Profiling of Potential Antibacterial Compounds of Lactic Acid Bacteria against Extremely Drug Resistant (XDR) Acinetobacter baumannii
Abstract
1. Introduction
2. Results
2.1. Antimicrobial Assay
2.2. Gas Chromatography Mass Spectrometry (GCMS) Analysis
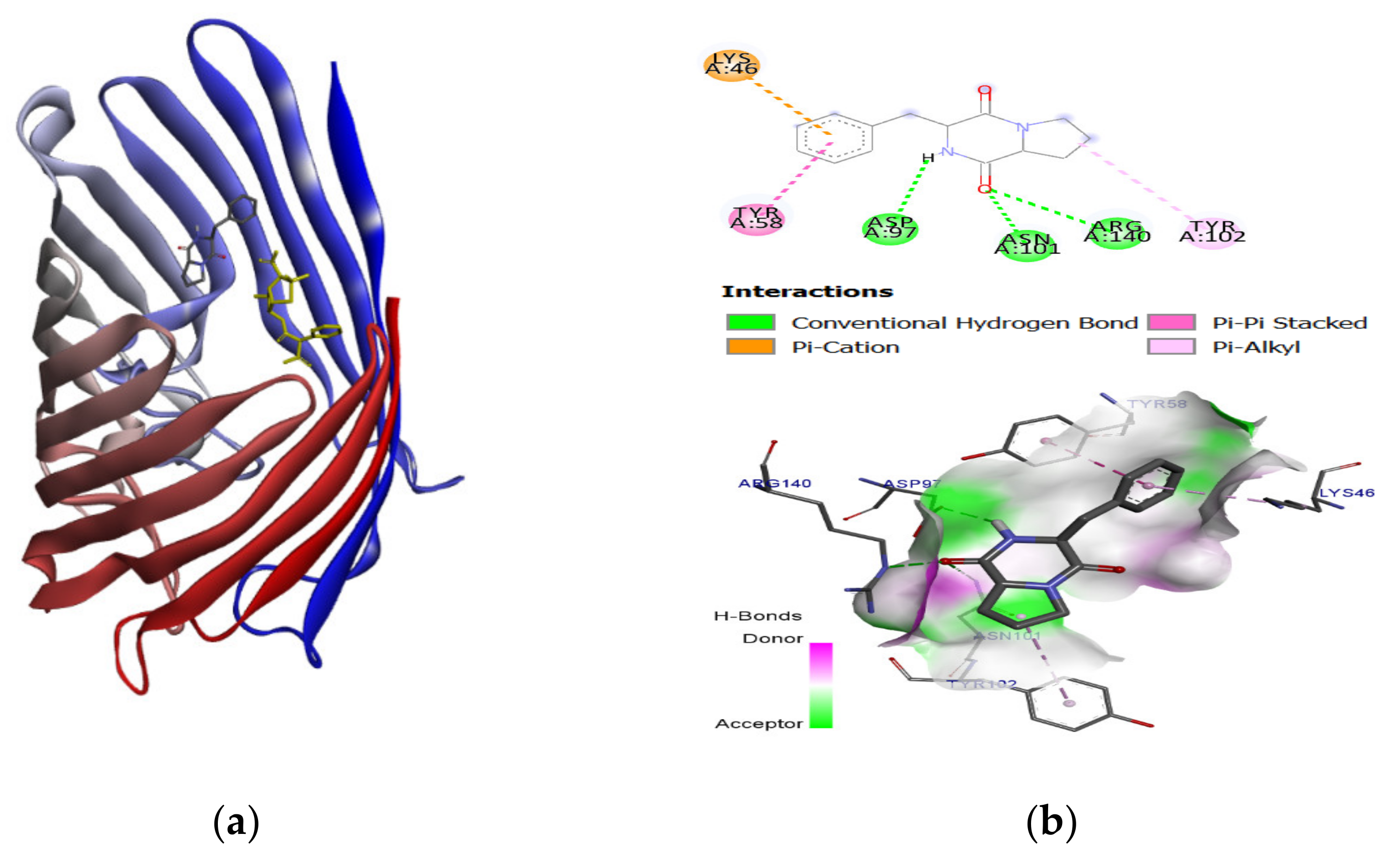
2.3. Molecular Docking and Binding Energy Evaluation
3. Discussion
4. Materials and Methods
4.1. Approval by Institutional Biosafety and Biosecurity Council and Ethics Committee
4.2. Sampling and Isolation of Lactic Acid Bacteria
4.3. Molecular Identification and Hydrogen Peroxide Production
4.4. Well-Diffusion Antimicrobial Assay
4.5. Screening Against Clinical Isolates of Extremely Drug Resistance (XDR) Acinetobacter baumannii
4.6. GCMS
4.7. In Silico Investigation toward Antimicrobial Multi-Protein Targets
- 4GCP: Crystal structure of E. coli OmpF porin in complex with ampicillin
- 4GCQ: Crystal structure of E. coli OmpF porin in complex with carbenicillin
- 4GCS: Crystal structure of E. coli OmpF porin in complex with ertapenem
- 1YRJ: Crystal structure of apramycin bound to a ribosomal RNA
- 1MWL: Crystal structure of geneticin bound to the eubacterial 16S rRNA
- 1J7T: Crystal structure of paromomycin and the 16S rRNA
- 1LC4: Crystal structure of tobramycin bound to the eubacterial 16S rRNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability: Sample of the compound L001 is available from the authors. |
References
- Rouse, S.; Harnett, D.; Vaughan, A.; Van Sinderen, D. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2007, 104, 915–923. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhang, L.-W.; Ma, W.; Yi, H.-X.; Yang, X.; Du, M.; Shan, Y.-J.; Han, X.; Zhang, L.-L. Screening of probiotic lactobacilli for inhibition of Shigella sonnei and the macromolecules involved in inhibition. Anaerobe 2012, 18, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bazukyan, I.; Matevosyan, L.; Toplaghaltsyan, A.; Trchounian, A. Antifungal activity of lactobacilli isolated from Armenian dairy products: An effective strain and its probable nature. AMB Express 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous probiotic Lactobacillus isolates presenting antibiotic like activity against human pathogenic bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, K.; Lee, Y.S.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.-W. Preliminary probiotic and technological characterization of Pediococcus pentosaceus strain KID7 and in vivo assessment of its cholesterol-lowering activity. Front. Microbiol. 2015, 6, 768. [Google Scholar] [CrossRef] [PubMed]
