A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System
Abstract
:1. Introduction: Overview of Red Yeast Rice, Its History, Production Method, and Use
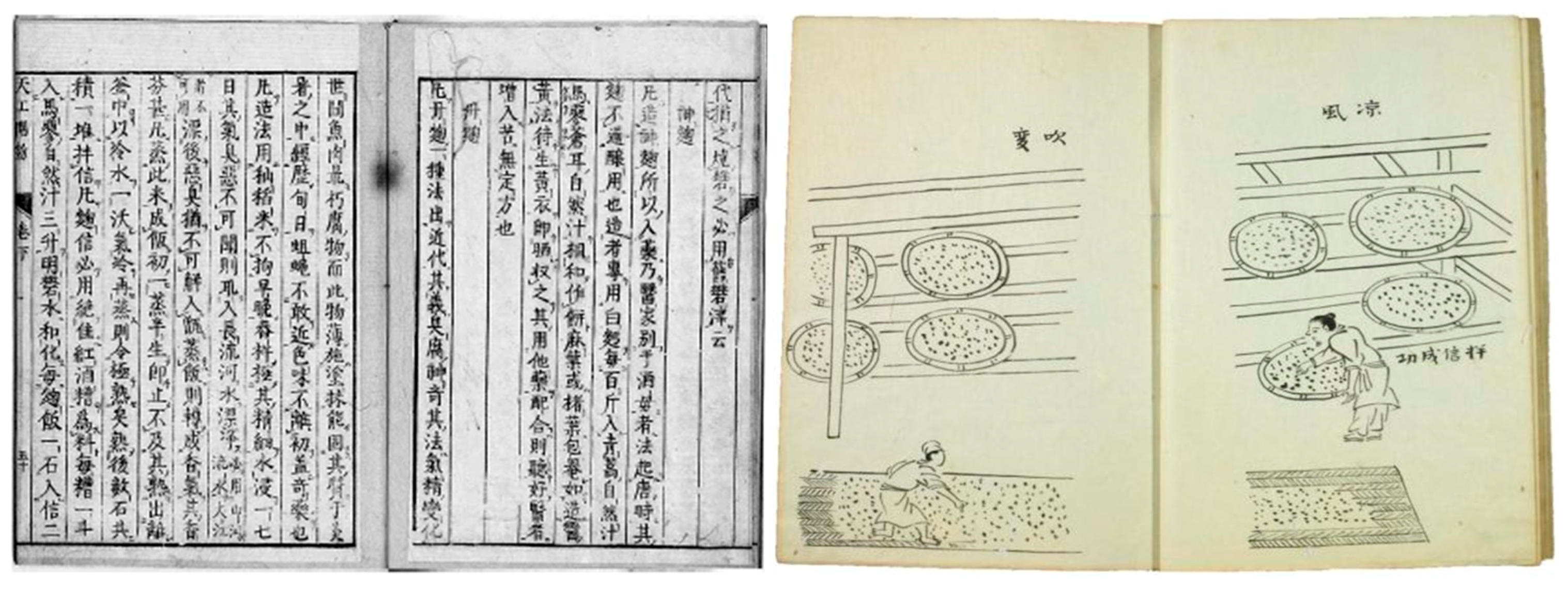
1.1. The History behind Koji (Yeast Grain) and Red Yeast Rice (Red Koji)
1.2. Monascus Fungi and Red Yeast Rice
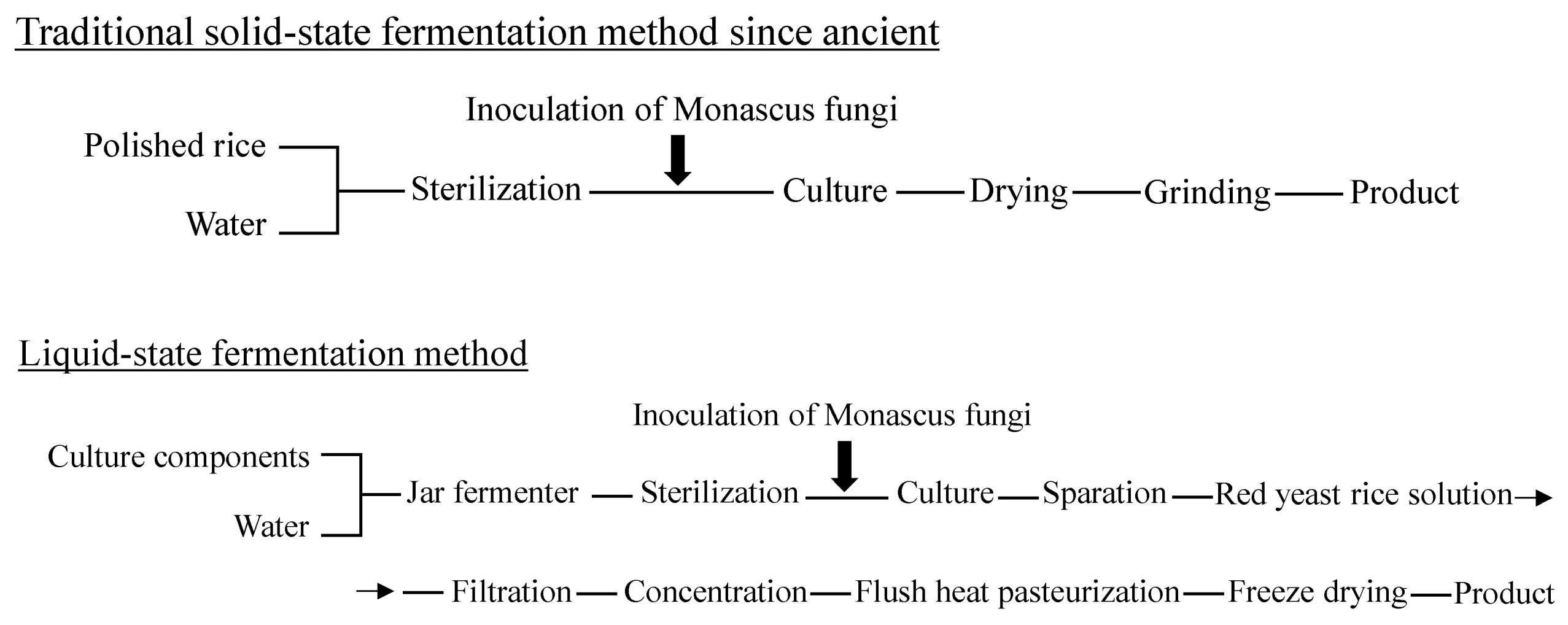
1.3. Production of Red Yeast Rice
1.4. Brewed Foods Produced Using Red Yeast Rice
2. Polyketides and Other Metabolites Produced in Monascus Fungi
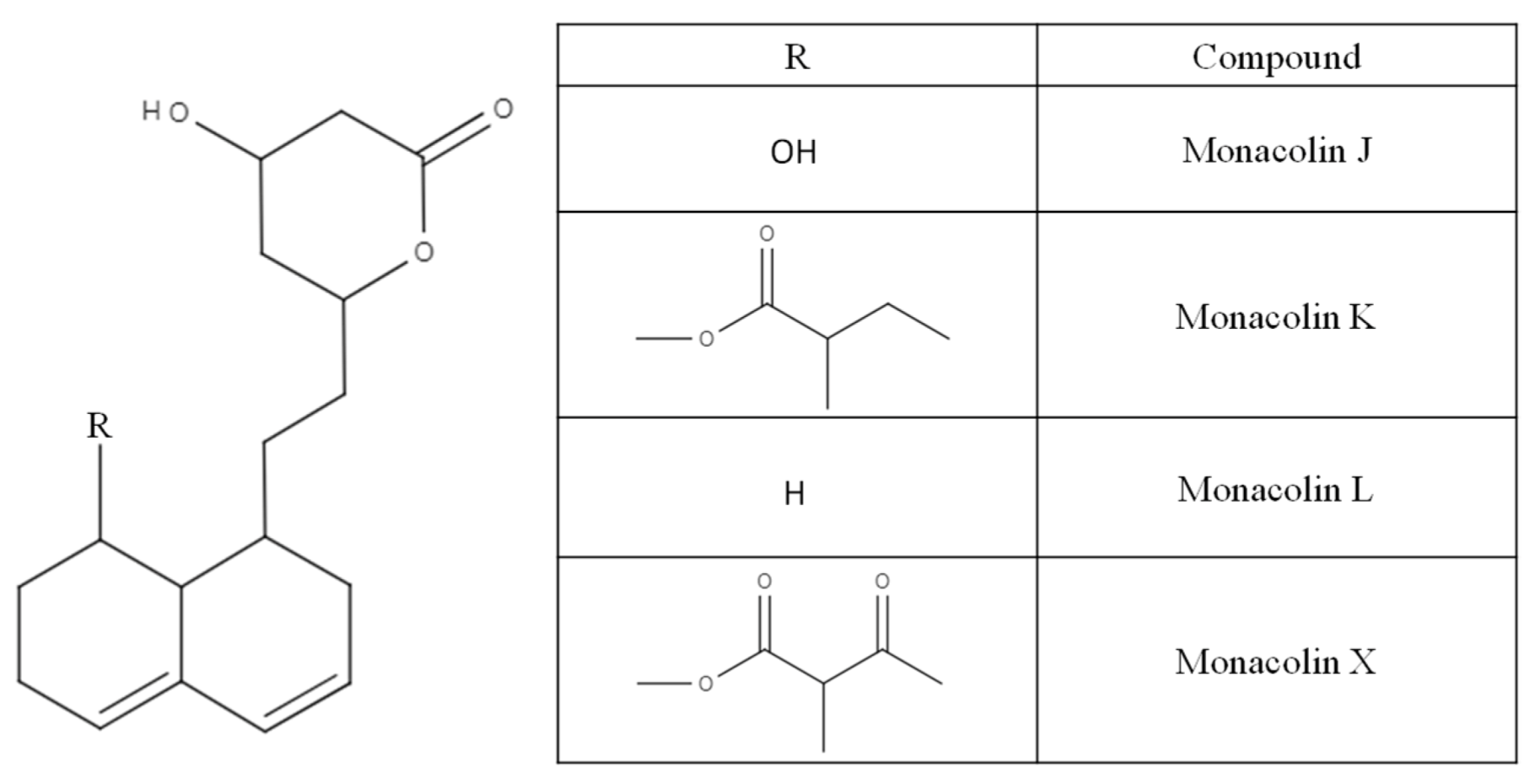
2.1. Polyketides Identified in Red Yeast Rice
2.2. Production of Metabolites, Including Representative Polyketides in Traditional Solid-State Fermentation
3. Effectiveness of Red Yeast Rice: Its Functional Ingredients and Physiological Actions on Lipid Metabolism and the Circulatory System
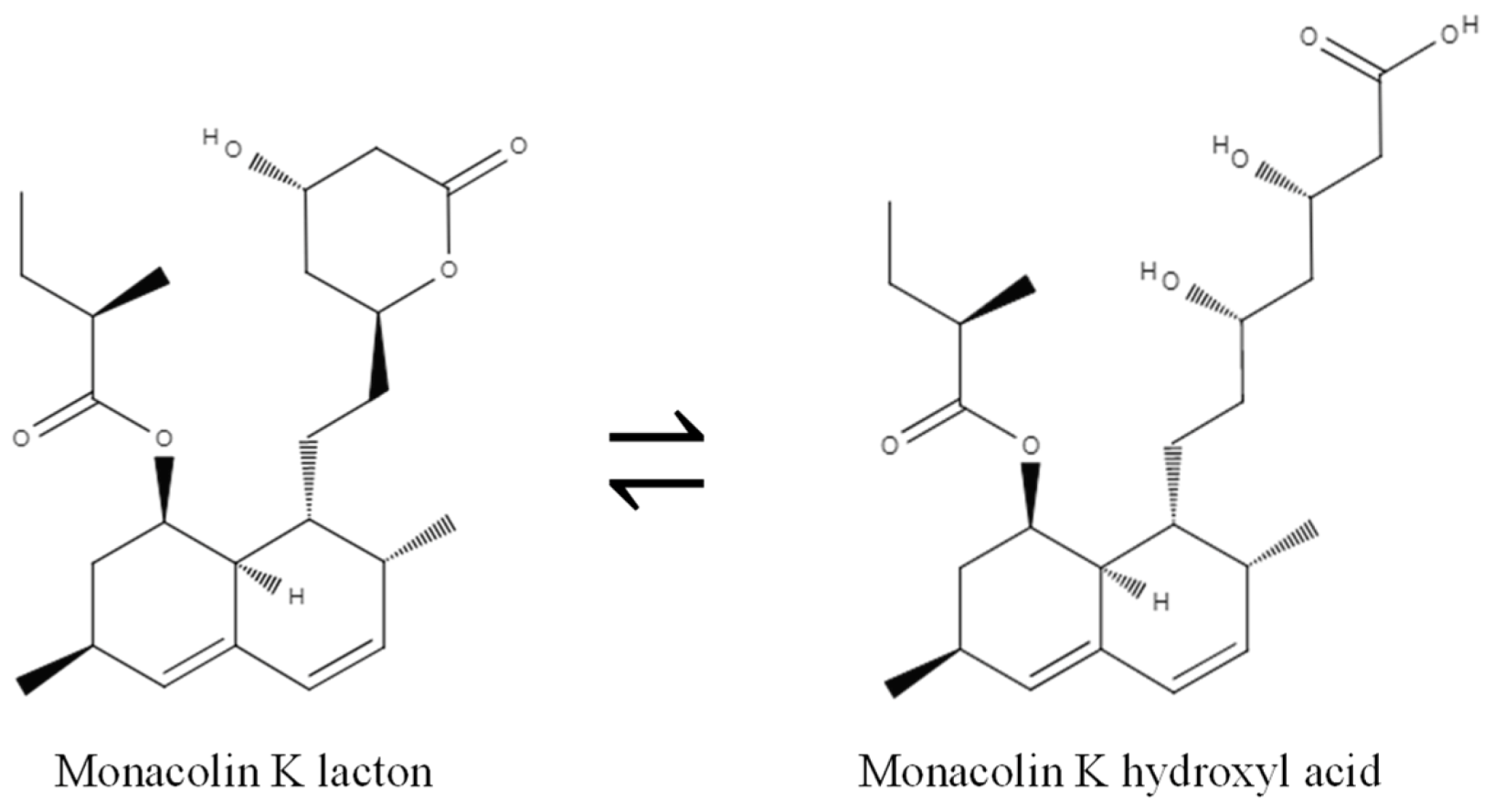
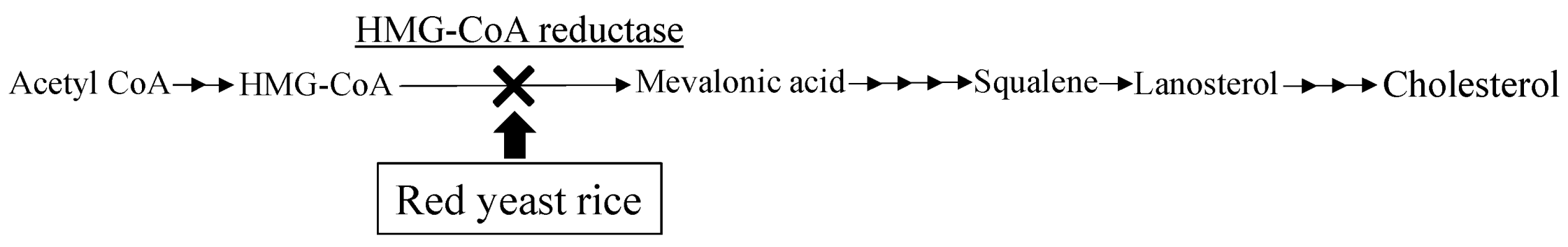
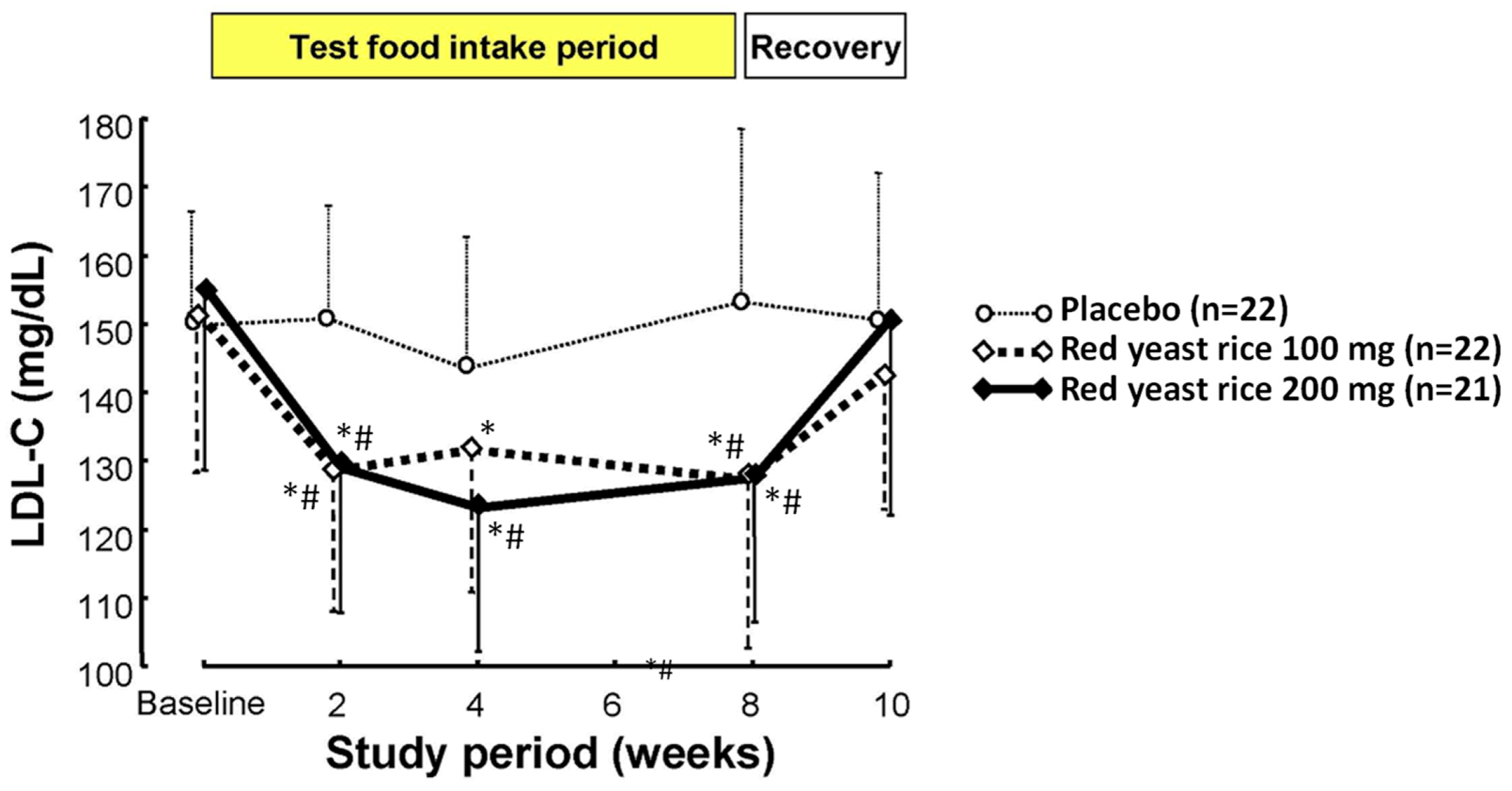
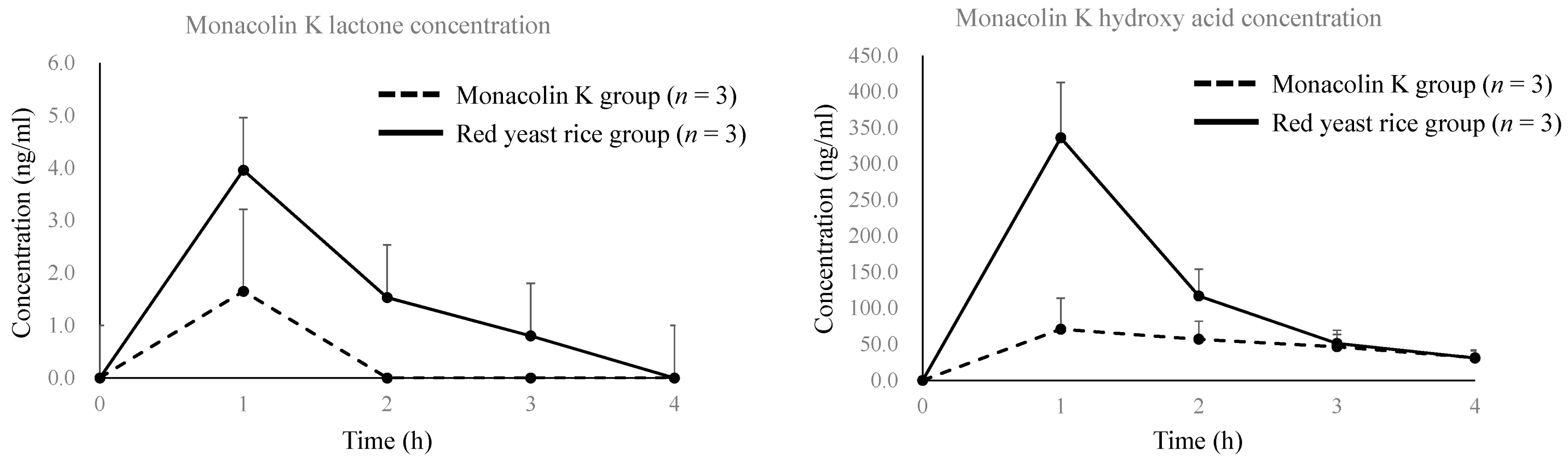
3.1. Monacolins and Their Cholesterol Lowering Effect
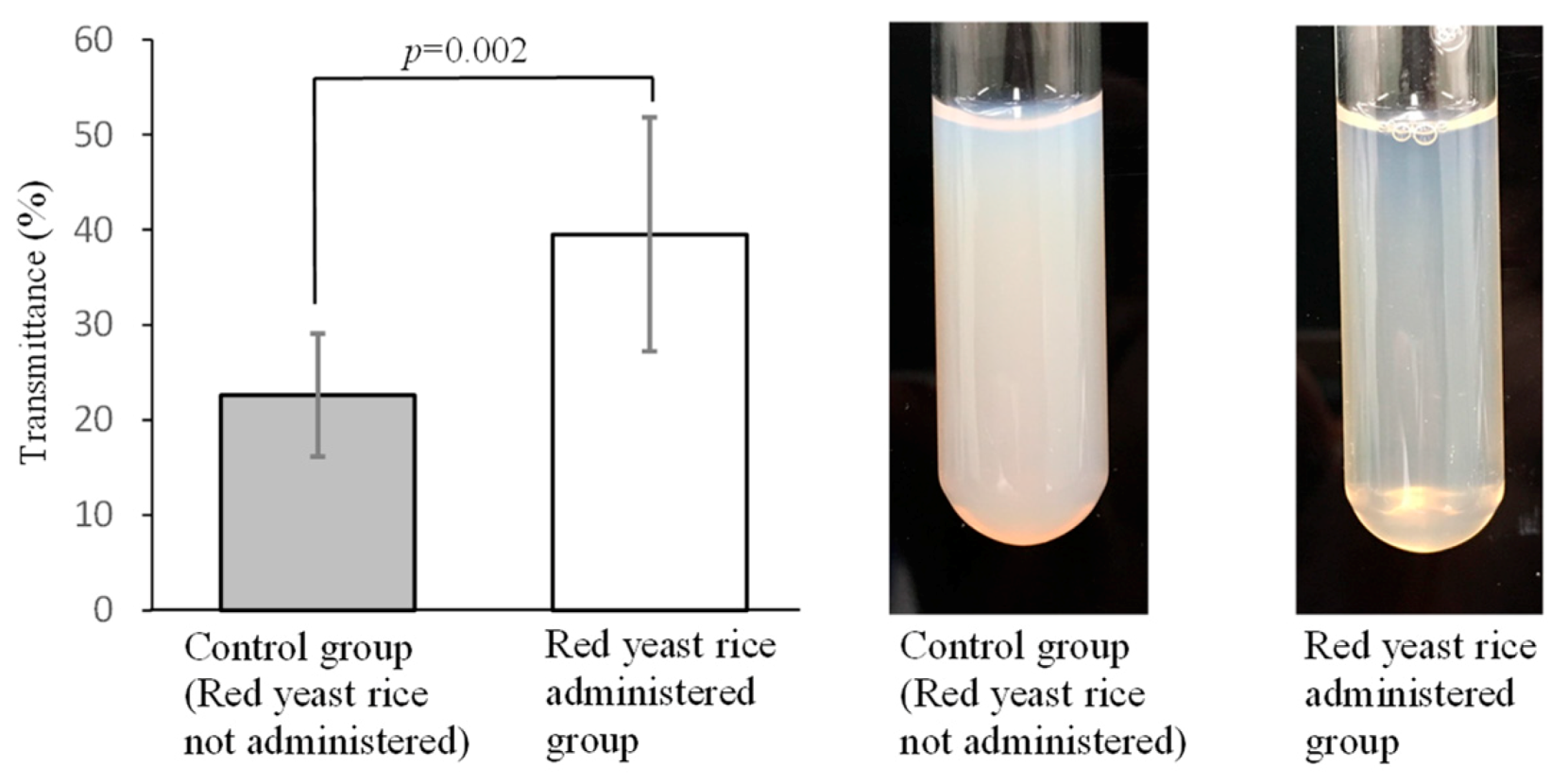
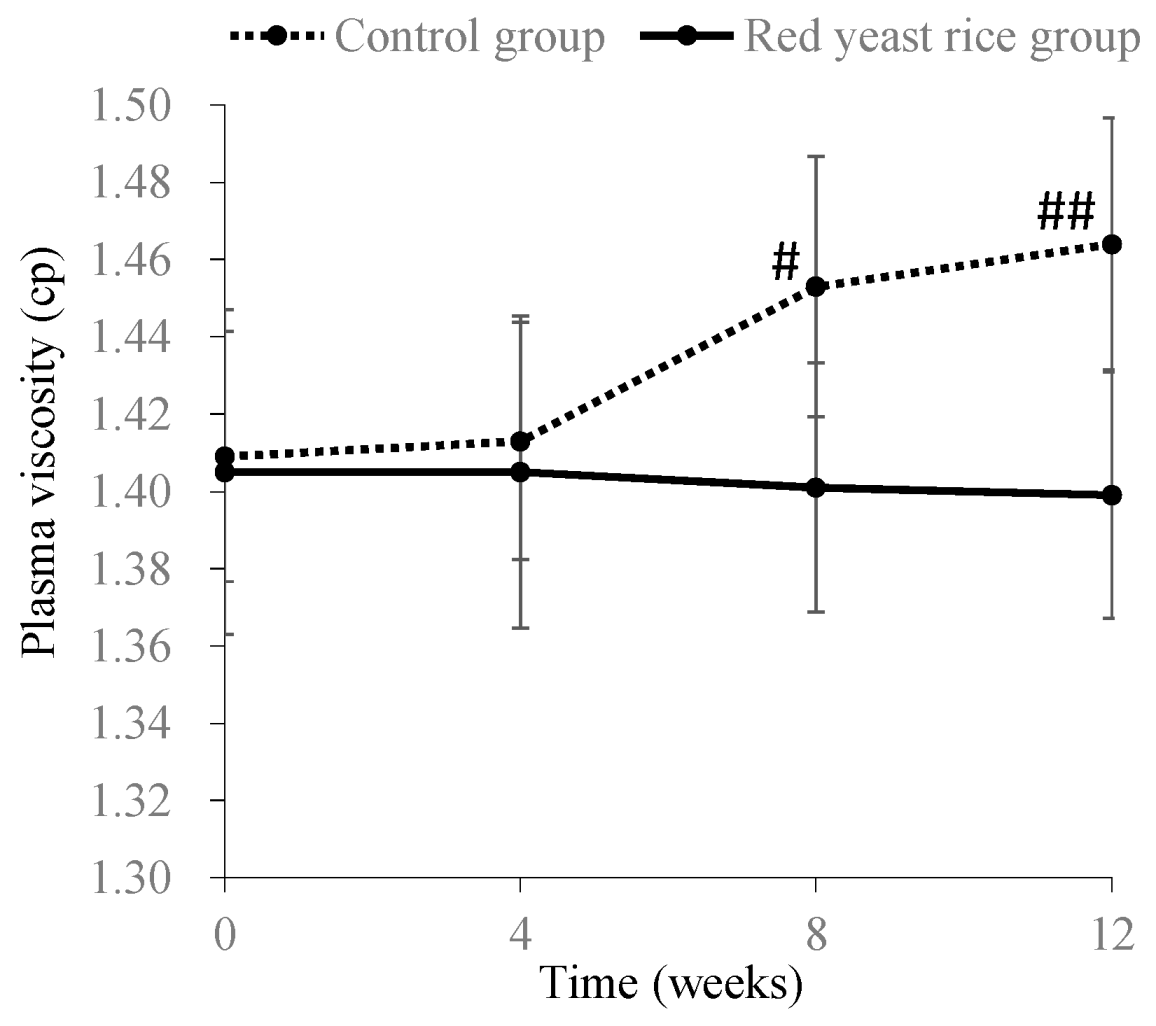
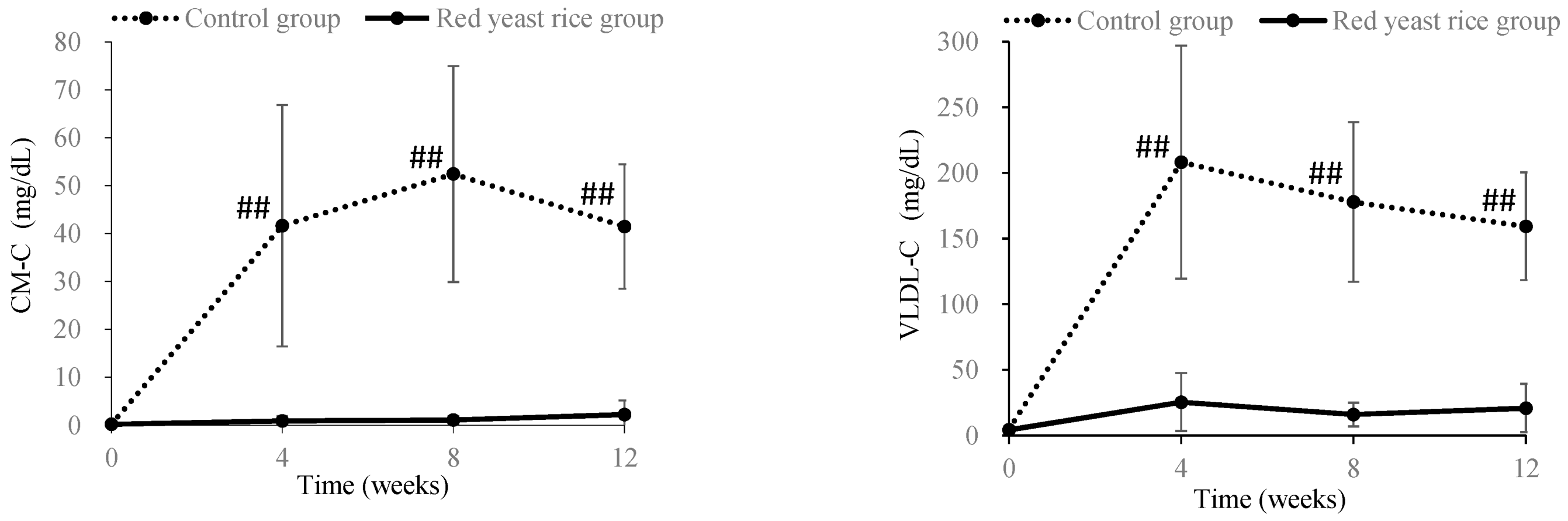
3.2. Improving Effects of Red Yeast Rice on Chyle in Blood and Increased Viscosity Caused by Hyperlipidemia
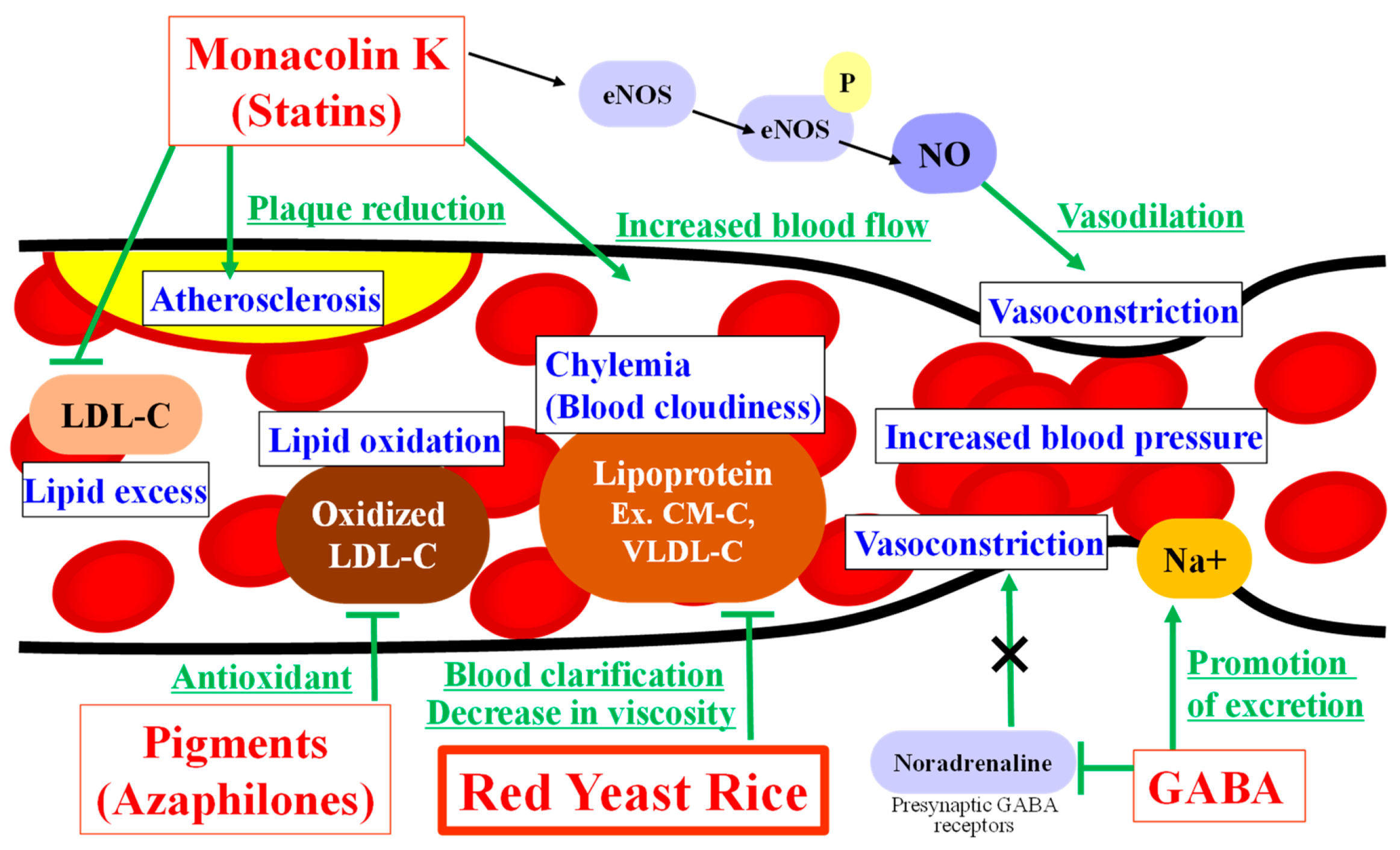
3.3. Promising Effects of Ingredients in Red Yeast Rice on Risk Reduction for Circulatory Diseases
4. Safety of Red Yeast Rice
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Endo, A.; Monacolin, K. A new hypocholesterolemic agent produced by a Monuscus species. J. Antibiot. 1979, 32, 852–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endo, A.; Monacolin, K. A new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J. Antibiot. 1980, 33, 334–336. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Negishi, Y.; Iwashita, T.; Mizukawa, K.; Hirama, M. Biosynthesis of ML-236B (compactin) and monacolin K. J. Antibiot. 1985, 38, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. History and recent trends about red koji and Monascus fungi. Ferment. Ind. 1985, 43, 544–552. (In Japanese) [Google Scholar]
- Tsukioka, A.; Hiroi, T.; Suzuki, T. Pigment Production by Mutants of Monascus anka. J. JSBBA 1986, 60, 451–455. (In Japanese) [Google Scholar]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Jpn. J. Complem. Altern. Med. 2015, 14, 555–567. [Google Scholar]
- Nishitani, M.; Inagaki, M. Red Koji (Red Mold Rice) for Complementary and Alternative Medicine as well as for Health Conditioning. Jpn. J. Complem. Altern. Med. 2009, 6, 45–51. [Google Scholar]
- Su, Y. Characteristics of Monascus fungi and their usage. J. Brew. Soc. Japan. 1975, 33, 28–36. (In Japanese) [Google Scholar]
- Su, Y. Proceeding of the Oriental Fermented Foods; Industry Research and Development Institute: Hsinchu, Taiwan, 1980; pp. 15–30. [Google Scholar]
- Itou, H. Utilization of Beni-koji (Monascus koji) for Miso and Soysauce. J. Brew. Soc. Japan. 1994, 89, 948–953. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Ysasuda, M. Tofuyo and red koji (2). J. Brew. Soc. Japan. 1983, 78, 912–915. (In Japanese) [Google Scholar]
