Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect
Abstract
1. Introduction
2. Results
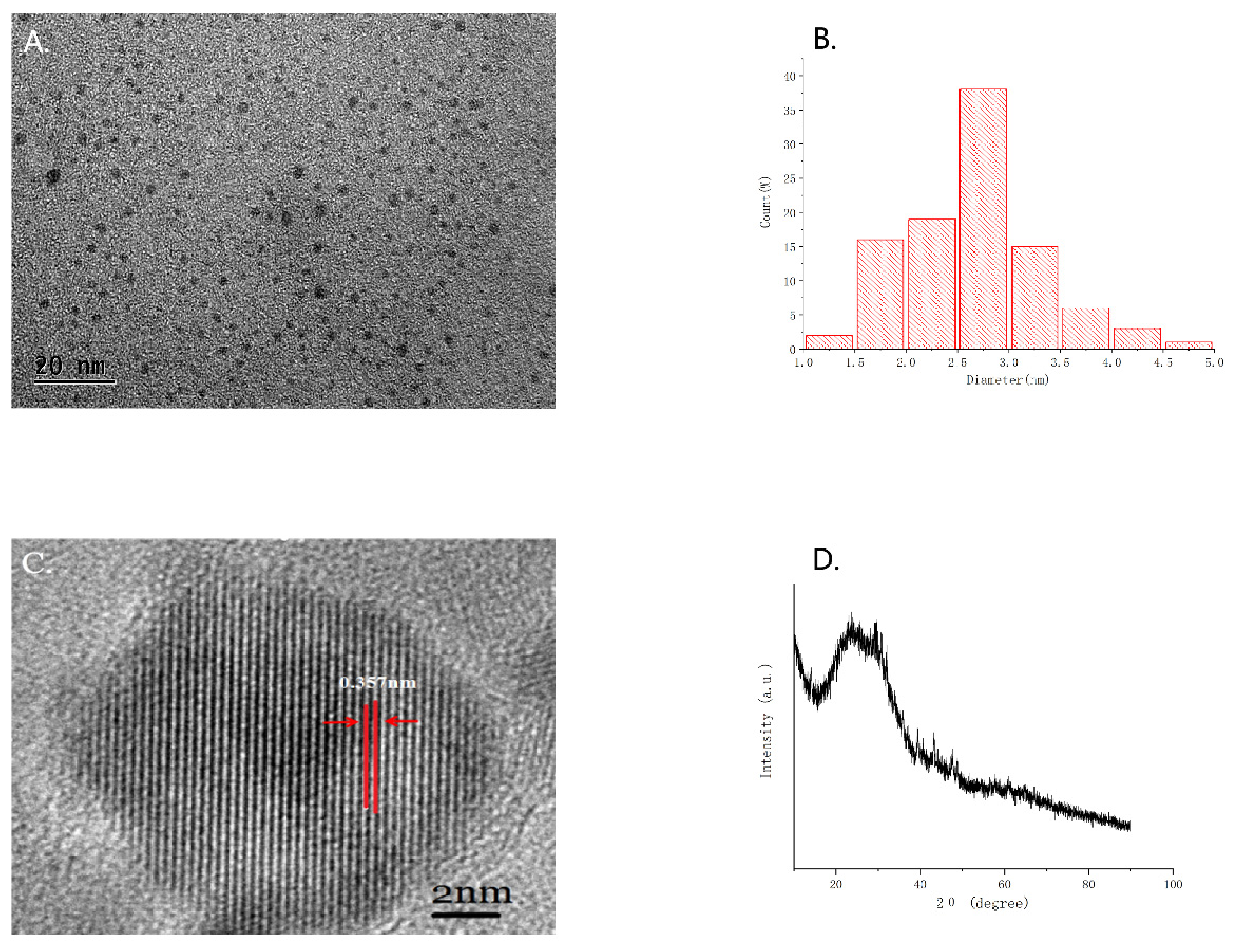
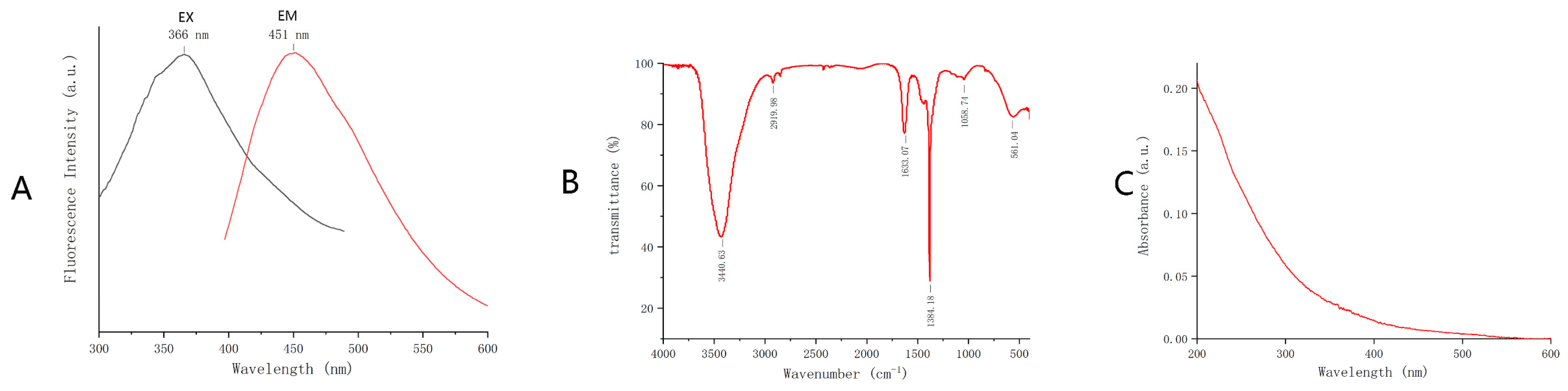
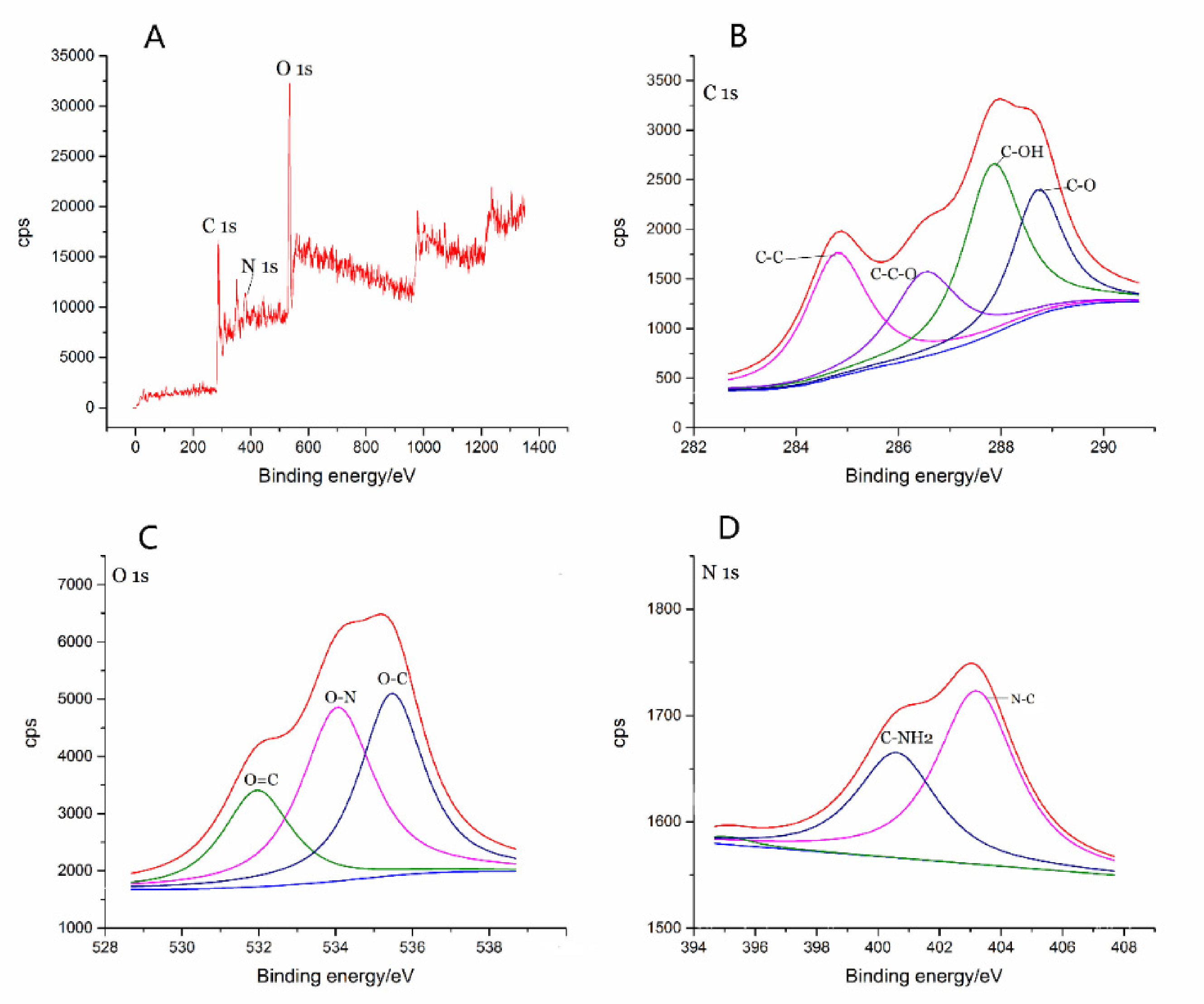
2.1. Characterization of GRR-CDs
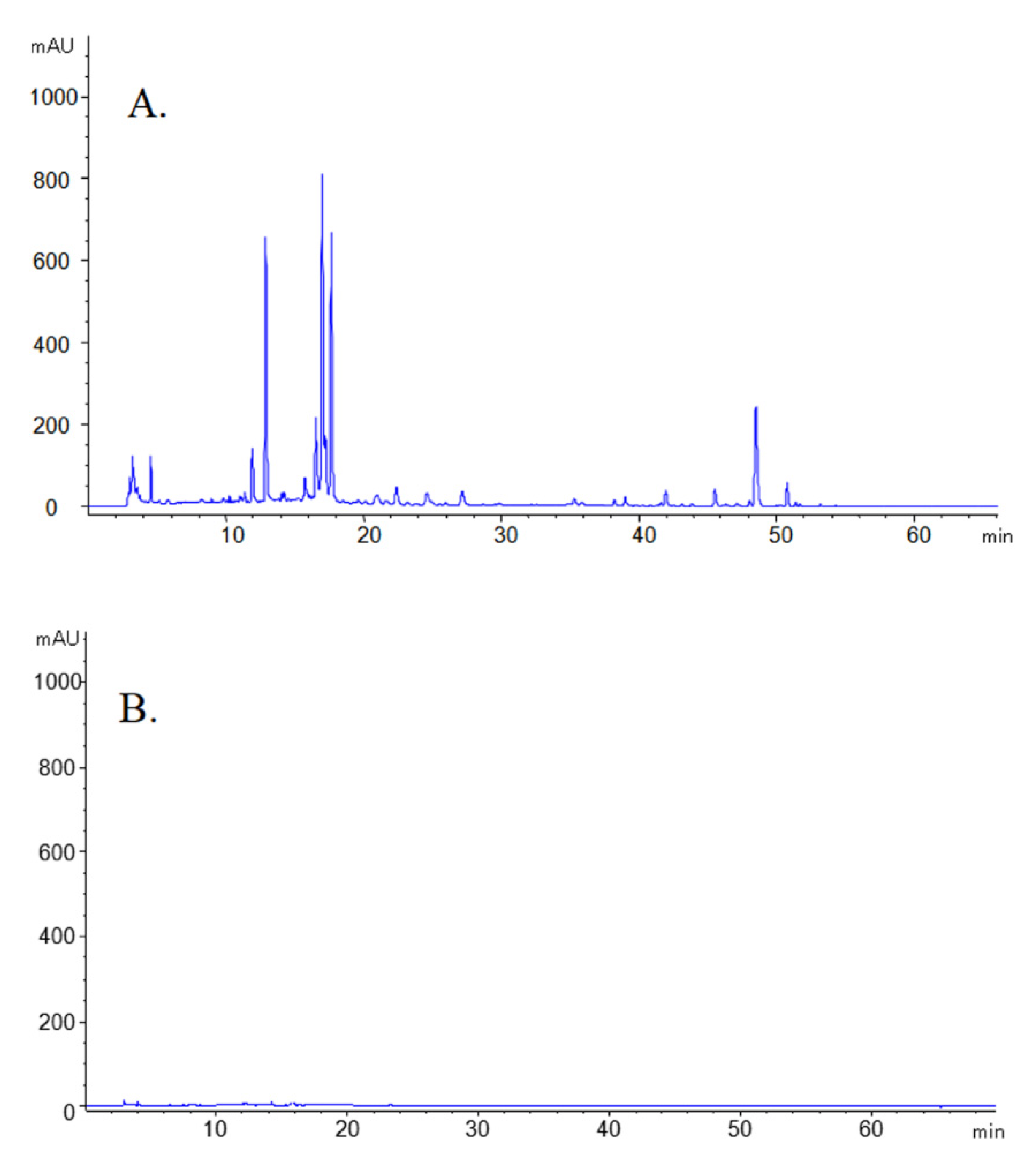
2.2. Fingerprint Profile of GRR and GRR-CDs
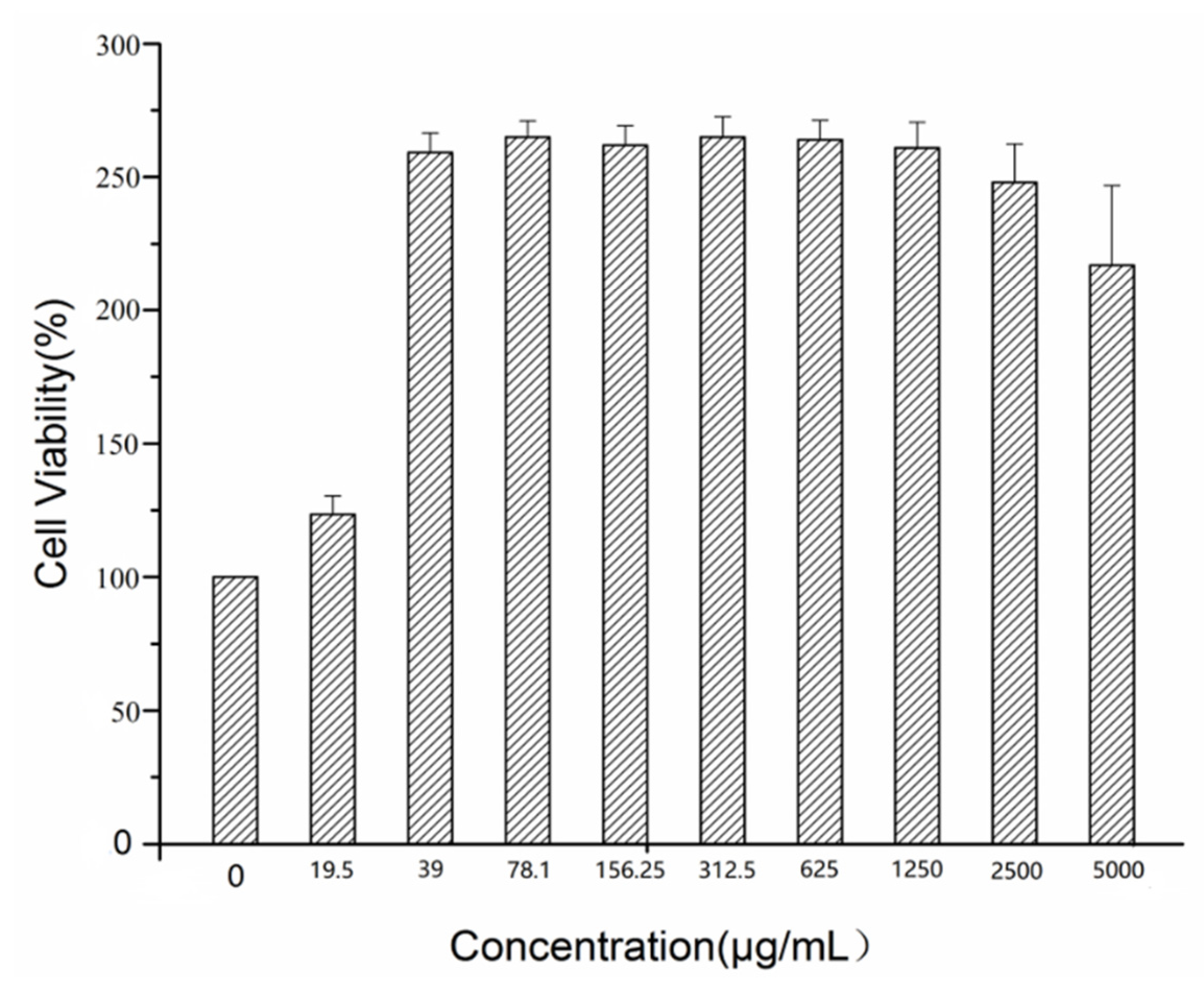
2.3. Cellular Toxicity of GRR-CDs
2.4. Anti-Gastric Ulcer Activity of GRR-CDs
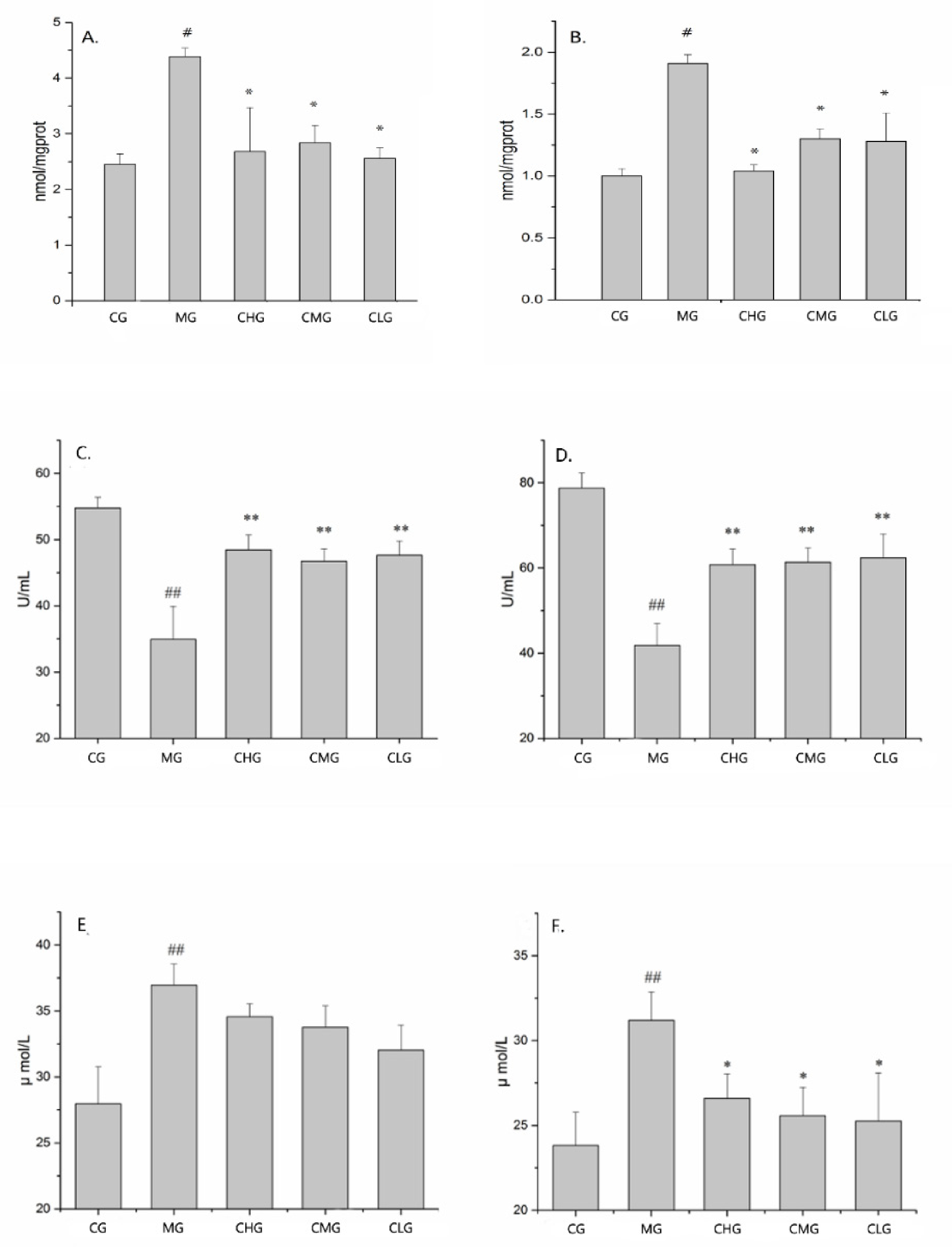
2.5. Effect of GRR-CDs on Biochemical Indexes in Serum and Tissues
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of GRR-CDs
4.4. Characterization of GRR-CDs
4.5. Fluorescence Quantum Yield of GRR-CDs
4.6. Fingerprint Analysis of GRR and GRR-CDs by High Performance Liquid Chromatography
4.7. Cytotoxicity Test of GRR-CDs
4.8. Study on Anti-Ulcer Activity of GRR-CDs
4.9. Detection of Biochemical Indexes in Serum and Tissues
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hou, Z.K.; Li, J.P.; Chen, Z.Q.; Liu, F.B. Clinical experience and academic thoughts of Professor LIU Feng-bin on case series of gastroesophageal reflux disease based on data mining. Zhongguo Zhong Yao Za Zhi 2018, 43, 1261–1267. [Google Scholar]
