An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections
Abstract
1. Introduction
2. Results
2.1. Biochemical Characterization of V. opulus Fruit and Bark Extracts
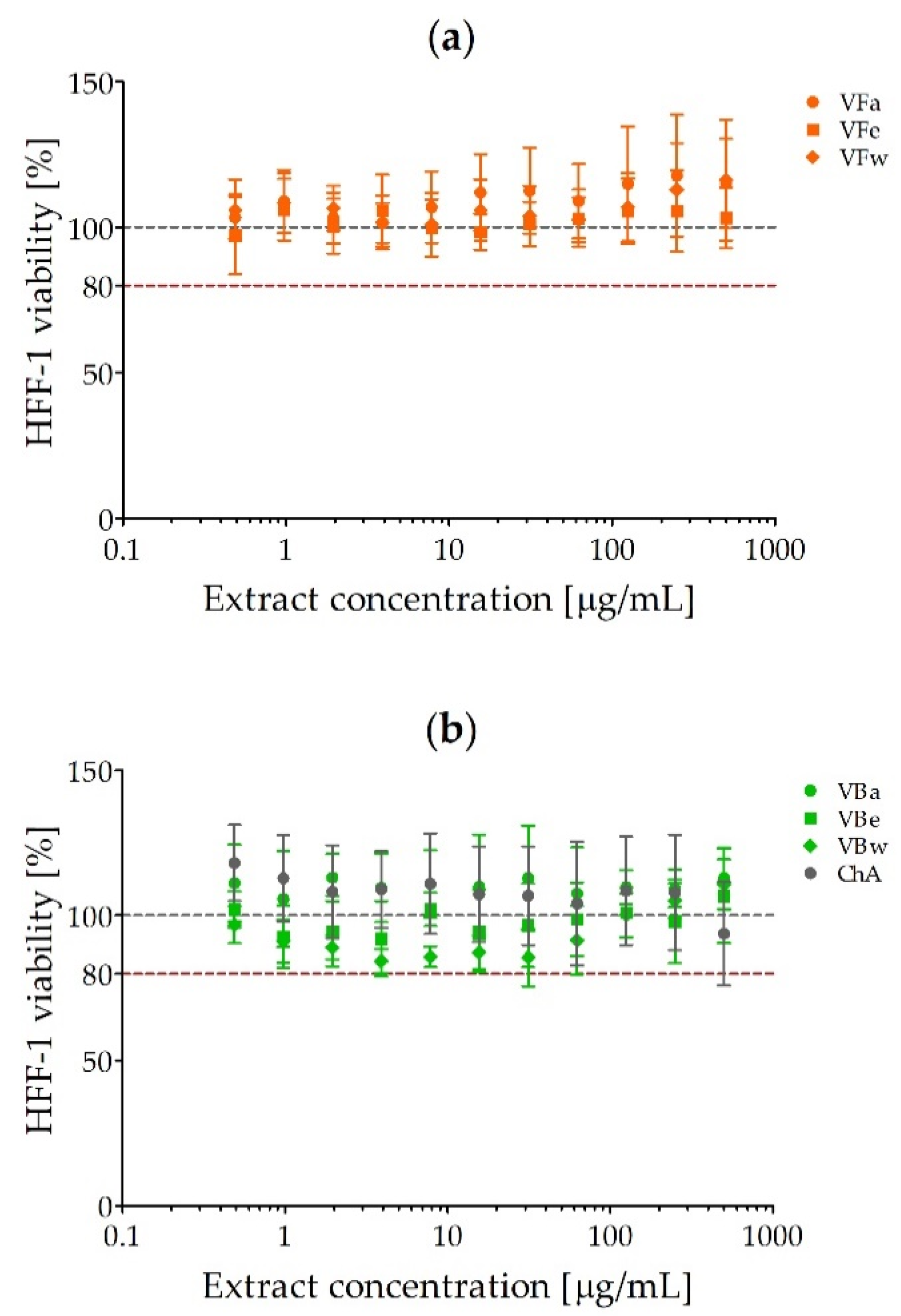
2.2. Cytotoxicity of V. opulus Fruit and Bark Extracts
2.3. Minimal Inhibitory/Bactericidal Concentration (MIC/MBC) of V. opulus Extract against S. aureus
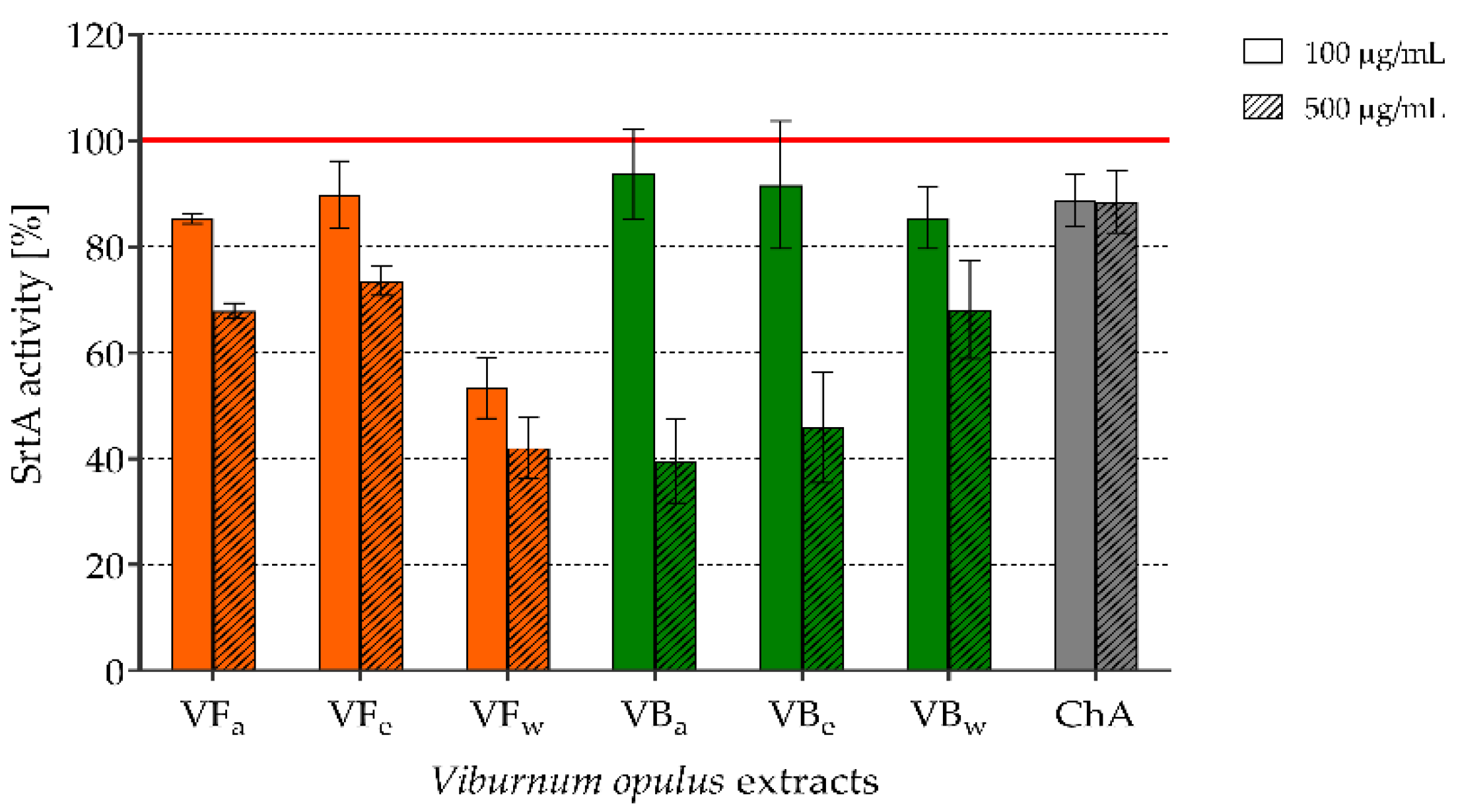
2.4. The Effect of V. opulus Extracts on SrtA Activity
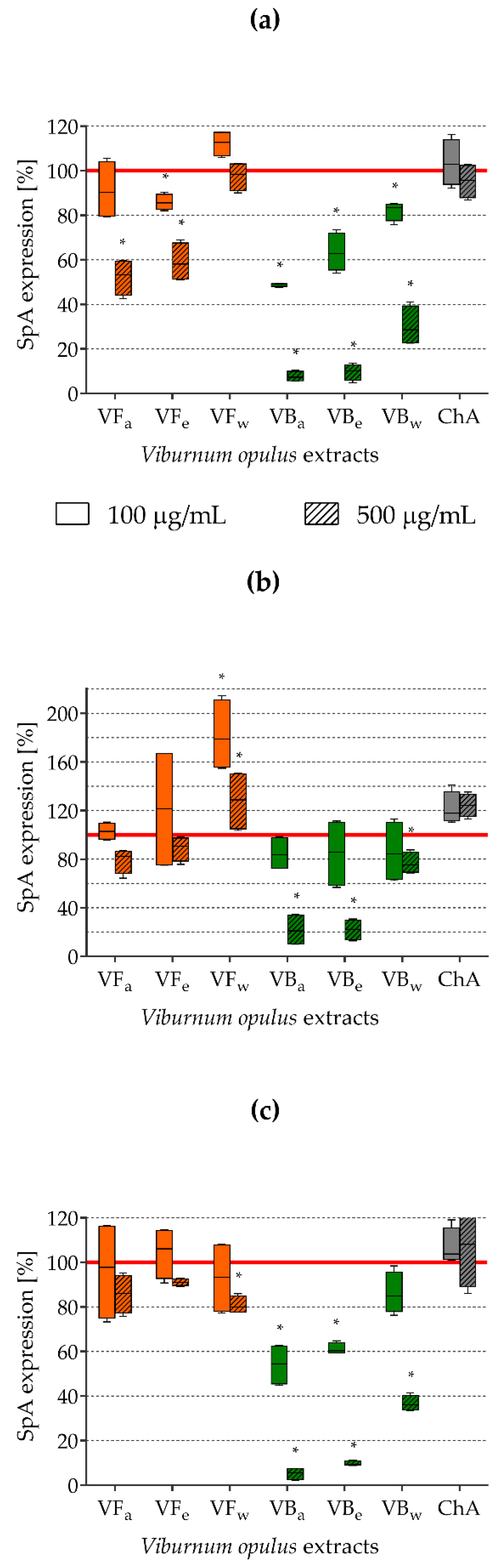
2.5. Impact of V. opulus Extracts on SpA Expression
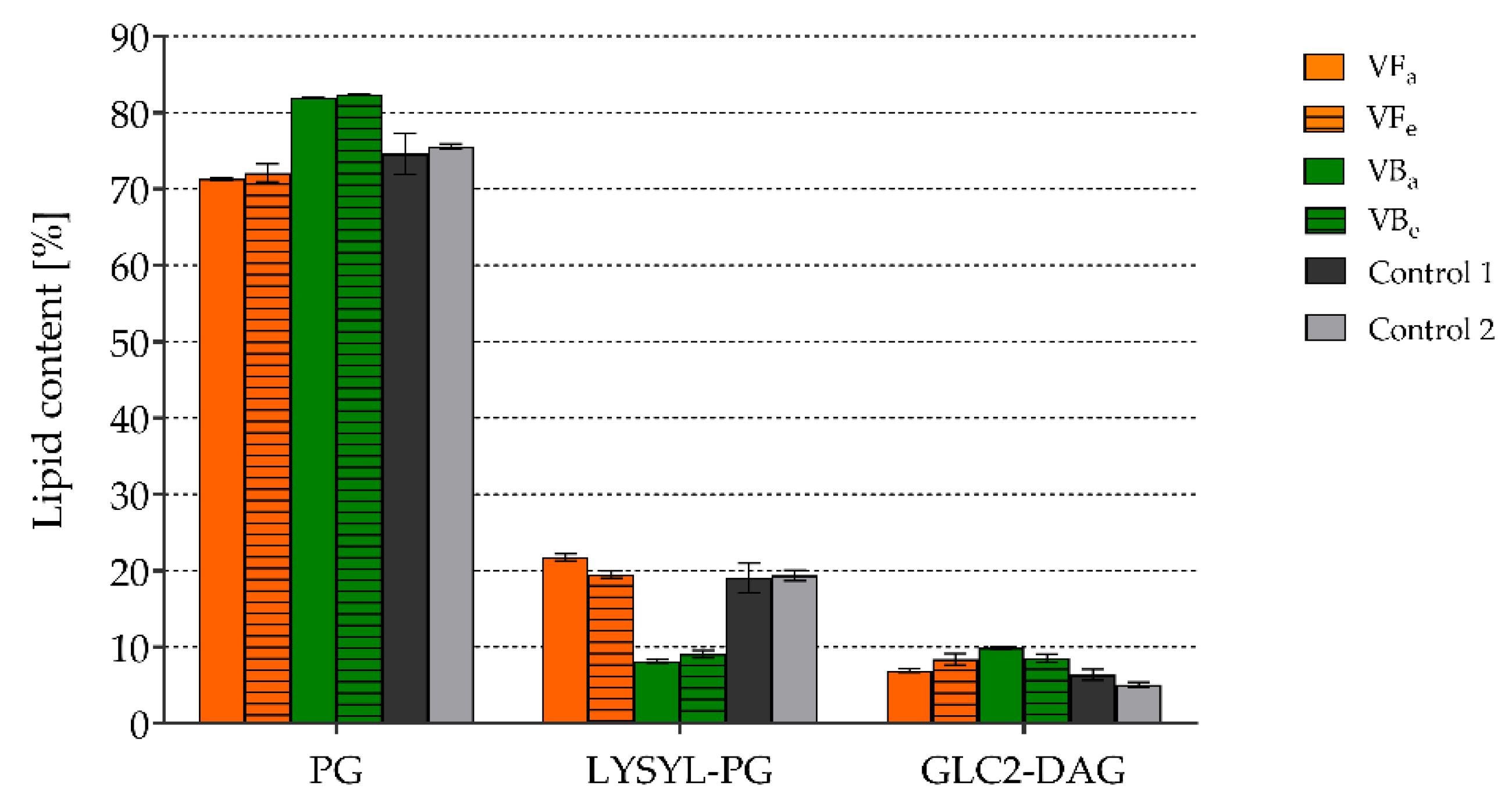
2.6. The Effects of the Tested Extracts on the Compositions of Phospholipids and Fatty Acids in Staphylococcal Membranes
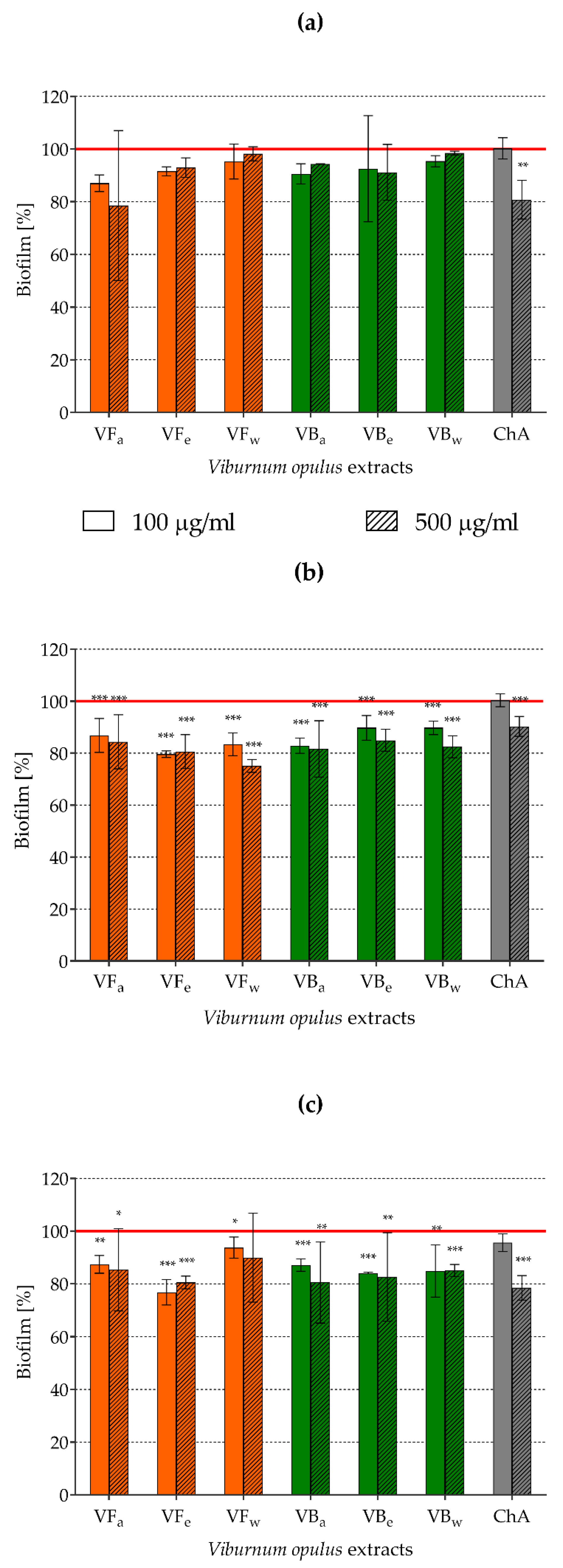
2.7. Assessment of Biofilm Formation by Staphylococci Pre-exposed to V. opulus Extracts
3. Discussion
4. Materials and Methods
4.1. Plant Material Preparation and Chemical Analysis
4.1.1. Standards and Reagents
4.1.2. Extract Preparation
4.1.3. Analysis of Chemical Composition of Extracts
4.1.4. Analysis of Phenolic Compounds Using Ultra-Performance Liquid Chromatography–Quadruple–Time of Flight Mass Spectrometry (UPLC–QTOF–MS)
4.2. Stock Solutions for Biological Tests
4.3. Cytotoxicity of V. opulus Fruit and Bark Extracts
4.4. Staphylococcal Strains and Culture Conditions
4.5. Minimal Inhibitory/Bactericidal Concentration (MIC/MBC)
4.6. SrtA Activity Testing
4.7. Assessment of SpA Expression
4.8. Extraction and Examination of the Composition of Glycolipids, Phospholipids, and Fatty Acids in Staphylococcal Membranes
4.8.1. Chemicals
4.8.2. Exposure of S. aureus to V. opulus Extracts
4.8.3. Extraction of the Lipids
4.8.4. Staphylococcal Lipid Analysis
4.8.5. Staphylococcal Fatty Acid Analysis
4.9. Assessment of Biofilm Formation by S. aureus
4.10. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chan, L.C.; Chaili, S.; Filler, S.G.; Miller, L.S.; Solis, N.V.; Wang, H.; Johnson, C.W.; Lee, H.K.; Diaz, L.F.; Yeaman, M.R. Innate immune memory contributes to host defense against recurrent skin and skin structure infections caused by methicillin-resistant Staphylococcus aureus. Infect. Immun. 2017, 85, e00876-16. [Google Scholar] [CrossRef]
- Herrera, A.; Kulhankova, K.; Sonkar, V.K.; Dayal, S.; Klingelhutz, A.J.; Salgado-Pabón, W.; Schlievert, P.M. Staphylococcal β-Toxin modulates human aortic endothelial cell and platelet function through sphingomyelinase and biofilm ligase activities. mBio 2017, 8, e00273-17. [Google Scholar] [CrossRef]
