Does the Phytochemical Diversity of Wild Plants Like the Erythrophleum genus Correlate with Geographical Origin?
Abstract
1. Introduction
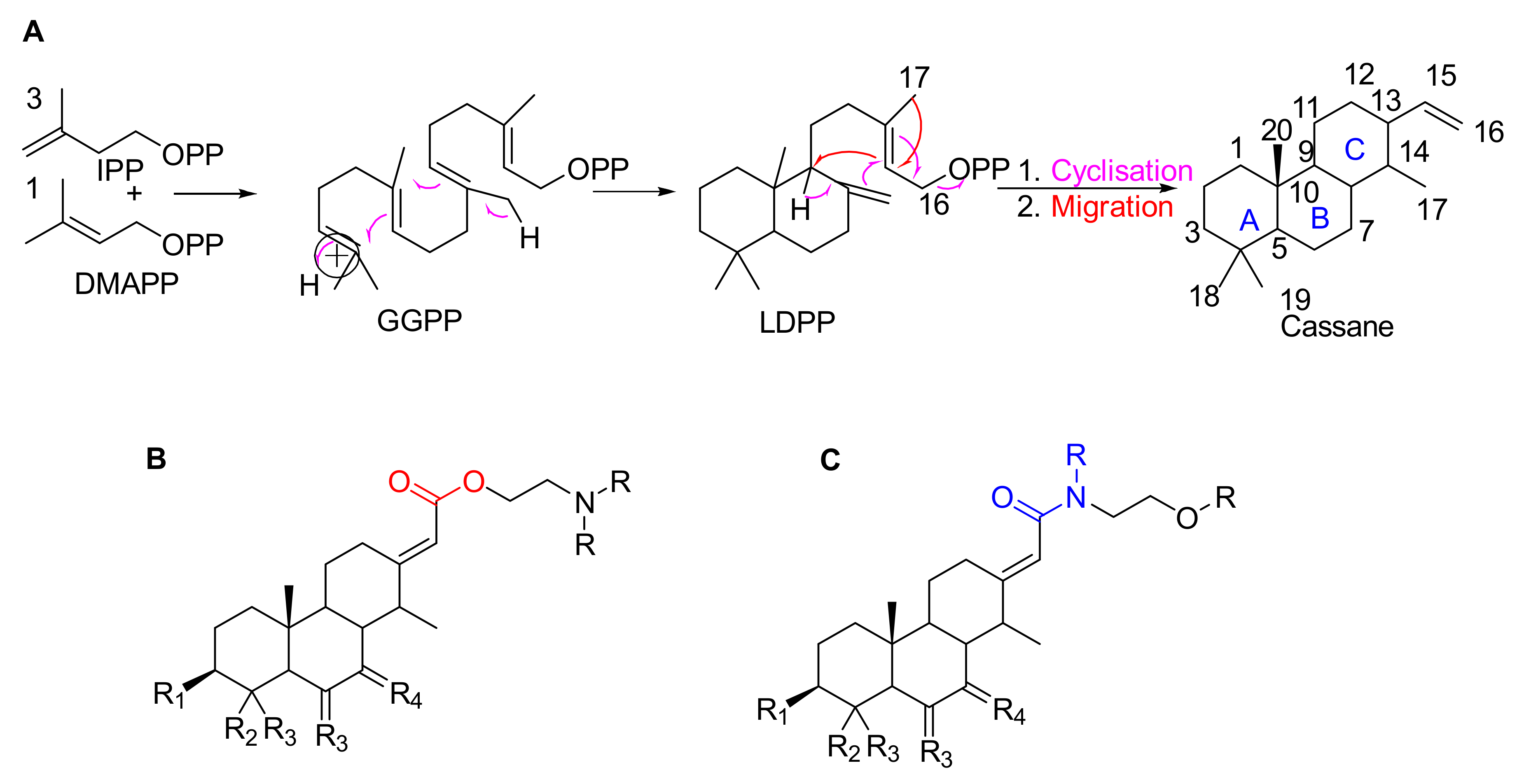
- Cassaine-type diterpenoids with an ester amine arm (Figure 1B) result from esterification between the carboxylic group of a cassane-type tricyclic diterpene acid and the alcohol group of an aminoethanol (often N-methylethanolamine (CH3-NH-CH2-CH2-OH) or N,N-dimethylethanolamine ((CH3)2N-CH2-CH2-OH)) [26]. Ethanolamine can be formed by decarboxylation of a serine or by a transamination reaction (exchange of an amine group) between a glycoaldehyde and a glutamic acid [24,25].