- Mukherjee, M.; Bhaskaran, N.; Srinath, R.; Shivaprasad, H.N.; Allan, J.J.; Shekhar, D.; Agarwal, A. Anti-ulcer and antioxidant activity of GutGard. Indian J. Exp. Biol. 2010, 48, 269–274. [Google Scholar]
- Aly, A.M.; Al-Alousi, L.; Salem, H.A. Licorice: A possible anti-inflammatory and anti-ulcer drug. AAPS PharmSciTech 2005, 6, E74–E82. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Liu, J.; Yang, Y.A.; Zhu, H.L. A Review: The Anti-inflammatory, Anticancer and Antibacterial Properties of Four Kinds of Licorice Flavonoids Isolated from Licorice. Curr. Med. Chem. 2020, 27, 1997–2011. [Google Scholar] [CrossRef] [PubMed]
- Olukoga, A.; Donaldson, D. Liquorice and its health implications. J. R. Soc. Promot. Health 2000, 120, 83–89. [Google Scholar] [CrossRef] [PubMed]
- García Rodríguez, L.A.; Hernández-Díaz, S. Risk of uncomplicated peptic ulcer among users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Am. J. Epidemiol. 2004, 159, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Clark-Wibberley, T.; Stamford, I.F.; Wright, J.E. Aspirin-induced gastric mucosal damage in rats: Cimetidine and deglycyrrhizinated liquorice together give greater protection than low doses of either drug alone. J. Pharm. Pharmacol. 1980, 32, 151. [Google Scholar] [CrossRef] [PubMed]
