Investigation into the Structural, Chemical and High Mechanical Reforms in B4C with Graphene Composite Material Substitution for Potential Shielding Frame Applications
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
2.2. Methods
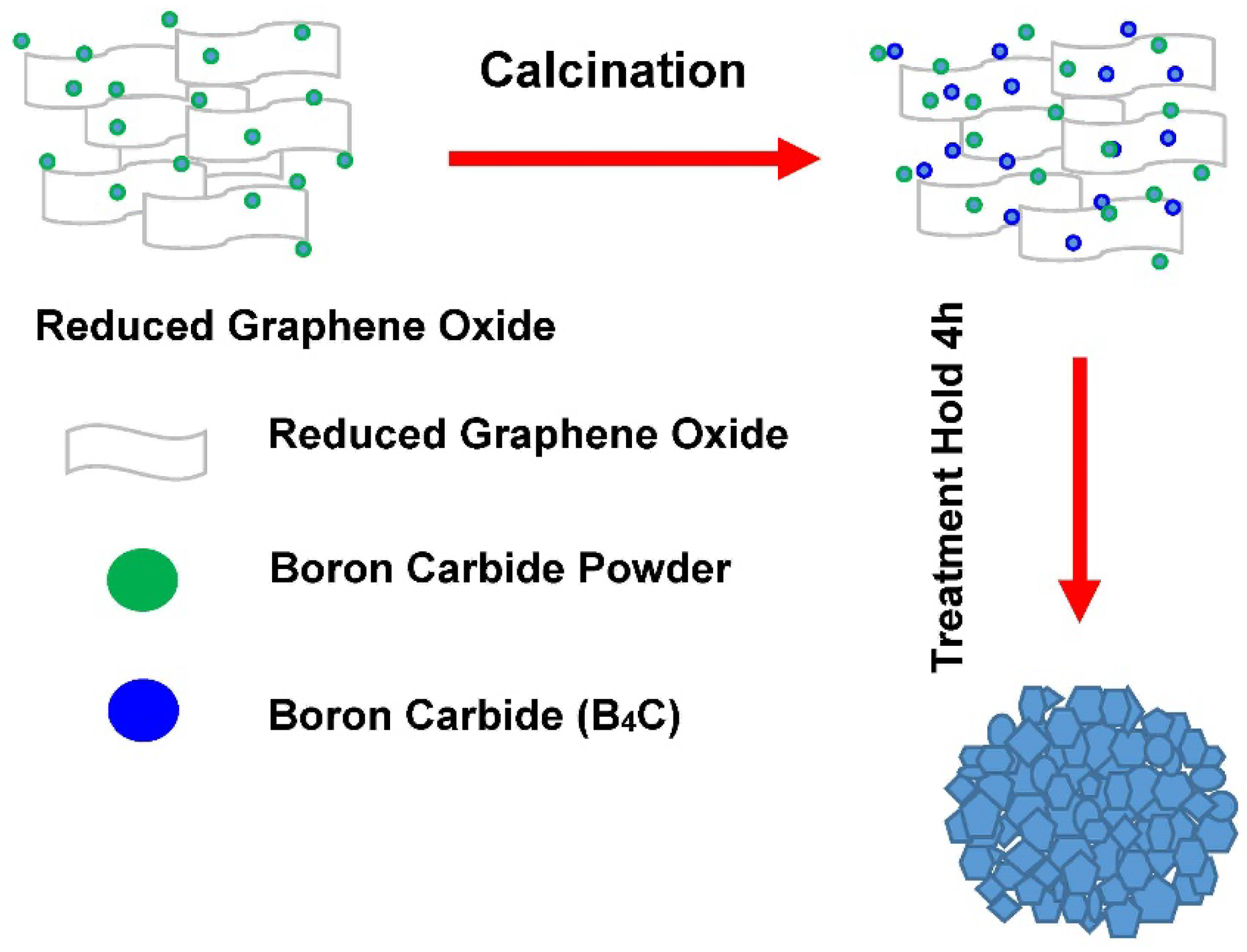
2.2.1. Heat Treatment
2.2.2. Preparation and Solidification
2.2.3. SEM + EDS Test
2.2.4. XRD Test
2.2.5. Raman Spectrum Test
2.2.6. FTIR Test
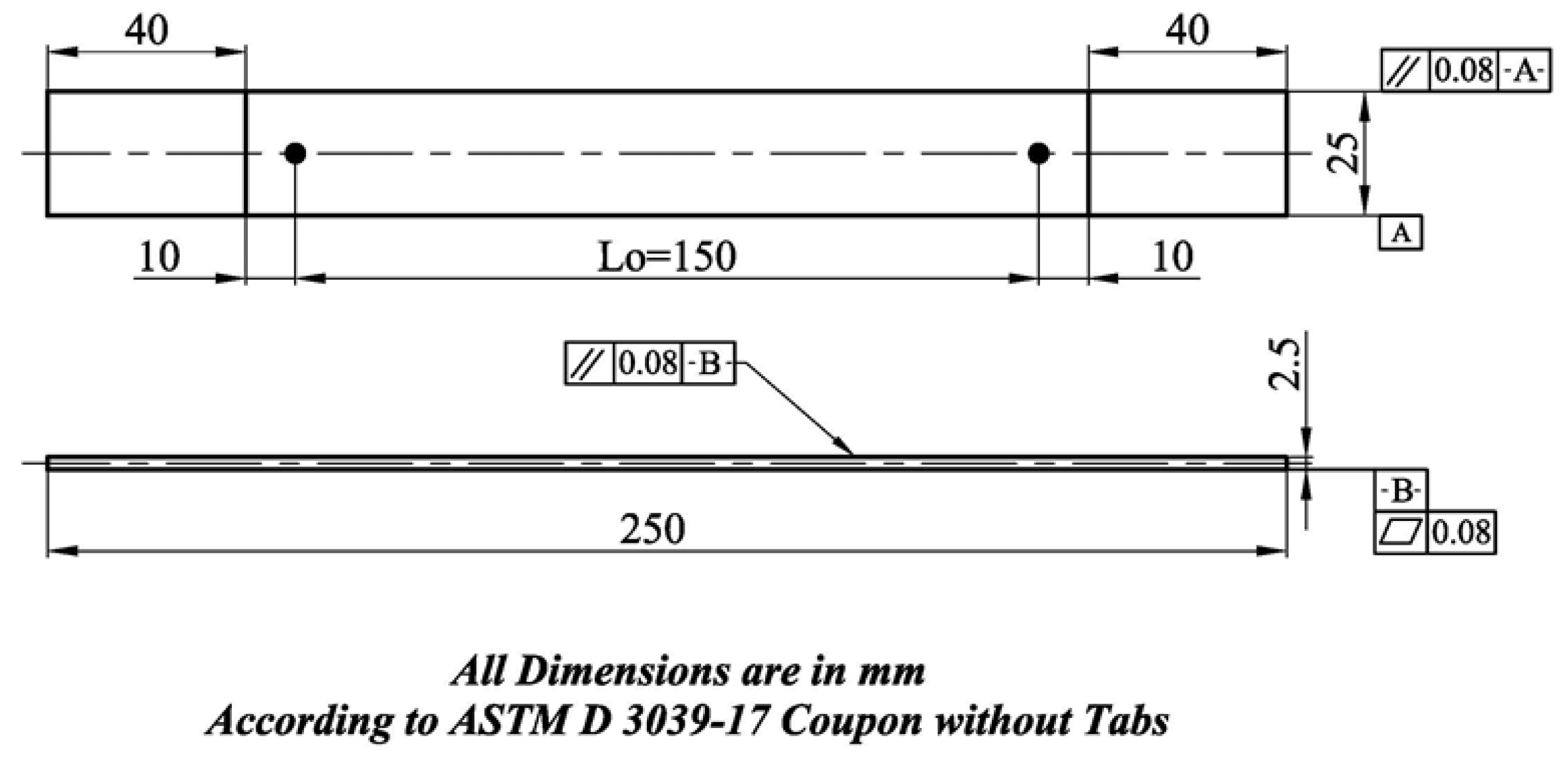
2.2.7. Tensile Test
2.2.8. Fracture Toughness Test
3. Results and Discussion
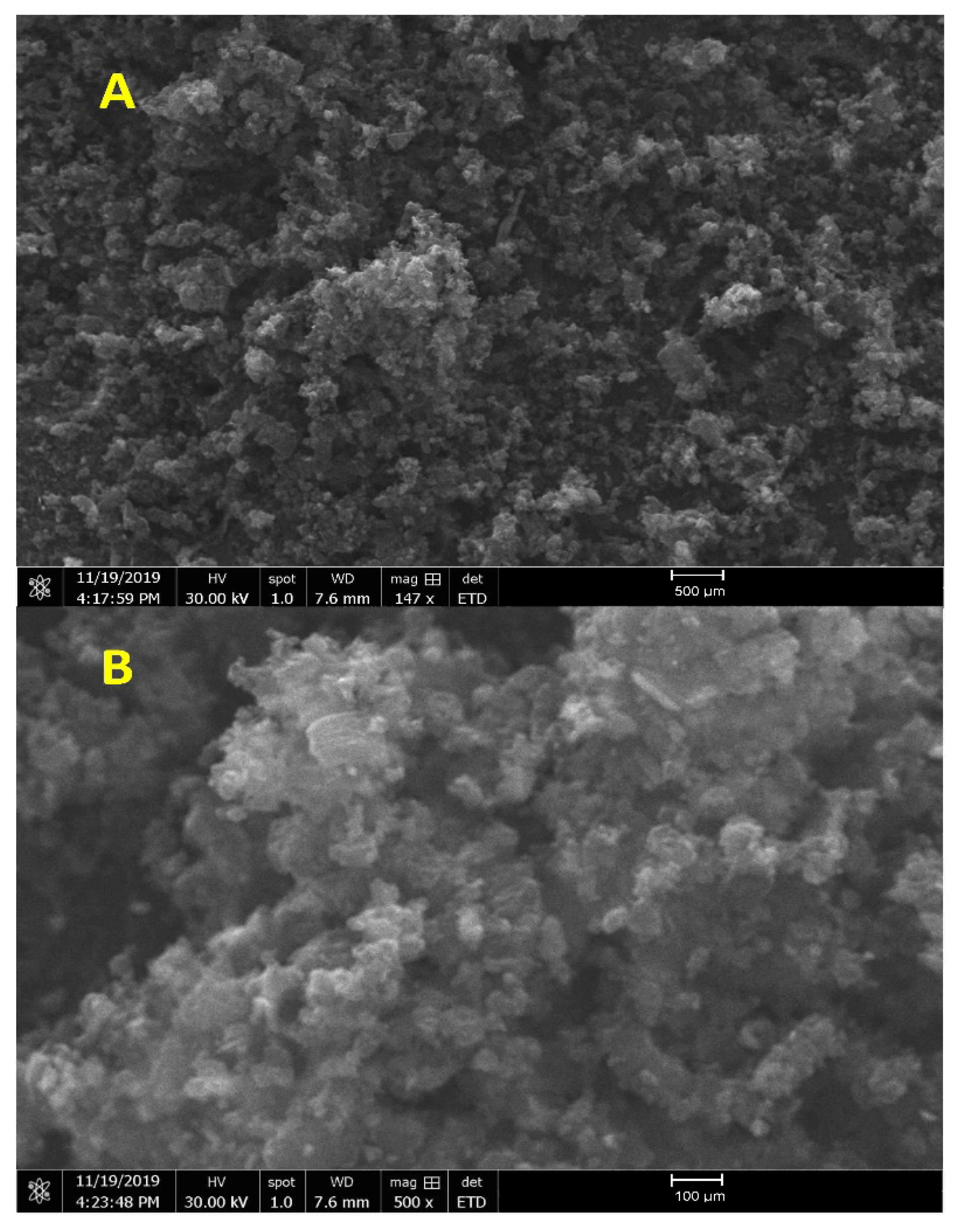
3.1. SEM Microstructure Analysis
3.2. SEM + EDS Morphology Analysis
3.3. XRD Analysis
3.4. Raman Analysis
3.5. FTIR Analysis
3.6. Tensile Analysis
3.7. Fracture Toughness Analysis
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Subramanian, C.; Suri, A.K.; Murthy, T.S. Development of Boron-based materials for nuclear applications. BARC Newsl. 2010, 313, 14. [Google Scholar]
- Kovalčíková, A.; Sedlák, R.; Rutkowski, P.; Dusza, J. Mechanical properties of boron carbide+ graphene platelet composites. Ceram. Int. 2016, 42, 2094–2098. [Google Scholar] [CrossRef]