- Tarui, S. New material trends of processed foods. (2). Character of Beni koji (red colored malted rice) and its use. Jpn. Food Science. 1993, 32, 35–42. (In Japanese) [Google Scholar]
- Mapari, S.A.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef]
- Patakova, P. Monascus secondary metabolites: Production and biological activity. J. Ind. Microbiol. Biotchnol. 2013, 40, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Mafune, S.; Ukiya, M.; Kimura, Y.; Yasukawa, K.; Suzuki, T.; Tokuda, H.; Tanabe, N.; Fukuoka, T. (+)- and (−)-syn-2-isobutyl-4-methylazetidine-2,4-dicarboxylic acids from the extract of Monascus pilosus-fermented rice (red-mold rice). J. Nat. Prod. 2004, 67, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Tokuda, H.; Yasukawa, K.; Ukiya, M.; Kiyota, A.; Sakamoto, N.; Suzuki, T.; Tanabe, N.; Nishino, H. Azaphilones, furanoisophthalides, and amino acids from the extracts of Monascus pilosus-fermented rice (red-mold rice) and their chemopreventive effects. J. Agric. Food Chem. 2005, 53, 562–565. [Google Scholar] [CrossRef]
- Su, Y.C.; Wang, J.J.; Lin, T.T.; Pan, T.M. Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J. Ind. Microbiol. Biotchnol. 2003, 30, 41–46. [Google Scholar] [CrossRef]
- Kim, C.; Jung, H.; Kim, Y.O.; Shin, C.S. Antimicrobial activities of amino acid derivatives of monascus pigments. Fems Microbiol. Lett. 2006, 264, 117–124. [Google Scholar] [CrossRef]
- Blanc, P.J.; Laussac, J.P.; Bars, J.L.; Bars, P.L.; Loret, M.O.; Pareilleux, A.; Prome, D.; Prome, J.C.; Santerre, A.L.; Goma, G. Characterization of monascidin A from Monascus as citrinin. Int. J. Food Microbiol. 1995, 27, 201–213. [Google Scholar] [CrossRef]
- Kohama, Y.; Matsumoto, S.; Mimura, T.; Tanabe, N.; Inada, A.; Nakanishi, T. Isolation and identification of hypotensive principles in red-mold rice. Chem. Pharm. Bull. 1987, 35, 2484–2489. [Google Scholar] [CrossRef] [Green Version]
- Razak, D.L.A.; Rashid, N.Y.A.; Jamaluddin, A.; Sharifudin, S.A.; Long, K. Enhancement of phenolic acid content and antioxidant activity of rice bran fermented with Rhizopus oligosporus and Monascus purpureus. Biocatal. Agric. Biotechnol. 2015, 4, 33–38. [Google Scholar] [CrossRef]
- Cheng, J.; Choi, B.; Yang, S.H.; Suh, J. Effect of Fermentation on the Antioxidant Activity of Rice Bran by Monascus pilosus KCCM60084. J. Appl. Biol. Chem. 2016, 59, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef] [Green Version]
- Endo, A. Compactin (ML-236B) and related compounds as potential cholesterol-lowering agents that inhibit HMG-CoA reductase. J. Med. Chem. 1985, 28, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Regulation of HMG-CoA Reductase; Preiss, B., Ed.; Academic Press: New York, NY, USA, 1985; pp. 49–78. [Google Scholar]