2. Results
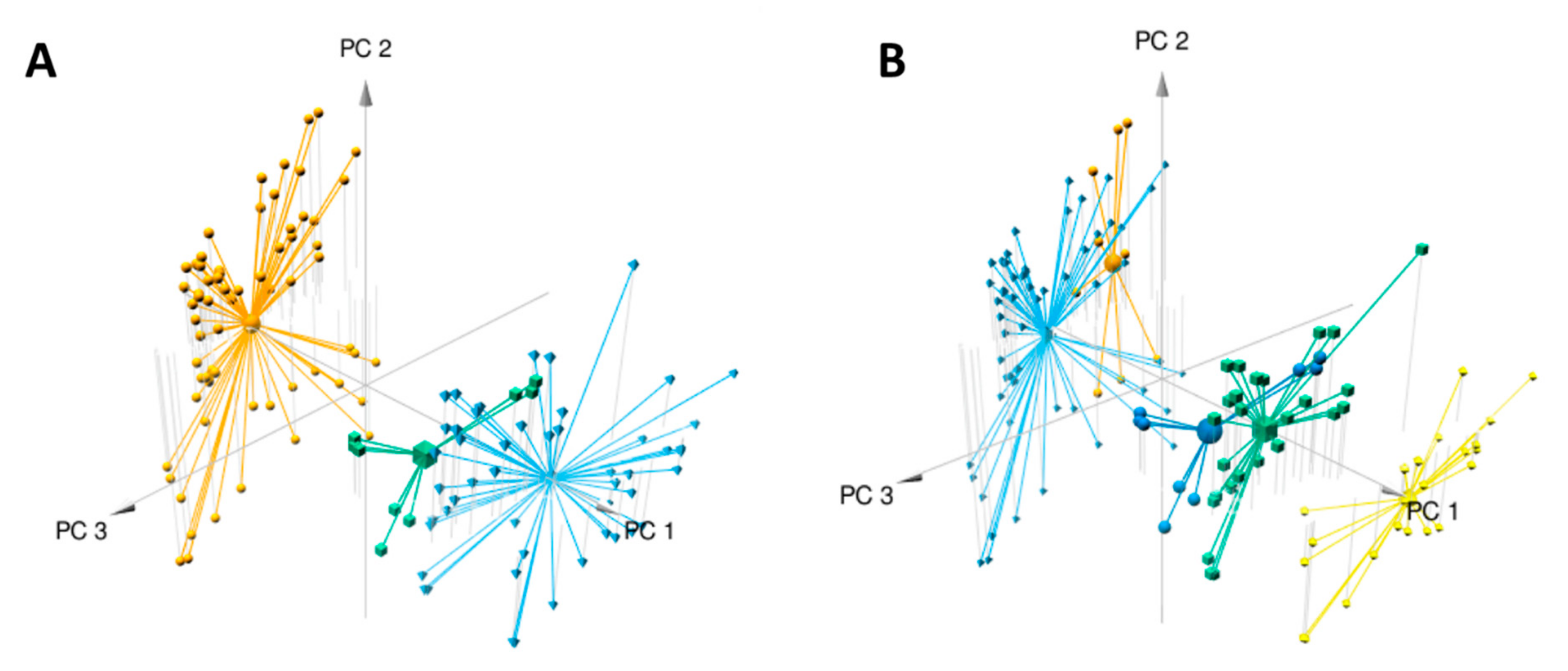
2.1. Metabolomics Analysis
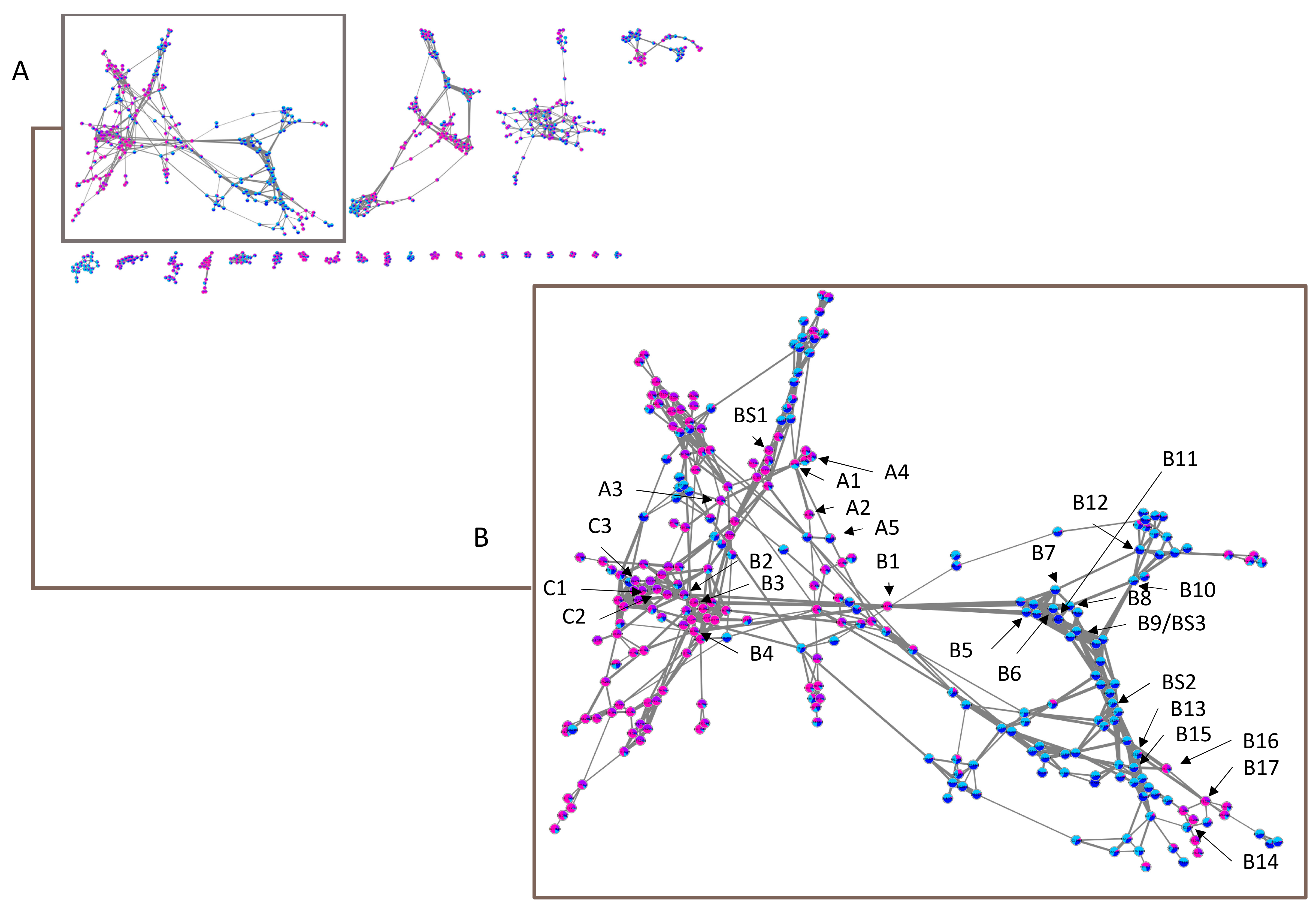
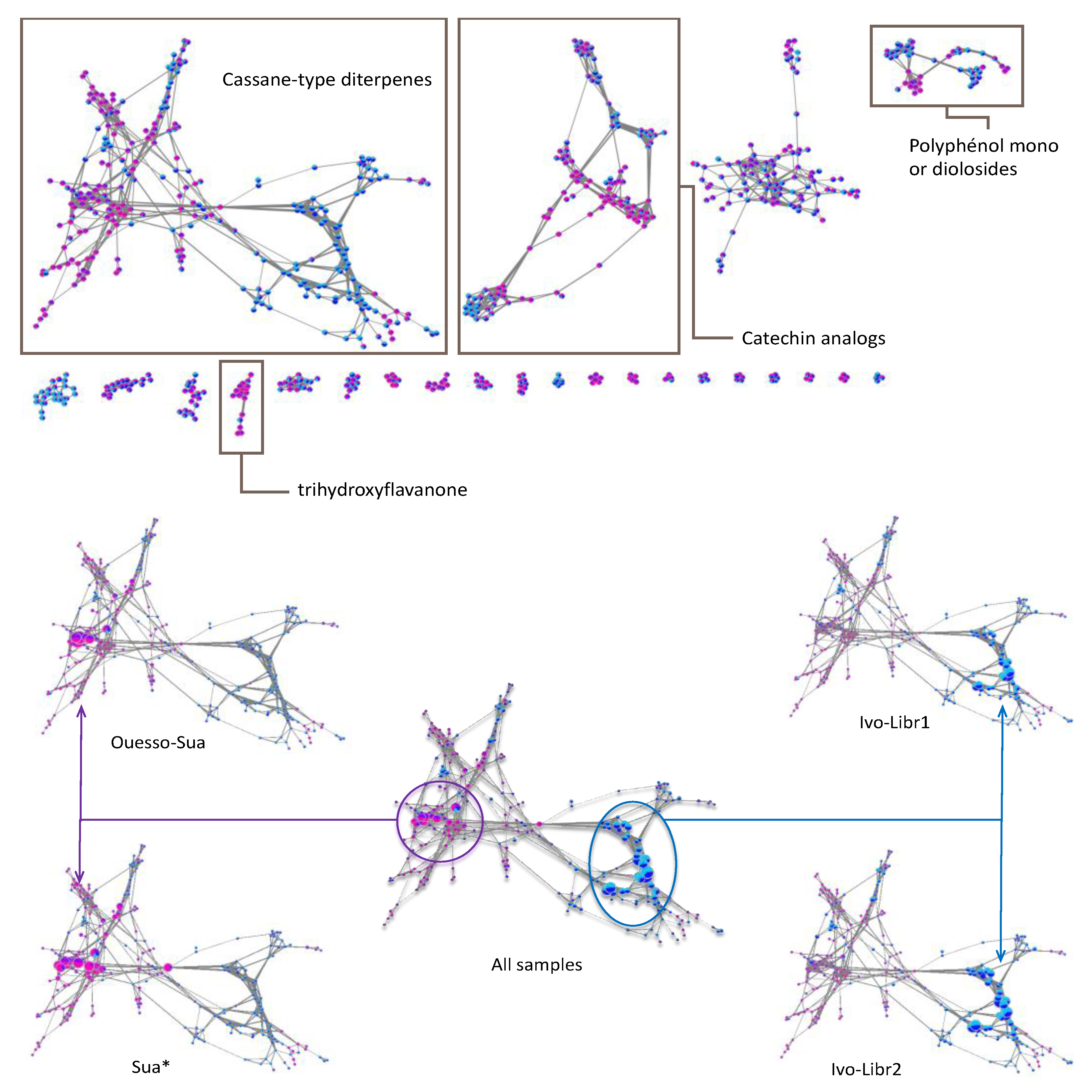
2.2. Molecular Networks
2.2.1. Sub-Cluster A
2.2.2. Sub-Cluster B
2.2.3. Sub-Cluster C
2.2.4. Other Networks
2.3. Species-Specific Metabolite Features and Identification
3. Discussion
4. Materials and Methods
4.1. Field Sampling
4.2. Controlling the Identification and Origin of Samples
4.3. Metabolomics Analyses
4.3.1. Sample Preparation
4.3.2. LC-HRMS(/MS) Analysis
4.3.3. Metabolomics Data
4.3.4. Data Processing and Chemometrics on W4M
4.3.5. Statistical Analyses
4.3.6. Data Preprocessing with MZmine 2 and Molecular Network Analysis with MetGem
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kami, C.; Lorrain, S.; Hornitschek, P.; Fankhauser, C. Light-Regulated Plant Growth and Development. Curr. Top. Dev. Biol. 2010, 91, 29–66. [Google Scholar] [CrossRef]
- Quint, M.; Delker, C.; Franklin, K.A.; Wigge, P.A.; Halliday, K.J.; van Zanten, M. Molecular and Genetic Control of Plant Thermomorphogenesis. Nat. Plants 2016, 2, 15190. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant Hormone-Mediated Regulation of Stress Responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Saito, K. Editorial: The Origin of Plant Chemodiversity - Conceptual and Empirical Insights. Front. Plant Sci. 2020, 11, 890. [Google Scholar] [CrossRef]
- Fang, C.; Fernie, A.R.; Luo, J. Exploring the Diversity of Plant Metabolism. Trends Plant Sci. 2019, 24, 83–98. [Google Scholar] [CrossRef]
- Jamil, I.N.; Remali, J.; Azizan, K.A.; Nor Muhammad, N.A.; Arita, M.; Goh, H.-H.; Aizat, W.M. Systematic Multi-Omics Integration (MOI) Approach in Plant Systems Biology. Front. Plant Sci. 2020, 11, 944. [Google Scholar] [CrossRef]
- Dixon, R.A.; Strack, D. Phytochemistry Meets Genome Analysis, and Beyond. Phytochemistry 2003, 62, 815–816. [Google Scholar] [CrossRef]
- Rai, A.; Saito, K.; Yamazaki, M. Integrated Omics Analysis of Specialized Metabolism in Medicinal Plants. Plant J. Cell Mol. Biol. 2017, 90, 764–787. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Kooke, R.; Keurentjes, J.J.B. Multi-Dimensional Regulation of Metabolic Networks Shaping Plant Development and Performance. J. Exp. Bot. 2012, 63, 3353–3365. [Google Scholar] [CrossRef]
- Macel, M.; Van Dam, N.M.; Keurentjes, J.J.B. Metabolomics: The Chemistry between Ecology and Genetics. Mol. Ecol. Resour. 2010, 10, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Marchev, A.S.; Koycheva, I.K.; Aneva, I.Y.; Georgiev, M.I. Authenticity and Quality Evaluation of Different Rhodiola Species and Commercial Products Based on NMR-Spectroscopy and HPLC. Phytochem. Anal. PCA 2020, 31, 756–769. [Google Scholar] [CrossRef]