- Saxena, S.; Gomber, C. Surmounting antimicrobial resistance in the Millennium superbug: Staphylococcus aureus. Cent. Eur. J. Med. 2010, 5, 12–29. [Google Scholar] [CrossRef]
- Strauß, L.; Stegger, M.; Akpaka, P.E.; Alabi, A.; Breurec, S.; Coombs, G.; Egyir, B.; Larsen, A.R.; Laurent, F.; Monecke, S.; et al. Origin, evolution, and global transmission of community-acquired Staphylococcus aureus ST8. Proc. Natl. Acad. Sci. USA 2017, 20, E10596–E10604. [Google Scholar] [CrossRef] [PubMed]
- Thammavongsa, V.; Kim, H.K.; Missiakas, D.; Schneewind, O. Staphylococcal manipulation of host immune responses. Nat. Rev. Microbiol. 2015, 13, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Claes, J.; Liesenborghs, L.; Peetermans, M.; Veloso, T.R.; Missiakas, D.; Schneewind, O.; Mancini, S.; Entenza, J.M.; Hoylaerts, M.F.; Heying, R.; et al. Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J. Thromb Haemost. 2017, 15, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Vlaeminck, J.; Raafat, D.; Surmann, K.; Timbermont, L.; Normann, N.; Sellman, B.; van Wamel, W.; Malhotra-Kumar, S. Exploring virulence factors and alternative therapies against Staphylococcus aureus pneumonia. Toxins 2020, 12, 721. [Google Scholar] [CrossRef]
- Hiltunen, A.K.; Savijoki, K.; Nyman, T.A.; Miettinen, I.; Ihalainen, P.; Peltonen, J.; Fallarero, A. Structural and functional dynamics of Staphylococcus aureus biofilms and biofilm matrix proteins on different clinical materials. Microorganisms 2019, 7, 584. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Intern. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microb. 2017, 104, 365–376. [Google Scholar] [CrossRef]
- Otto, M. Staphylococcal biofilms. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; He, L.; Asiamah, T.K.; Otto, M. Colonization of medical devices by staphylococci. Environ. Microbiol. 2018, 20, 3141–3153. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, K.; Moriwaki, M.; Miyake, R.; Hide, M. Staphylococcus aureus in atopic dermatitis: Strain-specific cell wall proteins and skin immunity. Allergol. Int. 2019, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Paharik, A.E.; Horswill, A.R. The staphylococcal biofilm: Adhesins, regulation, and host response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Askarian, F.; Uchiyama, S.; Valderrama, J.A.; Ajayi, C.; Sollid, J.; van Sorge, N.M.; Nizet, V.; van Strijp, J.; Johannessen, M. Serine-aspartate repeat protein D increases Staphylococcus aureus virulence and survival in blood. Infect. Immun. 