Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
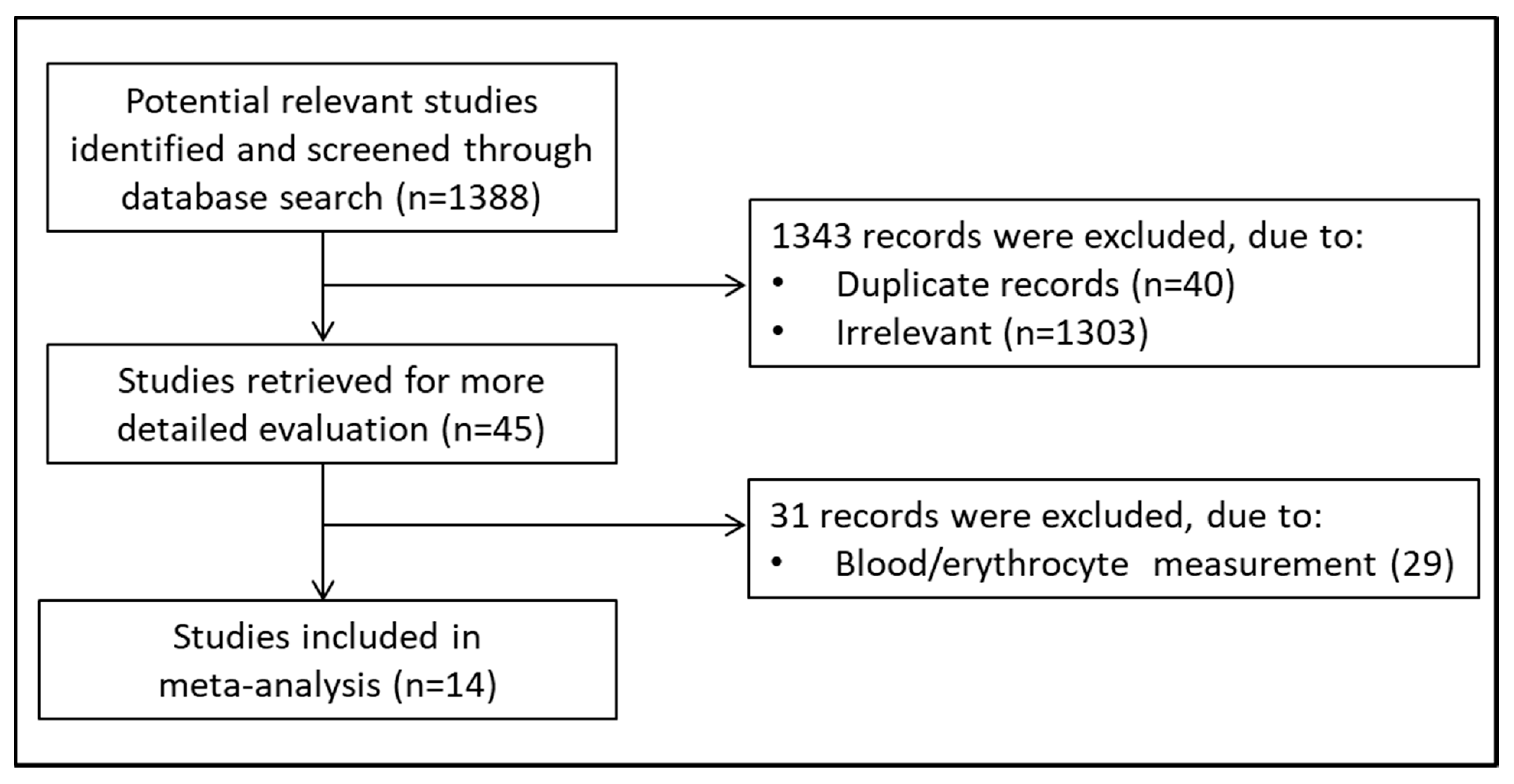
2.1. Search Strategy and Studies Selection
2.2. Statistical Analysis
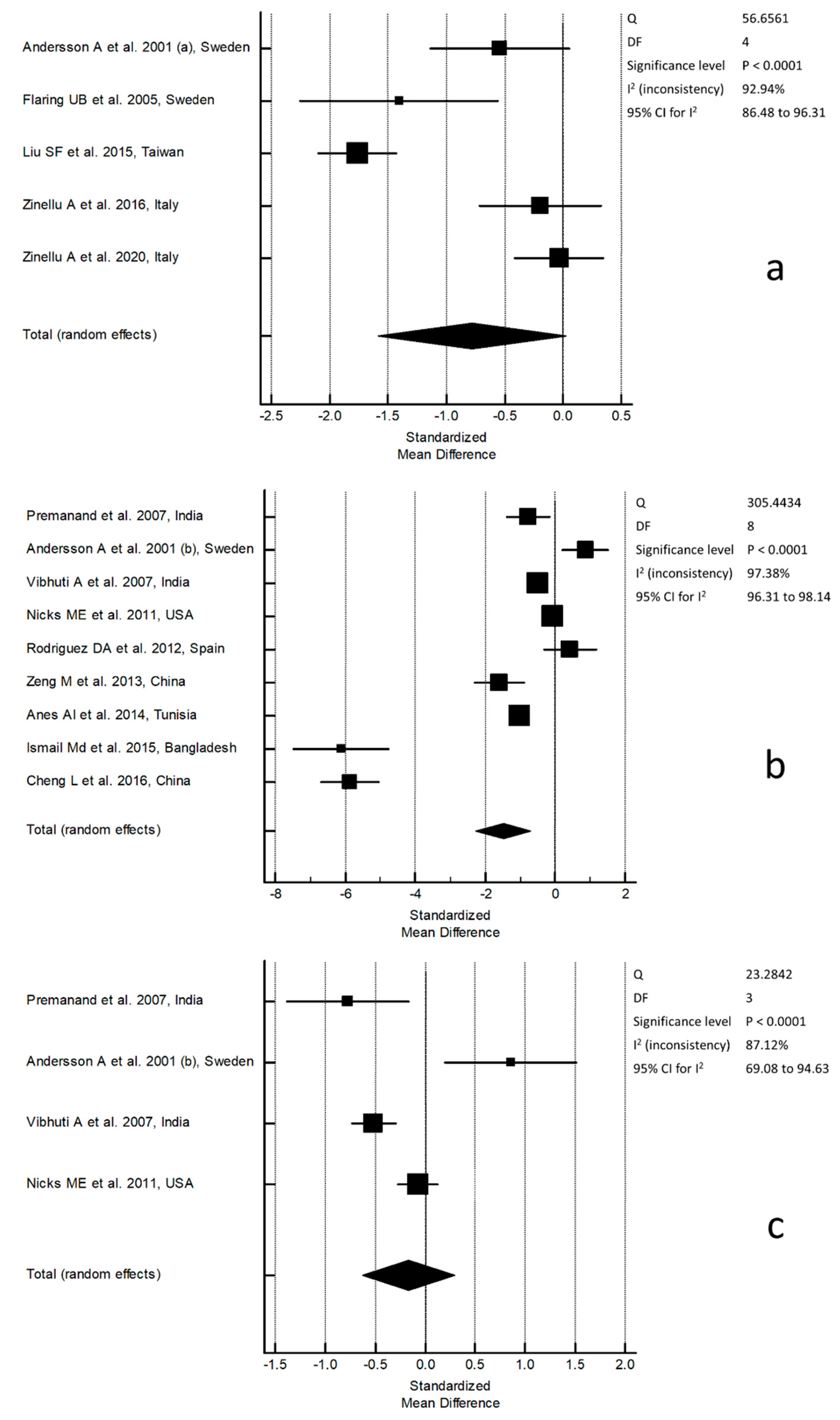
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meister, A. The gamma-glutamyl cycle. Diseases associated with specific enzyme deficiencies. Ann. Intern. Med. 1974, 81, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Role of transcription factors in inflammatory lung diseases. Thorax 1998, 53, 601–612. [Google Scholar] [CrossRef]
- Deneke, S.M.; Fanburg, B.L. Regulation of cellular glutathione. Am. J. Physiol. 1989, 257, L163–L173. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W.; Schulze-Osthoff, K.; Mihm, S.; Galter, D.; Schenk, H.; Eck, H.P.; Roth, S.; Gmünder, H. Functions of glutathione and glutathione disulfide in immunology and immunopathology. FASEB J. 1994, 8, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Donaldson, K.; Rahman, I.; MacNee, W. An investigation of the role of glutathione in the increased permeability induced by cigarette smoke in vivo and in vitro. Am. Rev. Respir. Crit. Care Med. 1994, 149, 1518–1525. [Google Scholar] [CrossRef]
- Lannan, S.; Donaldson, K.; Brown, D.; MacNee, W. Effects of cigarette smoke and its condensates on alveolar cell injury in vitro. Am. J. Physiol. 1994, 266, L92–L100. [Google Scholar] [CrossRef]
- van Klaveren, R.J.; Demedts, M.; Nemery, B. Cellular glutathione turnover in vitro, with emphasis on type II pneumocytes. Eur. Respir. J. 1997, 10, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; MacNee, W. Oxidative stress and regulation of glutathione in lung inflammation. Eur. Respir. J. 2000, 16, 534–554. [Google Scholar] [CrossRef]
- Oxman, A.D.; Muir, D.C.; Shannon, H.S.; Stock, S.R.; Hnizdo, E.; Lange, H.J. Occupational dust exposure and chronic obstructive pulmonary disease. A systematic overview of the evidence. Am. Rev. Respir. Dis. 1993, 148, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Forbes, L.J.L.; Kapetanakis, V.; Rudnicka, A.R.; Cook, D.G.; Bush, T.; Stedman, J.R.; Whincup, P.H.; Strachan, D.P.; Anderson, H.R. Chronic exposure to outdoor air pollution and lung function in adults. Thorax 2009, 64, 657–663. [Google Scholar] [CrossRef]
- Salvi, S.S.; Barnes, P.G. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009, 374, 733–743. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; U.S. Government Printing Office: Washington, DC, USA; Atlanta, GA, USA, 2010.