- Liu, F.; Meng, Y.; He, K.; Song, F.; Cheng, J.; Wang, H.; Huang, Z.; Luo, Z.; Yan, X. Comparative Analysis of Proteomic and Metabolomic Profiles of Different Species of Paris. J. Proteomics 2019, 200, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Gang, F.; Qi, L.I.; Xin-Mei, X.U.; Huan, D.U.; Tong, X.U.; Xian-Rong, L.; Lei-Lei, D.U. [Quality evaluation of different Berberidis Cortex species based on ~1H-NMR metabolomics and anti-diabetic activity]. Zhongguo Zhong Yao Za Zhi Zhongguo Zhongyao Zazhi China J. Chin. Mater. Medica 2020, 45, 4677–4685. [Google Scholar] [CrossRef]
- Duminil, J.; Brown, R.P.; Ewédjè, E.-E.B.; Mardulyn, P.; Doucet, J.-L.; Hardy, O.J. Large-Scale Pattern of Genetic Differentiation within African Rainforest Trees: Insights on the Roles of Ecological Gradients and Past Climate Changes on the Evolution of Erythrophleum Spp (Fabaceae). BMC Evol. Biol. 2013, 13, 195. [Google Scholar] [CrossRef]
- Dade, J.M.E.; Kablan, L.A.; Okpekon, T.A.; Say, M.; Yapo, K.D.; Komlaga, G.; Boti, J.B.; Koffi, A.P.; Guei, L.E.; Djakoure, L.A.; et al. Cassane Diterpenoids from Stem Bark of Erythrophleum Suaveolens [(Guill. et Perr.), Brenan]. Phytochem. Lett. 2015, 12, 224–231. [Google Scholar] [CrossRef]
- Kablan, A.C.L.; Konan, J.D.; Komlaga, G.; Kabran, F.A.; Daouda, B.; N’Tamon, A.D.; Kouamé, T.; Jagora, A.; Leblanc, K.; Seon-Méniel, B.; et al. Five New Cassane Diterpenes from the Seeds and Bark of Erythrophleum Suaveolens. Fitoterapia 2020, 146, 104700. [Google Scholar] [CrossRef]
- Kablan, L.A.; Dade, J.M.E.; Say, M.; Okpekon, T.A.; Yapo, K.D.; Ouffoue, S.K.; Koffi, A.P.; Retailleau, P.; Champy, P. Four New Cassane Diterpenoid Amides from Erythrophleum Suaveolens [(Guill. et Perr.), Brenan]. Phytochem. Lett. 2014, 10, 60–64. [Google Scholar] [CrossRef]
- Son, N.T. Genus Erythrophleum: Botanical Description, Traditional Use, Phytochemistry and Pharmacology. Phytochem. Rev. 2019, 18, 571–599. [Google Scholar] [CrossRef]
- Dalma, G. Chapter 36 The Erythrophleum Alkaloids. In The Alkaloids: Chemistry and Physiology; Manske, R.H.F., Holmes, H.L., Eds.; Academic Press: Cambridge, MA, USA, 1954; Volume 4, pp. 265–273. [Google Scholar]
- Jing, W.; Zhang, X.; Zhou, H.; Wang, Y.; Yang, M.; Long, L.; Gao, H. Naturally Occurring Cassane Diterpenoids (CAs) of Caesalpinia: A Systematic Review of Its Biosynthesis, Chemistry and Pharmacology. Fitoterapia 2019, 134, 226–249. [Google Scholar] [CrossRef]
- Coates, R.M. Biogenetic-Type Rearrangements of Terpenes. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Cimino, G., Coates, R.M., De Stefano, S., Fontana, A., Hemmerich, P., Minale, L., Rinehart, K.L., Shield, L.S., Sodano, G., Toniolo, C., et al., Eds.; Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1976; pp. 73–230. ISBN 978-3-7091-3262-3. [Google Scholar]