- Domnich, V.; Reynaud, S.; Haber, R.A.; Chhowalla, M. Boron carbide: Structure, properties, and stability under stress. J. Am. Ceram. Soc. 2011, 94, 3605–3628. [Google Scholar] [CrossRef]
- Sheng, Y.; Li, G.; Meng, H.; Han, Y.; Xu, Y.; Wu, J.; Xu, J.; Sun, Z.; Liu, Y.; Zhang, X. An improved carbothermal process for the synthesis of fine-grained boron carbide microparticles and their photoelectrocatalytic activity. Ceram. Int. 2018, 44, 1052–1058. [Google Scholar] [CrossRef]
- Mortensen, M.W.; Sørensen, P.G.; Björkdahl, O.; Jensen, M.R.; Gundersen, H.J.; Bjørnholm, T. Preparation and characterization of Boron carbide nanoparticles for use as a novel agent in T cell-guided boron neutron capture therapy. Appl. Radiat. Isot. 2006, 64, 315–324. [Google Scholar] [CrossRef]
- Chen, S.; Wang, D.Z.; Huang, J.Y.; Ren, Z.F. Synthesis and characterization of boron carbide nanoparticles. Appl. Phys. A 2004, 79, 1757–1759. [Google Scholar]
- Chang, B.; Gersten, B.L.; Szewczyk, S.T.; Adams, J.W. Characterization of boron carbide nanoparticles prepared by a solid state thermal reaction. Appl. Phys. A 2007, 86, 83–87. [Google Scholar] [CrossRef]
- Seiler, N.; Bertrand, F.; Marchand, O.; Repetto, G.; Ederli, S. Investigations on boron carbide oxidation for nuclear reactors safety—General modelling for ICARE/CATHARE code applications. Nucl. Eng. Des. 2008, 238, 820–836. [Google Scholar] [CrossRef]
