Abstract
A highly specific and sensitive proton nuclear magnetic resonance (1H-NMR) method has been developed for the quantification of ephedrine alkaloid derivatives in Ephedra herbal commercial prescriptions. At the region of δ 4.0 to 5.0 ppm in the 1H NMR spectrum, the characteristic signals are separated well from each other, and six analogues in total, methylephedrine (ME), ephedrine (EP), norephedrine (NE), norpseudoephedrine (NP), pseudoephedrine (PE), and methylpseudoephedrine (MP) could be identified. The quantities of these compounds are calculated by the relative ratio of the integral values of the target peak for each compound to the known concentrations of the internal standard anthracene. The present method allows for a rapid and simple quantification of ephedrine alkaloid derivatives in Ephedra-related commercial prescriptions without any preliminary purification steps and standard compounds, and accordingly it can be a powerful tool to verify different Ephedra species. In comparison to conventional chromatographic methods, the advantages of this method include the fact that no standard compounds are required, the quantification can be directly performed on the crude extracts, a better selectivity for various ephedrine alkaloid derivatives, and the fact that a very significant time-gain may be achieved.
1. Introduction
The aerial parts of Ephedra have long been used as diaphoretics, antiasthmatics and diuretics, as well as for the treatment of bronchitis and acute nephritic edema, and to induce perspiration, reduce fever, and treat cough and asthma in traditional Chinese medicine. Ephedra-containing dietary supplements have been promoted for use as aids in dieting and as stimulants for boosting energy and athletic performance [1,2,3,4]. The activity of Ephedra species is attributed to the presence of optically active diastereomeric alkaloids (Figure 1), of which ephedrine (EP) and pseudoephedrine (PE) constitute the major fractions [4]. Ephedra plant materials used in oriental medicines show quite variable qualities since there are many species comprising the sources of the Ephedra on the market [5,6]. Moreover, the diverse geographical origins of the plant materials make the total content of the main active alkaloids quite different from species to species.
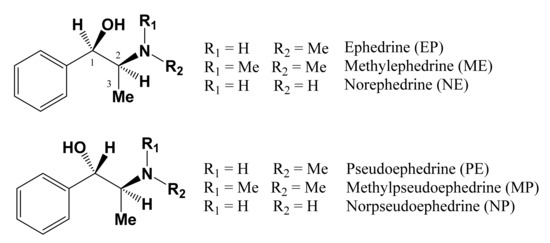
Figure 1.
Structures of the active alkaloids in Ephedra species.
The International Olympic Committee listed ephedrine and related compounds as stimulants in athletic sports in 2003 [7]. However, considering that these products are often used for the treatment of colds, the International Athletic Committee has made a quantitative limitation for ephedrine alkaloids. In December 2003, the FDA issued a rule to declare dietary supplements that contained ephedrine alkaloids to be adulterated [8]. This rule was based on the evidences of health risks associated with the uses of Ephedra and in effect banned the use of ephedrine alkaloids (regardless of their botanical origin) in dietary supplements. In addition, Ephedra was used extensively in various traditional Chinese medicine prescriptions, including Ding Chuan Tang, Shern Mih Tang, Ma Huang Tang, and Ma Xing Yin Gan Tang, which aroused our interests due to the quality of these prescriptions. Increasing concerns about the safety of Ephedra alkaloids from both the consumers and regulatory agencies have led to the development of several methods to detect these analytes in a range of complex matrices.