- Lee, C.; Wang, J.; Pan, T. Synchronous analysis method for detection of citrinin and the lactone and acid forms of monacolin K in red mold rice. J. Aoac Int. 2006, 89, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Endo, A. Chemistry, biochemistry and pharmacology of HMG-CoA reductase inhibitors. Klin. Wochenschr. 1988, 66, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Cao, X.; Chen, Z.; Xiong, Z.; Liu, J.; Cheng, Z.; Zheng, Z.; Long, C.; Zheng, B.; Huang, Z. An overview of Monascus fermentation processes for monacolin K production. Open Chem. 2020, 18, 10–21. [Google Scholar] [CrossRef]
- Kim, H.J.; Ji, G.E.; Lee, I.H. Natural Occurring Levels of Citrinin and Monacolin K in Korean Monascus Fermentation Products. Food Sci. Biotechnol. 2007, 16, 142–145. [Google Scholar]
- Virk, M.S.; Ramzan, R.; Virk, M.A.; Yuan, X.; Chen, F. Transfigured Morphology and Ameliorated Production of Six Monascus Pigments by Acetate Species Supplementation in Monascus ruber M7. Microorganisms 2020, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Mabuchi, H. Hyperlipidemia and atherosclerosis. J. Jpn. Soc. Intern. Med. 1998, 87, 950–957. (In Japanese) [Google Scholar] [CrossRef] [Green Version]
- Frishman, W.H.; Zimetbaum, P.; Nadelmann, J. Lovastatin and other HMG-CoA reductase inhibitors. J. Clin. Pharmacol. 1989, 29, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, H. Statin drugs. Clin. All-Round. 2007, 56, 2272–2280. (In Japanese) [Google Scholar]
- Kazi, D.S.; Penko, J.M.; Bibbins-Domingo, K. Statins for Primary Prevention of Cardiovascular Disease: Review of Evidence and Recommendations for Clinical Practice. Med. Clin. N. Am. 2017, 101, 689–699. [Google Scholar] [CrossRef]
- Shibasaki, M.; Saito, Y. Statin drugs and their pleiotropic effects. Prog. Med. 2004, 24, 15–20. (In Japanese) [Google Scholar]
- Blum, A.; Shamburek, R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 2009, 203, 325–330. [Google Scholar] [CrossRef]
- Shoji, T.; Fujii, H.; Tokai, H.; Fujimoto, S.; Wada, T.; Kawano, N.; Tsuchikura, S.; Eiko, L.; Otsuka, Y.; Teramura, M.; et al. A Randomized, Double-blinded, Comparative, Dose-finding Trial to Examine the Cholesterol-lowering Effect of Red Koji in Healthy Volunteers. J. Jpn. Soc. Clin. Nutr. 2008, 29, 425–433. (In Japanese) [Google Scholar]
- Shoji, T.; Fukui, M.; Fujii, H. LDL-C lowering effect of red yeast rice-Stratified analysis of a randomized controlled trial including healthy volunteers with borderline hyper-LDL cholesterolemia. Anti Aging Med. 2018, 14, 533–541. (In Japanese) [Google Scholar]
- Takemoto, M.; Yoshino, G. Effect of Lovastatin-Containing Red Koji on Plasma Lipid Levels in Hyperlipidemic Subjects. J. Jpn. Soc. Clin. Nutr. 2000, 22, 39–42. (In Japanese) [Google Scholar]
- Heinz, T.; Schuchardt, J.P.; Möller, K.; Hadji, P.; Hahn, A. Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention. Nutr. Res. 2016, 36, 1162–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicero, A.F.G.; Fogacci, F.; Banach, M. Red Yeast Rice for Hypercholesterolemia. Methodist Debakey Cardiovasc. J. 2019, 15, 192–199. [Google Scholar] [PubMed]
