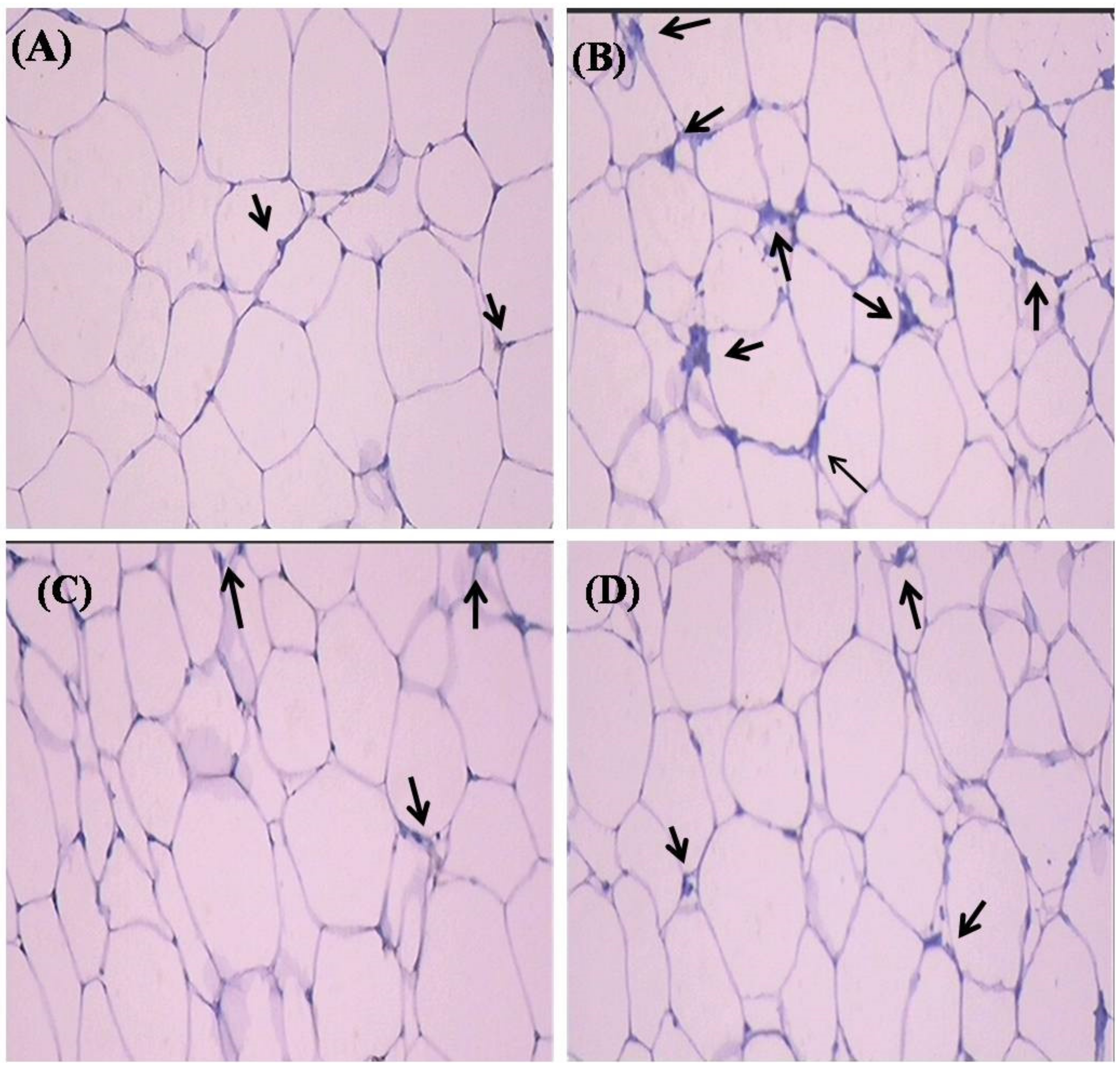
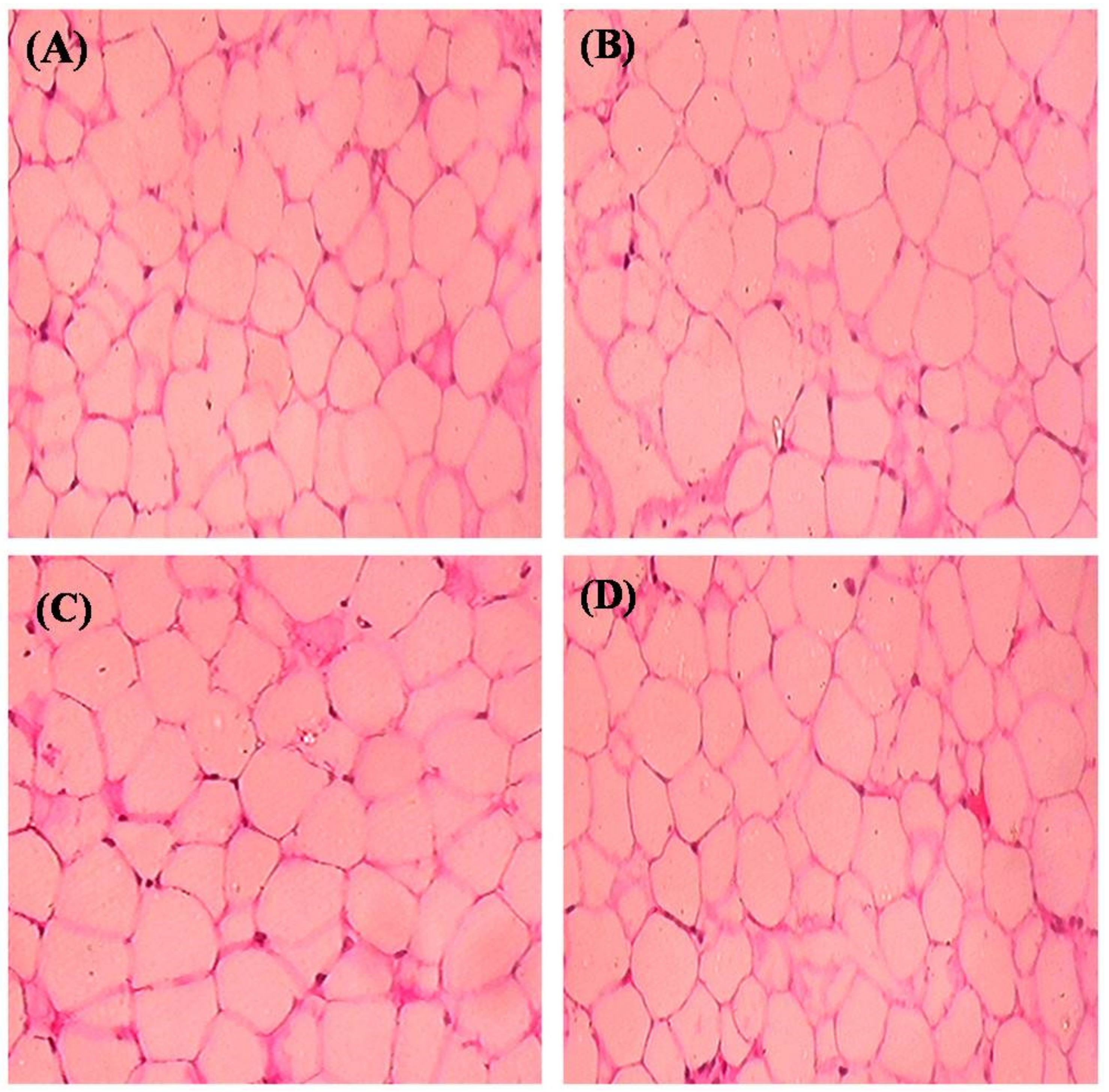
2.7. Histopathological Observation
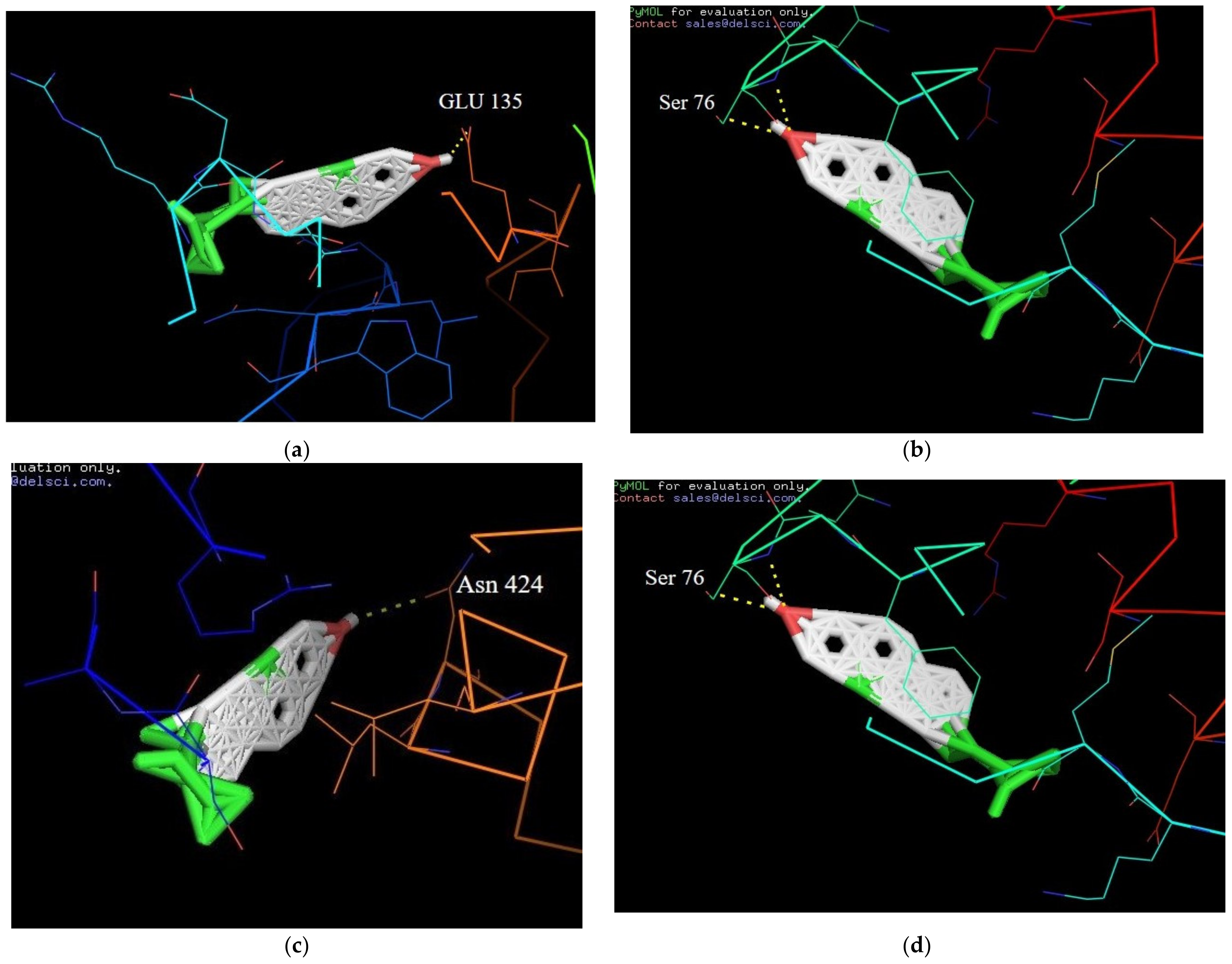
2.8. Molecular Docking Interactions of β-Sitosterol with Target Proteins
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Induction of Type-2 Diabetes
4.4. Experimental Design
4.5. Estimation of Serum Adiponectin, Leptin and Resistin
4.6. Analysis of the Protein of Proinflammatory Cytokines and Transcriptions Factors
4.7. Total RNA, cDNA Synthesis and Real-Time PCR
4.8. Protein Isolation and Western Blot Analysis
4.9. Histopathology of Adipose Tissue
4.10. Immunohistochemical Analysis
4.11. Statistical Analysis
4.12. Molecular Docking
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. 2014, 7, 587–591. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 23 October 2020).
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Invest. 2000, 106, 473–481. [Google Scholar] [CrossRef]
- Ye, J. Mechanisms of insulin resistance in obesity. Front Med. 2013, 7, 14–24. [Google Scholar] [CrossRef]
- Makki, K.; Froguel, P.; Wolowczuk, I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013, 2013, 139239. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. The endocrine function of adipose tissues in health and cardiometabolic disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Rehman, K.; Akash, M.S. Mechanisms of inflammatory responses and development of insulin resistance: How are they interlinked? J. Biomed. Sci. 2016, 23, 87. [Google Scholar] [CrossRef]
- Boucher, J.; Kleinridders, A.; Kahn, C.R. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb. Perspect. Biol. 2014, 6, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic agents and potential anti-inflammatory activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The mechanisms of action of metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Li, J.; Shen, X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol. Metab. Syndr. 2019, 11, 35. [Google Scholar] [CrossRef]
- Nutrition data: Foods highest in beta-sitosterol per 200 calorie serving; Conde Nast: New York, NY, US, 2014; SR-21.