A number of methods for the quantitative analysis of Ephedra alkaloids have been reported, including thin-layer chromatography (TLC) [9], gas-chromatography (GC) [10,11], GC-mass spectrometry (GC-MS) [12,13], high-performance liquid chromatography (HPLC) [14,15,16,17,18], and capillary electrophoresis (CE) [19]. HPLC had been extensively used for ephedrine alkaloids analysis, and in many cases SDS was applied into the mobile phase to improve the resolution. However, it also increased the difficulties in separating the amphiphilic compounds. The lack of a specific and strong chromophore for detection was also a problem when a conventional HPLC–UV detector was used. The GC method was usually admired as the most popular technique for the quantitation of ephedrine analogs. In order to enhance the sensitivity and to remove interference compounds, complex clean-up procedures and precolumn derivatization were required before analysis, leading to time-consuming protocols. Therefore, developing a simple and accurate method for the simultaneous detection of Ephedra alkaloids for the quality control of Ephedra raw materials and for commercial pharmaceutical prescriptions is strongly required.
Recently, 1H-NMR spectroscopy was developed as an important tool for the quality control of phytochemical preparations [20,21,22,23,24,25,26,27,28], clinical diagnosis, and monitoring of treatment [29]. The advantages of the quantitative NMR (qNMR) method are manifold. For example, it is rapid, noninvasive, and does not require any sample pretreatment steps. In addition, no standard compounds are required to prepare the calibration curves, and it detects all the components presented in herbal preparations simultaneously in a single measurement. Although there are still some defects for qNMR, such as a high cost of instruments and lower sensitivity compared to the traditional chromatographic methods, more and more reports related to the qNMR methods are published due to the lack of many certified reference materials. Therefore, in this study we described a 1H NMR spectroscopic method for the quantitative analysis of ephedrine alkaloid derivatives in Ephedra species and related commercial traditional Chinese medicine prescriptions, including Ding Chuan Tang, Shern Mih Tang, Ma Huang Tang, Ma Xing Yin Gan Tang, Ye Jiao Teng, and Gui Pi Tang. This method would allow for the rapid and simultaneous determination of these ephedrine alkaloid derivatives to be performed without any pretreatment steps.
2. Results and Discussion
The extraction of alkaloids was performed according to the reported method [30]. Considering the solubility of ephedrine alkaloids, CDCl3 was used as the solvent to ensure that all the extract could be dissolved. The 1H NMR spectra of the extracts of E. sinica, E. intermedia, and E. equisetina were well documented in CDCl3 (Figure 2), and the NMR signals of these alkaloids were provided in Table 1. For the quantitative analysis of these alkaloids, including methylephedrine (ME), ephedrine (EP), norephedrine (NE), norpseudoephedrine (NP), pseudoephedrine (PE), and methylpseudoephedrine (MP), the H-1 signals of these compounds were selected as the target signals since they were well separated in the region of δ 4.0–5.0 ppm and no significant interferences were observed (Figure 3). According to the literature reports and compared with the provided spectra, the target protons (H-1) of ephedrine and pseudoephedrine are resonated at δ 4.76 (d, J = 3.9 Hz) and 4.17 (d, J = 8.2 Hz) [31]. The proton signals of methylephedrine and methylpseudoephedrine are also well separated from each other and observed at δ 4.96 (d, J = 3.8 Hz) and 4.19 (d, J = 6.4 Hz) [32]. For the third pair of diastereomers, norephedrine and norpseudoephedrine, the H-1 signals are located at δ 4.52 (d, J = 4.8 Hz) and 4.24 (d, J = 6.8 Hz) [31,33], respectively. Therefore, these data suggest that the H-1 signal is suitable for use as a target peak for quantification.
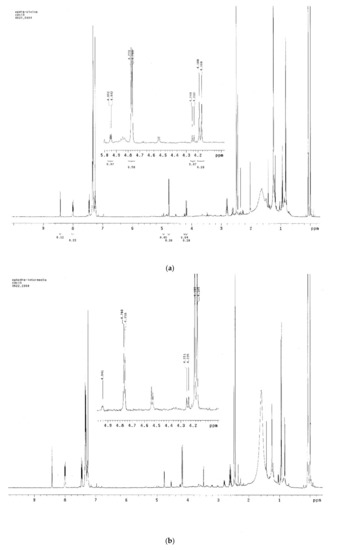

Figure 2.