- Wu, Y.; Zhao, J.; Henion, J.; Korfmacher, W.A.; Lapiguera, A.P.; Lin, C.C. Microsample determination of lovastatin and its hydroxy acid metabolite in mouse and rat plasma by liquid chromatography/ionspray tandem mass spectrometry. J. Mass Spectrom. 1997, 32, 379–387. [Google Scholar] [CrossRef]
- Fukami, H.; Ueda, T.; Matsuoka, N. Pharmacokinetic Study of Compound K in Japanese Subjects After Ingestion of Panax ginseng Fermented by Lactobacillus paracasei A221 Reveals Significant Increase of Absorption into Blood. J. Med. Food. 2019, 22, 257–263. [Google Scholar] [CrossRef]
- Taco-Vasquez, E.D.; Barrera, F.; Serrano-Duenas, M.; Jimenez, E.; Rocuts, A.; Perez, E.R. Association between Blood Viscosity and Cardiovascular Risk Factors in Patients with Arterial Hypertension in a High Altitude Setting. Cureus 2019, 11, e3925. [Google Scholar] [CrossRef] [Green Version]
- Destiana, D.; Timan, I.S. The relationship between hypercholesterolemia as a risk factor for stroke and blood viscosity measured using Digital Microcapillary. J. Phys. 2018, 1073, 042045. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.I.; Cho, D.J. Hemorheology and Microvascular Disorders. Korean Circ. J. 2011, 41, 287–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, L.Y.; Lee, S.R.; Jung, J.M.; Kim, Y.S.; Lee, S.H.; Rhee, K.S.; Chae, J.K.; Lee, D.H.; Kim, D.S.; Kim, W.H.; et al. Rosuvastatin Reduces Blood Viscosity in Patients with Acute Coronary Syndrome. Korean Circ. J. 2016, 46, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Sipahioglu, N.; Karis, D.; Uzun, H.; Sipahioglu, F.; Ercan, S.; Ercan, A. The Effect of Ezetimibe on Plasma Viscosity, Fibrinogen and Lipid Profile. Med. Sci. Discov. 2015, 2, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [Green Version]
- Usui, N.; Iwata, K.; Miyachi, T.; Takagai, S.; Wakusawa, K.; Nara, T.; Tsuchiya, K.J.; Matsumoto, K.; Kurita, D.; Kameno, Y.; et al. VLDL-specific increases of fatty acids in autism spectrum disorder correlate with social interaction. EBioMedicine 2020, 58, 102917. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Sievers, R.E.; Sun, Y.; Isenberg, W.M.; Parmley, W.W. Effect of lovastatin on suppression and regression of atherosclerosis in lipid-fed rabbits. J. Cardiovasc. Pharmacol. 1992, 19, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Hussein, O.; Rosenblat, M.; Schlezinger, S.; Hayek, T.; Keidar, S. Interactions of platelets, macrophages, and lipoproteins in hypercholesterolemia: Antiatherogenic effects of HMG-CoA reductase inhibitor therapy. J. Cardiovasc. Pharmacol. 1998, 31, 39–45. [Google Scholar] [CrossRef]
- Lin, R.; Liu, J.; Peng, N.; Yang, G.; Gan, W.; Wang, W. Lovastatin reduces nuclear factor kappaB activation induced by C-reactive protein in human vascular endothelial cells. Biol. Pharm. Bull. 2005, 28, 1630–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, V.; Schneider, W.; Schunck, W.H.; Mervaala, E.; Luft, F.C. Chronic effects of lovastatin and bezafibrate on cortical and medullary hemodynamics in deoxycorticosterone acetate-salt hypertensive mice. J. Am. Soc. Nephrol. 1999, 10, 1430–1439. [Google Scholar]