- Cross, C.E.; van der Vliet, A.; O’Neill, C.A.; Louie, S.; Halliwell, B. Oxidants, antioxidants, and respiratory tract lining fluids. Environ. Health Perspect. 1994, 102, 185–191. [Google Scholar]
- Cantin, A.M.; North, S.L.; Habbard, R.C. Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987, 63, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.S.; Min, E.; Gauthier, S.; Martin, R.J.; Day, B.J. Lung glutathione adaptive responses to cigarette smoke exposure. Respir. Res. 2011, 12, 133. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hubbard, R.C.; Crystal, R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989, 139, 370–372. [Google Scholar] [CrossRef]
- Baz, M.A.; Tapson, V.F.; Roggli, V.L.; Van Trigt, P.; Piantadosi, C.A. Glutathione depletion in epithelial lining fluid of lung allograft patients. Am. J. Respir. Crit. Care Med. 1996, 153, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Moss, M.; Guidot, D.M.; Wong-Lambertina, M.; Ten Hoor, T.; Perez, R.L.; Brown, L.A. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am. J. Respir. Crit. Care Med. 2000, 161, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Roum, J.H.; Buhl, R.; McElvaney, N.G.; Borok, Z.; Crystal, R.G. Systemic deficiency of glutathione in cystic fibrosis. J. Appl. Physiol. 1993, 75, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A. Antioxidant Defenses in The Lung. In Comparative Biology of The Normal Lung; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Franciosi, L.; Govorukhina, N.; Ten Hacken, N.; Postma, D.; Bischoff, R. Proteomics of epithelial lining fluid obtained by bronchoscopic microprobe sampling. Methods Mol. Biol. 2011, 790, 17–28. [Google Scholar]
- Snell, N.; Newbold, P. The clinical utility of biomarkers in asthma and COPD. Curr. Opin. Pharmacol. 2008, 8, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Freels, J.L.; Robbins, R.A.; Campbell, S.C. Exhaled Breath Condensate The Past, Present, and Future. Clin. Pulm. Med. 2003, 10, 263–268. [Google Scholar] [CrossRef]