- Ishii, Y.; Fujii, Y. Effects of FM100, a fraction of licorice root, on serum gastrin concentration in rats and dogs. Jpn. J. Pharmacol. 1982, 32, 23–27. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Li, G.; Zhao, C.; Liao, X. Green Synthesis of Fluorescent Carbon Dots for Selective Detection of Tartrazine in Food Samples. J. Agric. Food Chem. 2015, 63, 6707–6714. [Google Scholar] [CrossRef]
- Li, S.; Guo, Z.; Zhang, Y.; Xue, W.; Liu, Z. Blood Compatibility Evaluations of Fluorescent Carbon Dots. ACS Appl. Mater. Interfaces 2015, 7, 19153–19162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Y.; Teng, X.; Yan, M.; Bi, H.; Morais, P.C. Mitochondria-targeting nanoplatform with fluorescent carbon dots for long time imaging and magnetic field-enhanced cellular uptake. ACS Appl. Mater. Interfaces 2015, 7, 10201–10212. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Talib, A.; Wu, H.F. One pot synthesis of gold—Carbon dots nanocomposite and its application for cytosensing of metals for cancer cells. Talanta 2017, 166, 357–363. [Google Scholar] [CrossRef]
- Gowthaman, N.S.K.; Sinduja, B.; Karthikeyan, R.; Rubini, K.; Abraham John, S. Fabrication of nitrogen-doped carbon dots for screening the purine metabolic disorder in human fluids. Biosens. Bioelectron. 2017, 94, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.B.; Oliva, M.M.; Contreras-Cáceres, R.; Rodriguez-Castellón, E.; Jiménez-Jiménez, J.; da Silva, J.C.; Algarra, M. Carbon dots on based folic acid coated with PAMAM dendrimer as platform for Pt(IV) detection. J. Colloid Interface Sci. 2016, 465, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. (Int. Ed. Engl.) 2012, 51, 12215–12218. [Google Scholar]
- Wu, Y.; Wei, P.; Pengpumkiat, S.; Schumacher, E.A.; Remcho, V.T. Development of a carbon dot (C-Dot)-linked immunosorbent assay for the detection of human α-fetoprotein. Anal. Chem. 2015, 87, 8510–8516. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Kong, H.; Zhang, M.; Cheng, J.; Wang, X.; Lu, F.; Qu, H.; Zhao, Y. Antihyperuricemic and anti-gouty arthritis activities of Aurantii fructus immaturus carbonisata-derived carbon dots. Nanomedicine 2019, 14, 2925–2939. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, J.; Sun, Z.; Kong, H.; Zhang, Y.; Wang, S.; Wang, X.; Zhao, Y.; Qu, H. Protective Effects of Carbon Dots Derived from Phellodendri Chinensis Cortex Carbonisata against Deinagkistrodon acutus Venom-Induced Acute Kidney Injury. Nanoscale Res. Lett. 2019, 14, 377. [Google Scholar] [CrossRef]