2016, 85, e00559-16. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti. Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Otto, M.; Reppschläger, K.; Iqbal, J.; Holtfreter, S. Fighting Staphylococcus aureus biofilms with monoclonal antibodies. Trends Microbiol. 2019, 27, 303–322. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A review of phytochemistry and biological effects. Nutrients 2020, 12, 3398. [Google Scholar] [CrossRef]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of phenolic compounds in the reduction of risk factors of cardiovascular diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Polka, D.; Podsedek, A. Phenolics composition and antioxidant capacity of guelder rose fruit, flower and bark extracts. Biotechnol. Food Sci. 2019, 83, 37–46. [Google Scholar] [CrossRef]
- Rasamiravaka, T.; Ngezahayo, J.; Pottier, L.; Ribeiro, S.O.; Souard, F.; Hari, L.; Stévigny, C.; Jaziri, M.E.; Duez, P. Terpenoids from Platostoma rotundifolium (Briq.) A. J. Paton alter the expression of quorum sensing-related virulence factors and the formation of biofilm in Pseudomonas aeruginosa PAO1. Int. J. Mol. Sci. 2017, 18, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Howell, A.B.; Botto, H.; Combescure, C.; Blanc-Potard, A.B.; Gausa, L.; Matsumoto, T.; Tenke, P.; Sotto, A.; Lavigne, J.-P. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: A multicentric randomized double blind study. BMC Infect. Dis. 2010, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Laskowski, D.; Bernat, P.; Micota, B.; Więckowska-Szakiel, M.; Podsędek, A.; Różalska, B. Molecular mechanisms of Leonurus cardiaca L. extract activity in prevention of staphylococcal endocarditis—Study on in vitro and ex vivo models. Molecules 2019, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Różalska, B.; Sadowska, B.; Żuchowski, J.; Więckowska-Szakiel, M.; Budzyńska, A.; Wójcik, U.; Stochmal, A. Phenolic and nonpolar fractions of Elaeagnus rhamnoides (L.) A. Nelson extracts as virulence modulators—In vitro study on bacteria, fungi, and epithelial cells. Molecules 2018, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, B.; Wójcik, U.; Krzyżanowska-Kowalczyk, J.; Kowalczyk, M.; Stochmal, A.; Rywaniak, J.; Burzyńska, J.; Różalska, B. The pros and cons of cystic fibrosis (CF) patient use of herbal supplements containing Pulmonaria officinalis L. extract: The evidence from an in vitro study on Staphylococcus aureus CF clinical isolates. Molecules 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Suliman, M.; Santosh, V.; Seegar, T.; Dalton, A.C.; Schultz, K.M.; Klug, C.S.; Barton, W.A. Directed evolution provides insight into conformational substrate sampling by SrtA. PLoS ONE 2017, 12, e0184271. [Google Scholar] [CrossRef]
- Schneewind, O.; Missiakas, D. Sortases, surface proteins, and their roles in Staphylococcus aureus disease and vaccine development. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Zhang, B.; Teng, Z.; Xianhe, L.; Lu, G.; Deng, X.; Niu, X.; Wang, J. Chalcone attenuates Staphylococcus aureus virulence by targeting sortase A and alpha-hemolysin. Front. Microbiol. 2017, 8, 1715. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.-G.; Lee, T.-H.; Oh, K.-B. Flavonols inhibit sortases and sortase-mediated Staphylococcus aureus clumping to fibrinogen. Biol. Pharm. Bull. 2006, 29, 1751–1755. [Google Scholar] [CrossRef]
- Liu, B.; Chen, F.; Bi, C.; Wang, L.; Zhong, X.; Cai, H.; Deng, X.; Niu, X.; Wang, D. Quercitrin, an inhibitor of sortase A, interferes with the adhesion of Staphylococcal aureus. Molecules 2015, 20, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, P.; He, X.; Yuan, Z.W.; Yin, Z.Q.; Fu, H.; Lin, J.; He, C.; Liang, X.; Lv, C.; Shu, G.; et al. Erianin against Staphylococcus aureus infection via inhibiting sortase A. Toxins 2018, 10, 385. [Google Scholar] [CrossRef]
- Sarıözkan, S.; Türk, G.; Eken, A.; Bayram, L.; Baldemir, A.; Doğan, G. Gilaburu (Viburnum opulus L.) fruit extract alleviates testis and sperm damages induced by taxane-based chemotherapeutics. Biomed. Pharmacother. 2017, 95, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef]
- Barak, T.H.; Celep, E.; İnan, Y.; Yesilada, E. Influence of in vitro human digestion on the bioavailability of phenolic content and antioxidant activity of Viburnum opulus L. (European cranberry) fruit extracts. Ind. Crop. Prod. 2019, 131, 62–69. [Google Scholar] [CrossRef]
- Bujor, A.; Miron, A.; Luca, S.V.; Skalicka-Wozniak, K.; Silion, M.; Ancuceanu, R.; Dinu, M.; Girard, C.; Demougeot, C.; Totoson, P. Metabolite profiling, arginase inhibition and vasorelaxant activity of Cornus mas, Sorbus aucuparia and Viburnum opulus fruit extracts. Food Chem. Toxicol. 2019, 133, 1–2. [Google Scholar] [CrossRef]