- Kovziridze, Z.D.; Mestvirishvili, Z.; Tabatadze, G.; Nizharadze, N.S.; Mshvildadze, M.; Nikoleishvili, E. Improvement of Boron Carbide Mechanical Properties in B 4 C-TiB 2 and B 4 C-ZrB 2 Systems. J. Electron. Cool. Therm. Control 2006, 3, 43–48. [Google Scholar] [CrossRef]
- ASTM C750-18. Standard Specification for Nuclear-Grade Boron Carbide Powder. In Annual Book of ASTM Standards; Part 12.01; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Liu, L.; Wang, Y.; Li, X.; Xu, L.; Cao, X.; Wang, Y.; Wang, Z.; Meng, C.; Zhu, W.; Ouyang, X. Enhancing toughness in boron carbide with reduced graphene oxide. J. Am. Ceram. Soc. 2016, 99, 257–264. [Google Scholar] [CrossRef]
- Cheng, C.; Reddy, K.M.; Hirata, A.; Fujita, T.; Chen, M. Structure and mechanical properties of boron-rich boron carbides. J. Eur. Ceram. Soc. 2017, 37, 4514–4523. [Google Scholar] [CrossRef]
- Buyuk, B.; Tugrul, A.B. Gamma and neutron attenuation behaviours of boron carbide–silicon carbide composites. Ann. Nucl. Energy. 2014, 71, 46–51. [Google Scholar] [CrossRef]