The 1H-NMR spectra of three Ephedra extracts. (a) E. sinica (ES); (b) E. intermedia (EI); and (c) E. equisetina (EE).

Table 1.
1H-NMR signal assignments for the ephedrine alkaloid derivatives (δ in ppm).
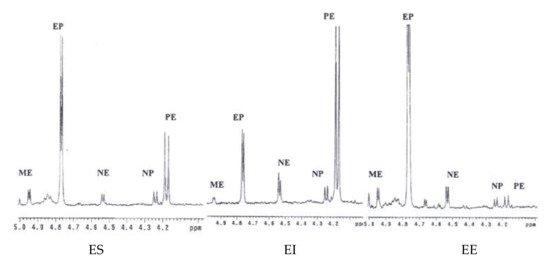
Figure 3.
The H-1 signals of ephedrine alkaloids in the 1H-NMR spectra of three Ephedra extracts [E. sinica (ES), E. intermedia (EI), and E. equisetina (EE)].
A suitable internal standard should preferably be a stable compound with a signal in a noncrowded region of the 1H-NMR spectrum. For this purpose, anthracene, with a signal at δ 8.41 and the integral value staying constant within 48 h, has been designated. In the case of the qNMR analysis, calibration curves of standard compounds were not necessary for quantification since the integration ratios between any proton signals and internal standard were always proportional to their concentration ratio. Therefore, the unknown concentrations of ephedrine alkaloids in the tested samples could be afforded by the simple calculation of the integral area ratio between anthracene and each target peak. The NMR signals can be improved by increasing the number of scans, and the validation parameters may therefore be varied. However, the ephedrine standard was applied to check the accuracy and limit of detection in the current NMR parameters. The accuracy of this method was determined by adding a known concentration of ephedrine (0.5, 1.0, 2.0 mg, respectively) to extract samples. The peak area corresponding to ephedrine was found to increase proportionally to the added concentration of the standard, and the average recovery percentage was 96.5% (three samples measured in triplicate). The limit of quantification (LOQ) and limit of detection (LOD) for ephedrine under the present experimental condition was determined to be 0.2 and 0.05 mg/mL at the signal-to-noise ratios of 10 and 3, respectively. Since we did not have the standards for other alkaloids, the LOD and LOQ of these compounds were not examined.
Moreover, an HPLC-UV analysis of the ephedrine standard was also performed. The calibration curve for ephedrine was established in the range between 0.2 and 2.0 mg/mL and possessed good linearity (R2 = 0.9991) in order to compare the results afforded by the NMR method with those from the chromatographic technique. The examined samples exhibited comparable data with these two analytical methods (data not shown). Compared with the previously reported data [16], the ephedrine content in E. sinica was in the range of 5–10 mg/g and was also comparable with the present analytical results.
Three Ephedra materials, including E. sinica, E. intermedia, and E. equisetina, were analyzed for the contents of ephedrine alkaloids using this 1H-NMR method. In the 1H-NMR spectra of these extracts, the H-1 signals at δ 4.96, 4.76, 4.52, 4.24, and 4.17 ppm for ME, EP, NE, NP, and PE, respectively, were well separated from other signals. These H-1 peaks were well assigned, according to the literature reports [31,32,33]. However, the lack of H-1 signal of MP indicated that the quantity of MP in Ephedra materials was low. These results were identical to the reports [15,16,17,18] and were further confirmed by using the published method [16], which showed that the amounts of alkaloids in the E. sinica extract were in the order of EP > PE > ME > NP > NE, while MP was trace. The quantification of these five alkaloids by 1H-NMR was feasible by the calculation of the ratio of the integral area between well-separated specific proton (H-1) signals of these compounds and the internal standard. The quantitatively analytical data of ME, EP, NE, NP, and PE in three Ephedra species determined by this 1H-NMR method are shown in Table 2. Ephedrine (EP) was found to be the major constituent in E. sinica, and the amount of EP was about two to three times that of pseudoephedrine (PE). Meanwhile, in E. intermedia, the major constituent was PE, and its amount was about two to three times that of EP. In addition, EP was the major component in E. equisetina, and other alkaloids were relatively few.