- Chun, S.; Muthu, M.; Gansukh, E.; Thalappil, P.; Gopal, J. The ethanopharmacological aspect of carbon nanodots in turmeric smoke. Sci. Rep. 2016, 6, 35586. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, Y.; Luo, J.; Xiong, W.; Liu, X.; Cheng, J.; Wang, Y.; Zhang, M.; Qu, H. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J. Nanobiotechnol. 2017, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yan, X.; Zhang, M.; Zhang, Y.; Cheng, J.; Lu, F.; Qu, H.; Wang, Q.; Zhao, Y. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine 2018, 13, 391–405. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Sun, Z.; Lu, F.; Xiong, W.; Luo, J.; Kong, H.; Wang, Q.; Qu, H.; Zhao, Y. Hemostatic and hepatoprotective bioactivity of Junci Medulla Carbonisata-derived Carbon Dots. Nanomedicine 2019, 14, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, M.; Cheng, J.; Zhang, Y.; Luo, J.; Liu, Y.; Kong, H.; Qu, H.; Zhao, Y. Effect of Lonicerae japonicae Flos Carbonisata-Derived Carbon Dots on Rat Models of Fever and Hypothermia Induced by Lipopolysaccharide. Int. J. Nanomed. 2020, 15, 4139–4149. [Google Scholar] [CrossRef]
