Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of UPLC Q-Exactive Orbitrap MS
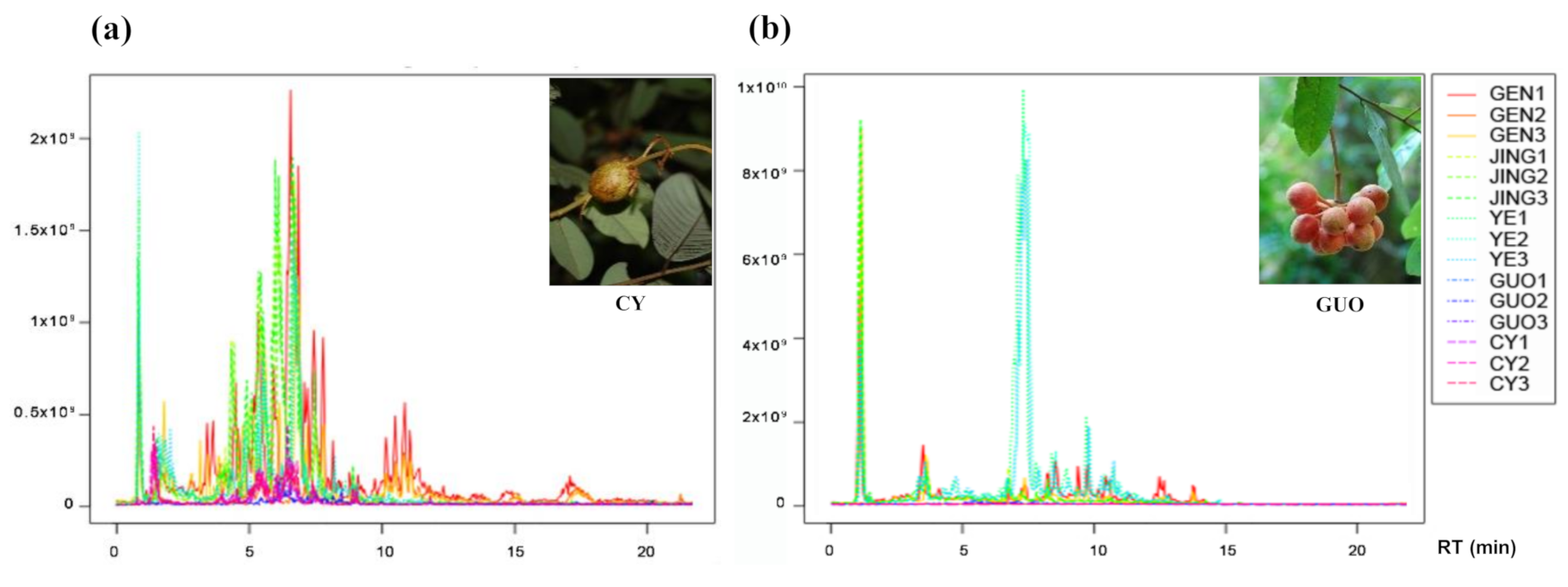
2.2. Total Ion Chromatogram Comparison and Qualitative Analysis of FO Parts
2.3. Different Components among FO Parts Screened by XCMS
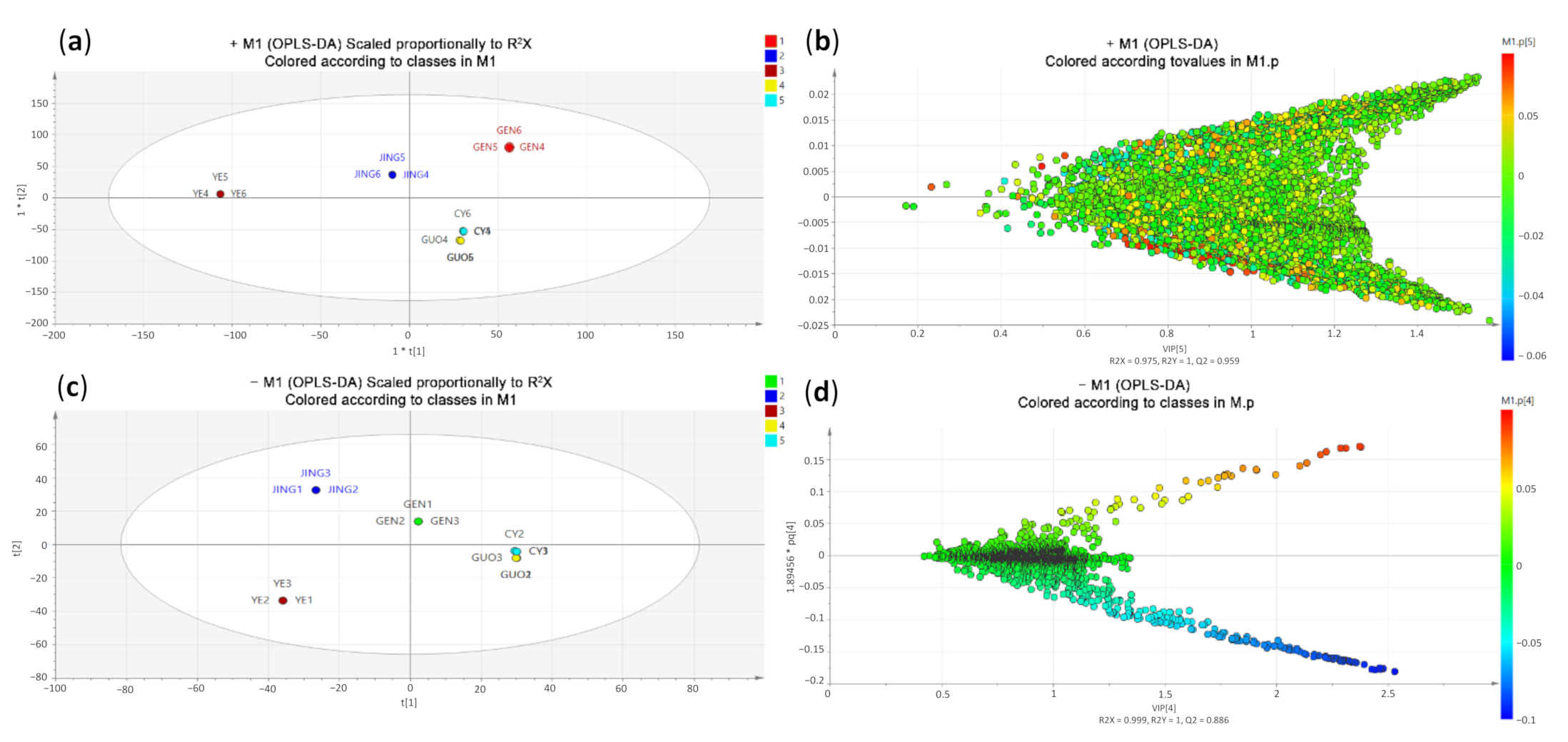
2.4. Multivariate Statistical Analysis and Comparison
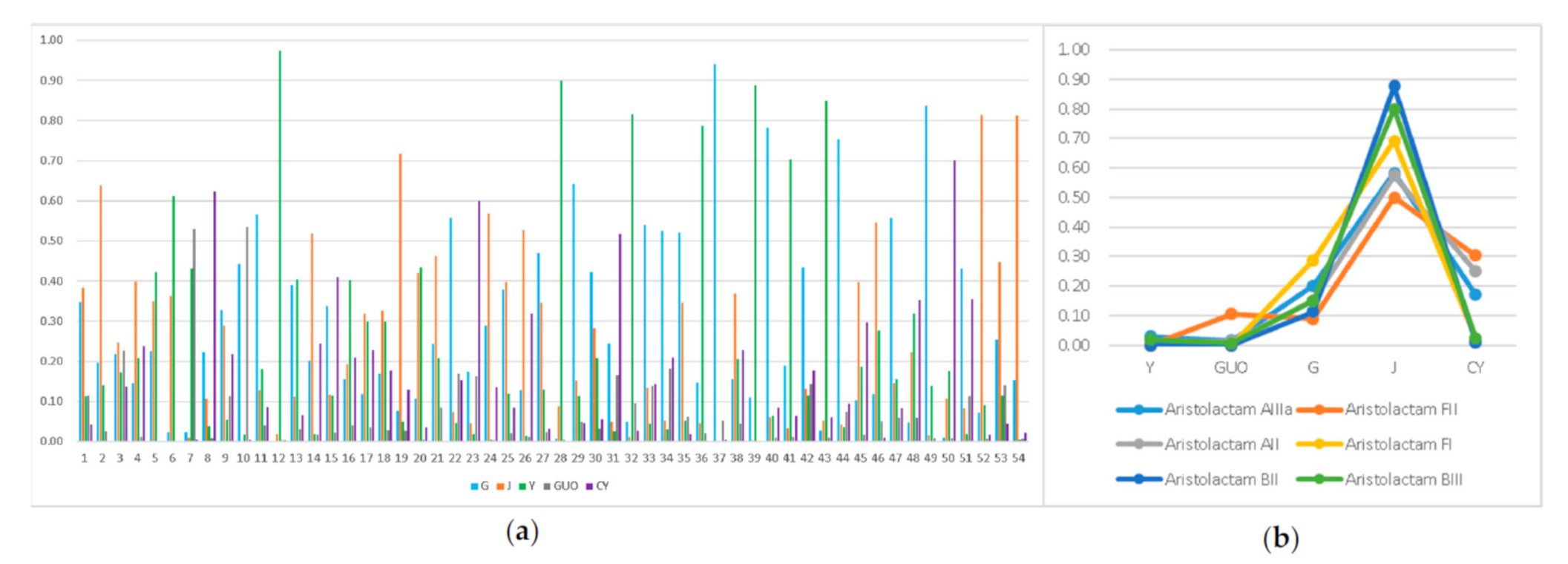
2.5. Semi-Quantitative Analysis
2.6. Aristolactams Differences
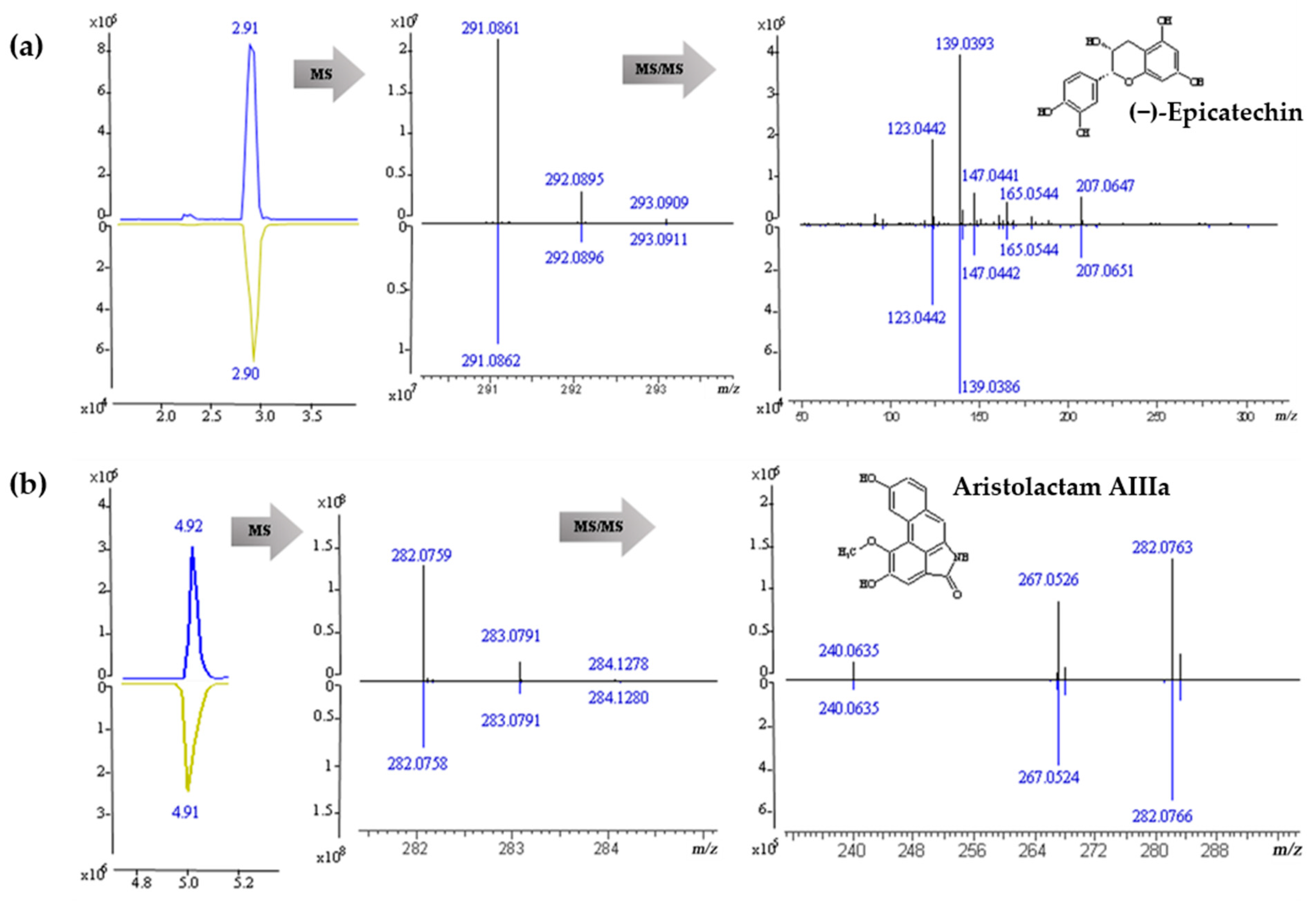
2.7. Identification and Component Pyrolysis Based on MS Fragmentater
2.8. Method Validation
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of Standard and Sample Solution
3.3. UHPLC and MS/MS Conditions
3.4. Data Processing and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chinese Flora Editorial Committee of Chinese Academy of Sciences. Flora of China (Chinese Version); Science Press: Beijing, China, 1979; Volume 30, pp. 162–163. [Google Scholar]
- Wu, Q. Zhi Wu Ming Shi Tu Kao; Zhonghua Book Company: Beijing, China, 1963; pp. 473–474. [Google Scholar]
- The Editorial Committee of Jiangxi Flora. Jiangxi Flora; China Science and Technology Press: Beijing, China, 2004; Volume 2, pp. 73–74. [Google Scholar]
- Chinese Materia Medica Editorial Committee of National Administration of TCM Chinese Materia Medica. Chinese Materia Medica; Shanghai science and Technology Press: Shanghai, China, 1999; Volume 3, pp. 495–502. [Google Scholar]
- Haibo, H.; Yang, Y.; Hao, H.; Longhuo, W.; Linfu, L.; Liping, Z. Study on pharmacognostic characteristics and microscopic characteristics of Xiangteng. LISHIZHEN Med. Mater. MEDICA Res. 2015, 26, 1653–1654. [Google Scholar]
- Minru, J.; Xingwei, L. Zhongguo Mingzu Yaozhi Yao; China Medical Science and Technology Press: Beijing, China, 2005; p. 278. [Google Scholar]
- Wei, X.C.; Cao, B.; Luo, C.H.; Huang, H.Z.; Tan, P.; Xu, X.R.; Xu, R.C.; Yang, M.; Zhang, Y.; Han, L.; et al. Recent advances of novel technologies for quality consistency assessment of natural herbal medicines and preparations. Chin. Med. Kingd. 2020, 15, 1–24. [Google Scholar] [CrossRef]
- Zhang, T.; Bai, G.; Han, Y.; Xu, J.; Gong, S.; Li, Y.; Zhang, H.; Liu, C. The method of quality marker research and quality evaluation of traditional Chinese medicine based on drug properties and effect characteristics. Phytomedicine 2018, 44, 204–211. [Google Scholar] [CrossRef]
- Shenghai, Z. Studies on the Chemical Constituents and Bioactivities of Fissistigma Oldhamii. Master’s Thesis, Degree-Hainan Normal University, Haikou, China, 2016. [Google Scholar]