- Pessione, E. Lactic acid bacteria contribution to gut microbiota complexity: Lights and shadows. Front. Cell. Infect. Microbiol. 2012, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Putting microbes to work: Dairy fermentation, cell factories and bioactive peptides. Part II: Bioactive peptide functions. Biotechnol. J. 2007, 2, 435–449. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Working Group Report on Drafting Guidelines for the Evalution of Probiotics in Food; FAO/WHO: London, ON, Canada, 2002. [Google Scholar]
- Ahmadi, S.; Soltani, M.; Shamsaie, M.; Islami, H.R.; Peyghan, R. Comparative effect of Pediococcus acidilactici as probiotic and vitamin C on survival, growth performance and enzyme activities of white leg shrimp (Litopenaeus vannamei). J. Anim. Vet. Adv. 2014, 13, 877–885. [Google Scholar] [CrossRef]
- Bassetti, M.; De Waele, J.J.; Eggimann, P.; Garnacho-Montero, J.; Kahlmeter, G.; Menichetti, F.; Nicolau, D.P.; Paiva, J.A.; Tumbarello, M.; Welte, T.; et al. Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Intensiv. Care Med. 2015, 41, 776–795. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, M.K.; Joseph, M.R.P.; Assiry, M.M.; Hamid, M.E. Multidrug-Resistant Acinetobacter baumannii: An emerging health threat in Aseer Region, Kingdom of Saudi Arabia. Can. J. Infect. Dis. Med. Microbiol. 2018, 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Alvi, I.A.; Rehman, S.U. Insight into Acinetobacter baumannii: Pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect. Drug Resist. 2018, 11, 1249–1260. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Hashemi, F.B.; Pourakbari, B.; Aziemzadeh, M.; Bahador, A. Antimicrobial resistance of Acinetobacter baumannii to imipenem in Iran: A systematic review and meta-analysis. Open Microbiol. J. 2016, 10, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Li, H.; Zhang, C.; Liang, B.; Li, J.; Wang, L.; Du, X.; Liu, X.; Qiu, S.; Song, H. Relationship between antibiotic resistance, biofilm formation, and biofilm-specific resistance in Acinetobacter baumannii. Front. Microbiol. 2016, 7, 483. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and pathophysiological overview of Acinetobacter infections: A century of challenges. Clin. Microbiol. Rev. 2016, 30, 409–447. [Google Scholar] [CrossRef]