Table 2.
The concentrations (mg/g) of ephedrine alkaloid derivatives, including methylephedrine (ME), ephedrine (EP), norephedrine (NE), norpseudoephedrine (NP), pseudoephedrine (PE), and methylpseudoephedrine (MP), in E. sinica, E. intermedia, and E. equisetina extracts and several Ephedra-related commercial prescriptions (companies A~E) a.
This developed method can be applied for the identification of the presence of Ephedra species in related commercial traditional Chinese medicinal preparations (Table 2). In the present study, all the Ephedra prescriptions (Ding Chuan Tang, Shern Mih Tang, Ma Huang Tang, and Ma Xing Yin Gan Tang purchased from companies A–D) were identified as containing E. sinica as their source rather than E. intermedia or E. equisetina, since ephedrine and pseudoephedrine were the major principles in their 1H NMR spectra (Figure 4a–d), which were identical with the profile of E. sinica. The commercial Ephedra prescriptions Ding Chuan Tang produced by different companies (A~D) showed similar contents of the ephedrine alkaloid derivatives. The prescriptions Shern Mih Tang showed a significantly different composition of ephedrine alkaloid derivatives. In particular, in the prescriptions provided by company C, the contents of ephedrine alkaloid derivatives were about two times higher than those in the prescriptions produced by companies A, B, and D. Similar situations were observed in the Ma Huang Tang prescriptions. The contents of ephedrine alkaloid derivatives in the prescription produced by company D were two times higher than those provided in companies A, B, and C. In addition, the alkaloid contents in Ma Xing Yin Gan Tang produced by companies A and D were somewhat lower than those in the other two companies (B and C). These variations may be due to the use of different stock materials by different companies or the abnormal addition of Ephedra plant materials to enhance the activity. Therefore, the developed 1H-NMR method met the analytical criteria for commercial Ephedra prescriptions, and the results were reproducible for Ephedra pharmaceutical preparations from various sources.
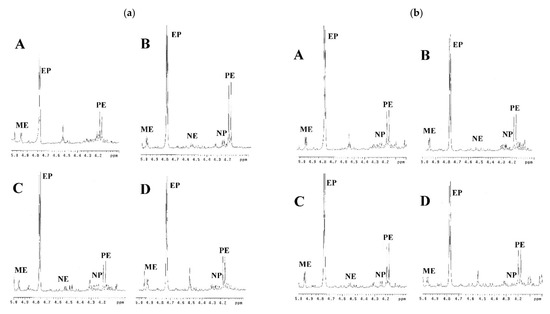
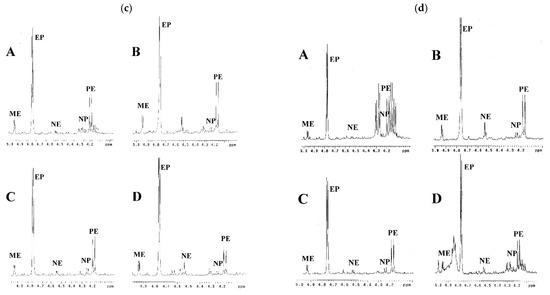
Figure 4.
1H-NMR spectra of the Ephedra commercial prescriptions in the range of δ 4.0~5.0 ppm. (a) Ding Chuan Tang, (b) Shern Mih Tang, (c) Ma Huang Tang, and (d) Ma Xing Yin Gan Tang. A~D: Samples from companies A~D.