- Sato, N.; Takahashi, D.; Tsuchiya, R.; Mukoyama, T.; Yamagata, S.; Satoh, N.; Ueda, S.; Chen, S.-M.; Ogawa, M.; Yoshida, M.; et al. Acute nephrotoxicity of aristolochic acids in mice. J. Pharm. Pharmacol. 2004, 56, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jelaković, B.; Karanović, S.; Vuković-Lela, I.; Miller, F.; Edwards, K.L.; Nikolić, J.; Tomić, K.; Slade, N.; Brdar, B.; Turesky, R.J.; et al. Aristolactam-DNA adducts are a biomarker of environmental exposure to aristolochic acid. Kidney Int. 2012, 81, 559–567. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Jiang, Z.; He, X.; Zhang, L.; Xu, M. Expression of Renal Aquaporins in Aristolochic Acid I and Aristolactam I-Induced Nephrotoxicity. Nephron 2016, 133, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Eiler, J.; Cesar, J.; Chimiak, L.; Dallas, B.; Grice, K.; Griep-Raming, J.; Juchelka, D.; Kitchen, N.; Lloyd, M.; Makarov, A.; et al. Analysis of molecular isotopic structures at high precision and accuracy by Orbitrap mass spectrometry. Int. J. Mass Spectrom. 2017, 422, 126–142. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Zhu, D.; Pang, X.; Liu, Y.; Frew, R.; Chen, G. Lipidomics profiling of goat milk, soymilk and bovine milk by UPLC-Q-Exactive Orbitrap Mass Spectrometry. Food Chem. 2017, 224, 302–309. [Google Scholar] [CrossRef]
- Sun, Z.; Zuo, L.; Sun, T.; Tang, J.; Ding, D.; Zhou, L.; Kang, J.; Zhang, X. Chemical profiling and quantification of XueBiJing injection, a systematic quality control strategy using UHPLC-Q Exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci. Rep. 2017, 7, 16921. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Gao, G.; Liu, P.; Pan, M.; Chai, Y.; Liu, X.; Lu, C. Development and validation of an ultra performance liquid chromatography Q-Exactive Orbitrap mass spectrometry for the determination of fipronil and its metabolites in tea and chrysanthemum. Food Chem. 2018, 246, 328–334. [Google Scholar] [CrossRef]
- Zheng, G.; Yang, X.; Chen, B.; Chao, Y.; Hu, P.; Cai, Y.; Wu, B.; Wei, M. Identification and determination of chemical constituents of Citrus reticulata semen through ultra high performance liquid chromatography combined with Q Exactive Orbitrap tandem mass spectrometry. J. Sep. Sci. 2020, 43, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.J.; Yang, J.; Brantner, A.H.; Lower-Nedza, A.D.; Si, N.; Song, J.F.; Bai, B.; Zhao, H.Y.; Bian, B.L. Chemical profiling and quantification of Chinese medicinal formula Huang-Lian-Jie-Du decoction, a systematic quality control strategy using ultra high performance liquid chromatography combined with hybrid quadrupole-orbitrap and triple quadrupole mass spectrometers. J. Chromatogr. A 2013, 1321, 88–99. [Google Scholar] [CrossRef]
- Liu, L.; Cui, Z.; Zhang, Y.; Xu, W.; Yang, X.; Zhong, L.; Zhang, P.; Gong, Y. Identification and quantification analysis of the chemical constituents from Mahonia fortune using Q-Exactive HF Mass Spectrometer and UPLC–ESI-MS/MS. J. Pharm. Biomed. Anal. 2021, 196, 113903. [Google Scholar] [CrossRef]