- Gould, N.S.; Day, B.J. Targeting maladaptive glutathione responses in lung disease. Biochem. Pharmacol. 2011, 81, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Zinellu, E.; Fois, A.G.; Pirina, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Systematic Review and Meta-Analysis of the Blood Glutathione Redox State in Chronic Obstructive Pulmonary Disease. Antioxidants 2020, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Ankerst, J.; Lindgren, A.; Larsson, K.; Hultberg, B. Hyperhomocysteinemia and changed plasma thiol redox status in chronic obstructive pulmonary disease. Clin. Chem. Lab. Med. 2001, 39, 229. [Google Scholar] [CrossRef]
- Fläring, U.B.; Rooyackers, O.E.; Hebert, C.; Bratel, T.; Hammarqvist, F.; Wernerman, J. Temporal changes in whole-blood and plasma glutathione in ICU patients with multiple organ failure. Intensive Care Med. 2005, 31, 1072. [Google Scholar] [CrossRef]
- Liu, S.F.; Kuo, H.C.; Tseng, C.W.; Huang, H.T.; Chen, Y.C.; Tseng, C.C.; Lin, M.C. Leukocyte Mitochondrial DNA Copy Number Is Associated with Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 22, e0138716. [Google Scholar] [CrossRef] [PubMed]
- Zinellu, A.; Fois, A.G.; Sotgia, S.; Zinellu, E.; Bifulco, F.; Pintus, G.; Mangoni, A.A.; Carru, C.; Pirina, P. Plasma protein thiols: An early marker of oxidative stress in asthma and chronic obstructive pulmonary disease. Eur. J. Clin. Investig. 2016, 46, 181. [Google Scholar] [CrossRef]
- Zinellu, A.; Zinellu, E.; Sotgiu, E.; Fois, A.G.; Paliogiannis, P.; Scano, V.; Piras, B.; Sotgia, S.; Mangoni, A.A.; Carru, C.; et al. Systemic transsulfuration pathway thiol concentrations in chronic obstructive pulmonary disease patients. Eur. J. Clin. Investig. 2020, 7, e13267. [Google Scholar] [CrossRef] [PubMed]
- Premanand, R.; Kumar, S.; Mohan, A. Study of thiobarbituric reactive substances and total reduced glutathione as indices of oxidative stress in chronic smokers with and without chronic obstructive pulmonary disease. Indian J. Chest Dis. Allied Sci. 2007, 49, 9. [Google Scholar]
- Vibhuti, A.; Arif, E.; Deepak, D.; Singh, B.; Qadar Pasha, M.A. Correlation of oxidative status with BMI and lung function in COPD. Clin. Biochem. 2007, 40, 958. [Google Scholar] [CrossRef] [PubMed]
- Nicks, M.E.; O’Brien, M.M.; Bowler, R.P. Plasma antioxidants are associated with impaired lung function and COPD exacerbations in smokers. COPD 2011, 8, 264. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Kalko, S.; Puig-Vilanova, E.; Perez-Olabarría, M.; Falciani, F.; Gea, J.; Cascante, M.; Barreiro, E.; Roca, J. Muscle and blood redox status after exercise training in severe COPD patients. Free Radic. Biol. Med. 2012, 52, 88. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Li, Y.; Jiang, Y.; Lu, G.; Huang, X.; Guan, K. Local and systemic oxidative stress status in chronic obstructive pulmonary disease patients. Can. Respir. J. 2013, 20, 35. [Google Scholar] [CrossRef]