With the assistance of this 1H-NMR method, it would be efficient to check the adulterates of the ephedrine alkaloid derivatives or Ephedra materials in traditional Chinese medicine prescriptions. For example, the ephedrine alkaloid derivatives should not be detected in the prescriptions Ye Jiao Teng and Gui Pi Tang since there were not any Ephedra plant materials included. Accordingly, the prescriptions from companies A, B, and C did not show any signals at the region of δ 4.0 to 5.0 ppm in their 1H-NMR spectra, indicating that these prescriptions do not contain ephedrine alkaloid derivatives. However, the prescription Ye Jiao Teng produced by company E showed significant appearances of ephedrine (EP) and pseudoephedrine (PE) (1.3091 mg/g for EP and 1.4822 mg/g for PE), indicating that it may be adulterated by synthetic products or raw materials (Figure 5). Similarly, the 1H-NMR spectra of the prescription Gui Pi Tang produced by company E also displayed some quantities of EP (0.2148 mg/g) and PE (0.1236 mg/g) at a ratio of about 2:1 (Figure 5). This composition was similar to the profile of E. sinica and suggested that Gui Pi Tang of company E was adulterated with the plant materials of E. sinica or that the prescription may have been cross-polluted by the Ephedra preparations in the production processes.
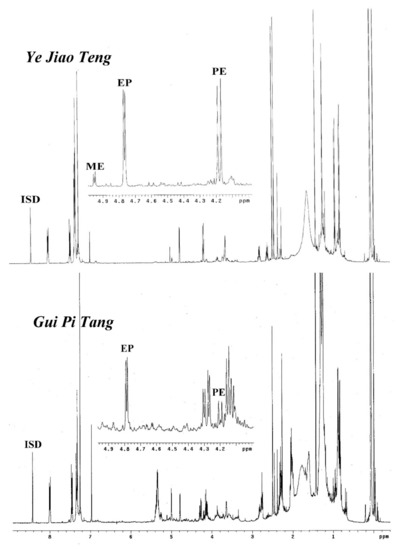
Figure 5.
1H-NMR spectra of the commercial prescriptions Ye Jiao Teng and Gui Pi Tang from company E. ISD: anthracene.
3. Materials and Methods
3.1. Chemicals and Materials
First grade sulfuric acid (98%), ether, methanol, sodium chloride, and anthracene were purchased from E. Merck (Darmstadt, Germany). CDCl3 (99.9%) was obtained from Aldrich (Milwaukee, WI, USA). The reference compound ephedrine was isolated from the stems of E. sinica in our previous study, and its purity was checked by the NMR and HPLC methods (>99.5%). The plant materials of E. sinica, E. intermedia, and E. equisetina were purchased at an herb shop in Tainan (August 2005) and authenticated by Prof. C. S. Kuoh (Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan). The commercial traditional Chinese medicine prescriptions (Ding Chuan Tang, Shern Mih Tang, Ma Huang Tang, and Ma Xing Yin Gan Tang) were purchased from four pharmaceutical companies (A–D) in Taiwan. In addition, Ye Jiao Teng and Gui Pi Tang were also purchased from four pharmaceutical companies (A–C and E) in Taiwan.
3.2. Sample Extraction
The extraction of alkaloids was performed according to the method of Cui et al. [30] with minor modifications. 0.4 g of powdered plant material or 1.0 g of prescription was weighed and transferred to a 15-mL centrifuge tube and mixed with 8 mL of 0.5 M H2SO4 solution. The mixture was shaken and sonicated for 1 h and then centrifuged for 20 min at 3000 rpm. The supernatant layer was transferred at 4.0 mL to another tube and 1.2 mL of 5 M KOH solution, 2.4 g NaCl and 5 mL of diethyl ether were added to the tube. The mixture was shaken for 20 min and then centrifuged for 20 min at 3000 rpm. Extraction with diethyl ether was performed twice. Diethyl ether layers were combined and evaporated after the addition of internal standard. The dried sample was dissolved in 1 mL of CDCl3 and used for the 1H-NMR measurement.