- Bin Sayeed, M.S.; Karim, S.M.R.; Sharmin, T.; Morshed, M.M. Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef]
- Ponnulakshmi, R.; Shyamaladevi, B.; Vijayalakshmi, P.; Selvaraj, J. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol. Mech. Methods 2019, 29, 276–290. [Google Scholar] [CrossRef]
- Deng, Y.; Scherer, P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010, 1212, E1–E19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Pessin, J.E. Adipokines mediate inflammation and insulin resistance. Front. Endocrinol. 2013, 4, 71. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in obesity and metabolic disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Hassan, W.; Abdelkader, D.; Shang, J. Adipokines and hepatic insulin resistance. J. Diabetes Res. 2013, 2013, 170532. [Google Scholar] [CrossRef]
- Osegbe, I.; Okpara, H.; Azinge, E. Relationship between serum leptin and insulin resistance among obese Nigerian women. Ann. Afr. Med. 2016, 15, 14–19. [Google Scholar] [CrossRef]
- Lustig, R.H.; Sen, S.; Soberman, J.E.; Velasquez-Mieyer, P.A. Obesity, leptin resistance, and the effects of insulin reduction. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kataria, M.A.; Saini, V.; Yadav, A. Role of leptin and adiponectin in insulin resistance. Clin. Chim. Acta. 2013, 17, 80–84. [Google Scholar] [CrossRef]
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research. Am. J. Clin. Nutr. 2010, 91, 258S–261S. [Google Scholar] [CrossRef]
- Aleidi, S.; Issa, A.; Bustanji, H.; Khalil, M.; Bustanji, Y. Adiponectin serum levels correlate with insulin resistance in type 2 diabetic patients. Saudi Pharm. J. 2015, 23, 250–256. [Google Scholar] [CrossRef]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18, 1–17. [Google Scholar]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Goyal, R.; Faizy, A.F.; Siddiqui, S.S.; Singhai, M. Evaluation of TNF-α and IL-6 Levels in Obese and Non-obese Diabetics: Pre- and Postinsulin Effects. N. Am. J. Med. Sci. 2012, 4, 180–184. [Google Scholar]
- Mirza, S.; Hossain, M.; Mathews, C.; Martinez, P.; Pino, P.; Gay, J.L.; Rentfro, A.; McCormick, J.B.; Fisher-Hoch, S.P. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: A cross-sectional study. Cytokine 2012, 57, 136–142. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Liaqat, A. Tumor Necrosis Factor-Alpha: Role in Development of Insulin Resistance and Pathogenesis of Type 2 Diabetes Mellitus. J. Cell Biochem. 2018, 119, 105–110. [Google Scholar] [CrossRef]
- Kim, J.H.; Bachmann, R.A.; Chen, J. Interleukin-6 and insulin resistance. Vitam. Horm. 2009, 80, 613–633. [Google Scholar]
- Hoene, M.; Weigert, C. The role of interleukin-6 in insulin resistance, body fat distribution and energy balance. Obes. Rev. 2008, 9, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, I.; Hammer, R.E.; Richardson, J.A.; Ikemoto, S.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Insulin resistance and diabetes mellitus in transgenicmiceexpressingnuclearSREBP-1cinadipose tissue:modelforcongenital generalized lipodystrophy. Genes Dev. 1998, 12, 3182–3194. [Google Scholar] [CrossRef]
- Sewter, C.; Berger, D.; Considine, R.V.; Medina, G.; Rochford, J.; Ciaraldi, T.; Henry, R.; Dohm, L.; Flier, J.S.; O’Rahilly, S.; et al. Human obesity and type 2 diabetes are associated with alterations in SREBP1 isoform expression that are reproduced ex vivo by tumor necrosis factor-alpha. Diabetes 2002, 51, 1035–1041. [Google Scholar] [CrossRef]