- Giannopoulos, S.; Katsanos, A.H.; Tsivgoulis, G.; Marshall, R.S. Statins and cerebral hemodynamics. J. Cereb. Blood Flow Metab. 2012, 32, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.; Michel, L.Y.M.; Balligand, J.L. Nitric oxide signalling in cardiovascular health and disease. Nat. Rev. Cardiol. 2018, 15, 292–316. [Google Scholar] [CrossRef]
- Merhi, M.; Dusting, G.J.; Khalil, Z. CGRP and nitric oxide of neuronal origin and their involvement in neurogenic vasodilatation in rat skin microvasculature. Br. J. Pharmacol. 1998, 123, 863–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeji, K.; Umemoto, S.; Itoh, S.; Tanaka, M.; Kawahara, S.; Fukai, T.; Matsuzaki, M. Comparative effects of pitavastatin and probucol on oxidative stress, Cu/Zn superoxide dismutase, PPAR-gamma, and aortic stiffness in hypercholesterolemia. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2522–H2532. [Google Scholar] [CrossRef] [PubMed]
- Shafi, O. Switching of vascular cells towards atherogenesis, and other factors contributing to atherosclerosis: A systematic review. Thromb J. 2020, 18, 28. [Google Scholar] [CrossRef]
- Ikeda, U.; Shimpo, M.; Ikeda, M.; Minota, S.; Shimada, K. Lipophilic statins augment inducible nitric oxide synthase expression in cytokine-stimulated cardiac myocytes. J. Cardiovasc. Pharmacol. 2001, 38, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Le Francois, B.G.; Goss, G.; Ding, K.; Bradbury, P.A.; Dimitroulakos, J. Lovastatin inhibits EGFR dimerization and AKT activation in squamous cell carcinoma cells: Potential regulation by targeting rho proteins. Oncogene 2010, 29, 4682–4692. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Chen, J.; Cong, X.; Hu, S.; Chen, X. Lovastatin protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt and ERK1/2. J. Cell Biochem. 2008, 103, 256–269. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Li, P.; Yang, Y.B.; Liu, M.L. Xuezhikang, extract of red yeast rice, improved abnormal hemorheology, suppressed caveolin-1 and increased eNOS expression in atherosclerotic rats. PLoS ONE 2013, 8, e62731. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, S.; Shimakage, H.; Tanioka, H.; Yoshida, M.; Suzuki-Kusaba, M.; Hisa, H.; Satoh, S. Effects of GABA on noradrenaline release and vasoconstriction induced by renal nerve stimulation in isolated perfused rat kidney. Br. J. Pharmacol. 1999, 127, 109–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, K.; Kimura, M.; Kamata, K. Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2002, 438, 107–113. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kimura, M.; Yamori, Y. Role of the renal nerves in gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. Eur. J. Pharmacol. 2005, 524, 120–125. [Google Scholar] [CrossRef]
- Monasterolo, L.A.; Trumper, L.; Elías, M.M. Effects of gamma-aminobutyric acid agonists on the isolated perfused rat kidney. J. Pharmacol. Exp. Ther. 1996, 279, 602–607. [Google Scholar]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef]