- Maurya, R.; Ravi, M.; Singh, S.; Yadav, P.P. A Review on Cassane and Norcassane Diterpenes and Their Pharmacological Studies. Fitoterapia 2012, 83, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Yu, S.; Tang, W.; Liu, Y.; Liu, Y.; Liu, J. Progress on Cassaine-Type Diterpenoid Ester Amines and Amides ( Erythrophleum Alkaloids). Nat. Prod. Commun. 2006, 1, 1934578X0600101. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.-H.; Li, J.-B.; Yu, S.-S.; Li, Y.; Liu, Y.-B. Rapid Structural Determination of New Trace Cassaine-Type Diterpenoid Amides in Fractions from Erythrophleum Fordii by Liquid Chromatography-Diode-Array Detection/Electrospray Ionization Tandem Mass Spectrometry and Liquid Chromatography/Nuclear Magnetic Resonance. Rapid Commun. Mass Spectrom. 2007, 21, 2109–2119. [Google Scholar] [CrossRef] [PubMed]
- Giacomoni, F.; Le Corguillé, G.; Monsoor, M.; Landi, M.; Pericard, P.; Pétéra, M.; Duperier, C.; Tremblay-Franco, M.; Martin, J.-F.; Jacob, D.; et al. Workflow4Metabolomics: A Collaborative Research Infrastructure for Computational Metabolomics. Bioinformatics 2015, 31, 1493–1495. [Google Scholar] [CrossRef]
- Olivon, F.; Elie, N.; Grelier, G.; Roussi, F.; Litaudon, M.; Touboul, D. MetGem Software for the Generation of Molecular Networks Based on the T-SNE Algorithm. Anal. Chem. 2018, 90, 13900–13908. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular Framework for Processing, Visualizing, and Analyzing Mass Spectrometry-Based Molecular Profile Data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Kang, K.B.; Caraballo-Rodríguez, A.M.; Nothias, L.-F.; Wandy, J.; Chen, C.; Wang, M.; Rogers, S.; Medema, M.H.; Dorrestein, P.C.; et al. MolNetEnhancer: Enhanced Molecular Networks by Integrating Metabolome Mining and Annotation Tools. Metabolites 2019, 9, 144. [Google Scholar] [CrossRef]
- Armah, F.A.; Amponsah, I.K.; Mensah, A.Y.; Dickson, R.A.; Steenkamp, P.A.; Madala, N.E.; Adokoh, C.K. Leishmanicidal Activity of the Root Bark of Erythrophleum Ivorense (Fabaceae) and Identification of Some of Its Compounds by Ultra-Performance Liquid Chromatography Quadrupole Time of Flight Mass Spectrometry (UPLC-QTOF-MS/MS). J. Ethnopharmacol. 2018, 211, 207–216. [Google Scholar] [CrossRef]
- Friedrich-Fiechtl, J.; Spiteller, G. [Use of Mass Spectrum Analysis for Structure Elucidation of Alkaloids. X. New Alkaloids from Erythrophleum Guineense and about Muawin]. Chem. Ber. 1971, 104, 3335–3348. [Google Scholar] [CrossRef] [PubMed]
- Konan, J.D.; Attioua, B.K.; Kablan, C.L.A.; Kabran, F.A.; Koffi, P.A.; Any-Grah, S.A.; Drissa, S.; Seon-Meniel, B.; LeBlanc, K.; Jullian, J.-C.; et al. New Cassane Diterpenoids from the Root Bark of Erythrophleum Suaveolens. Phytochem. Lett. 2019, 31, 166–169. [Google Scholar] [CrossRef]