- Qin, L.-J.; Wang, X. A review on Acinetobacter baumannii. J. Acute Dis. 2019, 8, 16. [Google Scholar] [CrossRef]
- Manzoor, A.; Ul-Haq, I.; Baig, S.; Qazi, J.I.; Niazi, R.K. Efficacy of locally isolated lactic acid bacteria against antibiotic-resistant uropathogens. Jundishapur J. Microbiol. 2015, 8, 18952. [Google Scholar] [CrossRef]
- Zheng, P.-X.; Fang, H.-Y.; Yang, H.-B.; Tien, N.-Y.; Wang, M.-C.; Wu, J.-J. Lactobacillus pentosus strain LPS16 produces lactic acid, inhibiting multidrug-resistant Helicobacter pylori. J. Microbiol. Immunol. Infect. 2016, 49, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Buyana, B.; Alven, S.; Nqoro, X.; Aderibigbe, B. Antibiotics encapsulated scaffolds as potential wound dressings. In Antibiotic Materials in Healthcare; Kokkarachedu, V., Kanikireddy, V., Sadiku, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 111–128. [Google Scholar]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, antibiotic resistance mechanisms, and prospective treatment options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, V.; Sinha, S.; Singh, N.P. Multidrug resistant Acinetobacter. J. Glob. Infect. Dis. 2010, 2, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Choi, J.; Choi, S.-J.; Baek, K.-H. Cyclodipeptides: An overview of their biosynthesis and biological activity. Molecules 2017, 22, 1796. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mohandas, C. Antimycobacterial activity of cyclic dipeptides isolated from Bacillus sp. N strain associated with entomopathogenic nematode. Pharm. Biol. 2013, 52, 91–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, S.N.; Mohandas, C.; Nambisan, B. Purification of an antifungal compound, cyclo(l-Pro-d-Leu) for cereals produced by Bacillus cereus subsp. thuringiensis associated with entomopathogenic nematode. Microbiol. Res. 2013, 168, 278–288. [Google Scholar] [CrossRef]