- Sugii, S.; Olson, P.; Sears, D.D.; Saberi, M.; Atkins, A.R.; Barish, G.D.; Hong, S.H.; Castro, G.L.; Yin, Y.Q.; Nelson, M.C.; et al. Evans RM. PPAR gammaactivationin adipocytes is sufficient for systemic insulin sensitization. Proc. Natl. Acad. Sci. USA 2009, 106, 22504–22509. [Google Scholar] [CrossRef] [PubMed]
- Dumasia, R.; Eagle, K.A.; Kline-Rogers, E.; May, N.; Cho, L.; Mukherjee, D. Role of PPAR- gamma agonist thiazolidinediones in treatment of pre-diabetic and diabetic individuals: A cardiovascular perspective. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Ye, J. Regulation of PPAR gamma function by TNF-alpha. Biochem. Biophys. Res. Commun. 2008, 374, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, R.; Wang, H.; Liang, F. Mechanisms Linking Inflammation to Insulin Resistance. Int. J. Endocrinol. 2015, 2015, 508409. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X. Advances and Challenges in Protein-Ligand Docking. Int. J. Mol. Sci. 2010, 11, 3016–3034. [Google Scholar] [CrossRef]
- Ziamajidi, N.; Nasiri, A.; Abbasalipourkabir, R.; Sadeghi Moheb, S. Effects of garlic extract on TNF-α expression and oxidative stress status in the kidneys of rats with STZ + nicotinamide-induced diabetes. Pharm. Biol. 2017, 55, 526–531. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Q.; Pu, L.J.; Xu, X.W.; Zhang, R.Y.; Zhang, J.S.; Hu, J.; Yang, Z.K.; Lü, A.K.; Ding, F.H.; et al. Elevation of tumor necrosis factor-alpha, interleukin-1beta and interleukin-6 levels in aortic intima of Chinese Guizhouminipigs with streptozotocin-induced diabetes. Chin. Med. J. (Engl). 2007, 120, 479–484. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abdel-Rahman, M.M.; Bastawy, N.A.; Eissa, H.M. Modulatory effect of berberine on adipose tissue PPARγ, adipocytokines and oxidative stress in high fat diet/streptozotocin-induced diabetic rats. J. App. Pharm. Sci. 2017, 7, 001–010. [Google Scholar]
- Bizeau, M.E.; MacLean, P.S.; Johnson, G.C.; Wei, Y. Skeletal Muscle Sterol Regulatory Element Binding Protein-1c Decreases with Food Deprivation and Increases with Feeding in Rats. J. Nutr. 2003, 133, 1787–1792. [Google Scholar] [CrossRef][Green Version]
- Zhou, H.; Li, Y.J.; Wang, M.; Zhang, L.H.; Guo, B.Y.; Zhao, Z.S.; Meng, F.L.; Deng, Y.G.; Wang, R.Y. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol. Sin. 2011, 32, 999–1008. [Google Scholar] [CrossRef]
- Qiu, L.L.; Wang, C.; Yao, S.; Li, N.; Hu, Y.; Yu, Y.; Xia, R.; Zhu, J.; Ji, M.; Zhang, Z.; et al. Fenvalerate induces oxidative hepatic lesions through an overload of intracellular calcium triggered by the ERK/IKK/NF-κB pathway. FASEB J. 2019, 33, 2782–2795. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Fadda, L.M.; Al-Rasheed, N.M.; Ali, H.M.; Yacoub, H.I. Down-Regulation of NFkB, Bax, TGF-β, Smad-2mRNA expression in the Livers of Carbon Tetrachloride Treated Rats using Different Natural Antioxidants. Braz. Arch. Biol. Technol. 2016, 59, e16150553. [Google Scholar] [CrossRef]
- Peinnequin, A.; Mouret, C.; Birot, O.; Alonso, A.; Mathieu, J.; Clarençon, D.; Agay, D.; Chancerelle, Y.; Multon, E. Rat pro-inflammatory cytokine and cytokine related mRNA quantification by real-time polymerase chain reaction using SYBR green. BMC Immunol. 2004, 3, 1–10. [Google Scholar]