- Loder, J.W.; Culvenor, C.C.J.; Nearn, R.H.; Russel, G.B.; Stanton, D.W. Isolation of Norcassamidide and Authentic Norcassamidine from Erythrophleum Chlorostachys. Structural Revision of the Alkaloids Previously Known as Norcassamidine, Norcassamine, Norethythrosuamine and Dehydro-Norerythrosuamine. Tetrahedron Lett. 1972, 13, 5069–5072. [Google Scholar] [CrossRef]
- Arya, V.P.; Engel, B.G.; Ronco, A. Zur Kenntnis Der Erythrophleum-Alkaloide. 17. Mitteilung. Über Einige Für Die Konstitutionsbestimmung von Cassamin Wichtige Derivate Der Cassan-19-Säure. Helv. Chim. Acta 1961, 44, 1645–1650. [Google Scholar] [CrossRef]
- Arya, V.P.; Engel, B.G. Zur Kenntnis der Erythrophleum-Alkaloide. 18. Mitteilung. Die Struktur des Cassamins und des Erythrophlamins. Helv. Chim. Acta 1961, 44, 1650–1673. [Google Scholar] [CrossRef]
- Cronlund, A. Two New Alkaloids from Bark of Erythrophleum Ivorense. Acta Pharm. Suec. 1973, 10, 507–514. [Google Scholar]
- Cronlund, A. The Botanical and Phytochemical Differentiation between Erythrophleum Suaveolens and E. Ivorense. Planta Med. 1976, 29, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Cronlund, A.; Oguakwa, J.U. Alkaloids from the Bark of Erythrophleum Couminga. Acta Pharm. Suec. 1975, 12, 467–478. [Google Scholar] [PubMed]
- Hung, T.M.; Cuong, T.D.; Kim, J.A.; Tae, N.; Lee, J.H.; Min, B.S. Cassaine Diterpene Alkaloids from Erythrophleum Fordii and Their Anti-Angiogenic Effect. Bioorg. Med. Chem. Lett. 2014, 24, 168–172. [Google Scholar] [CrossRef]
- Sandberg, F. Medicinal and Toxic Plants from Equatorial Africa: A Pharmacologic Approach. J. Ethnopharmacol. 1980, 2, 105–108. [Google Scholar] [CrossRef]
- Albuquerque, U.P.; do Nascimento, A.L.B.; Silva Chaves, L.; Feitosa, I.S.; de Moura, J.M.B.; Gonçalves, P.H.S.; da Silva, R.H.; da Silva, T.C.; Ferreira Júnior, W.S. The Chemical Ecology Approach to Modern and Early Human Use of Medicinal Plants. Chemoecology 2020, 30, 89–102. [Google Scholar] [CrossRef]
- Loder, J.W.; Nearn, R.H. 3β-Acetoxynorerythrosuamine, a Highly Cytotoxic Alkaloid from Erythrophleum Chlorostachys. Chemistry 1975. [Google Scholar] [CrossRef]
- Du, D.; Qu, J.; Wang, J.-M.; Yu, S.-S.; Chen, X.-G.; Xu, S.; Ma, S.-G.; Li, Y.; Ding, G.-Z.; Fang, L. Cytotoxic Cassaine Diterpenoid-Diterpenoid Amide Dimers and Diterpenoid Amides from the Leaves of Erythrophleum Fordii. Phytochemistry 2010, 71, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, Z.; Zhou, S.; Huang, P.; Zhuo, Z.; Zeng, S.; Wang, L.; Wang, Y.; Xu, C.; Tian, H. Cassaine Diterpenoids from the Seeds of Erythrophleum Fordii and Their Cytotoxic Activities. Fitoterapia 2018, 127, 245–251. [Google Scholar] [CrossRef]
- Verotta, L.; Aburjai, T.; Rogers, C.B.; Dorigo, P.; Maragno, I.; Fraccarollo, D.; Santostasi, G.; Gaion, R.M.; Floreani, M.; Carpenedo, F. Chemical and Pharmacological Characterization of Erythrophleum Lasianthum Alkaloids. Planta Med. 1995, 61, 271–274. [Google Scholar] [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; et al. Reproducible Molecular Networking of Untargeted Mass Spectrometry Data Using GNPS. Nat. Protoc. 2020, 15, 1954–1991. [Google Scholar] [CrossRef] [PubMed]