- Allen, F.; Greiner, R.; Wishart, D. Competitive fragmentation modeling of ESI-MS/MS spectra for putative metabolite identification. Metabolomics 2014, 11, 98–110. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Weissberg, A.; Dagan, S. Interpretation of ESI(+)-MS-MS spectra-towards the identification of “unknowns”. Int. J. Mass Spectrom. 2011, 299, 158–168. [Google Scholar] [CrossRef]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed]
- Pelander, A.; Tyrkkö, E.; Ojanperä, I. In silico methods for predicting metabolism and mass fragmentation applied to quetiapine in liquid chromatography/time-of-flight mass spectrometry urine drug screening. Rapid Commun. Mass Spectrom. 2009, 23, 506–514. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Choi, J.-H.; Abd El-Aty, A.M.; Chung, H.S.; Lee, H.S.; Kim, S.-W.; Rahman, M.M.; Park, B.-J.; Kim, J.-E.; Shin, H.-C.; et al. Development of a single-run analytical method for the detection of ten multiclass emerging contaminants in agricultural soil using an acetate-buffered QuEChERS method coupled with LC-MS/MS. J. Sep. Sci. 2017, 40, 415–423. [Google Scholar] [CrossRef]
- Flores, M.I.A.; Romero-González, R.; Frenich, A.g.; Vidal, J.L.M. Analysis of phenolic compounds in olive oil by solid-phase extraction and ultra high performance liquid chromatography-tandem mass spectrometry. Food Chem. 2012, 134, 2465–2472. [Google Scholar] [CrossRef]
- Shi, F.; Guo, C.; Gong, L.; Li, J.; Dong, P.; Zhang, J.; Cui, P.; Jiang, S.; Zhao, Y.; Zeng, S. Application of a high resolution benchtop quadrupole-Orbitrap mass spectrometry for the rapid screening, confirmation and quantification of illegal adulterated phosphodiesterase-5 inhibitors in herbal medicines and dietary supplements. J. Chromatogr. A 2014, 1344, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hogenboom, A.C.; van Leerdam, J.A.; de Voogt, P. Accurate mass screening and identification of emerging contaminants in environmental samples by liquid chromatography-hybrid linear ion trap Orbitrap mass spectrometry. J. Chromatogr. A 2009, 1216, 510–519. [Google Scholar] [CrossRef]
- Makarov, A.; Denisov, E.; Kholomeev, A.; Balschun, W.; Lange, O.; Strupat, K.; Horning, S. Performance evaluation of a hybrid linear ion trap/orbitrap mass spectrometer. Anal. Chem. 2006, 78, 2113–2120. [Google Scholar] [CrossRef]
- Wang, J.R.; Yau, L.F.; Gao, W.N.; Liu, Y.; Yick, P.W.; Liu, L.; Jiang, Z.H. Quantitative comparison and metabolite profiling of saponins in different parts of the root of panax notoginseng. J. Agric. Food Chem. 2014, 62, 9024–9034. [Google Scholar] [CrossRef]
- XCMSOnline. Available online: https://xcmsonline.scripps.edu (accessed on 10 February 2021).