- Laufs, U.; Fata, V.L.; Plutzky, J.; Liao, J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 1998, 97, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.J.; Meredith, I.T.; Yeung, A.C.; Frei, B.; Selwyn, A.P.; Ganz, P. The effect of cholesterol-lowering and antioxidant therapy on endothelium-dependent coronary vasomotion. N. Engl. J. Med. 1995, 332, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Srianta, I.; Zubaidah, E.; Estiasih, T.; Iuchi, Y.; Harijono, H.; Yamada, M. Antioxidant activity of pigments derived from Monascus purpureusfermented rice, corn, and sorghum. Int. Food Res. J. 2017, 24, 1186–1191. [Google Scholar]
- Wada, M.; Kido, H.; Ohyama, K.; Ichibangase, T.; Kishikawa, N.; Ohba, Y.; Nakashima, M.N.; Kuroda, N.; Nakashima, K. Chemiluminescent screening of quenching effects of natural colorants against reactive oxygen species: Evaluation of grape seed, monascus, gardenia and red radish extracts as multi-functional food additives. Food Chem. 2007, 101, 980–986. [Google Scholar] [CrossRef]
- Lee, C.L.; Wen, J.Y.; Hsu, Y.W.; Pan, T.M. The blood lipid regulation of Monascus-produced monascin and ankaflavin via the suppression of low-density lipoprotein cholesterol assembly and stimulation of apolipoprotein A1 expression in the liver. J. Microbiol. Immunol. Infect. 2018, 51, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Hermans, N.; Van der Auwera, A.; Breynaert, A.; Verlaet, A.; De Bruyne, T.; Van Gaal, L.; Pieters, L.; Verhoeven, V. A red yeast rice-olive extract supplement reduces biomarkers of oxidative stress, OxLDL and Lp-PLA 2, in subjects with metabolic syndrome: A randomised, double-blind, placebo-controlled trial. Trials 2017, 18, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, I. Development of food materials that have the effect of improving vascular endothelial function. J. Brew. Soc. Jpn. 2012, 107, 750–759. (In Japanese) [Google Scholar] [CrossRef]
- Hsu, W.H.; Chen, T.H.; Lee, B.H.; Hsu, Y.W.; Pan, T.M. Monascin and ankaflavin act as natural AMPK activators with PPARα agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 2014, 64, 94–103. [Google Scholar] [CrossRef]
- Ting, H.H.; Timimi, F.K.; Boles, K.S.; Creager, S.J.; Ganz, P.; Creager, M.A. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1996, 97, 22–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Ueda, T.; Yoshimizu, A.; Kurisu, S.; Matsuura, H.; Kajiyama, G.; Oshima, T. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: A multicenter study. J. Am. Coll. Cardiol. 2000, 35, 284–291. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, S.; Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am. J. Hypertens. 2002, 15, 302–309. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the Bioactivity of monacolin K / lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef]