3.3. NMR Analysis and Identification of Ephedrine Alkaloid Derivatives
1H-NMR spectra were recorded in CDCl3 (99.9%) by using a Varian UNITY plus 400 MHz NMR spectrometer equipped with a Varian Indirect NMR™ (ID) probe. Each sample was dissolved by deuterated solvent in a Schotte economic NMR sample tube (5 mm o.d./178 mm length). For each sample, 128 scans were recorded with the following parameters: 0.187 Hz/point; spectra width, 3600 Hz; a 90° pulse was used to obtain the maximum sensitivity; relaxation delay, 10 s; acquisition time, 2.56 s. For the quantitative analysis, the peak area was used, and the start and end points of the integration of each peak were selected manually.
3.4. Recovery, Limit of Quantification (LOQ), and Limit of Detection (LOD) of Ephedrine
Recovery tests were selected to determine the accuracy of the method, in which three different concentrations of ephedrine (0.5, 1.0, 2.0 mg, respectively) were spiked into the sample and the recovery percentages were calculated using the measured contents divided by the contents of added standards and original sample obtained by 1H-NMR analysis. A blank recovery sample was prepared and analyzed for the comparison. The LOQ and LOD for ephedrine under the present NMR analytical condition (128 scans) were determined at signal-to-noise ratios of 10 and 3, respectively.
4. Conclusions
The present 1H-NMR method allows for the rapid and simultaneous determination of six alkaloids in Ephedra species, including methylephedrine (ME), ephedrine (EP), norephedrine (NE), norpseudoephedrine (NP), pseudoephedrine (PE), and methylpseudoephedrine (MP), without any pretreatment steps. With the assistance of this technique, the contents of the ephedrine alkaloid derivatives can be analyzed within a much shorter time than with conventional chromatographic methods. These alkaloids can be detected without any preliminary derivatization steps. The overall profiles of the plants or prescriptions can be quickly obtained, and it would therefore be a powerful tool for verifying different species. Hopefully, this method can be applied in the near future to the quality control of commercial pharmaceutics or preparations of Ephedra such as cough syrups and dietary supplements.
Author Contributions
Conceptualization, P.-C.K. and T.-S.W.; Data curation and Investigation, H.-Y.H., S.-M.L., and C.-Y.L.; Methodology, S.-H.L., Y.-Y.C., and M.-J.L.; Resources, P.-C.S. and F.-A.C.; Writing-original draft, H.-Y.H. and C.-Y.L.; Writing-review & editing, P.-C.K. and T.-S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology (MOST), Taiwan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors wish to thank C. S. Kuoh (Department of Life Sciences, National Cheng Kung University, Tainan, Taiwan) for the authentication of plant materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the crude extracts are available from the authors.
References
- Bensky, D.; Gamble, A. Chinese Herbal Medicine-Materia Medica; Eastland Press: Seattle, WA, USA, 1986. [Google Scholar]
- Nagai, W.N.; Kanao, S. Constituents of Chinese drug “Ma Huang” VI. J. Pharm. Soc. Jpn. 1928, 48, 845–851. [Google Scholar] [CrossRef][Green Version]
- Abourashed, E.A.; EI-Alfy, A.T.; Khan, I.A.; Walker, L. Ephedra in perspective—A current review. Phytother. Res. 2003, 17, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Jiangsu New Medical College. Dictionary of Chinese Traditional Medicine; People’s Publisher of Shanghai: Shanghai, China, 1977; pp. 2222–2225. [Google Scholar]
- Liu, Y.M.; Sheu, S.J.; Chiou, S.H.; Chang, H.C.; Chen, Y.P. A comparative study of commercial samples of ephedrae herba. Planta Med. 1993, 59, 376–378. [Google Scholar] [CrossRef]
- Caveney, S.; Charlet, D.A.; Freitag, H.; Maier-Stolte, M.; Strarratt, A.N. New observations on the secondary chemistry of world Ephedra (Ephedraceae). Am. J. Bot. 2001, 88, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- International Olympic Committee. List of Doping Classes and Methods (UK); International Olympic Committee: Lausanne, Switzerland, 2003. [Google Scholar]
- U.S. Food and Drug Administration. White Paper on Ephedra: Evidence on the Safety and Effectiveness of Ephedra: Implications for Regulations. Available online: http://www.fda.gov/bbs/topics/NEWS/ephedra/whitepaper.html (accessed on 12 March 2005).