- ben Anes, A.; Fetoui, H.; Bchir, S.; ben Nasr, H.; Chahdoura, H.; Chabchoub, E.; Yacoub, S.; Garrouch, A.; Benzarti, M.; Tabka, Z.; et al. Increased oxidative stress and altered levels of nitric oxide and peroxynitrite in Tunisian patients with chronic obstructive pulmonary disease: Correlation with disease severity and airflow obstruction. Biol. Trace Elem. Res. 2014, 161, 20. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Hossain, M.F.; Tanu, A.R.; Shekhar, H.U. Effect of spirulina intervention on oxidative stress, antioxidant status, and lipid profile in chronic obstructive pulmonary disease patients. Biomed. Res. Int. 2015, 2015, 486120. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Li, B.; Liu, S.; Li, X.; Tu, H. Cigarette Smoke-Induced Hypermethylation of the GCLC Gene is Associated with COPD. Chest 2016, 149, 474. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Usai, M.F.; Zinellu, E.; Deiana, L.; Carru, C. GSH depletion after erythrocytes acidic treatment is related to intracellular hemoglobin levels. Clin. Chim. Acta 2006, 366, 363. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Usai, M.F.; Chessa, R.; Deiana, L.; Carru, C. Thiol redox status evaluation in red blood cells by capillary electrophoresis-laser induced fluorescence detection. Electrophoresis 2005, 26, 1963–1968. [Google Scholar] [CrossRef]
- Carru, C.; Zinellu, A.; Sotgia, S.; Marongiu, G.; Farina, M.G.; Usai, M.F.; Pes, G.M.; Tadolini, B.; Deiana, L. Optimization of the principal parameters for the ultrarapid electrophoretic separation of reduced and oxidized glutathione by capillary electrophoresis. J. Chromatogr. A 2003, 1017, 233–238. [Google Scholar] [CrossRef]
- Svardal, A.M.; Mansoor, M.A.; Ueland, P.M. Determination of reduced, oxidized, and protein-bound glutathione in human plasma with precolumn derivatization with monobromobimane and liquid chromatography. Anal. Biochem. 1990, 184, 338–346. [Google Scholar] [CrossRef]