- Shimizu, T.; Kinoshita, H.; Ishihara, S.; Sakai, K.; Nagai, S.; Nihira, T. Polyketide synthase gene responsible for citrinin biosynthesis in Monascus purpureus. Appl. Environ. Microbiol. 2005, 71, 3453–3457. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Jiang, N.; Xian, H.; Wei, D.; Shi, L.; Feng, X. Effects of Light Intensity and Color on the Biomass, Extracellular Red Pigment, and Citrinin Production of Monascus ruber. J. Chromatogr. A 2016, 1429, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Higa, Y.; Kim, Y.; Altaf-Ul-Amin, M.; Huang, M.; Ono, N.; Kanaya, S. Divergence of metabolites in three phylogenetically close Monascus species (M. pilosus, M. ruber, and M. purpureus) based on secondary metabolite biosynthetic gene clusters. BMC Genom. 2020, 21, 679. [Google Scholar] [CrossRef] [PubMed]



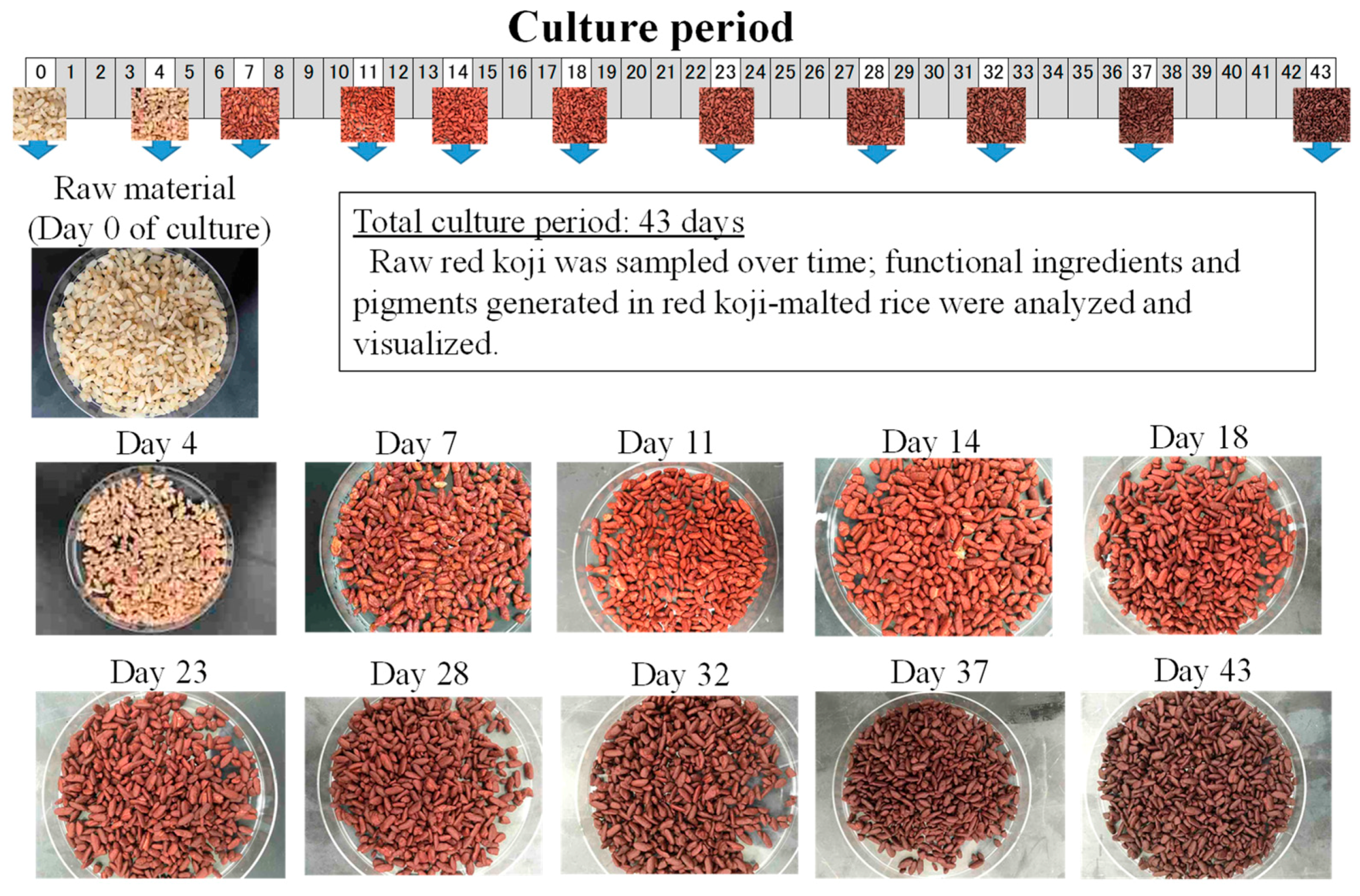
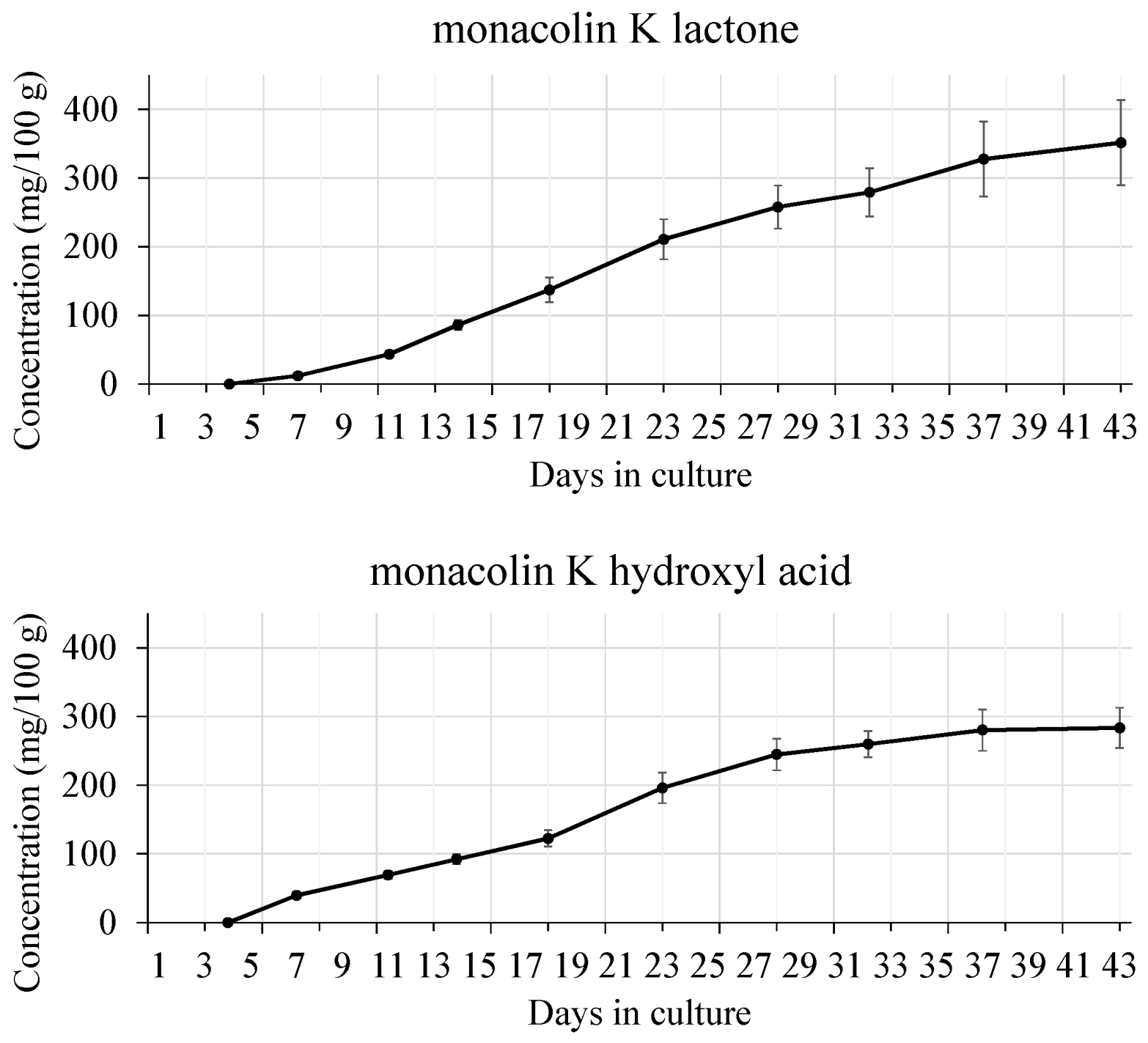
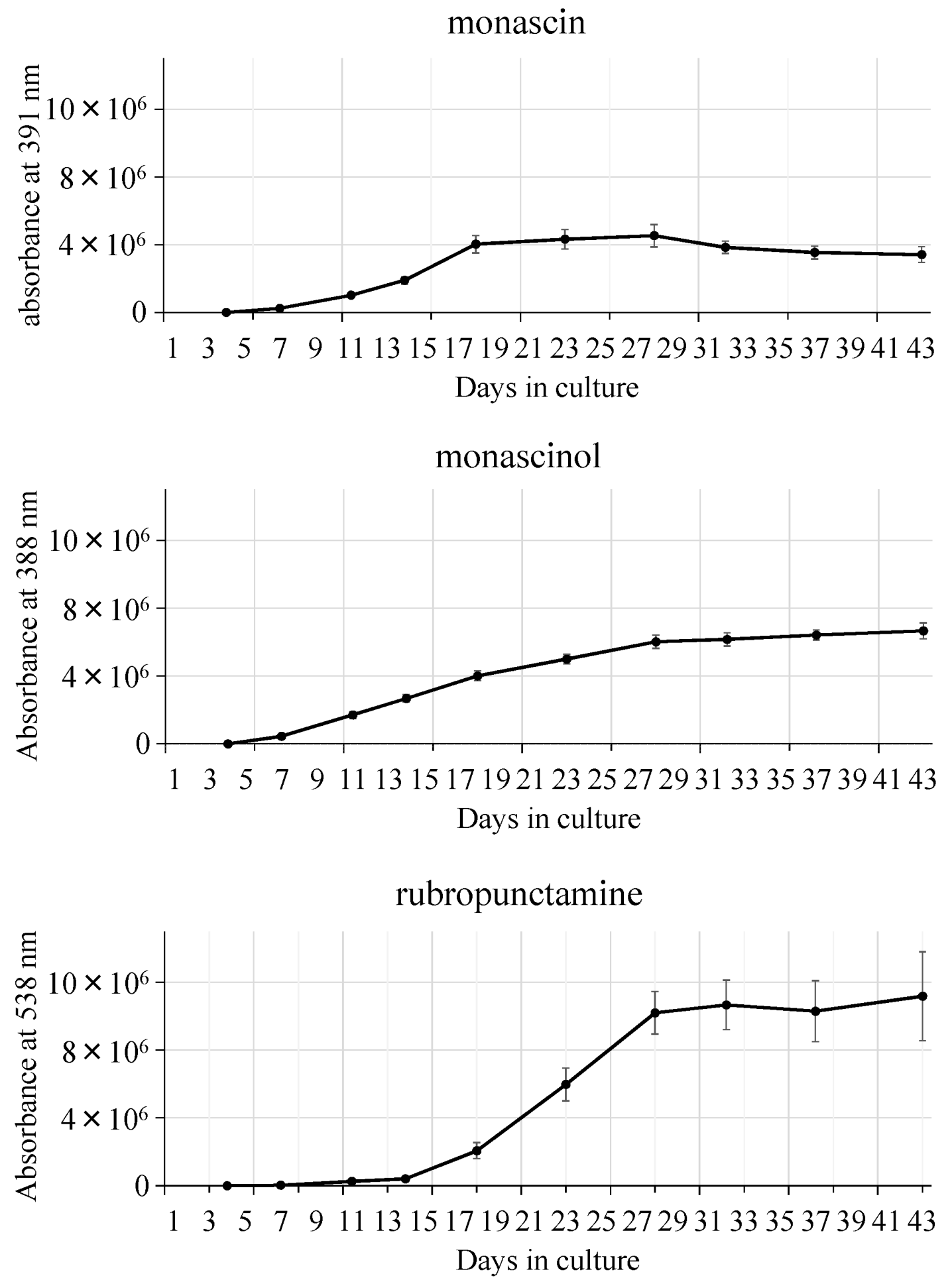
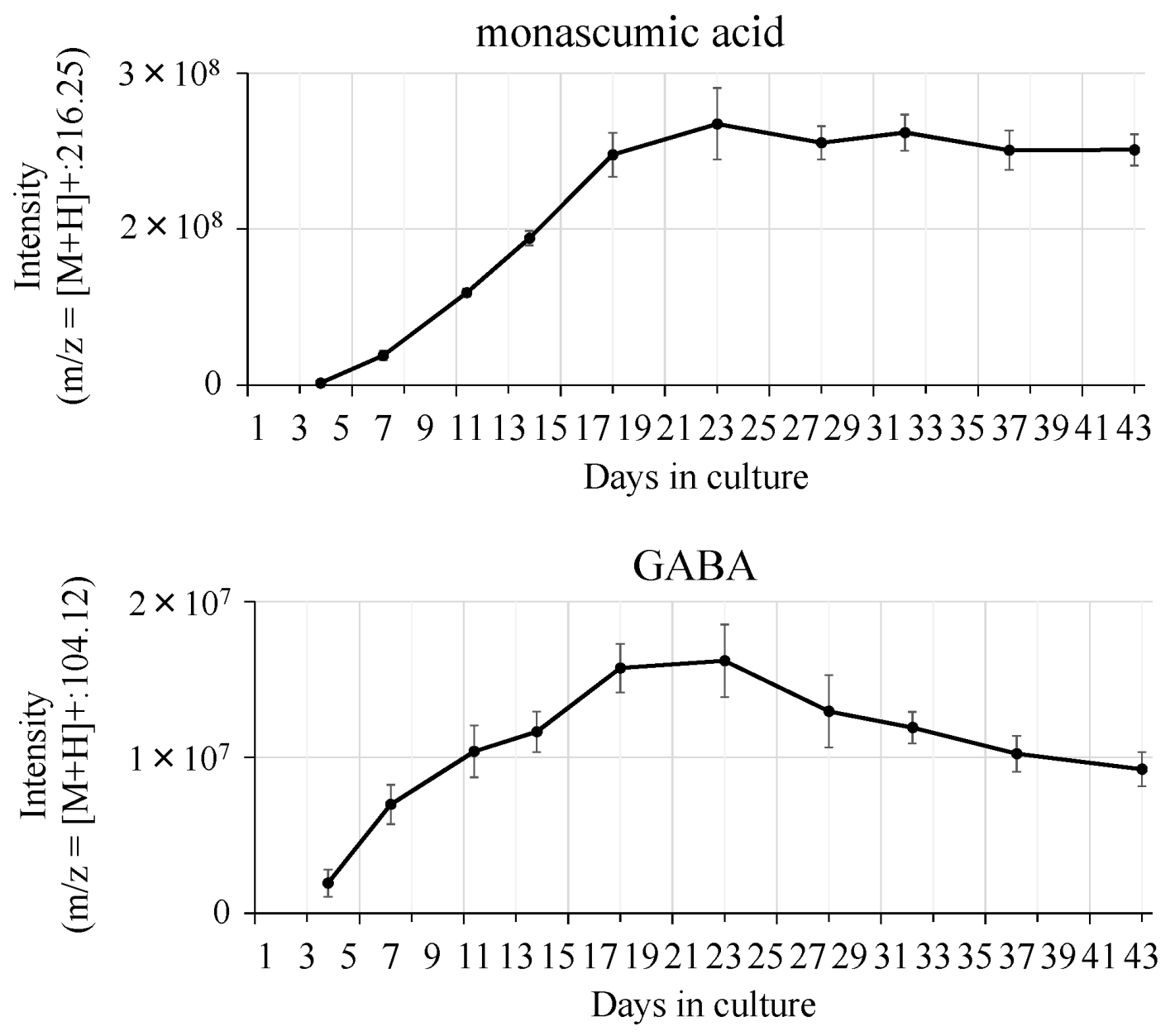
| Strain | Source |
|---|---|
| M. purpureus | Red yeast rice, malted rice (miquzi in Chinese) (China, Korea, Taiwan) |
| M. anka | Red yeast rice (Taiwan), malted rice of red nyufu |
| M. anka var. rubellus | Lees from the making process of red Laojiu (Chinese alcoholic beverage) |
| M. barkeri | Malted rice for samutu-syu (Chinese red alcoholic beverage) |
| M. albidus | Chantofu (Shanghai) |
| M. araneosus | Malted rice for gaoliangilu (Chinese distilled spirit, Northeastern China) |
| M. furiginosus | Malted rice (Guizhou Province, China) |
| M. major | Malted rice (Fuzhou, China) |
| M. albidus var. glaber | Malted rice (Fuzhou, China) |
| M. pilosus | Malted rice for gaoliangilu (Fengtian, China) |
| M. rubropanctatus | Powdered malted rice for medicinal wine (Incheon, Korea) |
| M. pubigerus | Malted rice for gaoliangilu (Liaoyang, China) |
| M. rubinosus | Malted rice (Guangdong Province) |
| M. serorubescens | Red funyu (Hong Kong) |
| M. vitreus | Red funyu (Hong Kong) |
| M. kaoliang | Malted rice for gaoliangilu (Taiwan) |
| M. ruber | Silage, soil, rotten fruit etc. |
| M. paxi | Dead branch and leaves of plants |
| Strain |
|---|
| M. anka Nakazawa et Sato NBRC 4478 |
| M. purpreus Went NBRC 4513 |
| M. anka Nakazawa et Sato (T. Hasegawa) NBRC 6540 |
| M. major Sato NBRC 4485 |
| M. ruber van Tieghem NBRC 4492 |
| M. pubigerus Sato NBRC 4521 |
| M. araneosus Sato NBRC 4482 |
| M. rubiginosus Sato NBRC 4484 |
| M. anka var. rubellus Sato NBRC 5965 |
| M. ruber van Tieghem (S. Udagawa) NBRC 9203 |
| M. pilosus Sato (FAT) NBRC 4520 |
| M. fuliginosus Sato NBRC 4483 |
| M. pilosus Sato NBRC 4480 |
| M. paxii Lingelsheim NBRC 8201 |
| M. vitreus Sato NBRC 4532 |
| M. vitreus Sato (J. Nicot) NBRC 7537 |
| M. albidus Sato NBRC 4489 |
| M. anka var. rubellus Sato (H. Iizuka) NBRC 6085 |
| M. serorubescens Sato NBRC 4487 |
| M. albidus var. glaber Sato NBRC 4486 |
| M. serorubescens Sato (FAT) NBRC 4525 |
| PK Parameter of Monacolin K Lactone | PK Parameter Of Monacolin K Hydroxy Acid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monacolin K Administration Group | Monacolin K Administration Group | ||||||||||
| PK Parameter | 101 | 102 | 103 | Mean | S.D. | PK Parameter | 101 | 102 | 103 | Mean | S.D. |
| t1/2 (h) | N.C. | N.C. | N.C. | - | - | t1/2 (h) | 2.7 | 1.7 | N.C. | 2.2 | - |