- Cam, M.; Hisil, Y.; Kuscu, A. Organic acid, phenolic content and antioxidant capacity of fruit flesh and seed of Viburnum opulus. Chem. Nat. Compd. 2007, 43, 379–380. [Google Scholar] [CrossRef]
- Ceylan, D.; Aksoy, A.; Ertekin, T.; Yay, A.H.; Nisari, M.; Karatoprak, G.Ş.; Ülger, H. The effects of gilaburu (Viburnum opulus) juice on experimentally induced Ehrlich ascites tumor in mice. J. Cancer Res. Ther. 2018, 14, 314–320. [Google Scholar] [CrossRef]
- Ilhan, M.; Ergene, B.; Süntar, I.; Özbilgin, S.; Çitoğlu, G.S.; Demirel, M.A.; Keleş, H.; Altun, L.; Akkol, E.K. Preclinical evaluation of antiurolithiatic activity of Viburnum opulus L. on sodium oxalate-induced urolithiasis rat model. Evidence-based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Paşayeva, L.; Arslan, A.K.K.; Kararenk, A.C. Viburnum opulus L. fruit extracts protect human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Proceedings 2019, 40, 5. [Google Scholar] [CrossRef]
- Podsędek, A.; Zakłos-Szyda, M.; Polka, D.; Sosnowska, D. Efects of Viburnum opulus fruit extracts on adipogenesis of 3T3-L1 cells and lipase activity. J. Funct. Foods 2020, 73, 104111. [Google Scholar] [CrossRef]
- Ulger, H.; Ertekin, T.; Karaca, O.; Canoz, O.; Nisari, M.; Unur, E.; Elmalı, F. Influence of gilaburu (Viburnum opulus) juice on 1,2-dimethylhydrazine (DMH)-induced colon cancer. Toxicol. Ind. Health 2013, 29, 824–829. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1 cells adipogenesis and pancreatic lipase activity. Nutrients 2020, 12, 2003. [Google Scholar] [CrossRef] [PubMed]
- Sagdic, O.; Ozturk, I.; Yapar, N.; Yetim, H. Diversity and probiotic potentials of lactic acid bacteria isolated from gilaburu, a traditional Turkish fermented European cranberrybush (Viburnum opulus L.) fruit drink. Food. Res. Internat. 2014, 64, 537–545. [Google Scholar] [CrossRef]
- Shikov, A.N.; Tsitsilin, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Heinrich, M. (2017) Traditional and Current Food Use of Wild Plants Listed in the Russian Pharmacopoeia. Front. Pharmacol. 2017, 8, 841. [Google Scholar] [CrossRef]
- Shikov, A.N.; Narkevich, I.A.; Flisyuk, E.V.; Luzhanin, V.G.; Pozharitskaya, O.N. Medicinal plants from the 14th edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharm. 2021, 268, 113685. [Google Scholar] [CrossRef]