- Kumar, S.N.; Mohandas, C.; Siji, J.; Rajasekharan, K.; Nambisan, B. Identification of antimicrobial compound, diketopiperazines, from a Bacillus sp. N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. J. Appl. Microbiol. 2012, 113, 914–924. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B.; Mohandas, C.; Sundaresan, A. In vitro synergistic activity of diketopiperazines alone and in combination with amphotericin B or clotrimazole against Candida albicans. Folia Microbiol. 2013, 58, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. dd-Diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yu, L.; Peng, C.; Li, Z.; Guo, Y. Diketopiperazines from two strains of South China Sea sponge-associated microorganisms. Biochem. Syst. Ecol. 2010, 38, 931–934. [Google Scholar] [CrossRef]
- De Carvalho, M.P. Antimicrobial and biofilm inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Kim, A.H.; Kwak, M.-K.; Kang, S.-O. Proline-based cyclic dipeptides from korean fermented vegetable kimchi and from Leuconostoc mesenteroides LBP-K06 have activities against multidrug-resistant bacteria. Front. Microbiol. 2017, 8, 761. [Google Scholar] [CrossRef]
- Lertcanawa, M.; Chawawisit, K.; Bhoopong, P.; Phupong, W. Combination effect between 2, 4-Di-tert-butylphenol produced by Streptomyces sp. KB1 TISTR 2304 and vancomycin against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Pharmacol. 2016, 12, 838–844. [Google Scholar] [CrossRef]
- Chawawisit, K.; Bhoopong, P.; Phupong, W.; Lertcanawa, M. Anti-MRSA activity, mode of action and cytotoxicity of 2, 4-Di-tert-butylphenol produced by Streptomyces sp. KB1. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 114–119. [Google Scholar]
- Aissaoui, N.; Mahjoubi, M.; Nas, F.; Mghirbi, O.; Arab, M.; Souissi, Y.; Hoceini, A.; Masmoudi, A.S.; Mosbah, A.; Cherif, A.; et al. Antibacterial potential of 2,4-Di-tert-Butylphenol and calixarene-based prodrugs from thermophilic Bacillus licheniformis isolated in Algerian Hot Spring. Geomicrobiol. J. 2018, 36, 53–62. [Google Scholar] [CrossRef]
- Viszwapriya, D.; Prithika, U.; Deebika, S.; Balamurugan, K.; Pandian, S.K. In vitro and in vivo antibiofilm potential of 2,4-Di- tert -butylphenol from seaweed surface associated bacterium Bacillus subtilis against group A Streptococcus. Microbiol. Res. 2016, 191, 19–31. [Google Scholar] [CrossRef]