- Annenkov, M.; Blank, V.; Kulnitskiy, B.; Larionov, K.; Ovsyannikov, D.; Perezhogin, I.; Popov, M.; Sorokin, P. Boron carbide nanoparticles for high-hardness ceramics: Crystal lattice defects after treatment in a planetary ball mill. J. Eur. Ceram. Soc. 2017, 37, 1349–1353. [Google Scholar] [CrossRef]
- ASTM WK56764. New Guide for Characterization of Graphene Flakes Produced by Exfoliation; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM D3039/D3039M-17. Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials; D3039M-17; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Quinn, G.D.; Salem, J.; Bar-On, I.; Cho, K.; Foley, M.; Fang, H. Fracture toughness of advanced ceramics at room temperature. J. Res. Natl. Inst. Stand. Technol. 1992, 97, 579. [Google Scholar] [CrossRef] [PubMed]
- ASTM C1327-15(2019). Standard Test Method for Vickers Indentation Hardness of Advanced Ceramics; C1327-15R19ASTM; International: West Conshohocken, PA, USA, 2019. [Google Scholar]
- ASTM Standards. American Society for Testing and Materials; Part 15.01; International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Park, J.B.; Kim, Y.J.; Kim, S.M.; Yoo, J.M.; Kim, Y.; Gorbachev, R.; Barbolina, I.I.; Kim, S.J.; Kang, S.; Yoon, M.H.; et al. Non-destructive electron microscopy imaging and analysis of biological samples with graphene coating. 2D Mater. 2016, 3, 045004. [Google Scholar] [CrossRef]