- Kraujalyte, V.; Venskutonis, P.R.; Pukalskas, A.; Cesoniene, L.; Daubaras, R. Antioxidant properties and polyphenolic compositions of fruits from different European cranberrybush (Viburnum opulus L.) genotypes. Food Chem. 2013, 141, 3695–3702. [Google Scholar] [CrossRef]
- Özrenk, M.; Gündoğdu, N.; Keskin, N.; Kaya, T. Some physical and chemical characteristics of gilaburu (Viburnum opulus L.) fruits in Erzincan region. Iğdır Univ. J. Inst. Sci. Technol. 2011, 1, 9–14. [Google Scholar]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G.V.; Samylina, I.A. Biologically active substances from European guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant. Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcal aureus infections through sortase A inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef]
- Bi, C.; Wang, L.; Niu, X.; Cai, H.; Zhong, X.; Deng, X.; Wang, T.; Wang, D. The use of chlorogenic acid and its analogues as inhibitors: An investigation of the inhibition of sortase A of Staphylococcal aureus using molecular docking and dynamic simulation. Biotechnol. Lett. 2016, 38, 1341–1347. [Google Scholar] [CrossRef]
- Arenz, S.; Wilson, D.N. Bacterial protein synthesis as a target for antibiotic inhibition. Cold Spring Harb. Perspect. Med. 2016, 6, a025361. [Google Scholar] [CrossRef]
- Hoppenbrouwers, T.; Sultan, A.R.; Abraham, T.E.; Lemmens-den Toom, N.A.; Hansenová Maňásková, S.; van Cappellen, W.A.; Houtsmuller, A.B.; van Wamel, W.; de Maat, M.; van Neck, J.W. Staphylococcal protein A is a key factor in neutrophil extracellular traps formation. Front. Immunol. 2018, 9, 165. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.C.; Leonard, A.; Schäuble, M.; Rieckmann, L.M.; Hoyer, J.; Maass, S.; Lalk, M.; Becher, D.; Pané-Farré, J.; Riedel, K. Virulence factors produced by Staphylococcus aureus biofilms have a moonlighting function contributing to biofilm integrity. Mol. Cell Proteomics 2019, 18, 1036–1053. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Dou, C.; Cao, Z.; Liu, C.; Dong, S.; Fei, J. Staphylococcal protein A promotes osteoclastogenesis through MAPK signaling during bone infection. J. Cell Physiol. 2017, 232, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Frankel, M.B.; Schneewind, O.; Missiakas, D. Release of protein A from the cell wall of Staphylococcus aureus. Proc. Natl. Acad. Sci. U S A. 2014, 111, 1574–1579. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharmaceut. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial effect and mode of action of chlorogenic acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Hewelt-Belka, W.; Nakonieczna, J.; Belka, M.; Bączek, T.; Namieśnik, J.; Kot-Wasik, A. Untargeted lipidomics reveals differences in the lipid pattern among clinical isolates of Staphylococcus aureus resistant and sensitive to antibiotics. J. Proteome Res. 2016, 15, 914–922. [Google Scholar] [CrossRef]