- Suedee, R.; Songkram, C.; Petmoreekul, A.; Sangkunakup, S.; Sankasa, S.; Kongyarit, N. Direct enantioseparation of adrenergic drugs via thin-layer chromatography using molecularly imprinted polymers. J. Pharm. Biomed. Anal. 1999, 19, 519–527. [Google Scholar] [CrossRef]
- Van Eenoo, P.; Delbeke, F.T.; Roels, K.; De Backer, P. Simultaneous quantitation of ephedrines in urine by gas chromatography-nitrogen-phosphorus detection for doping control purposes. J. Chromatogr. B 2001, 760, 255–261. [Google Scholar] [CrossRef]
- Lewis, R.J.; Huffine, E.F.; Chaturvedi, A.K.; Canfield, D.V.; Mattson, J. Formation of an interfering substance, 3,4-dimethyl-5-phenyl-1,3-oxazolidine, during a pseudoephedrine urinalysis. J. Forensic Sci. 2000, 45, 898–901. [Google Scholar] [CrossRef]
- Gentili, S.; Torresi, A.; Marsili, R.; Chiarotti, M.; Macchia, T. Simultaneous detection of amphetamine-like drugs with headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. B 2002, 780, 183–192. [Google Scholar] [CrossRef]
- El-Ha, B.M.; Al-Amri, A.M.; Hassan, M.H.; Ali, H.S.; Bin Khadem, R.K. The use of cyclohexanone as a “derivatizing” reagent for the GC-MS detection of amphetamines and ephedrines in seizures and the urine. Forensic Sci. Int. 2003, 135, 16–26. [Google Scholar] [CrossRef]
- Hood, D.J.; Cheung, H.Y. A chromatographic method for rapid and simultaneous analysis of codeine phosphate, ephedrine HCl and chlorpheniramine maleate in cough-cold syrup formulation. J. Pharm. Biomed. Anal. 2003, 30, 1595–1601. [Google Scholar] [CrossRef]
- Okamura, N.; Miki, H.; Harada, T.; Yamashita, S.; Masaoka, Y.; Nakamoto, Y.; Tsuguma, M.; Yoshitomi, H.; Yagi, A. Simultaneous determination of ephedrine, pseudoephedrine, norephedrine and methylephedrine in Kampo medicines by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 1999, 20, 363–372. [Google Scholar] [CrossRef]
- Lee, M.K.; Cheng, B.H.; Che, C.T.; Hsieh, D.P.H. Cytotoxicity assessment of Ma-huang (Ephedra) under different conditions of preparation. Toxicol. Sci. 2000, 56, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Ding, M.Y.; Lv, K.; Yu, J.Y. Simultaneous separation and determination of ephedrine alkaloids and tetramethylpyrazine in Ephedra sinica Stapf. by HPLC. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 313–320. [Google Scholar] [CrossRef]
- McCooeye, M.; Ding, L.; Gardner, G.J.; Fraser, C.A.; Lam, J.; Sturgeon, R.E.; Mester, Z. Separation and quantitation of the stereoisomers of ephedra alkaloids in natural health products using flow injection-electrospray ionization-high field asymmetric waveform ion mobility spectrometry-mass spectrometry. Anal. Chem. 2003, 75, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Mateus-Avois, L.; Mangin, P.; Saugy, M. Development and validation of a capillary zone electrophoresis method for the determination of ephedrine and related compounds in urine without extraction. J. Chromatogr. B 2003, 791, 203–216. [Google Scholar] [CrossRef]