- Manoratne, C.H.; Rosa, S.R.; Kottegoda, I.R. XRD-HTA, UV visible, FTIR and SEM interpretation of reduced graphene oxide synthesized from high purity vein graphite. Mater. Sci. Res. India 2017, 14, 19–30. [Google Scholar] [CrossRef]
- Herth, S.; Joost, W.J.; Doremus, R.H.; Siegel, R.W. New approach to the synthesis of nanocrystalline boron carbide. J. Nanosci. Nanotechnol. 2006, 6, 954–959. [Google Scholar] [CrossRef]
- Guan, Z.; Gutu, T.; Yang, J.; Yang, Y.; Zinn, A.A.; Li, D.; Xu, T.T. Boron carbide nanowires: Low temperature synthesis and structural and thermal conductivity characterization. J. Mater. Chem. 2012, 22, 9853–9860. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, J.; Huang, Z.; Yan, K.; Chen, F.; Jian, Y.; Yang, H.; Li, B. Preparation and properties of multilayer graphene reinforced binderless TiC nanocomposite cemented carbide through two-step sintering. Mater. Des. 2020, 188, 108495. [Google Scholar] [CrossRef]
- Song, N.; Gao, Z.; Li, X. Tailoring nanocomposite interfaces with graphene to achieve high strength and toughness. Sci. Adv. 2020, 6, eaba7016. [Google Scholar] [CrossRef] [PubMed]