- Young, S.A.; Desbois, A.P.; Coote, P.J.; Smith, T.K. Characterisation of Staphylococcus aureus lipids by nanoelectrospray ionisation tandem mass spectrometry (nESI-MS/MS). bioRxiv 2019. [Google Scholar] [CrossRef]
- Bayer, A.S.; Mishra, N.N.; Chen, L.; Kreiswirth, B.N.; Rubio, A.; Yang, S.-J. Frequency and distribution of single-nucleotide polymorphisms within mprF in methicillin-resistant Staphylococcus aureus clinical isolates and their role in cross-resistance to daptomycin and host defense antimicrobial peptides. Antimicrob. Agents Chemother. 2015, 59, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Kilelee, E.; Pokorny, A.; Yeaman, M.R.; Bayer, A.S. Lysyl-phosphatidylglycerol attenuates membrane perturbation rather than surface association of the cationic antimicrobial peptide 6W-RP-1 in a model membrane system: Implications for daptomycin resistance. Antim. Agents Chemother. 2010, 54, 4476–4479. [Google Scholar] [CrossRef]
- Diemond-Hernández, B.; Solórzano-Santos, F.; Leaños-Miranda, B.; Peregrino-Bejarano, L.; Miranda-Novales, G. Production of icaADBC-encoded polysaccharide intercellular adhesin and therapeutic failure in pediatric patients with staphylococcal device-related infections. BMC Infect. Dis. 2010, 10, 68–74. [Google Scholar] [CrossRef]
- Nguyen, H.; Nguyen, T.H.; Otto, M. The staphylococcal exopolysaccharide PIA-biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020, 18, 3324–3334. [Google Scholar] [CrossRef]
- Nasr, R.A.; AbuShady, H.M.; Hussein, H.S. Biofilm formation and presence of icaAD gene in clinical isolates of staphylococci. Egypt, J. Med. Hum. Genet. 2012, 13, 269–274. [Google Scholar] [CrossRef]
- Rohde, H.; Frankerberger, R.H.; Zähringer, U.; Mack, D. Structure, function and contribution of polysaccharide intercellular adhesion (PIA) to Staphylococcus epidermidis biofilm formation and pathogenesis of biomaterial-associated infections. Eur. J. Cell Biol. 2010, 89, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica I- the quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: http://www.eucast.org (accessed on 27 January 2021).