- Forsberg, E.M.; Huan, T.; Rinehart, D.; Benton, H.P.; Warth, B.; Hilmers, B.; Siuzdak, G. Data processing, multi-omic pathway mapping, and metabolite activity analysis using XCMS Online. Nat. Protoc. 2018, 13, 633–651. [Google Scholar] [CrossRef]
- Boyce, M.C.; Lawler, N.G.; Tu, Y.; Reinke, S.N. Introducing Undergraduate Students to Metabolomics Using Liquid Chromatography-High Resolution Mass Spectrometry Analysis of Horse Blood. J. Chem. Educ. 2019, 96, 745–750. [Google Scholar] [CrossRef]
- McCully, M. How do real roots work? Some new views of root structure. Plant Physiol. 1995, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Eissenstat, D. Root structure and function in an ecological context. New Phytol. 2000, 148, 353–354. [Google Scholar] [CrossRef]
- Foley, J.W.; Zhu, C.; Jolivet, P.; Zhu, S.X.; Lu, P.; Meaney, M.J.; West, R.B. Gene expression profiling of single cells from archival tissue with laser-capture microdissection and Smart-3SEQ. Genome Res. 2019, 29, 1816–1825. [Google Scholar] [CrossRef]
- Huang, P.; Kong, Q.; Gao, W.; Chu, B.; Li, H.; Mao, Y.; Cai, Z.; Xu, R.; Tian, R. Spatial proteome profiling by immunohistochemistry-based laser capture microdissection and data-independent acquisition proteomics. Anal. Chim. Acta 2020, 1127, 140–148. [Google Scholar] [CrossRef]
- Parker, D.; Beckmann, M.; Zubair, H.; Enot, D.P.; Caracuel-Rios, Z.; Overy, D.P.; Snowdon, S.; Talbot, N.J.; Draper, J. Metabolomic analysis reveals a common pattern of metabolic re-programming during invasion of three host plant species by Magnaporthe grisea. Plant J. 2009, 59, 723–737. [Google Scholar] [CrossRef]
- Roessner, U.; Luedemann, A.; Brust, D.; Fiehn, O.; Linke, T.; Willmitzer, L.; Fernie, A.R. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 2001, 13, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, Y.; Guo, H.; Sang, F.; Ma, H.; Peng, H.; Zheng, N.; Xu, L. Quality control of the traditional Chinese medicine Ruyi jinhuang powder based on high-throughput sequencing and real-time PCR. Sci. Rep. 2018, 8, 8261. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, W.; Li, H. Application of metabolomics for unveiling the therapeutic role of traditional Chinese medicine in metabolic diseases. J. Ethnopharmacol. 2019, 242, 112057. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Zhang, P.; Dai, X.; Gao, Y.; Lv, Y.; Qin, S.; Xu, F. Integrated Metabolomics and Network Pharmacology Strategy-Driven Active Traditional Chinese Medicine Ingredients Discovery for the Alleviation of Cisplatin Nephrotoxicity. Chem. Res. Toxicol. 2019, 32, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Allard, P.M.; Wolfrum, C.; Ke, C.; Tang, C.; Ye, Y.; Wolfender, J.L. Identification of chemotypes in bitter melon by metabolomics: A plant with potential benefit for management of diabetes in traditional Chinese medicine. Metabolomics 2019, 15, 104. [Google Scholar] [CrossRef]
- Hassan, H.A.; Ammar, N.M.; Serag, A.; Shaker, O.G.; El Gendy, A.N.; Abdel-Hamid, A.H.Z. Metabolomics driven analysis of obesity-linked colorectal cancer patients via GC-MS and chemometrics: A pilot study. Microchem. J. 2020, 155, 104742. [Google Scholar] [CrossRef]
- Senizza, B.; Rocchetti, G.; Ghisoni, S.; Busconi, M.; De Los Mozos Pascual, M.; Fernandez, J.A.; Lucini, L.; Trevisan, M. Identification of phenolic markers for saffron authenticity and origin: An untargeted metabolomics approach. Food Res. Int. 2019, 126, 108584. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.M.; Zha, Q.L.; Chen, T.B.; Xiao, S.Y.; Xie, Y.; Luo, P.; Wang, Y.P.; Liu, L.; Zhou, H. Discovery of markers for discriminating the age of cultivated ginseng by using UHPLC-QTOF/MS coupled with OPLS-DA. Phytomedicine 2018, 45, 8–17. [Google Scholar] [CrossRef]
- Triba, M.N.; Le Moyec, L.; Amathieu, R.; Goossens, C.; Bouchemal, N.; Nahon, P.; Rutledge, D.N.; Savarin, P. PLS/OPLS models in metabolomics: The impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol. Biosyst. 2015, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Haibo, H.; Yaoli, L.; Jinsheng, C.; Jialin, L.; Qilai, C.; Jingyi1, D.; Hao, H. Comparative Research on Volatile Components in Different Parts of Fissistigma oldhamii Using HS-GC-MS and Multivariate Statistical Analysis. Modemization Tradit. Chin. Med. Mater. Med. World Sci. Technol. 2019, 21, 1724–1730. [Google Scholar]
- Pickard, W.F. Xylem Structure and the Ascent of Sap. Martin H. Zimmermann. Q. Rev. Biol. 1984, 59, 475–476. [Google Scholar] [CrossRef]