- Choi, Y.H.; Choi, H.K.; Hazekamp, A.; Bermejo, P.; Schilder, Y.; Erkelens, C.; Verpoorte, R. Quantitative analysis of bilobalide and ginkgolides from Ginkgo biloba leaves and Ginkgo products using 1H-NMR. Chem. Pharm. Bull. 2003, 51, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Frédérich, M.; Choi, Y.H.; Verpoorte, R. Quantitative analysis of strychnine and brucine in Strychnos nux-vomica using 1H-NMR. Planta Med. 2003, 69, 1169–1171. [Google Scholar]
- Li, C.Y.; Lin, C.H.; Wu, C.C.; Lee, K.H.; Wu, T.S. Efficient 1H nuclear magnetic resonance method for improved quality control analyses of Ginkgo constituents. J. Agric. Food Chem. 2004, 52, 3721–3725. [Google Scholar] [CrossRef]
- Pauli, G.F.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR: Development and potential of a method for natural products analysis. J. Nat. Prod. 2005, 68, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Lin, C.H.; Wu, T.S. Quantitative analysis of camptothecin derivatives in Nothapodytes foetida using 1H-NMR method. Chem. Pharm. Bull. 2005, 53, 347–349. [Google Scholar] [CrossRef]
- Li, C.Y.; Lu, H.J.; Lin, C.H.; Wu, T.S. A rapid and simple determination of protoberberine alkaloids in cortex phellodendri by 1H NMR and its application for quality control of commercial traditional Chinese medicine prescriptions. J. Pharm. Biomed. Anal. 2006, 40, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Xu, H.X.; Han, Q.B.; Wu, T.S. Quality assessment of Radix Codonopsis by quantitative nuclear magnetic resonance. J. Chromatogr. A 2009, 1216, 2124–2129. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.Y.; Chan, H.H.; Hung, H.Y.; Kuo, C.L.; Wu, J.B.; Sun, I.W.; Kuo, P.C.; Wu, T.S. A rapid quantitative 1H NMR analysis of kinsenoside and other bioactive principles from Anoectochilus formosanus. Anal. Methods 2016, 8, 5645–5650. [Google Scholar] [CrossRef]
- Hsieh, L.Y.; Chan, H.H.; Kuo, P.C.; Hung, H.Y.; Li, Y.C.; Kuo, C.L.; Peng, Y.; Zhao, Z.Z.; Kuo, D.H.; Sun, I.W.; et al. A feasible and practical 1H NMR analytical method for the quality control and quantification of bioactive principles in Lycii Fructus. J. Food Drug Anal. 2018, 26, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Lindon, J.C.; Nicholson, J.K.; Everett, J.R. NMR spectroscopy of biofluids. Annu. Rep. NMR Spectrosc. 1999, 38, 1–88. [Google Scholar]
- Cui, J.F.; Zhou, T.H.; Zhang, J.S.; Lou, Z.C. Analysis of alkaloids in Chinese Ephedra species by gas chromatographic methods. Phytochem. Anal. 1991, 2, 116–119. [Google Scholar] [CrossRef]
- Gunderson, K.G.; Shapiro, M.J.; Doti, R.A.; Skiles, J.W. Simultaneous determination of enantiomeric purity and erythro/threo relationship in chiral 1,2-aminoalcohols by NMR using (R)-(+)-t-butylphenylphosphinothioic acid. Tetrahedron Asymmetry 1999, 10, 3263–3266. [Google Scholar] [CrossRef]
- Fujita, M.; Hiyama, T. Erythro-directive reduction of α-substituted alkanones by means of hydrosilanes in acidic media. J. Org. Chem. 1988, 53, 5415–5421. [Google Scholar] [CrossRef]
- Nishimura, M.; Minakata, S.; Takahashi, T.; Oderaotoshi, Y.; Komatsu, M. Asymmetric N1 unit transfer to olefins with a chiral nitridomanganese complex: novel stereoselective pathways to aziridines or oxazolines. J. Org. Chem. 2002, 67, 2101–2110. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).