- Modi, P.; Patel, S.; Chhabria, M.T. Identification of some novel pyrazolo[1,5-a]pyrimidine derivatives as InhA inhibitors through pharmacophore-based virtual screening and molecular docking. J. Biomol. Struct. Dyn. 2018, 37, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Hameed, H.; Islam, M.M.; Chhotaray, C.; Wang, C.; Liu, Y.; Tan, Y.; Li, X.; Tan, S.; Delorme, V.; Yew, W.W. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front. Cell. Infect. Microbiol. 2018, 8, 114. [Google Scholar] [CrossRef]
- Monika, S.; Kumar, V.; Kumari, A.; Angmo, K.; Bhalla, T.C. Isolation and characterization of lactic acid bacteria from traditional pickles of Himachal Pradesh, India. J. Food Sci. Technol. 2017, 54, 1945–1952. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Chai, L.-C.; Tang, S.-Y.; Jinap, S.; Ghazali, F.M.; Nakaguchi, Y.; Nishibuchi, M.; Son, R. Application of MPN-PCR in biosafety of Bacillus cereus s.l. for ready-to-eat cereals. Food Control. 2009, 20, 1068–1071. [Google Scholar] [CrossRef]
- Garcia, E.F.; Luciano, W.A.; Xavier, D.E.; Da Costa, W.C.A.; Oliveira, K.D.S.; Franco, O.L.; Júnior, M.A.D.M.; Lucena, B.T.L.; Picão, R.C.; Magnani, M.; et al. Identification of lactic acid bacteria in fruit pulp processing byproducts and potential probiotic properties of selected Lactobacillus Strains. Front. Microbiol. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Seki, M.; Iida, K.-I.; Nakayama, H.; Yoshida, S.-I. A novel agar medium to detect hydrogen peroxide-producing bacteria based on the prussian blue-forming reaction. Microbiol. Immunol. 2007, 51, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Hoover, D.G.; Harlander, S.K. CHAPTER 2—Screening methods for detecting bacteriocin activity. In Bacteriocins of Lactic Acid Bacteria; Hoover, D.G., Steenson, L.R., Eds.; Academic Press: Cambridge, MA, USA, 1993; pp. 23–39. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Biovia, D.S. Discovery Studio Modeling Environment; Dassault Systèmes: San Diego, CA, USA, 2018. [Google Scholar]
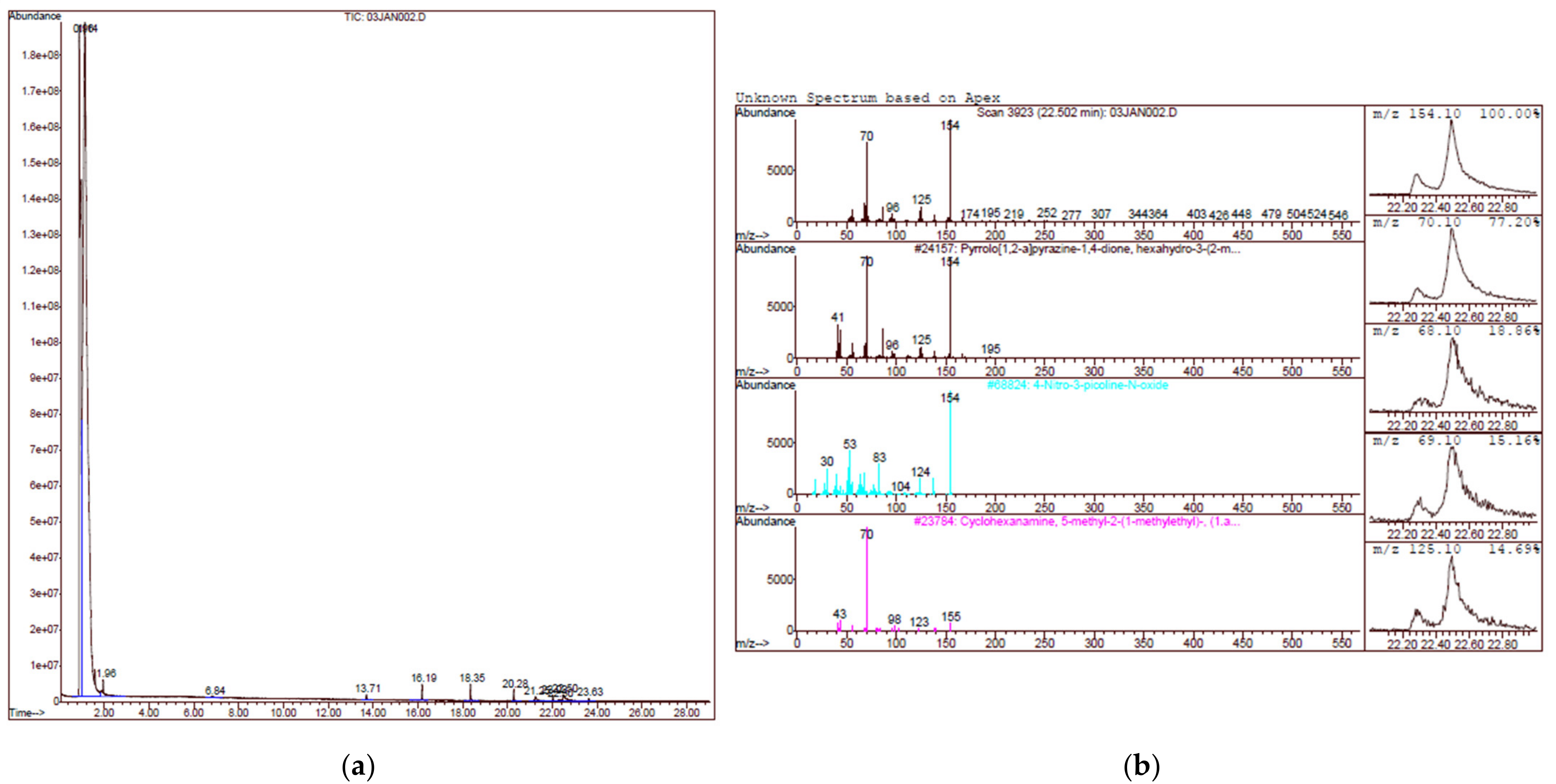
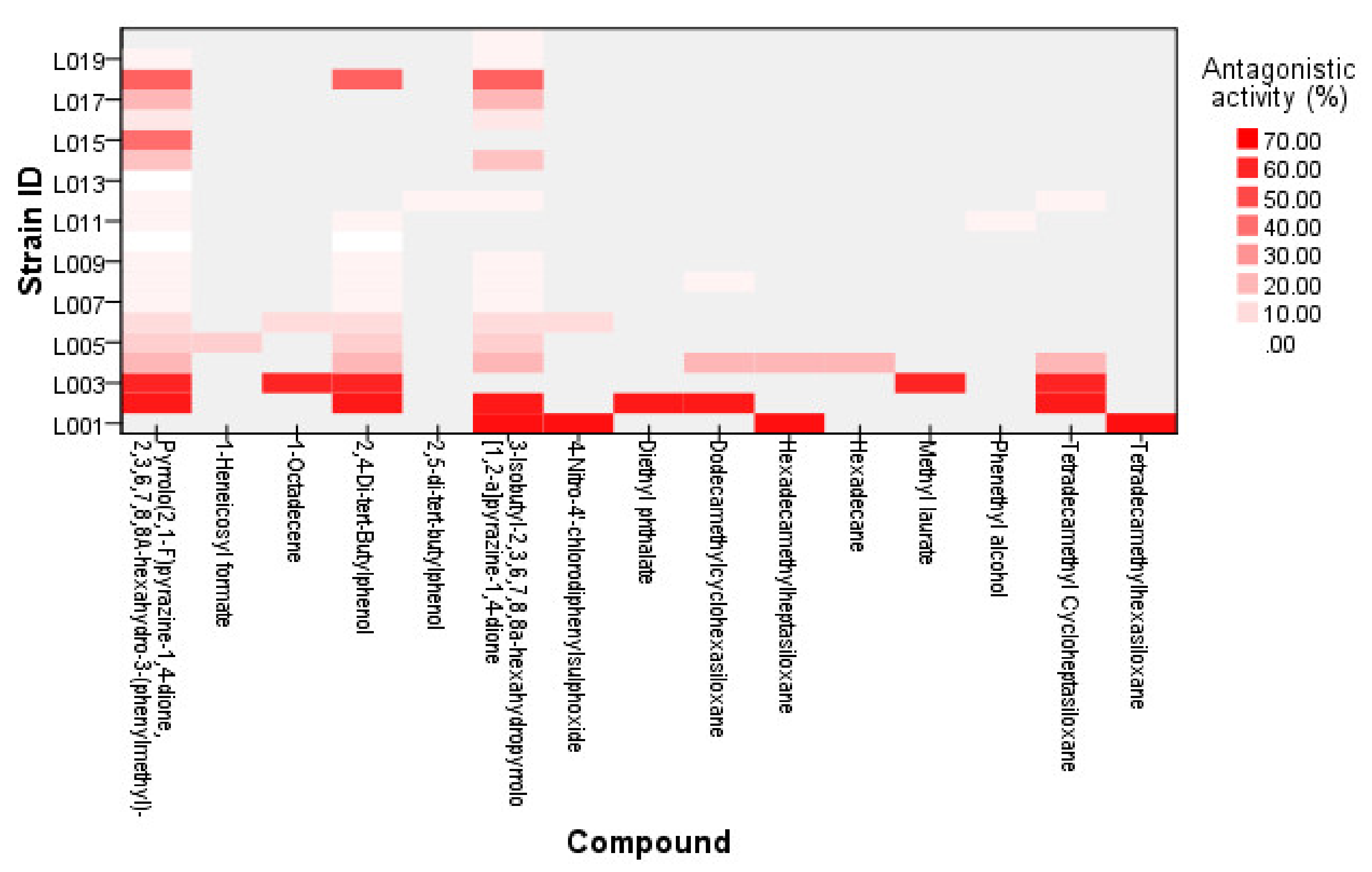

| Sample ID | Antagonistic Activity in 30 XDR A. Baumannii Strains (%) | CAS NO. | Compound ID |
|---|---|---|---|
| L001 (Lactobacillus plantarum) | 20/30 (66.0) | 000541-01-5 | Hexadecamethylheptasiloxane |
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 024535-53-3 | 4-Nitro-4′-chlorodiphenylsulfoxide | ||
| 000107-52-8 | Tetradecamethylhexasiloxane | ||