| Tmax (h) | 1.0 | 1.0 | N.C. | 1.0 | - | Tmax (h) | 1.0 | 1.0 | 2.0 | 1.3 | 0.6 |
| Cmax (ng/mL) | 3.11 | 1.85 | N.C. | 2.48 | - | Cmax (ng/mL) | 72.6 | 113 | 55.5 | 80.4 | 29.5 |
| AUC0–4h (ng·h/mL) | 3.11 | 1.85 | N.C. | 2.48 | - | AUC0–4h (ng·h/mL) | 143 | 274 | 153 | 190 | 73 |
| Red yeast rice administration group | Red yeast rice administration group | ||||||||||
| PK parameter | 201 | 202 | 203 | Mean | S.D. | PK parameter | 201 | 202 | 203 | Mean | S.D. |
| t1/2 (h) | N.C. | N.C. | N.C. | - | - | t1/2 (h) | 1.4 | 1.1 | 0.98 | 1.1 | 0.2 |
| Tmax (h) | 1.0 | 1.0 | 1.0 | 1.0 | 0.0 | Tmax (h) | 1.0 | 1.0 | 1.0 | 1.0 | 0.0 |
| Cmax (ng/mL) | 4.32 | 4.03 | 3.54 | 3.96 | 0.39 | Cmax (ng/mL) | 254 | 349 | 406 | 336 | 77 |
| AUC0–4h (ng·h/mL) | 7.20 | 5.22 | 6.46 | 6.29 | 1.00 | AUC0–4h (ng·h/mL) | 457 | 470 | 632 | 520 | 98 |
| S.D.: Standard deviation | |||||||||||
| Evaluation Target | Study Subjects or Study Materials | Effect of Monacolin K | Ref. |
|---|---|---|---|
| Atheroma formation | Rabbits fed on a high-lipid diet | Decrease in number of plaques in the aorta and lung artery | [52] |
| Patients with hypercholesterolemia | Inhibition of platelet aggregation, macrophage foam cell formation and LDL oxidation | [53] | |
| Human umbilical vein endothelial cells (HUVECs) | Inhibition of NF-KB activation by CRP | [54] | |
| Blood circulation | Hypertensive model mice | Increase in renal blood flow | [55] |
| Several studies using experimental animals | Increase in cerebral blood flow and cerebral vasomotor response | [56] | |
| NOS signaling pathway | Cardiac myocytes | Increase of IL-1 induced production of nitrite (Increase in NOS expression and NO production mediated by Rho inhibition) | [61] |
| Squamous epithelial cancer cells | Induction of expression of Rho family proteins | [62] | |
| Mesenchymal stem cells derived from rat bone marrow | Activation (phosphorylation) of AI3K/Akt pathway and MEK 1/2 pathway | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukami, H.; Higa, Y.; Hisano, T.; Asano, K.; Hirata, T.; Nishibe, S. A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules 2021, 26, 1619. https://doi.org/10.3390/molecules26061619
Fukami H, Higa Y, Hisano T, Asano K, Hirata T, Nishibe S. A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules. 2021; 26(6):1619. https://doi.org/10.3390/molecules26061619
Chicago/Turabian StyleFukami, Hiroyuki, Yuki Higa, Tomohiro Hisano, Koichi Asano, Tetsuya Hirata, and Sansei Nishibe. 2021. "A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System" Molecules 26, no. 6: 1619. https://doi.org/10.3390/molecules26061619
APA StyleFukami, H., Higa, Y., Hisano, T., Asano, K., Hirata, T., & Nishibe, S. (2021). A Review of Red Yeast Rice, a Traditional Fermented Food in Japan and East Asia: Its Characteristic Ingredients and Application in the Maintenance and Improvement of Health in Lipid Metabolism and the Circulatory System. Molecules, 26(6), 1619. https://doi.org/10.3390/molecules26061619