- Carru, C.; Deiana, L.; Sotgia, S.; Pes, G.M.; Zinellu, A. Plasma thiols redox status by laser-induced fluorescence capillary electrophoresis. Electrophoresis 2004, 25, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Lindgren, A.; Hultberg, B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin. Chem. 1995, 41, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.A.; Svardal, A.M.; Ueland, P.M. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal. Biochem. 1992, 200, 218–229. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar] [PubMed]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef]
- Zinellu, A.; Carru, C.; Sotgia, S.; Deiana, L. Plasma D-penicillamine redox state evaluation by capillary electrophoresis with laser-induced fluorescence. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 803, 299–304. [Google Scholar] [CrossRef]
- Zinellu, A.; Carru, C.; Sotgia, S.; Porqueddu, E.; Enrico, P.; Deiana, L. Separation of aceclofenac and diclofenac in human plasma by free zone capillary electrophoresis using N-methyl-D-glucamine as an effective electrolyte additive. Eur. J. Pharm. Sci. 2005, 24, 375–380. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Usai, M.F.; Fois, A.G.; Spada, V.; Deledda, A.; Deiana, L.; Pirina, P.; Carru, C. Simultaneous detection of N-acetyl-L-cysteine and physiological low molecular mass thiols in plasma by capillary electrophoresis. Amino Acids 2009, 37, 395–400. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Scanu, B.; Pisanu, E.; Sanna, M.; Sati, S.; Deiana, L.; Sengupta, S.; Carru, C. Determination of homocysteine thiolactone, reduced homocysteine, homocystine, homocysteine-cysteine mixed disulfide, cysteine and cystine in a reaction mixture by overimposed pressure/voltage capillary electrophoresis. Talanta 2010, 82, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Arru, D.; Sotgiu, E.; Mangoni, A.A.; Forteschi, M.; Pintus, G.; Carru, C.; Zinellu, A. Simultaneous determination of the main amino thiol and thione in human whole blood by CE and LC. Bioanalysis 2016, 8, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Fois, A.G.; Paliogiannis, P.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: A systematic review. Respir. Res. 2018, 27, 51. [Google Scholar] [CrossRef] [PubMed]
| Control Group | COPD Group | |||||||
|---|---|---|---|---|---|---|---|---|
| First Author, Year, and Country | Assay Type | Derivatization Reagent | GSH Form | Measure Units | n | GSH Mean ± SD | n | GSH Mean ± SD |
| Andersson A et al., 2001, Sweden [28] | HPLC | dithiopyridine | t | μmol/L | 29 | 5.69 ± 1.33 | 19 | 4.93 ± 1.43 |