- Nothias, L.-F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Protsyuk, I.; Ernst, M.; Tsugawa, H.; Fleischauer, M.; et al. Feature-Based Molecular Networking in the GNPS Analysis Environment. Nat. Methods 2020, 17, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Phelan, V.V. Feature-Based Molecular Networking for Metabolite Annotation. Methods Mol. Biol. Clifton NJ 2020, 2104, 227–243. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and Community Curation of Mass Spectrometry Data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, P.; Boudah, S.; Junot, C.; Thévenot, E.A. Biosigner: A New Method for the Discovery of Significant Molecular Signatures from Omics Data. Front. Mol. Biosci. 2016, 3, 26. [Google Scholar] [CrossRef]
- Ha, M.T.; Tran, M.H.; Phuong, T.T.; Kim, J.A.; Woo, M.H.; Choi, J.S.; Lee, S.; Lee, J.H.; Lee, H.K.; Min, B.S. Cytotoxic and Apoptosis-Inducing Activities against Human Lung Cancer Cell Lines of Cassaine Diterpenoids from the Bark of Erythrophleum Fordii. Bioorg. Med. Chem. Lett. 2017, 27, 2946–2952. [Google Scholar] [CrossRef]
- Guitton, Y.; Tremblay-Franco, M.; Le Corguillé, G.; Martin, J.-F.; Pétéra, M.; Roger-Mele, P.; Delabrière, A.; Goulitquer, S.; Monsoor, M.; Duperier, C.; et al. Create, Run, Share, Publish, and Reference Your LC–MS, FIA–MS, GC–MS, and NMR Data Analysis Workflows with the Workflow4Metabolomics 3.0 Galaxy Online Infrastructure for Metabolomics. Int. J. Biochem. Cell Biol. 2017, 93, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hardy, O.J.; Delaide, B.; Hainaut, H.; Gillet, J.-F.; Gillet, P.; Kaymak, E.; Vankerckhove, N.; Duminil, J.; Doucet, J.-L. Seed and Pollen Dispersal Distances in Two African Legume Timber Trees and Their Reproductive Potential under Selective Logging. Mol. Ecol. 2019, 28, 3119–3134. [Google Scholar] [CrossRef] [PubMed]


| Species | Population/ Country (*) | GPS Coordinates (Long/Lat) | Genetic Groups (**) | Block Abbreviation (Number of Individuals) | n |
|---|---|---|---|---|---|
| E. ivorense | Libreville/C | 9.3727/0.6187 | Ivo | A(5), B(6), C(7), D(5) | 23 |
| E. suaveolens | Ouesso/RC | 16.4892/1.2926 | Ouesso-Sua (N) | A(4), 2B(1), C(2), D(3) | 10 |
| UFA30/C | 13.9128/3.4287 | Sua (N) | A(1), B(2), C(1), D(4) | 8 | |
| Lastourville & Mekambo/G | 12.952/−0.5345 & 13.9396/0.9618 | Sua (S) | C(1), D(2) | 3 |
| Scaffold | 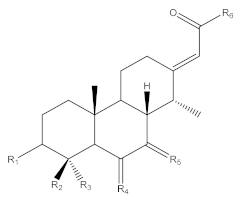 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Molecular Formula/RT (min) | m/z/δ (ppm) | R1 | R2 | R3 | R4 | R5 | R6 | Ref | More Abundant in: | Relative Abundance in Ouesso/ Sua */ ivo-Libr 1/ ivo-Libr 2 |