- Yang, D.L.; Mei, W.L.; Zeng, Y.B.; Guo, Z.K.; Wei, D.J.; Liu, S.B.; Wang, Q.H.; Dai, H.F. A new antibacterial denitroaristolochic acid from the tubers of Stephania succifera. J. Asian Nat. Prod. Res. 2013, 15, 315–318. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, Y.; Luo, H.; Zhang, M.; Qin, X.; Li, L. Antimicrobial activity of extract and two alkaloids from traditional Chinese medicinal plant Stephania dielsiana. Food Chem. 2011, 124, 1556–1560. [Google Scholar] [CrossRef]
- Vanherweghem, J.L. Misuse of herbal remedies: The case of an outbreak of terminal renal failure in Belgium (Chinese herbs nephropathy). J. Altern. Complement. Med. 1998, 4, 9–13. [Google Scholar] [CrossRef]
- Michl, J.; Ingrouille, M.J.; Simmonds, M.S.J.; Heinrich, M. Naturally occurring aristolochic acid analogues and their toxicities. Nat. Prod. Rep. 2014, 31, 676–693. [Google Scholar] [CrossRef]
- Nortier, J.L.; Martinez, M.-C.M.; Schmeiser, H.H.; Arlt, V.M.; Bieler, C.A.; Petein, M.; Depierreux, M.F.; De Pauw, L.; Abramowicz, D.; Vereerstraeten, P.; et al. Urothelial Carcinoma Associated with the Use of a Chinese Herb (Aristolochia fangchi ). N. Engl. J. Med. 2000, 342, 1686–1692. [Google Scholar] [CrossRef]
- Grollman, A.P.; Shibutani, S.; Moriya, M.; Miller, F.; Wu, L.; Moll, U.; Suzuki, N.; Fernandes, A.; Rosenquist, T.; Medverec, Z.; et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. USA 2007, 104, 12129–12134. [Google Scholar] [CrossRef] [PubMed]
- De Broe, M.E. On a nephrotoxic and carcinogenic slimming regimen. Am. J. Kidney Dis. 1999, 33, 1171–1173. [Google Scholar] [CrossRef]
- Stiborová, M.; Frei, E.; Breuer, A.; Bieler, C.A.; Schmeiser, H.H. Aristolactam I a metabolite of aristolochic acid I upon activation forms an adduct found in DNA of patients with chinese herbs nephropathy. Exp. Toxicol. Pathol. 1999, 51, 421–427. [Google Scholar] [CrossRef]
- Sidorenko, V.S.; Yeo, J.E.; Bonala, R.R.; Johnson, F.; Schärer, O.D.; Grollman, A.P. Lack of recognition by global-genome nucleotide excision repair accounts for the high mutagenicity and persistence of aristolactam-DNA adducts. Nucleic Acids Res. 2012, 40, 2494–2505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Zhong, X.G.; Zheng, Z.P.; Hu, X.D.; Zuo, J.P.; Hu, L.H. Discovery and synthesis of new immunosuppressive alkaloids from the stem of Fissistigma oldhamii (Hemsl.) Merr. Bioorg. Med. Chem. 2007, 15, 988–996. [Google Scholar] [CrossRef]
- Chia, Y.C.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Aristolactams and dioxoaporphines from Fissistigma balansae and Fissistigma oldhamii. J. Nat. Prod. 2000, 63, 1160–1163. [Google Scholar] [CrossRef]
- Ge, Y.W.; Zhu, S.; Shang, M.Y.; Zang, X.Y.; Wang, X.; Bai, Y.J.; Li, L.; Komatsu, K.; Cai, S.Q. Aristololactams and aporphines from the stems of Fissistigma oldhamii (Annonaceae). Phytochemistry 2013, 86, 201–207. [Google Scholar] [CrossRef]
- Zhou, J.; Weber, R.J.M.; Allwood, J.W.; Mistrik, R.; Zhu, Z.; Ji, Z.; Chen, S.; Dunn, W.B.; He, S.; Viant, M.R. HAMMER: Automated operation of mass frontier to construct in silico mass spectral fragmentation libraries. Bioinformatics 2014, 30, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, Z.; Rong, C.; Shao, Y.; Wang, Y.; Yu, K. Transformation of antibacterial agent roxithromycin in sodium hypochlorite disinfection process of different water matrices. Sep. Purif. Technol. 2019, 212, 528–535. [Google Scholar] [CrossRef]
- Changfu, X.; Cunheng, H.; Guanghong, B.; Shantian, M. Crystallography of Fissistigine C. Acta Acad. Med. Sin. 1985, 3, 187–190. [Google Scholar]

| No. | Name | RT | VIP | Formula | Ion Mode | Observed m/z | Calculated m/z | Diff. (ppm) | MS/MS Fragments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Glucosyringic Acid | 1.45 | 1.32 | C15H20O10 | [M − H]− | 359.0986 | 359.0984 | 0.64 | 198.0490, 197.0456, 182.0222, 153.0557, 138.0322, 89.0243 |
| 2 | Dehydrodeguelin | 1.86 | 2.00 | C23H20O6 | [M + H]+ | 393.1335 | 393.1333 | 0.56 | 393.1335, 377.1386, 351.0865, 175.0752 |
| 3 | Syringic acid | 2.51 | 3.07 | C9H10O5 | [M + H]+ | 199.0599 | 199.0601 | −1.00 | 171.0650,156.0416,140.0480,125.0223 |
| 4 | (−)-Epicatechin | 2.90 | 2.16 | C15H14O6 | [M + H]+ | 291.0861 | 291.0863 | −0.69 | 207.0647, 165.0544, 147.0441, 139.0393, 123.0442 |
| 5 | Stigmahamone I | 2.98 | 2.53 | C19H20O7 | [M − H]− | 359.1136 | 359.1136 | −0.11 | 313.0718, 299.0561, 297.0405, 282.0171, 166.9985 |
| 6 | Astilbin | 3.08 | 1.72 | C21H22O11 | [M − H]− | 449.1092 | 449.1089 | 0.64 | 449.1080, 343.0826, 303.0804, 299.0926, 285.0421, 151.0032 |
| 7 | Lactucin | 3.71 | 2.53 | C15H16O5 | [M + H]+ | 277.1063 | 277.1071 | −2.71 | 259.1221, 245.0456, 173.0611, 99.0087, 91.0572 |