| Duration | GROUP I Control (Weight in Grams) | GROUP II Diabetes (Weight in Grams) | GROUP III Diab + β-Sitosterol (Weight in Grams) | GROUP IV Diab + Metformin (Weight in Grams) | GROUP V Control + β-Sitosterol (Weight in Grams) |
|---|---|---|---|---|---|
| Initial | 185.5 ± 5.32 | 192.5 ± 6.74 | 195.4 ± 9.23 a | 189.5 ± 6.72 | 201.3 ± 6.62 a |
| 2nd week | 192.2 ± 7.45 1 | 220.3 ± 8.55 1, a | 210.3 ± 10.56 1, a, b | 206.2 ± 8.62 1, b | 200.5 ± 7.75 b |
| 4th week | 203.5 ± 8.25 1 | 230.2 ± 9.23 1, a | 215.3 ± 8.56 1, a, b | 210.3 ± 9.23 1, b | 209.3 ± 8.45 b |
| 6th week | 209.3 ± 6.78 1,2 | 270.5 ± 6.62 1,2,3, a, b | 225.2 ± 6.56 1,2,3, a, b | 220.5 ± 10.56 1,2,3, a, b | 215.4 ± 12.67 1,2,3, b, c |
| 8th week | 210.5 ± 5.78 1,2 | 310.4 ± 7.82 1,2,3,4, a, b | 230.3 ± 7.8 1,2,3,4, a, b | 210.3 ± 11.21 1,2,3,4, a, b, c | 195.5 ± 8.56 1,2,3,4, a, b, c |
| Serial No | Compounds | Receptor Name and Its Protein Data Bank ID | Binding Affinity (Kcal/mol) | H-Bond Interaction |
|---|---|---|---|---|
| 1 | β-Sitosterol | PPAR-GAMMA (1I7I) | −6.7 | Asn 424 |
| 2 | β-Sitosterol | IL-6 (1ALU) | −8.6 | Ser 76 |
| 3 | β-Sitosterol | JNK-3 (4WHZ) | 2.5 | Lys 93 |
| 4 | β-Sitosterol | TN-ALPHA (5MU8) | −7.4 | Glu 135 |
| Gene Name | Primer Sequence | Reference |
|---|---|---|
| TNF-α | FW: 5′-GTCGTAGCAAACCACCAAGC-3′ RW: 5′-CTCCTGGTATGAA ATGGCAAA-3′ | [43] |
| IL-6 | FW: 5′-GTGAGAAGTATGAGAAGTGTGA-3′ RW: 5′-GCAGGATGAGAATGATCTTTG-3′ | [44] |
| PPARγ | FW: 5′-CCTGAAGCTCCAAGAATACC-3′ RW: 5′-GATGCTTTATCCCCACAGAC-3′ | [45] |
| SREBP-1c | FW: 5′-GGAGCCATGGATTGCACATT-3′ RW: 5′-GCTTCCAGAGAGGAGCCCAG-3′ | [46] |
| JNK | FW: 5′-TCAGAATCCGAACGAGACAAAAT-3′ RW: 5′-AAGCCAGAGTCCTTCACAGACAA-3′ | [47] |
| IKKB | FW: 5′-TGGCATGGAAACGGATAACTGA-3′ RW: 5′-CTGGAACTCTGTGCCTGTGGAA-3′ | [48] |
| NFκB | FW: 5′-CATGAAGAGAAGACACTGACCATGGAAA-3′ RW: 5′-TGGATAGAGGCTAAGTGT AGACACG-3′ | [49] |
| β-actin | FW: 5′-AAG TCC CTC ACC CTC CCA AAA G-3′ RW: 5′-AAG CAA TGC TGT CAC CTT CCC-3′ | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaraman, S.; Devarajan, N.; Rajagopal, P.; Babu, S.; Ganesan, S.K.; Veeraraghavan, V.P.; Palanisamy, C.P.; Cui, B.; Periyasamy, V.; Chandrasekar, K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules 2021, 26, 2101. https://doi.org/10.3390/molecules26072101
Jayaraman S, Devarajan N, Rajagopal P, Babu S, Ganesan SK, Veeraraghavan VP, Palanisamy CP, Cui B, Periyasamy V, Chandrasekar K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules. 2021; 26(7):2101. https://doi.org/10.3390/molecules26072101
Chicago/Turabian StyleJayaraman, Selvaraj, Nalini Devarajan, Ponnulakshmi Rajagopal, Shyamaladevi Babu, Senthil Kumar Ganesan, Vishnu Priya Veeraraghavan, Chella Perumal Palanisamy, Bo Cui, Vijayalakshmi Periyasamy, and Kirubhanand Chandrasekar. 2021. "β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats" Molecules 26, no. 7: 2101. https://doi.org/10.3390/molecules26072101
APA StyleJayaraman, S., Devarajan, N., Rajagopal, P., Babu, S., Ganesan, S. K., Veeraraghavan, V. P., Palanisamy, C. P., Cui, B., Periyasamy, V., & Chandrasekar, K. (2021). β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKβ/NF-κB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules, 26(7), 2101. https://doi.org/10.3390/molecules26072101