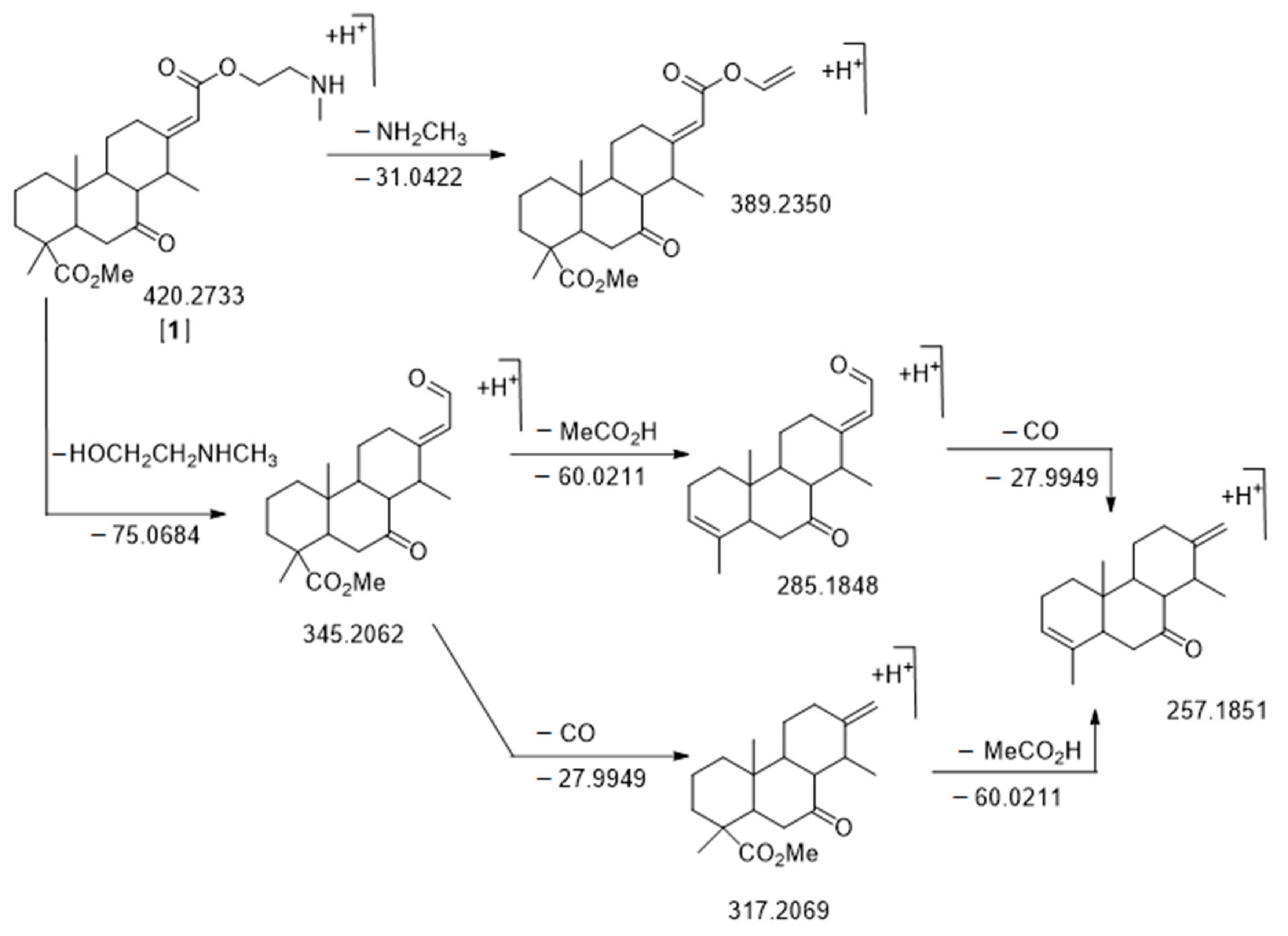
| [A1] | C24H37NO5/16.97 | 420.2733/2.74 | H | COOCH3 | CH3 | H,H | O | OCH2CH2 NHCH3 | [17,32,33,34] | Sua * | 9/48/33/11 |
| [A2] | C23H35NO5/16.75 | 406.2569/4.69 | H | COOCH3 | CH3 | H,H | O | NHCH2CH2OH/OCH2CH2NH2 | NEW | Sua * | 21/71/4/4 |
| [A3] | C24H35NO5/14.54 | 434.2549/2.74 | H | COOCH3 | CH3 | O | O | OCH2CH2NHCH3 | [32,34] | Ouesso-Sua | 53/36/6/5 |
| [A4] | C25H39NO5/17.16 | 434.2855/10.59 | H | COOCH3 | CH3 | H,H | O | OCH2CH2N(CH3)2 | [31,37,41,52] | Sua * | 24/52/14/11 |
| [A5] | C24H35NO6/15.77 | 434.2555/4.11 | H | COOCH3 | CH3 | O | O | OCH2CH2NHCH3 | [32,34] | Ivo-Libr 2 | 9/11/28/53 |
| [B1] | C26H39NO8/14.44 | 494.2757/1.74 | OCOCH3 | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NHCH3 | [34] | Sua * | 10/82/3/5 |
| [B2] | C24H37NO7/12.62 | 452.2664/4.7 | OH | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NHCH3 | [43,46] | Ouesso-Sua | 45/26/19/9 |
| [B3] | C25H37NO8/14.34 | 480.2607/7.32 | OCOCH3 | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NH2 | NEW | Sua * | 16/83/0/0 |
| [B4] | C25H37NO8/14.51 | 480.2828/49 | OCOCH3 | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NH2 | NEW | Sua * | 24/70/4/3 |
| [B5] | C27H41NO8/14.40 | 508.2980/10.64 | OCOCH3 | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | [43] | Ivo-Libr 2 | 2/4/39/55 |
| [B6] | C30H45NO8/17.04 | 548.3212/1.09 | OCOC(CH3)=CHCH3 (tigloyl) or OCOCH=C(CH3)2 (methylcrotonyl) | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | NEW | Ivo | 0/0/48/52 |
| [B7] | C30H47NO8/17.28 | 550.3350/ 4.45 | OCOCH(CH3)CH2CH3 or OCOCH2CH(CH3)2 | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | NEW | Ivo | 0/0/50/50 |
| [B8] | C30H47NO9/14.05 | 566.3312/ 2.69 | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2 | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | NEW | Ivo | 0/1/51/48 |
| [B9] | C28H43NO7/15.65 | 506.3102/ 2.04 | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2 | CH3 | CH3 | O | O | OCH2CH2NHCH3 | NEW | Ivo | 0/0/52/48 |
| [B10] | C28H41NO6/17.87 | 488.2988/ 3.83 | OCOC(CH3)=CHCH3 or OCOCH=C(CH3)2 | CH3 | CH3 | O | O | OCH2CH2NHCH3 | NEW | Ivo-Libr 1 | 1/1/59/39 |
| [B11] | C30H48NO9/14.17 | 566.3308/2.76 | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2 | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | NEW | Ivo-Libr 2 | 1/1/3/95 |
| [B12] | C31H49NO12/10.40 | 628.3323/0.72 | O-glucopyrannosyl | COOCH3 | CH3 | OMe (or O) | O (or OMe) | OCH2CH2NHCH3 | NEW | Ivo-Libr 2 | 1/0/35/65 |
| [B13] | C23H37NO4/13.27 | 392.2802/1.70 | OH | CH3 | CH3 | H,H | O | OCH2CH2NHCH3 | NEW | Ivo-Libr 1 | 4/7/63/27 |
| [B14] | C23H37NO4/12.66 | 392.2789/1.62 | OH | CH3 | CH3 | O | H,H | OCH2CH2NHCH3 | NEW | Ivo-Libr1 | 5/6/62/27 |