| 8 | N-Caffeoyl-O-methyltyramine | 4.04 | 1.95 | C18H19NO4 | [M + H]+ | 314.1384 | 314.1387 | −0.95 | 297.1176, 265.0858, 237.0908, 233.0597,205.0646 |
| 9 | Corytuberine | 4.13 | 2.23 | C19H21NO4 | [M + H]+ | 328.1539 | 328.1543 | −1.22 | 297.1118, 282.0884, 265.0856, 237.0907 |
| 10 | 4,6-dimethoxy-2,5-quinodihydrochalcone | 4.26 | 2.08 | C17H16O5 | [M − H]− | 299.0930 | 299.0925 | 1.68 | 173.0607, 164.0107, 125.0243, 108.0209, 91.0541 |
| 11 | Haplotubinone | 4.32 | 1.24 | C19H23NO4 | [M + H]+ | 330.1695 | 330.1700 | −1.51 | 299.1274, 239.1066, 192.1018, 175.0752, 151.0754, 143.0492, 137.0597 |
| 12 | Afzelin | 4.91 | 2.43 | C21H20O10 | [M − H]− | 431.0984 | 431.0984 | 0.07 | 431.0984, 399.0730, 371.0767, 341.0667, 311.0562, 283.0614 |
| 13 | (−)-Epicatechin gallate | 4.93 | 1.68 | C22H18O10 | [M − H]− | 441.0827 | 441.0827 | −0.05 | 39.0930, 382.0699, 295.0615, 278.0071, 250.0122, 173.0609 |
| 14 | Aristolactam AIIIa | 4.94 | 3.41 | C16H11NO4 | [M + H]+ | 282.0759 | 282.0761 | −0.71 | 282.0763, 267.0526, 240.0635 |
| 15 | Coclaurine | 5.05 | 1.04 | C17H19NO3 | [M + H]+ | 286.1435 | 286.1438 | −1.05 | 267.0527, 239.0581, 211.0629,183.0682 |
| 16 | Isoboldine | 5.22 | 2.43 | C19H21NO4 | [M + H]+ | 328.1542 | 328.1543 | −0.30 | 328.1543, 329.1577, 311.1288, 297.1124, 265.0858,237.0910, 192.1023 |
| 17 | Naringin dihydrochalcone | 5.36 | 1.48 | C27H34O14 | [M − H]− | 581.1882 | 581.1876 | 1.07 | 301.0312, 300.0278, 274.0806, 273.0772, 167.0349, 125.0244 |
| 18 | Proanthocyanidin A2 | 5.17 | 2.89 | C30H24O12 | [M − H]− | 575.1202 | 575.1195 | 1.22 | 575.1204, 449.0883, 423.0727, 407.0773, 394.0696, 271.0253, 243.0302, 161.0245, 137.0245, 125.0245 |
| 19 | Thaipetaline | 5.75 | 2.67 | C20H23NO5 | [M + H]+ | 358.1643 | 358.1649 | −1.68 | 358.1647, 341.1625,311.1277, 299.1299, 192.1012, 74.0606, 60.0442 |
| 20 | Demethylmoracin I | 5.83 | 2.39 | C19H18O4 | [M + H]+ | 311.1283 | 311.1278 | 1.61 | 296.1047, 295.0962, 280.1093, 265.0857, 237.0909, 219.0796, 92.0014 |
| 21 | 2,5,6,7-Tetramethoxyflavan | 6.15 | 2.67 | C19H22O5 | [M + H]+ | 331.1541 | 331.1540 | 0.30 | 328.0938, 313.0702, 287.0554, 231.0654, 131.0491, 91.0538 |
| 22 | Calycinine | 6.24 | 1.76 | C18H17NO4 | [M + H]+ | 312.1229 | 312.1230 | −0.32 | 295.0963,294.1123, 0858, 265.0858, 237.0912, 236.4374 |
| 23 | Oxodiscoguattine | 6.31 | 2.13 | C19H13NO5 | [M + H]+ | 336.0856 | 336.0866 | −2.98 | 336.0862, 321.0627, 318.0764,263.0581, 246.0548, 178.0657 |
| 24 | noraristolodione | 6.43 | 1.18 | C17H11NO4 | [M − H]− | 292.0620 | 292.0615 | 1.61 | 278.0415, 277.0382, 250.0463, 249.0432, 221.0481, 140.4708, 91.9955 |
| 25 | Asimilobine | 6.69 | 2.38 | C17H17NO2 | [M + H]+ | 268.1329 | 268.1332 | −1.12 | 252.1100, 251.1065, 236.0830, 219.0803, 191.0855, 103.6783 |
| 26 | Aristolactam AII | 6.71 | 1.24 | C16H11NO3 | [M + H]+ | 266.0809 | 266.0812 | −1.13 | 251.0581, 249.0433, 238.0861, 223.0628, 221.0482, 195.0679 |
| 27 | Glaucine | 6.89 | 2.34 | C21H25NO4 | [M + H]+ | 356.1852 | 356.1856 | −1.12 | 325.1431, 310.1196, 295.1282, 294.1248, 251.1062 |
| 28 | Aloenin | 6.91 | 1.36 | C19H22O10 | [M − H]− | 409.1140 | 409.1140 | 0.00 | 229.0354, 214.0121, 170.9937, 142.9987, 111.0087, 83.0138 |
| 29 | N-Jasmonoyl-L-isoleucine | 7.12 | 2.12 | C18H29NO4 | [M + H]+ | 322.2025 | 322.2024 | 0.37 | 322.2026, 271.0607, 165.1286, 130.0874, 128.1082, 58.0299 |
| 30 | Fissistigmatin A | 7.31 | 2.36 | C33H42O5 | [M + H]+ | 519.3104 | 519.3105 | −0.19 | 519.3105, 501.2999, 487.2843, 469.2737 |
| 31 | Duguevanine | 7.31 | 2.28 | C20H21NO5 | [M + H]+ | 356.1491 | 356.1492 | −0.28 | 325.1068, 310.1197, 294.1249, 267.1013, 255.1013, 81.4017 |
| 32 | Licocoumarin A | 7.34 | 2.28 | C25H26O5 | [M + H]+ | 405.1708 | 405.1707 | 0.13 | 343.1705, 293.1143, 292.1108, 274.1003, 256.1107, 201.0058, 157.0657 |
| 33 | Globulixanthone A | 7.38 | 3.15 | C19H16O5 | [M + H]+ | 325.1063 | 325.1071 | −2.46 | 296.0995, 295.0963, 267.1016, 265.0856, 253.0858, 225.0907 |
| 34 | Clausamine F | 7.78 | 3.41 | C19H19NO4 | [M + H]+ | 326.1382 | 326.1387 | −1.53 | 310.1152, 309.1119, 294.0884, 279.1014, 265.0862, 251.1061, 248.0830 |
| 35 | Crebanine | 7.87 | 2.19 | C20H21NO4 | [M + H]+ | 340.1546 | 340.1543 | 0.88 | 309.1118, 308.1062, 294.0884, 279.1013, 239.1063, 236.0833 |
| 36 | Hippeastrine | 7.96 | 2.67 | C17H17NO5 | [M + H]+ | 314.1036 | 314.1034 | 0.65 | 299.0802, 284.0568, 267.0540, 255.0302, 208.0252, 179.9940 |
| 37 | Ammidin | 8.44 | 2.26 | C16H14O4 | [M − H]− | 269.0818 | 269.0819 | −0.37 | 269.0820, 254.0586, 226.0636, 171.0451, 165.0191, 122.0008 |