- Luo, J.; Kong, H.; Zhang, M.; Cheng, J.; Sun, Z.; Xiong, W.; Zhu, Y.; Zhao, Y.; Qu, H. Novel Carbon Dots-Derived from Radix Puerariae Carbonisata Significantly Improve the Solubility and Bioavailability of Baicalin. J. Biomed. Nanotechnol. 2019, 15, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Kong, H.; Zhang, M.; Cheng, J.; Luo, J.; Zhao, Y.; Qu, H. Haemostatic Nanoparticles-Derived Bioactivity of from Selaginella tamariscina Carbonisata. Molecules 2020, 25, 446. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Denev, P.; Eftimov, M.; Georgieva, A.; Kuzmanova, V.; Kuzmanov, A.; Kuzmanov, K.; Tzaneva, M. Protective effects of Aronia melanocarpa juices either alone or combined with extracts from Rosa canina or Alchemilla vulgaris in a rat model of indomethacin-induced gastric ulcers. Food Chem. Toxicol. 2019, 132, 110739. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, Y.; Zhang, J.; Wang, L.; Jin, Z.; Huang, H.; Man, S.; Gao, W. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem.-Biol. Interact. 2016, 250, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, F.; Gan, S.; He, Y.; Chen, Z.; Liu, X.; Fu, C.; Qu, Y.; Zhang, J. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem. Toxicol. 2019, 131, 110539. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.M.; Pezzini, B.C.; Somensi, L.B.; Bolda Mariano, L.N.; Mariott, M.; Boeing, T.; Dos Santos, A.C.; Longo, B.; Cechinel-Filho, V.; de Souza, P.; et al. Hesperidin, a citrus flavanone glycoside, accelerates the gastric healing process of acetic acid-induced ulcer in rats. Chem.-Biol. Interact. 2019, 308, 45–50. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Dekanski, D.; Ristić, S.; Radonjić, N.V.; Petronijević, N.D.; Giampieri, F.; Astolfi, P.; González-Paramás, A.M.; Santos-Buelga, C.; Tulipani, S.; et al. Strawberry polyphenols attenuate ethanol-induced gastric lesions in rats by activation of antioxidant enzymes and attenuation of MDA increase. PLoS ONE 2011, 6, e25878. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, J.; Wang, S.; Zhao, Y.; Liu, Z.; Li, J.; Ren, W.; Tang, S.; Xie, L.; Huang, Y.; et al. Evaluation of Transabdominal Ultrasound with Oral Cellulose-Based Contrast Agent in the Detection and Surveillance of Gastric Ulcer. Ultrasound Med. Biol. 2017, 43, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, Anti-inflammatory, and Antiulcer Potential of Manuka Honey against Gastric Ulcer in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef] [PubMed]
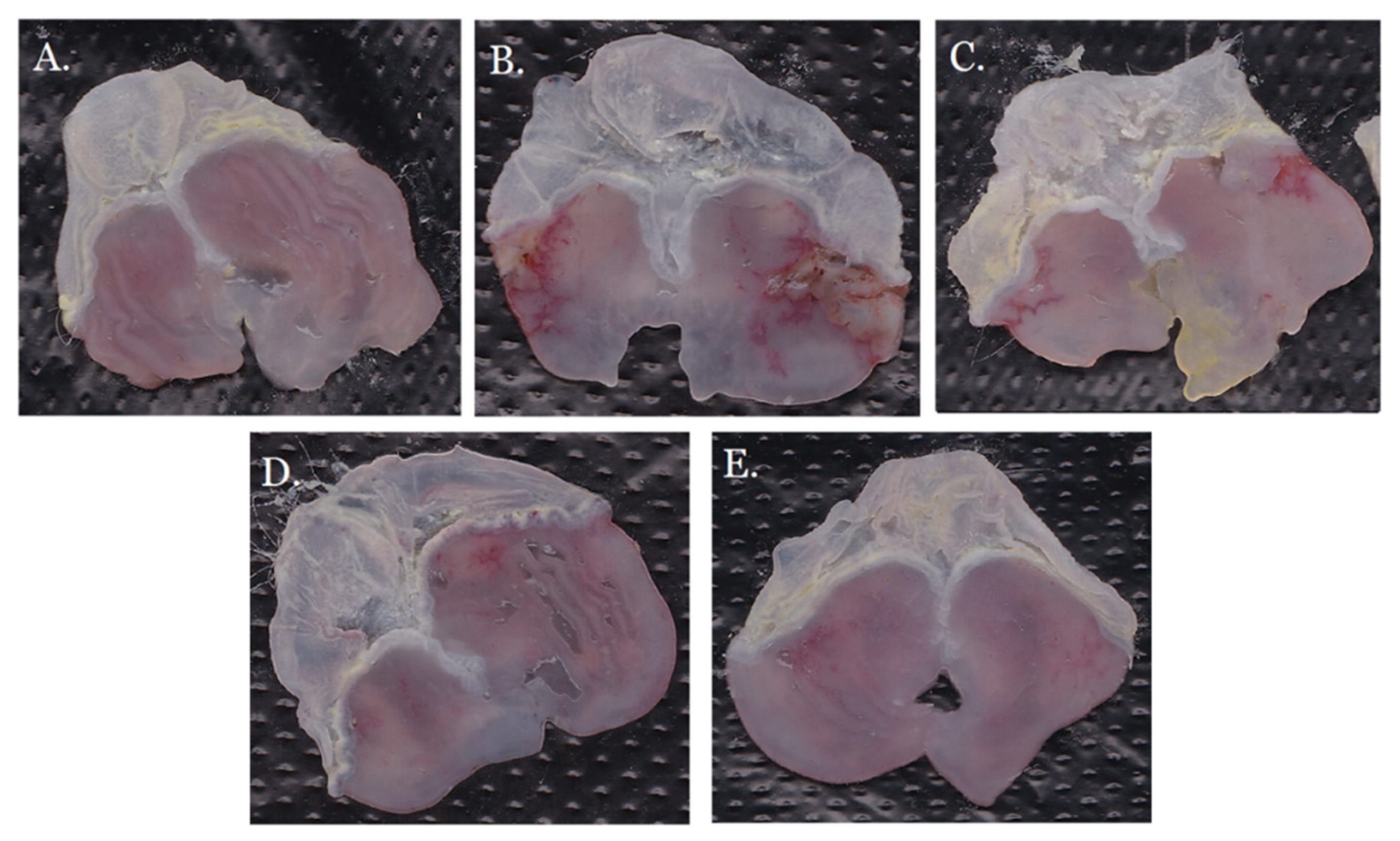