| [B15] | C28H45NO6/15.83 | 492.3314/1.15 | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2OCOCH2C(OH) (CH3)2 | CH3 | CH3 | H,H | O | OCH2CH2NHCH3 | [26] | Ivo-Libr1 | 1/3/53/43 |
| [B16] | C27H43NO6/15.61 | 478.3067/20.1 | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2OCOCH2C(OH) (CH3)2 | CH3 | CH3 | H,H | O | OCH2CH2NH2 | NEW | Sua * | 21/61/7/11 |
| [B17] | C22H35NO4/13.10 | 378.2625/3.67 | OH | CH3 | CH3 | H,H | O (or OH) | OCH2CH2NH2 | NEW | Sua * | 33/62/3/2 |
| [C1] | C24H37NO7/11.17 | 452.2636/1.50 | OH | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NHCH3 | [43,46] | Ouesso-Sua | 60/36/1/3 |
| [C2] | C23H35NO7/10.97 | 438.2501/3.36 | OH | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NH2 | NEW | Ouesso-Sua | 60/40/0/0 |
| [C3] | C25H39NO7/11.31 | 466.2787/2.64 | OH | COOCH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NH(CH3)2 | NEW | Ouesso-Sua | 69/24/2/5 |
| [BS1] | C24H35NO7/12.84 | 450.2477/1.27 (Biosigner 450.2492/2.07) | OH | COOCH3 | CH3 | O | O | OCH2CH2NHCH3 | NEW | Sua * | 40/59/0/1 |
| [BS2] | C28H43NO6/18.48 | 490.3145/3.71 (Biosigner 490.3158/1.05) | OCOC(CH3)=CHCH3 or OCOCH=C(CH3)2 | CH3 | CH3 | OH (or O) | O (or OH) | OCH2CH2NHCH3 | NEW | Ivo-Libr 1 | 0/0/65/35 |
| [BS3] | C28H43NO7/15.65 | 506.3102/2.04 (Biosigner 506.3113/0.14) | OCOC(OH)(CH3)CH2CH3 or OCOCH(OH) CH(CH3)2 | CH3 | CH3 | O | O | OCH2CH2NHCH3 | NEW | Ivo | 0/0/52/48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delporte, C.; Noret, N.; Vanhaverbeke, C.; Hardy, O.J.; Martin, J.-F.; Tremblay-Franco, M.; Touboul, D.; Gorel, A.; Faes, M.; Stévigny, C.; et al. Does the Phytochemical Diversity of Wild Plants Like the Erythrophleum genus Correlate with Geographical Origin? Molecules 2021, 26, 1668. https://doi.org/10.3390/molecules26061668
Delporte C, Noret N, Vanhaverbeke C, Hardy OJ, Martin J-F, Tremblay-Franco M, Touboul D, Gorel A, Faes M, Stévigny C, et al. Does the Phytochemical Diversity of Wild Plants Like the Erythrophleum genus Correlate with Geographical Origin? Molecules. 2021; 26(6):1668. https://doi.org/10.3390/molecules26061668
Chicago/Turabian StyleDelporte, Cédric, Nausicaa Noret, Cécile Vanhaverbeke, Olivier J. Hardy, Jean-François Martin, Marie Tremblay-Franco, David Touboul, Anais Gorel, Marie Faes, Caroline Stévigny, and et al. 2021. "Does the Phytochemical Diversity of Wild Plants Like the Erythrophleum genus Correlate with Geographical Origin?" Molecules 26, no. 6: 1668. https://doi.org/10.3390/molecules26061668
APA StyleDelporte, C., Noret, N., Vanhaverbeke, C., Hardy, O. J., Martin, J.-F., Tremblay-Franco, M., Touboul, D., Gorel, A., Faes, M., Stévigny, C., Van Antwerpen, P., & Souard, F. (2021). Does the Phytochemical Diversity of Wild Plants Like the Erythrophleum genus Correlate with Geographical Origin? Molecules, 26(6), 1668. https://doi.org/10.3390/molecules26061668