| 38 | Thunberginol C | 8.58 | 1.51 | C15H12O5 | [M + H]+ | 271.0614 | 271.0612 | 0.75 | 271.0615, 227.0716, 151.0037, 119.0502, 107.0138, 93.0345 |
| 39 | [3-3″]bi-2-hydroxy-4,5,6-trimethoxydihydrochalcone | 8.59 | 1.35 | C36H38O10 | [M + H]+ | 631.2534 | 631.2538 | −0.63 | 631.2538, 615.2589, 599.2639, 467.1700 |
| 40 | 5, 6, 7, 8-tetramethoxyflavone | 8.66 | 1.72 | C19H18O6 | [M + H]+ | 343.1174 | 343.1176 | −0.63 | 343.1172, 328.0938, 314.0737, 313.0702, 282.0884 |
| 41 | 1, 2-Dihydrotan-shinquinone | 8.68 | 3.42 | C18H14O3 | [M + H]+ | 279.1011 | 279.1016 | −1.79 | 279.1012, 249.0907, 221.0958, 206.0728, 178.0776 |
| 42 | Oxocrebanine | 8.69 | 3.51 | C19H13NO5 | [M + H]+ | 336.0865 | 336.0866 | −0.30 | 336.0864, 321.0629, 319.0793, 306.0406, 278.0453, 250.0503 |
| 43 * | CAS 1391982-39-0 | 8.71 | 1.69 | C17H18O5 | [M + H]+ | 303.1221 | 303.1227 | −1.98 | 285.1126, 253.0861, 225.0912, 169.0496, 105.0700, 91.0543 |
| 44 | Anolobine | 8.87 | 2.22 | C17H15NO3 | [M + H]+ | 282.1121 | 282.1125 | −1.42 | 265.0855, 235.0750, 209.0957, 207.0802, 121.2282 |
| 45 | Procyanidin B | 9.148 | 2.90 | C30H26O12 | [M + H]+ | 577.1347 | 577.1351 | −0.78 | 407.0771, 289.0719, 245.0819, 161.0243, 137.0243, 125.0243 |
| 46 | Isolaureline | 9.51 | 3.65 | C19H19NO3 | [M + H]+ | 310.1437 | 310.1438 | −0.32 | 310.1432, 280.1047, 279.1014, 249.0908, 221.0957 |
| 47 | Xylopine | 9.56 | 1.51 | C18H17NO3 | [M + H]+ | 296.1278 | 296.1281 | −1.01 | 280.1041, 279.1014, 249.0912, 240.0747, 102.7522, 95.9003 |
| 48 | Fissilandione | 9.6 | 3.14 | C19H15NO5 | [M + H]+ | 338.1019 | 338.1023 | −1.18 | 338.1020, 308.0918, 280.1045, 279.1014, 249.0908, 221.0961, 178.0770, 92.0010 |
| 49 | Daphmanidin E | 10.65 | 2.11 | C25H31NO5 | [M + H]+ | 426.2273 | 426.2275 | −0.47 | 426.2274, 410.2325, 408.2167, 392.2223 |
| 50 | Fissistigmatin C | 11.34 | 1.38 | C33H40O4 | [M + H]+ | 501.2996 | 501.2999 | −0.60 | 501.2996, 473.2684, 469.2732, 365.1751 |
| 51 | Atherospermidine | 12.52 | 2.04 | C18H11NO4 | [M + H]+ | 306.0753 | 306.0761 | −2.61 | 306.0758, 307.0791, 291.0523, 278.0815, 263.0575,76.3926 |
| 52 | Fissistigine C | 14.42 | 1.24 | C20H23NO4 | [M + H]+ | 342.1695 | 342.1700 | −1.46 | 342.1697, 311.1278, 296.1043,285.1120, 280.1092, 191.0940, 162.0915, 58.0658 |
| 53 | Byakangelicin | 15.93 | 2.42 | C17H18O7 | [M − H]− | 333.0983 | 333.0980 | 0.97 | 289.1085, 258.0854, 257.0821, 205.0871, 173.0607, 125.0243, 101.0243, 92.9981 |
| 54 | Aristolactam BIII | 17.07 | 2.10 | C18H15NO4 | [M + H]+ | 310.1075 | 310.1074 | 0.32 | 310.1072, 295.0838, 280.0603, 277.0732, 248.0702, 98.5990 |
| No. | Name | RT | Formula | Ion Species | Observed m/z | Calculated m/z | Diff. (ppm) | MS/MS Fragments | GEN | JING | YE | GUO | CY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 55 | Artabotryside A | 4.35 | C26H28O15 | [M − H]− | 579.1359 | 579.1355 | 0.62 | 579.1361, 301.0314, 300.0277, 271.0250, 255.0301, 178.9986, 151.0035 | - | ✓ | ✓ | ✓ | ✓ |
| 56 | Apiin | 4.76 | C26H28O14 | [M − H]− | 563.1410 | 563.1406 | 0.66 | 563.1411, 503.1199, 473.1091, 443.0988, 383.0775, 354.0703, 353.0669 | - | ✓ | ✓ | ✓ | ✓ |
| 57 | Nicotiflorin | 4.90 | C27H30O15 | [M − H]− | 593.1511 | 593.1512 | −0.16 | 593.1514, 503.1198, 473.1090, 383.0773, 353.0667 | - | ✓ | ✓ | ✓ | ✓ |
| 58 | Eupatolin | 4.94 | C23H24O12 | [M − H]− | 491.1198 | 491.1195 | 0.61 | 491.1200, 330.0703, 329.0656, 328.0590, 314.0424, 313.0357,282.0535 | - | ✓ | ✓ | ✓ | ✓ |
| 59 | Aristolactam FII | 5.59 | C17H13NO4 | [M − H]− | 294.0776 | 294.0772 | 1.42 | 294.0779, 280.0572, 279.0539, 265.0338, 264.0304 | ✓ | ✓ | - | ✓ | ✓ |
| 60 | Quercetin-3-O-rhamn-oside | 6.33 | C21H20O11 | [M − H]− | 447.0937 | 447.0933 | 0.93 | 447.0934, 358.0652, 357.0618, 328.0547, 327.0513, 299.0562, 285.0408 | - | ✓ | ✓ | ✓ | ✓ |
| 61 | Aristolactam FI | 6.80 | C16H11NO3 | [M + H]+ | 266.0810 | 266.0812 | −0.64 | 266.0807, 251.0582, 249.0909, 219.0803, 191.0857,84.4612 | ✓ | ✓ | - | - | ✓ |
| 62 | Cnidimol B | 7.04 | C15H16O6 | [M − H]− | 291.0878 | 291.0874 | 1.33 | 219.1027, 188.0798, 187.0765, 172.0530, 155.0503, 145.0659, 83.0139 | - | - | ✓ | ✓ | - |
| 63 | Isoquercitrin | 7.33 | C21H20O12 | [M − H]− | 463.0888 | 463.0882 | 1.30 | 463.0886, 301.0341, 300.0277, 271.0251, 255.1028, 178.9986, 151.0036 | - | - | ✓ | - | - |
| 64 | Stigmahamone II | 7.45 | C18H18O7 | [M − H]− | 345.0982 | 345.0980 | 0.58 | 330.0747, 315.0513, 298.0483, 283.0249, 255.0300, 239.0198, 200.0717 | ✓ | - | - | ✓ | - |
| 65 | Claussequinone | 7.81 | C16H14O5 | [M + H]+ | 287.0908 | 287.0914 | −2.09 | 287.0910, 271.0966, 257.0819, 183.0291,149.0591, 131.0492, 91.0542 | ✓ | - | ✓ | ✓ | - |