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef] [PubMed]
| Components | Extraction Solvent | Fruit (VF) | Bark (VB) |
|---|---|---|---|
| Protein (mg/g) | 70% acetone | 11.38 ± 0.88 Aa | 13.14 ± 0.90 Ab |
| 70% ethanol | 9.63 ± 0.88 Aa | 8.64 ± 0.01 Aa | |
| water | 14.29 ± 0.15 Ab | 16.13 ± 0.93 Bc | |
| Sugars (mg/g) | 70% acetone | 606.87 ± 9.97 Bb | 186.00 ± 0.52 Ab |
| 70% ethanol | 674.69 ± 18.67 Bc | 192.95 ± 1.45 Ac | |
| water | 541.79 ± 30.58 Ba | 162.38 ± 2.18 Aa | |
| Organic acids (mg/g) | 70% acetone | 79.05 ± 1.24 Bb | 6.26 ± 0.21 Ab |
| 70% ethanol | 86.36 ± 0.48 Bc | 7.24 ± 0.04 Ac | |
| water | 71.51 ± 4.89 Ba | 4.26 ± 0.09 Aa | |
| Total phenolics 1 (mg GAE/g) | 70% acetone | 80.47 ± 3.86 Ac | 254.97 ± 1.54 Bc |
| 70% ethanol | 71.76 ± 3.02 Ab | 218.53 ± 2.07 Bb | |
| water | 50.64 ± 1.53 Aa | 171.02 ± 3.16 Ba | |
| Flavanols 2 (mg CE/g) | 70% acetone | 18.58 ± 0.19 Ac | 91.51 ± 2.38 Ba |
| 70% ethanol | 14.44 ± 0.73 Ab | 96.69 ± 2.59 Bab | |
| water | 11.82 ± 1.19 Aa | 97.79 ± 3.71 Bb | |
| Proanthocyanidins (mg CYE/g) | 70% acetone | 12.26 ± 0.46 Ac | 63.83 ± 4.60 Bc |
| 70% ethanol | 10.06 ± 0.46 Ab | 56.67 ± 1.41 Bb | |
| water | 5.81 ± 0.31 Aa | 50.29 ± 1.80 Ba | |
| Total hydroxycinnamic acids 4 (mg CA/g) | 70% acetone | 65.04 ± 0.49 Bb | 26.20 ± 0.02 Ab |
| 70% ethanol | 65.64 ± 0.29 Bb | 26.65 ± 0.01 Ac | |
| water | 60.37 ± 0.04 ba | 14.24 ± 0.02 Aa | |
| Total flavanols4 (mg CE/g) | 70% acetone | 11.58 ± 0.07 Ac | 132.15 ± 0.13 Bc |
| 70% ethanol | 10.33 ± 0.07 Ab | 127.25 ± 0.12 Bb | |
| water | 8.84 ± 0.02 Aa | 119.13 ± 0.08 Ba | |
| Total flavonols 4 (mg QG/g) | 70% acetone | 0.56 ± 0.01 b | - |
| 70% ethanol | 0.61 ± 0.01 c | - | |
| water | 0.48 ± 0.01 a | - | |
| Total flavalignans 4 (mg CIN/g) | 70% acetone | 1.13 ± 0.03 Aa | 3.93 ± 0.03 Ba |
| 70% ethanol | 1.92 ± 0.09 Ab | 4.07 ± 0.10 Ba | |
| water | 4.30 ± 0.04 Ac | 6.04 ± 0.01 Bb | |
| Total iridoids 4 (mg QR/g) | 70% acetone | - | 15.05 ± 0.23 a |
| 70% ethanol | - | 15.44 ± 0.19 a | |
| water | - | 23.66 ± 0.51 b |
| Concentration [mg/mL] | VFa | VFe | VFw | VBa | VBe | VBw | ChA |
|---|---|---|---|---|---|---|---|
| MIC | 2 | 2 | >2 | 1 | 2 | 2 | >2 |
| MBC | >2 | 2 | >2 | 1 | >2 | >2 | >2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Bojek, U.; Rywaniak, J.; Bernat, P.; Podsędek, A.; Kajszczak, D.; Sadowska, B. An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections. Molecules 2021, 26, 1758. https://doi.org/10.3390/molecules26061758
Wójcik-Bojek U, Rywaniak J, Bernat P, Podsędek A, Kajszczak D, Sadowska B. An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections. Molecules. 2021; 26(6):1758. https://doi.org/10.3390/molecules26061758
Chicago/Turabian StyleWójcik-Bojek, Urszula, Joanna Rywaniak, Przemysław Bernat, Anna Podsędek, Dominika Kajszczak, and Beata Sadowska. 2021. "An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections" Molecules 26, no. 6: 1758. https://doi.org/10.3390/molecules26061758
APA StyleWójcik-Bojek, U., Rywaniak, J., Bernat, P., Podsędek, A., Kajszczak, D., & Sadowska, B. (2021). An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections. Molecules, 26(6), 1758. https://doi.org/10.3390/molecules26061758