| L002 (Lactobacillus plantarum) | 19/30 (63.3) | 000084-66-2 | Diethyl phthalate |
| 000096-76-4 | 2,4-Di-tert-Butylphenol | ||
| 000540-97-6 | Dodecamethylcyclohexasiloxane | ||
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| 000107-50-6 | Tetradecamethyl Cycloheptasiloxane | ||
| L003 (Lactobacillus plantarum) | 18/30 (60.0) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 000112-88-9 | 1-Octadecene | ||
| 000111-82-0 | Methyl laurate | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| 000107-50-6 | Tetradecamethyl Cycloheptasiloxane | ||
| L004 (Pediococcus pentosaceus) | 5/30 (16.7) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 000540-97-6 | Dodecamethylcyclohexasiloxane | ||
| 000541-01-5 | Hexadecamethylheptasiloxane | ||
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 000544-76-3 | Hexadecane | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| 000107-50-6 | Tetradecamethyl Cycloheptasiloxane | ||
| L005 (Pediococcus pentosaceus) | 4/30 (13.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 077899-03-7 | 1-Heneicosyl formate | ||
| 014705-60-3 | Pyrrolo (2,1-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| L006 (Pediococcus pentosaceus) | 3/30 (10.0) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 000112-88-9 | 1-Octadecene | ||
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 024535-53-3 | 4-Nitro-4′-chlorodiphenylsulphoxide | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L007 (Pediococcus pentosaceus) | 1/30 (3.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 014705-60-3 | Pyrrolo (2,1-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L008 (Pediococcus pentosaceus) | 1/30 (3.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 000540-97-6 | Dodecamethylcyclohexasiloxane | ||
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo [1,2-a] pyrazine-1,4-dione | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L009 (Pediococcus acidilactici) | 1/30 (3.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| L010 (Pediococcus spp.) | 0/30 (0) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| L011 (Pediococcus acidilactici) | 1/30 (3.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 000060-12-8 | Phenethyl alcohol | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L012 (Pediococcus pentosaceus) | 1/30 (3.3) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione |
| 005875-45-6 | 2,5-di-tert-butylphenol | ||
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| 000107-50-6 | Tetradecamethyl Cycloheptasiloxane | ||
| L013 (Pediococcus pentosaceus) | 0/30 (0) | 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) |
| L014 (Pediococcus pentosaceus) | 5/30 (16.7) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione |
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- | ||
| L015 (Pediococcus pentosaceus) | 12/30 (40.0) | 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl)- |
| L016 (Enterococcus spp.) | 2/30 (6.7) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione |
| 014705-60-3 | Pyrrolo(2,1-F)pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L017 (Pediococcus acidilactici) | 6/30 (20.0) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-a]pyrazine-1,4-dione |
| 014705-60-3 | Pyrrolo (2,1-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L018 (Pediococcus pentosaceus) | 13/20 (43.3) | 000096-76-4 | 2,4-Di-tert-Butylphenol |
| 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo [1,2-a] pyrazine-1,4-dione | ||
| 014705-60-3 | Pyrrolo (2,1-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L019 (Lactobacillus paraplantarum) | 1/30 (3.3) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo [1,2-a] pyrazine-1,4-dione |
| 014705-60-3 | Pyrrolo (2,1-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) | ||
| L020 (Enterococcus spp.) | 1/30 (3.3) | 005654-86-4 | 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo [1,2-a] pyrazine-1,4-dione |
| Compound ID (CAS NO.) | Autodock Vina Binding Affinity (kcal/mol) | ||||||
|---|---|---|---|---|---|---|---|
| OmpF Porin Protein | 16S rRNA | ||||||
| 4GCP | 4GCQ | 4GCS | 1YRJ | 1MWL | 1J7T | 1LC4 | |
| Tetradecamethylhexasiloxane (107-52-8 Si) | −4.8 | −4.6 | −4.5 | −6.3 | −5.6 | −5.9 | −5.3 |
| Dodecamethylcyclohexasiloxane (540-97-6 Si) | −5.8 | −6.4 | −5.6 | −6.0 | −6.2 | −6.1 | −6.6 |
| Hexadecamethylheptasiloxane (541-01-5 Si) | −5.0 | -4.9 | −4.2 | −6.1 | −5.3 | −5.8 | −6.2 |
| 3-Isobutyl-2,3,6,7,8,8a-hexahydropyrrolo[1,2-A] Pyrazine-1,4-Dione (5654-86-4) | −5.4 | −5.4 | −5.5 | −6.1 | −5.7 | −6.0 | −5.9 |
| 4-Nitro-4′-Chlorodiphenylsulphoxide (24535-53-3) | −6.1 | −6.7 | −6.1 | −6.4 | −6.6 | −6.5 | −6.3 |