| 66 | Oxoglaucine | 8.40 | C20H17NO5 | [M − H]− | 350.1034 | 350.1034 | 0.01 | 335.0801,307.0852, 279.0902, 231.0173, 231.0173, 203.0226, 175.0274 | ✓ | ✓ | ✓ | - | ✓ |
| 67 | Methyl 3-(2-oxo- 2-prop-2-enoxyethyl)-1-benzofuran-2-carboxylate | 8.64 | C15H14O5 | [M + H]+ | 273.0772 | 273.0768 | 1.29 | 259.0568, 258.0534, 168.0018, 166.9985, 139.0037 | ✓ | - | ✓ | ✓ | - |
| 68 | Morusin | 8.73 | C25H24O6 | [M − H]− | 419.1501 | 419.1500 | 0.24 | 386.1161, 276.1635, 375.1602, 357.1498, 270.0855, 269.0821 | - | - | ✓ | ✓ | - |
| 69 | Kwangsienin A | 8.77 | C18H18O6 | [M + H]+ | 331.1168 | 331.1176 | −2.42 | 230.0416, 228.0580, 227.0546, 212.0312, 197.0079, 169.0142, 139.0389, 85.0285 | ✓ | ✓ | - | ✓ | ✓ |
| 70 | Isopedicin | 9.44 | C18H18O6 | [M + H]+ | 331.1172 | 331.1176 | −1.25 | 316.0949, 227.0557, 212.0320, 197.0084, 169.0136, 113.0233, 85.0280 | ✓ | ✓ | - | ✓ | ✓ |
| 71 | Kanakugiol | 9.52 | C19H20O6 | [M + H]+ | 345.1330 | 345.1333 | −0.77 | 242.0736, 241.0702, 226.0468, 211.0235, 183.0287, 131.0490 | ✓ | - | - | ✓ | - |
| 72 | Rottlerin | 9.91 | C30H28O8 | [M + H]+ | 517.1853 | 517.1857 | −0.77 | 499.1742, 485.1605, 468.2096, 385.1286, 231.1024, 184.0736, 105.0700, 91.0541 | ✓ | - | ✓ | ✓ | ✓ |
| 73 | Nomilin | 9.96 | C28H34O9 | [M − H]− | 513.2126 | 513.2130 | −0.79 | 481.1867, 453.1920, 307.1704, 289.0717, 178.9985, 151.0399,123.0450 | ✓ | - | - | ✓ | - |
| 74 | Proceranolide | 10.59 | C27H34O7 | [M + H]+ | 471.2376 | 471.2377 | −0.21 | 403.1756, 353.1379, 297.0758, 261.1484, 233.0807, 221.0807, 201.0544, 193.0858 | - | - | ✓ | ✓ | ✓ |
| 75 | Aristolactam BII | 12.35 | C17H13NO3 | [M + H]+ | 280.0965 | 280.0968 | −1.14 | 280.0965, 265.0732, 264.0652, 240.0629, 236.0703, 149.0234 | ✓ | ✓ | - | - | ✓ |
| 76 | Fissohamione | 12.72 | C16H18O5 | [M + H]+ | 291.1226 | 291.1227 | −0.34 | 273.1904, 141.0574, 105.0721 | - | - | ✓ | ✓ | - |
| 77 | Norfissilandione | 17.78 | C18H13NO5 | [M + H]+ | 324.0866 | 324.0866 | −0.15 | 324.0864, 312.1001, 309.1121, 295.0960, 266.0810 | ✓ | - | - | - | - |
| 78 | Norcepharadione B | 19.83 | C18H13NO4 | [M + H]+ | 308.0910 | 308.0917 | −2.27 | 308.0911, 293.0678, 279.0839, 250.0856, 156.3622 | ✓ | - | - | - | - |
| 79 | Gedunin | 19.88 | C28H34O7 | [M − H]− | 481.2235 | 481.2232 | 0.67 | 449.1976, 434.1734, 419.1509, 401.1396, 391.1556, 373.1449, 198.0172, 182.9936, 152.9830 | ✓ | - | - | - | - |
| Standards | Precision (RSD, n = 6) | Repeatability | Stability | Regression Equation (n = 3) | Linear Range * | R2 | LOQ * | LOD * | Added (ng) | Detected (ng) | Recovery (%) | RSD (%) n = 3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra-day | Inter-day | (RSD, n = 6) | |||||||||||
| Syringic acid | 2.2 | 4.6 | 3.8 | 2.8 | y = 0.688x + 0.0147 | 0.03–4 | 0.9994 | 3.2 | 1 | 100 | 99.5 ± 0.5 | 99.5 | 0.50 |
| (−)-Epicatechin | 3.2 | 4.4 | 2.3 | 3.3 | y = 0.10x − 0.0022 | 0.03–1 | 0.9997 | 2.2 | 0.5 | 50 | 51.75 ± 0.72 | 103.5 | 1.39 |
| Crebanine | 2.9 | 3.1 | 3.3 | 3.2 | y = 0.773x − 0.010 | 0.06–4 | 0.9999 | 0.7 | 0.2 | 25 | 25.17 ± 0.90 | 100.68 | 3.58 |
| Corytuberine | 3.8 | 4.3 | 2.9 | 4.6 | y = 0.318x + 0.004 | 0.06–1 | 0.9991 | 1.3 | 0.4 | 50 | 50.9 ± 0.68 | 101.8 | 1.34 |
| Isopedicin | 2.7 | 4.5 | 3.2 | 2.6 | y = 0.500x − 0.083 | 0.03–4 | 0.9999 | 1.5 | 0.4 | 50 | 52.0 ± 0.91 | 104 | 1.75 |
| Aristolactam BII | 1.1 | 4 | 2.8 | 2.8 | y = 0.172x − 0.007 | 0.125–4 | 0.9988 | 2.2 | 0.5 | 100 | 100.2 ± 0.93 | 100.2 | 0.93 |
| Aristolactam FI | 2.3 | 1.8 | 3.2 | 3.2 | y = 0.720x − 0.026 | 0.125–1 | 0.9991 | 0.8 | 0.2 | 50 | 50.6 ± 0.54 | 101.2 | 1.07 |
| Aristolactam AIIIa | 4.8 | 4.9 | 4.5 | 4.8 | y = 0.793x − 0.029 | 0.06–4 | 0.9992 | 0.1 | 0.03 | 50 | 49.9 ± 0.38 | 99.8 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Lee-Fong, Y.; Peng, J.; Hu, B.; Li, J.; Li, Y.; Huang, H. Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry. Molecules 2021, 26, 960. https://doi.org/10.3390/molecules26040960
Hu H, Lee-Fong Y, Peng J, Hu B, Li J, Li Y, Huang H. Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry. Molecules. 2021; 26(4):960. https://doi.org/10.3390/molecules26040960
Chicago/Turabian StyleHu, Haibo, Yau Lee-Fong, Jinnian Peng, Bin Hu, Jialin Li, Yaoli Li, and Hao Huang. 2021. "Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry" Molecules 26, no. 4: 960. https://doi.org/10.3390/molecules26040960
APA StyleHu, H., Lee-Fong, Y., Peng, J., Hu, B., Li, J., Li, Y., & Huang, H. (2021). Comparative Research of Chemical Profiling in Different Parts of Fissistigma oldhamii by Ultra-High-Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap Mass Spectrometry. Molecules, 26(4), 960. https://doi.org/10.3390/molecules26040960