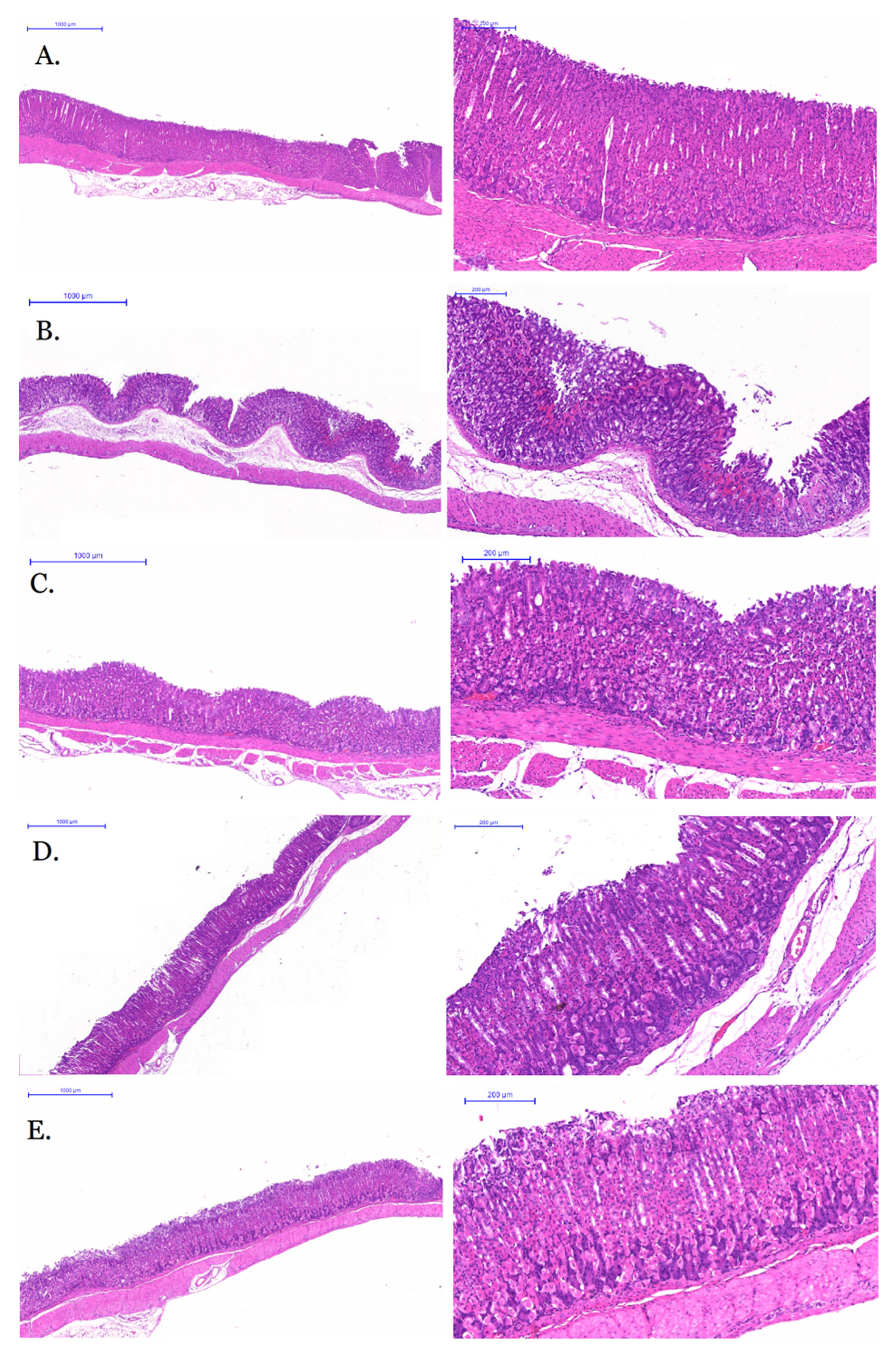
| Groups | Gastric Ulcer Index | Gastric Ulcer Inhibition Rate (%) |
|---|---|---|
| control | 0 | 100 |
| model | 47.00 ± 4.60 # | 0 |
| GRR-CDs high-dose | 18.17 ± 3.81 * | 61.34 |
| GRR-CDs medium-dose | 12.83 ± 3.97 * | 71.17 |
| GRR-CDs low-dose | 15.17 ± 3.37 * | 67.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, M.; Cheng, J.; Zhang, Y.; Kong, H.; Zhao, Y.; Qu, H. Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect. Molecules 2021, 26, 1512. https://doi.org/10.3390/molecules26061512
Liu Y, Zhang M, Cheng J, Zhang Y, Kong H, Zhao Y, Qu H. Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect. Molecules. 2021; 26(6):1512. https://doi.org/10.3390/molecules26061512
Chicago/Turabian StyleLiu, Yuhan, Meiling Zhang, Jinjun Cheng, Yue Zhang, Hui Kong, Yan Zhao, and Huihua Qu. 2021. "Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect" Molecules 26, no. 6: 1512. https://doi.org/10.3390/molecules26061512
APA StyleLiu, Y., Zhang, M., Cheng, J., Zhang, Y., Kong, H., Zhao, Y., & Qu, H. (2021). Novel Carbon Dots Derived from Glycyrrhizae Radix et Rhizoma and Their Anti-Gastric Ulcer Effect. Molecules, 26(6), 1512. https://doi.org/10.3390/molecules26061512