| Diethyl Phthalate (84-66-2) | −5.3 | −5.6 | −5.1 | −5.3 | −5.7 | −5.8 | −5.3 |
| 2,4-Di-Tert-Butylphenol (96-76-4) | −5.9 | −6.0 | −5.9 | −5.3 | −5.1 | −5.7 | −5.2 |
| Pyrrolo (1,2-F) pyrazine-1,4-dione,2,3,6,7,8,8A-hexahydro-3-(phenylmethyl) (14705-60-3) | −6.4 | −6.8 | −6.4 | −7.1 | −6.5 | −7.0 | −6.5 |
| 1-Octadecene (112-88-9) | −4.2 | −4.9 | −4.1 | −3.6 | −3.2 | −4.0 | −3.4 |
| Hexadecane (544-76-3) | −4.1 | −4.6 | −4.7 | −3.5 | −3.2 | −3.5 | −3.0 |
| PhenethylAlcohol (60-12-8) | −4.3 | −4.9 | −4.3 | −4.0 | −4.3 | −4.4 | −4.2 |
| 1-Heneicosylformate (77899-03-7) | −4.5 | −4.2 | −4.2 | −4.0 | −3.9 | −4.4 | −3.9 |
| Methyl laurate (111-82-0) | −4.5 | −4.7 | −4.7 | −4.1 | −3.7 | −4.2 | −3.7 |
| 2,5-di-tert-butylphenol (5875-45-6) | −5.8 | −6.0 | −5.8 | −5.7 | −5.3 | −5.6 | −5.6 |
| Xray Ligand | Ampicillin | Carbenicillin | Ertapenem | Apramycin | Geneticin | Paromomycin | Tobramycin |
| −7.1 | −7.1 | −6.1 | −8.2 | −7.2 | −8.6 | −7.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yap, P.-C.; Ayuhan, N.; Woon, J.J.; Teh, C.S.J.; Lee, V.S.; Azman, A.S.; AbuBakar, S.; Lee, H.Y. Profiling of Potential Antibacterial Compounds of Lactic Acid Bacteria against Extremely Drug Resistant (XDR) Acinetobacter baumannii. Molecules 2021, 26, 1727. https://doi.org/10.3390/molecules26061727
Yap P-C, Ayuhan N, Woon JJ, Teh CSJ, Lee VS, Azman AS, AbuBakar S, Lee HY. Profiling of Potential Antibacterial Compounds of Lactic Acid Bacteria against Extremely Drug Resistant (XDR) Acinetobacter baumannii. Molecules. 2021; 26(6):1727. https://doi.org/10.3390/molecules26061727
Chicago/Turabian StyleYap, Phui-Chyng, Noorfazlin Ayuhan, Jia Jie Woon, Cindy Shuan Ju Teh, Vannajan Sanghiran Lee, Adzzie Shazleen Azman, Sazaly AbuBakar, and Hai Yen Lee. 2021. "Profiling of Potential Antibacterial Compounds of Lactic Acid Bacteria against Extremely Drug Resistant (XDR) Acinetobacter baumannii" Molecules 26, no. 6: 1727. https://doi.org/10.3390/molecules26061727
APA StyleYap, P.-C., Ayuhan, N., Woon, J. J., Teh, C. S. J., Lee, V. S., Azman, A. S., AbuBakar, S., & Lee, H. Y. (2021). Profiling of Potential Antibacterial Compounds of Lactic Acid Bacteria against Extremely Drug Resistant (XDR) Acinetobacter baumannii. Molecules, 26(6), 1727. https://doi.org/10.3390/molecules26061727