- Junaid, M.; Khir, M.H.; Witjaksono, G.; Tansu, N.; Saheed, M.S.; Kumar, P.; Ullah, Z.; Yar, A.; Usman, F. Boron-doped reduced Graphene Oxide with tunable bandgap and enhanced surface plasmon resonance. Molecules 2020, 25, 3646. [Google Scholar] [CrossRef]
- Xia, K.; Zhan, H.; Wei, Y.; Gu, Y. Tensile properties of a boron/nitrogen-doped carbon nanotube–graphene hybrid structure. Beilstein J. Nanotechnol. 2014, 5, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wang, J. Fabrication and tensile properties of graphene/copper composites prepared by electroless plating for structrual applications. Phys. Status Solidi 2014, 211, 2878–2885. [Google Scholar] [CrossRef]
- Koo, J.H.; Paek, S.M. Microwave-Assisted Synthesis of Ge/GeO2-Reduced Graphene Oxide Nanocomposite with Enhanced Discharge Capacity for Lithium-Ion Batteries. Nanomater 2021, 11, 319. [Google Scholar] [CrossRef]
- Lee, B.; Lee, D.; Lee, J.H.; Ryu, H.J.; Hong, S.H. Enhancement of toughness and wear resistance in Boron Nitride nanoplatelet (BNNP) reinforced Si3N4 nanocomposites. Sci. Rep. 2016, 6, 1–2. [Google Scholar] [CrossRef]
- Kvetková, L.; Duszová, A.; Hvizdoš, P.; Dusza, J.; Kun, P.; Balázsi, C. Fracture toughness and toughening mechanisms in graphene platelet reinforced Si3N4 composites. Scr. Mater. 2012, 66, 793–796. [Google Scholar] [CrossRef]