| Flaring UB et al., 2005, Sweden [29] | HPLC | monobromobimane | t | μmol/L | 10 | 5.9 ± 1.03 | 21 | 4.6 ± 0.95 |
| Liu SF et al., 2015, Taiwan [30] | Spectr | DTNB | t | mU/mL | 110 | 6.8 ± 1.3 | 86 | 4.5 ± 1.3 |
| Zinellu A et al., 2016, Italy [31] | CE | 5-IAF | t | μmol/L | 29 | 7.2 ± 2.6 | 29 | 6.7 ± 2.4 |
| Zinellu A et al., 2020, Italy [32] | CE | 5-IAF | t | μmol/L | 54 | 7.5 ± 2.9 | 54 | 7.4 ± 3.0 |
| Premanand et al., 2007, India [33] | Spectr | DTNB | r | μmol/ mg of protein | 20 | 4.85 ± 0.97 | 20 | 5.72 ± 1.02 |
| Andersson A et al., 2001, Sweden [28] | HPLC | dithiopyridine | r | μmol/L | 29 | 1.58 ± 0.53 | 19 | 1.23 ± 0.26 |
| Vibhuti A et al., 2007, India [34] | Spectr | DTNB | r | μmol/L | 136 | 4.10 ± 2.64 | 202 | 3.06 ± 1.45 |
| Nicks ME et al., 2011, USA [35] | Spectr | M2VP | r | μmol/L | 136 | 0.53 ± 0.34 | 367 | 0.50 ± 0.42 |
| Rodriguez DA et al., 2012, Spain [36] | Kit | NR | r | μmol/L | 12 | 0.47 ± 0.04 | 18 | 0.49 ± 0.05 |
| Zeng M et al., 2013, China [37] | Kit | NR | r | mg/L (μmol/L) | 14 | 413 ± 8 # (1.340 ± 30) | 35 | 352 ± 44 # (1.147 ± 143) |
| Ben Anes AI et al., 2014, Tunisia [38] | Spectr | DTNB | r | μmol/L | 229 | 96 ± 58 | 153 | 47 ± 26 |
| Ismail Md et al., 2015, Bangladesh [39] | Spectr | DTNB | r | μmol/L | 20 | 415 ± 20 | 30 | 279 ± 23 |
| Cheng L et al., 2016, China [40] | Spectr | DTNB | r | mg/L (μmol/L) | 64 | 17.2 ± 1.2 # (56 ± 4) | 58 | 10.6 ± 0.8 # (35 ± 3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotgia, S.; Fois, A.G.; Paliogiannis, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease. Molecules 2021, 26, 1572. https://doi.org/10.3390/molecules26061572
Sotgia S, Fois AG, Paliogiannis P, Carru C, Mangoni AA, Zinellu A. Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease. Molecules. 2021; 26(6):1572. https://doi.org/10.3390/molecules26061572
Chicago/Turabian StyleSotgia, Salvatore, Alessandro G. Fois, Panagiotis Paliogiannis, Ciriaco Carru, Arduino A. Mangoni, and Angelo Zinellu. 2021. "Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease" Molecules 26, no. 6: 1572. https://doi.org/10.3390/molecules26061572
APA StyleSotgia, S., Fois, A. G., Paliogiannis, P., Carru, C., Mangoni, A. A., & Zinellu, A. (2021). Methodological Fallacies in the Determination of Serum/Plasma Glutathione Limit Its Translational Potential in Chronic Obstructive Pulmonary Disease. Molecules, 26(6), 1572. https://doi.org/10.3390/molecules26061572