- Liao, Z.; Standke, Y.; Gluch, J.; Balázsi, K.; Pathak, O.; Höhn, S.; Herrmann, M.; Werner, S.; Dusza, J.; Balázsi, C.; et al. Microstructure and fracture mechanism investigation of porous silicon Nitride–Zirconia–Graphene composite using multi-Scale and In-Situ microscopy. Nanomaterials 2021, 11, 285. [Google Scholar] [CrossRef]
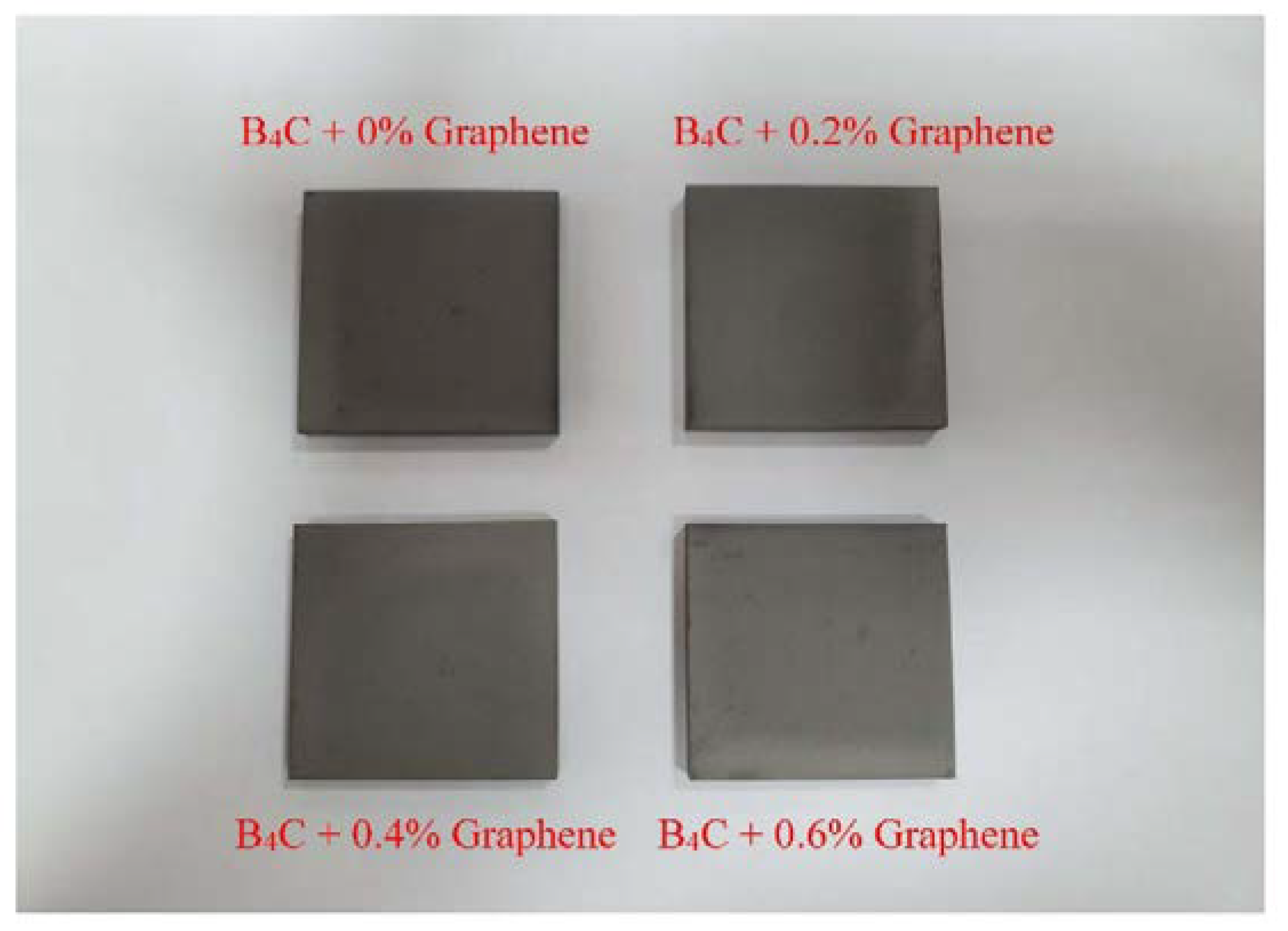

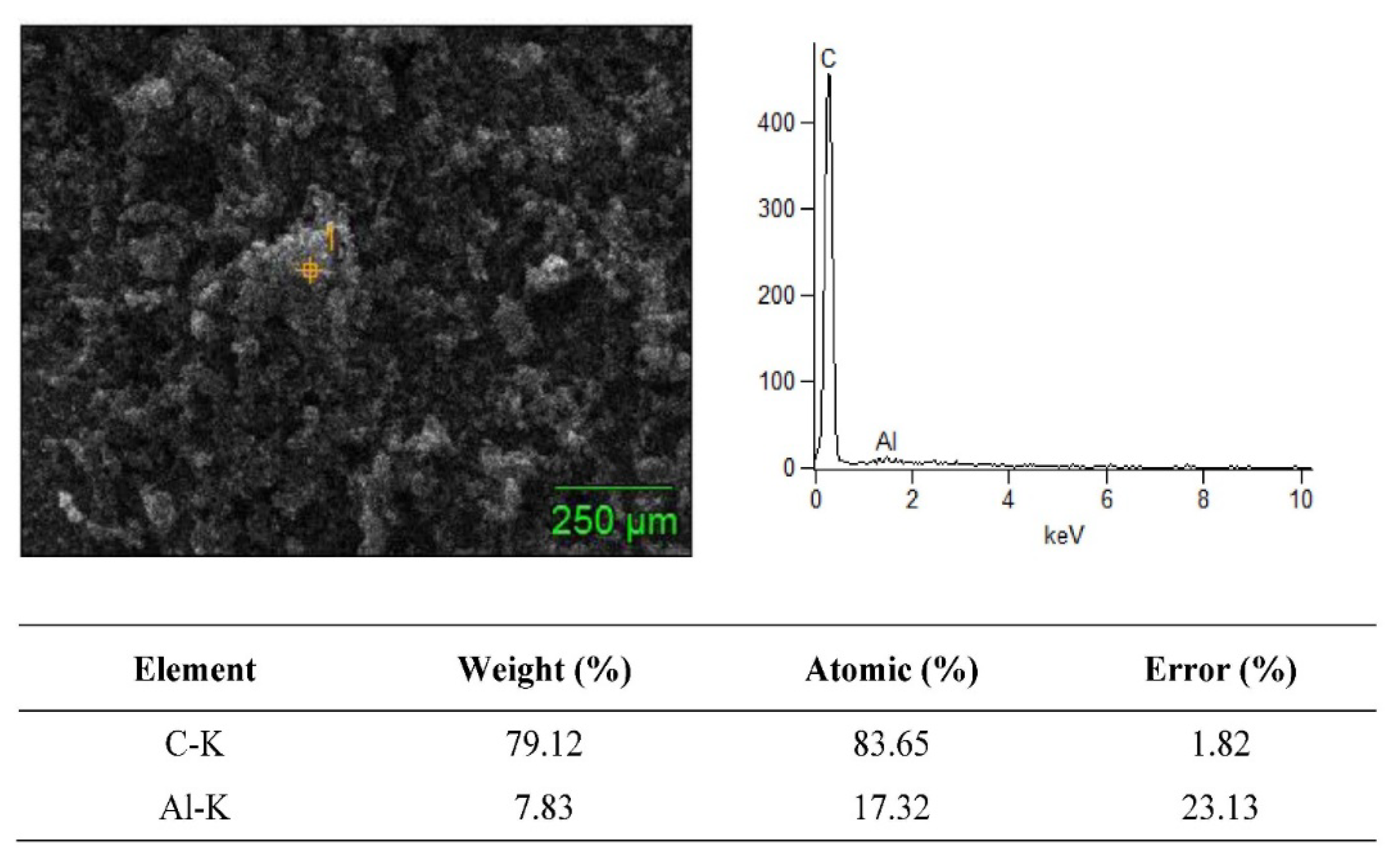
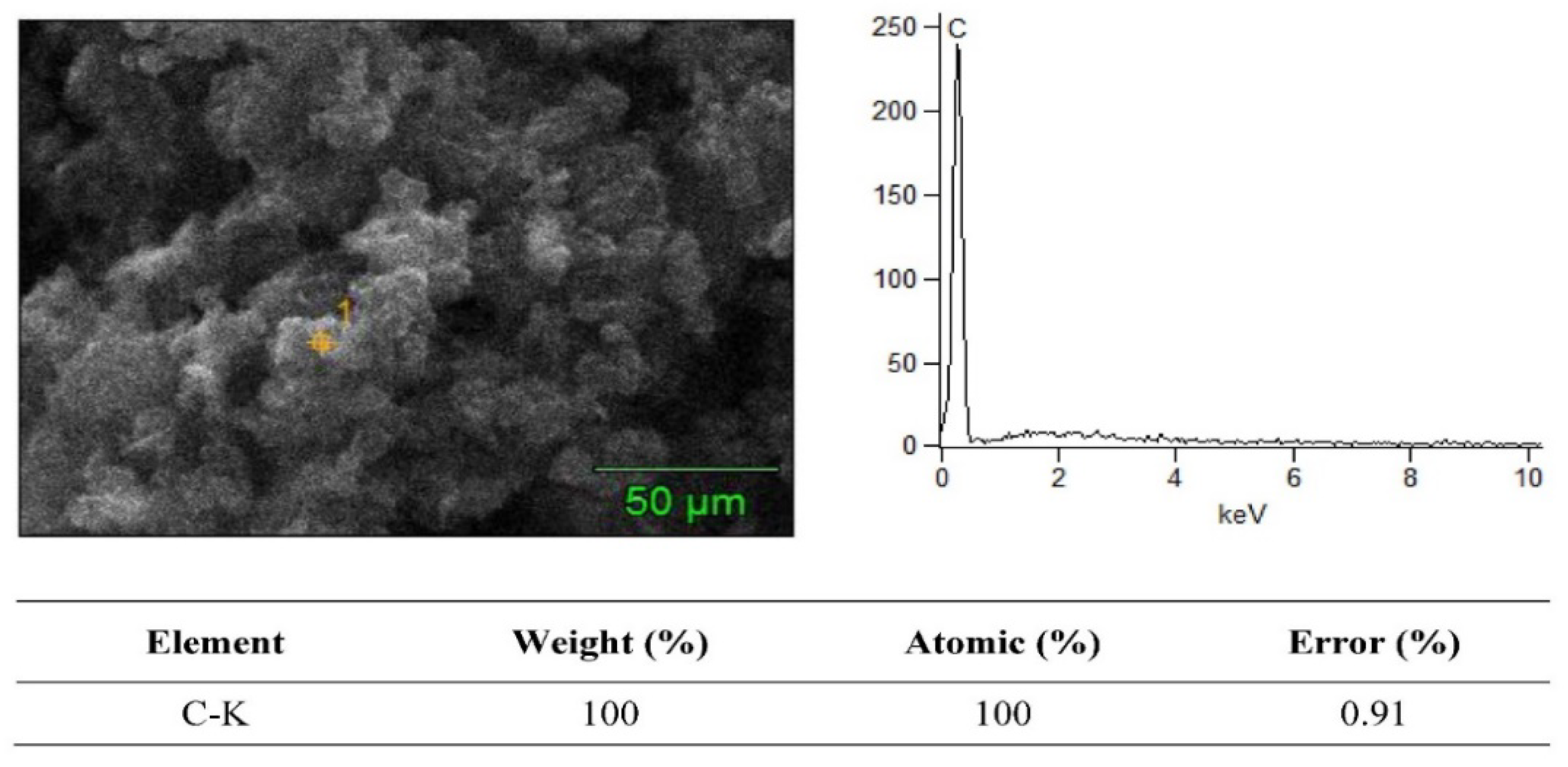
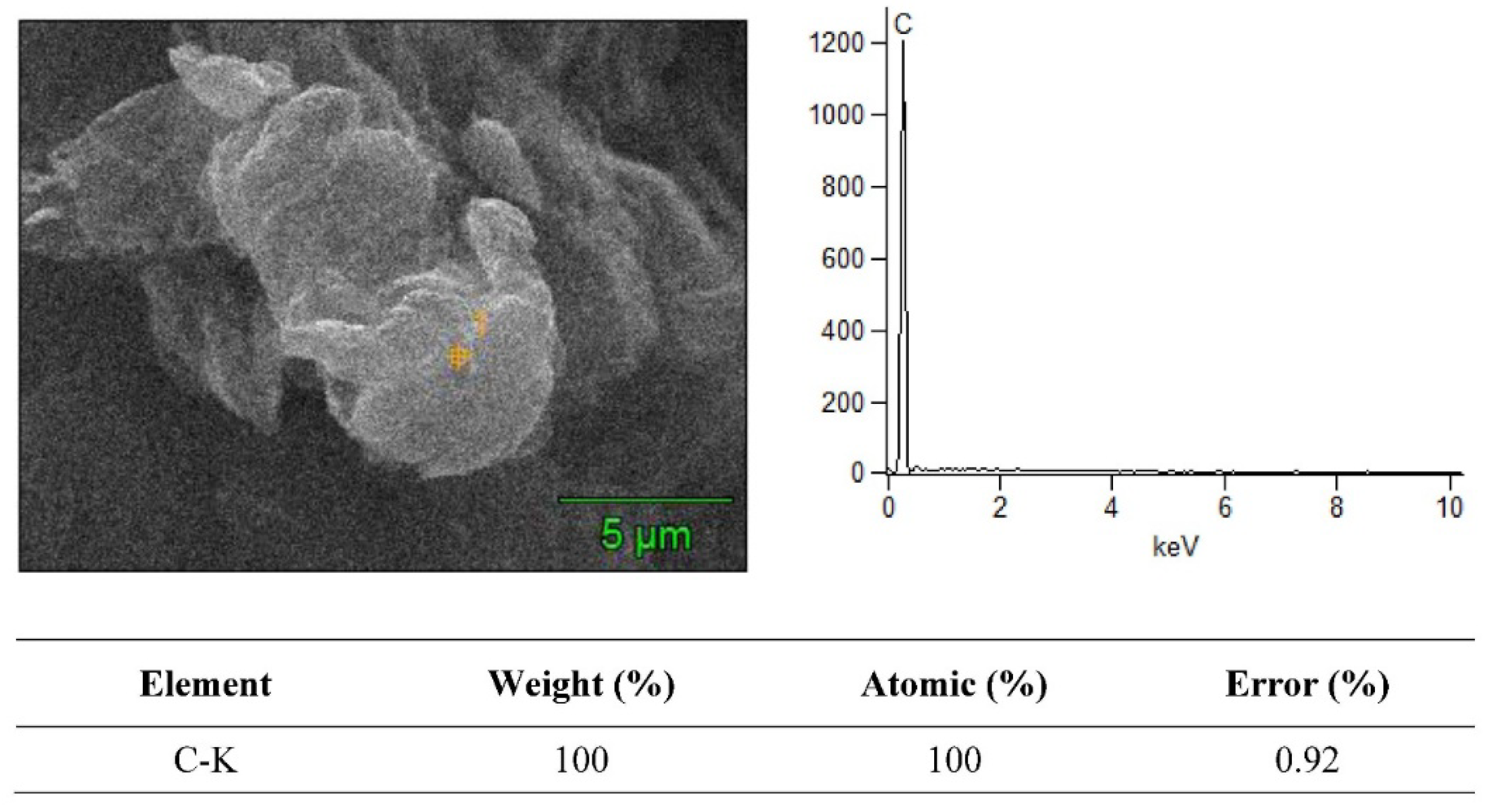
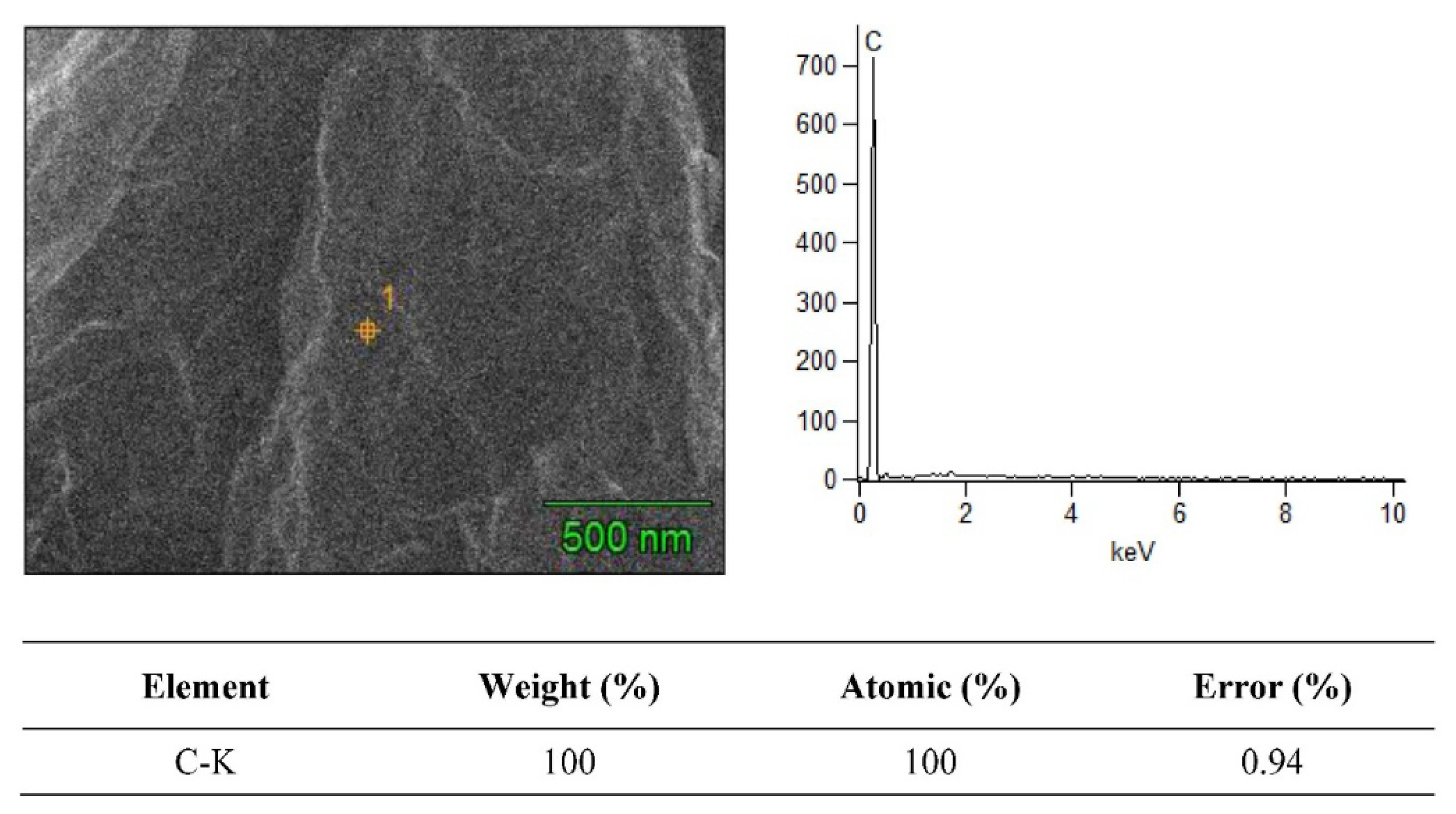


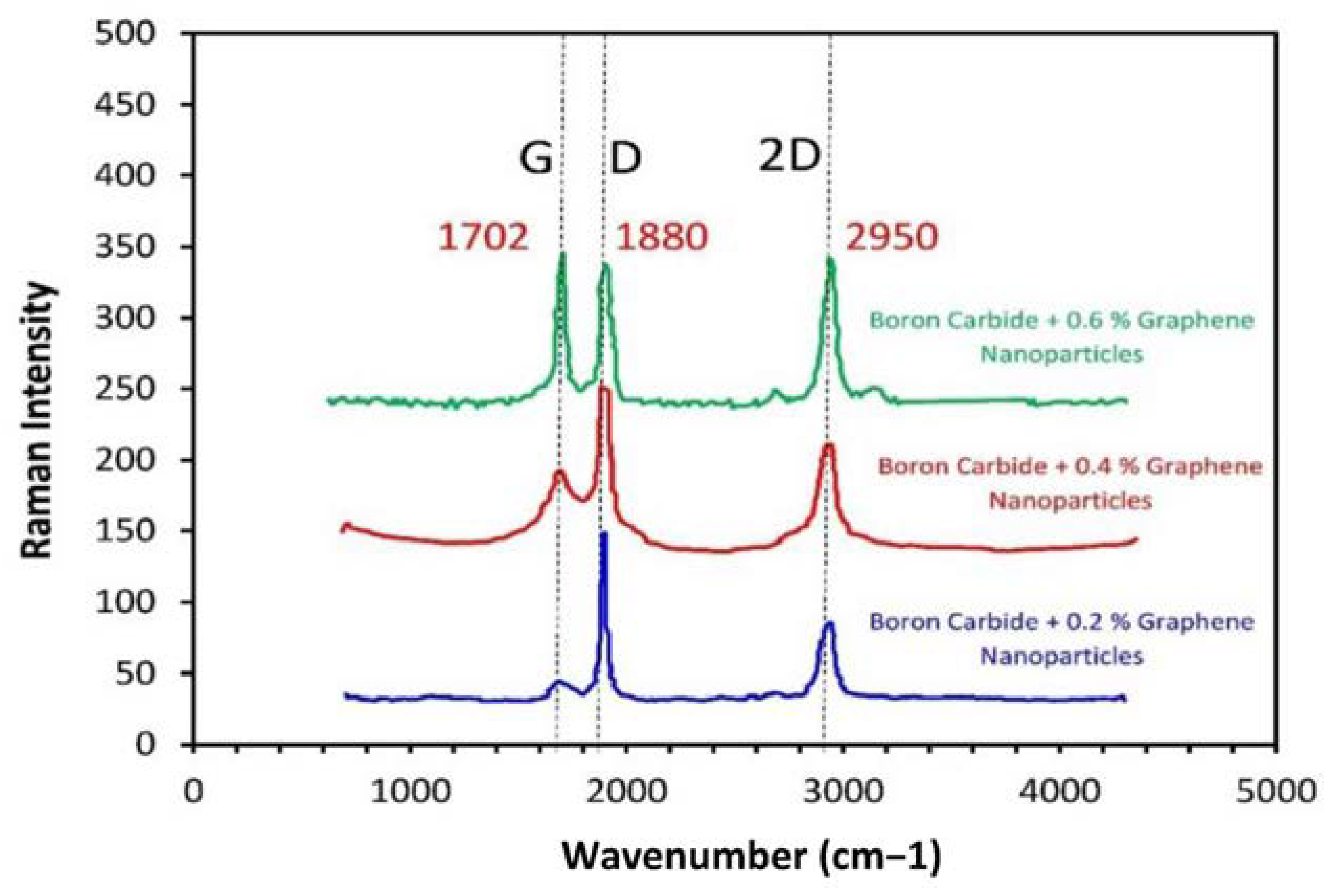
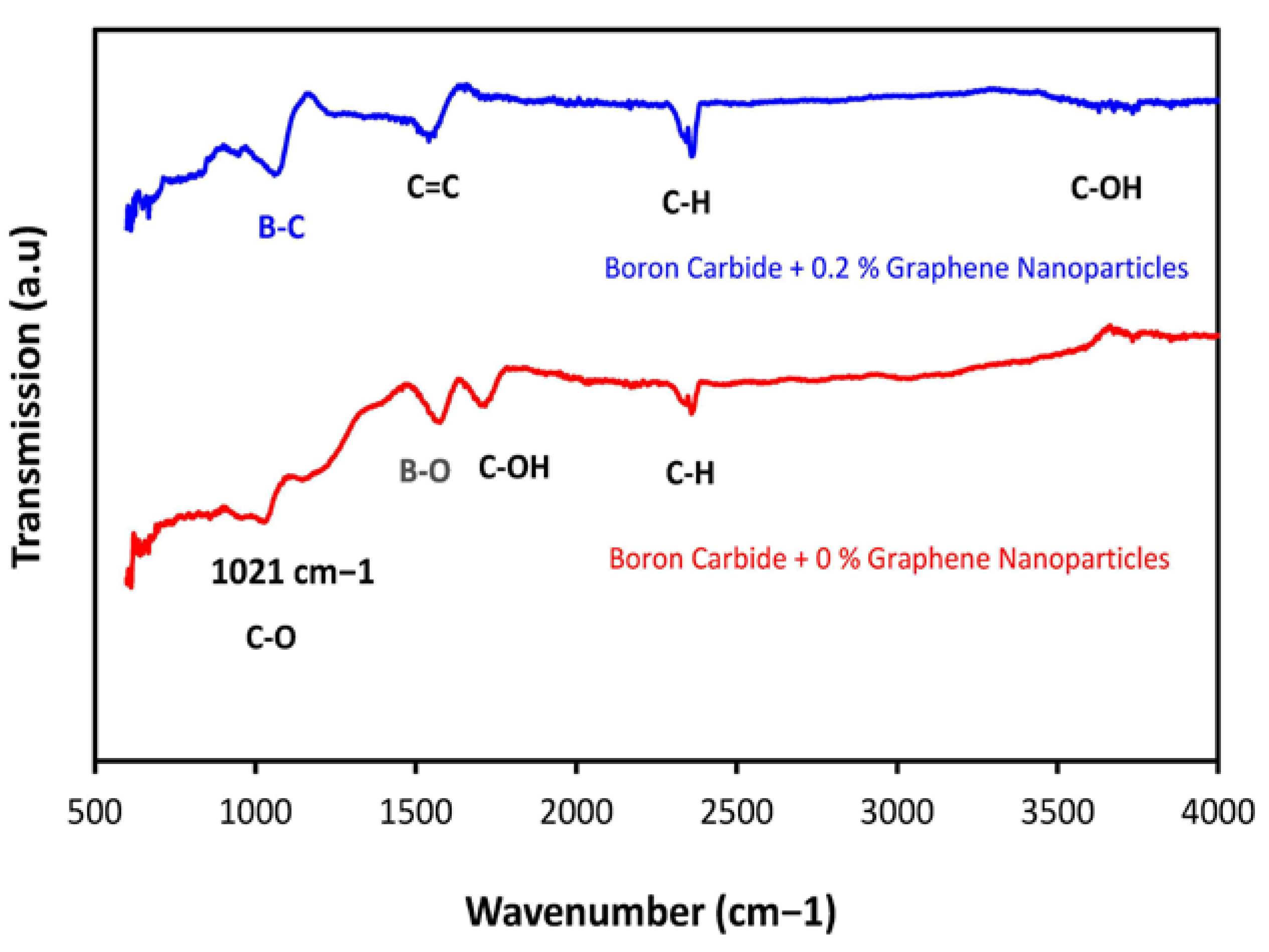
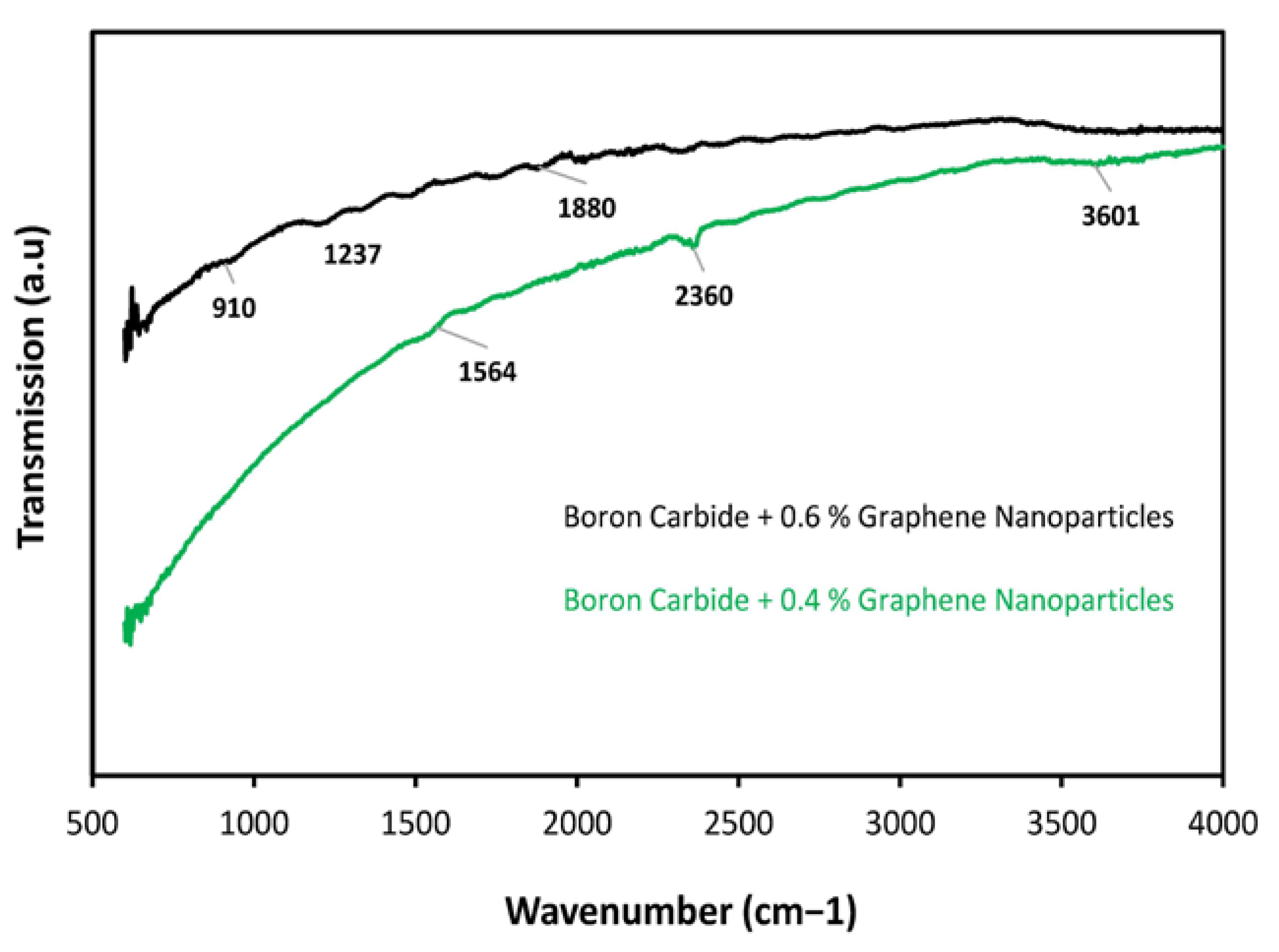

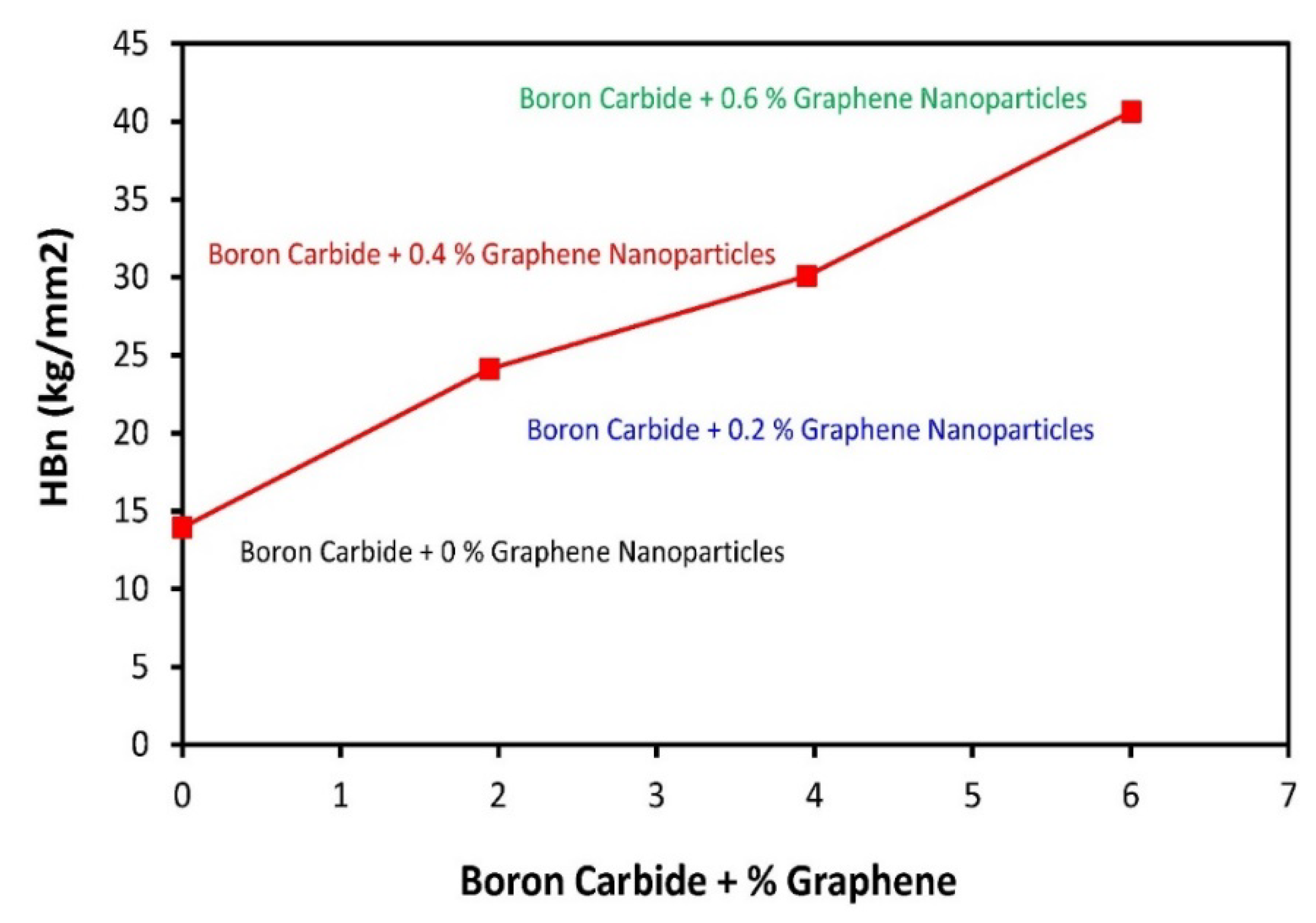
| Material Composition | B4C (Vol.%) | Graphene (Vol.%) | Experimental Mechanical Tests |
|---|---|---|---|
| 100–0% | 100 | 0 | Tensile, hardness, Indentation Fracture Toughness |
| 99.80–0.2% | 99.80 | 0.2 | |
| 99.60–0.4% | 99.60 | 0.4 | |
| 99.60–0.6% | 99.40 | 0.6 |
| Material Composition (B4C–G%) (Peak) | 2 Ө deg. | dhk1 (Å) | ||||
|---|---|---|---|---|---|---|
| 100–0% | 5.61 | 20.32 | 23.43 | 0.53 | 2.46 | 3.87 |
| 99.80–0.2% | - | 22.34 | - | - | 2.78 | - |
| 99.60–0.4% | - | 22.34 | - | - | 2.78 | - |
| 99.60–0.6% | - | 21.54 | 52.23 | - | 2.54 | 4.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alarifi, I.M. Investigation into the Structural, Chemical and High Mechanical Reforms in B4C with Graphene Composite Material Substitution for Potential Shielding Frame Applications. Molecules 2021, 26, 1921. https://doi.org/10.3390/molecules26071921
Alarifi IM. Investigation into the Structural, Chemical and High Mechanical Reforms in B4C with Graphene Composite Material Substitution for Potential Shielding Frame Applications. Molecules. 2021; 26(7):1921. https://doi.org/10.3390/molecules26071921
Chicago/Turabian StyleAlarifi, Ibrahim M. 2021. "Investigation into the Structural, Chemical and High Mechanical Reforms in B4C with Graphene Composite Material Substitution for Potential Shielding Frame Applications" Molecules 26, no. 7: 1921. https://doi.org/10.3390/molecules26071921
APA StyleAlarifi, I. M. (2021). Investigation into the Structural, Chemical and High Mechanical Reforms in B4C with Graphene Composite Material Substitution for Potential Shielding Frame Applications. Molecules, 26(7), 1921. https://doi.org/10.3390/molecules26071921






