The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples from Different Botanical, Entomological, and Geographical Origins
2.2. UV Spectra Data Acquisition
2.3. Chemometrics Analysis
3. Results and Discussion
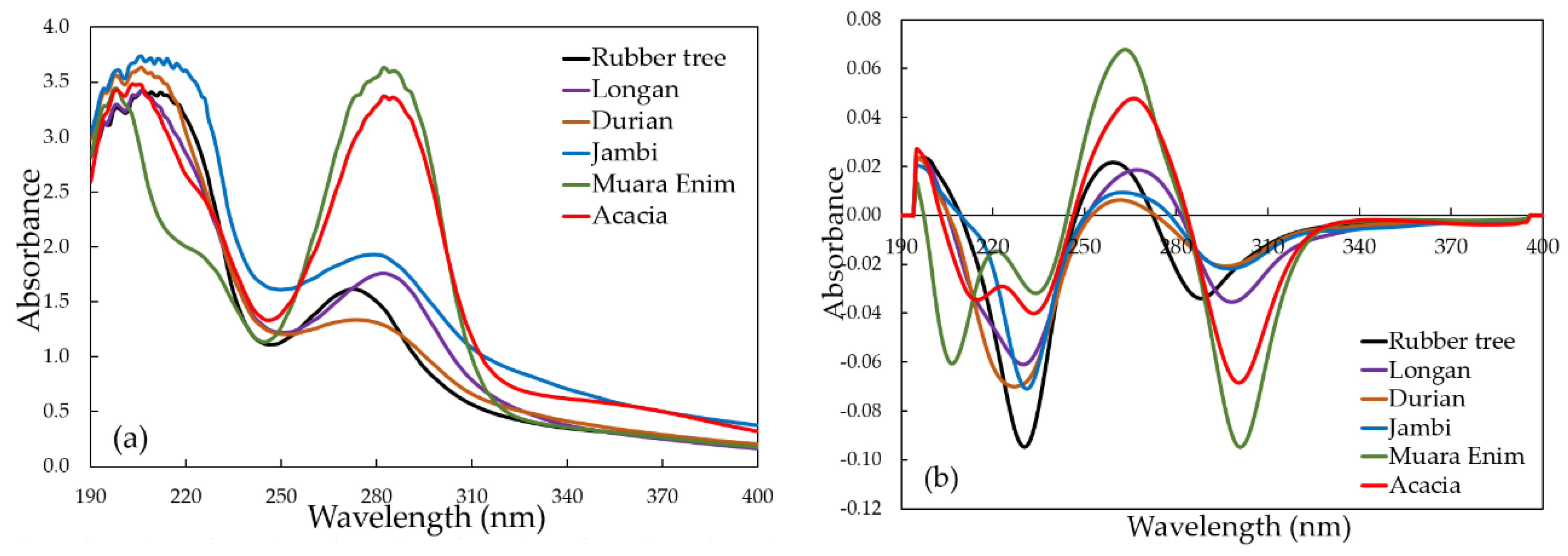
3.1. Analysis of UV Spectra
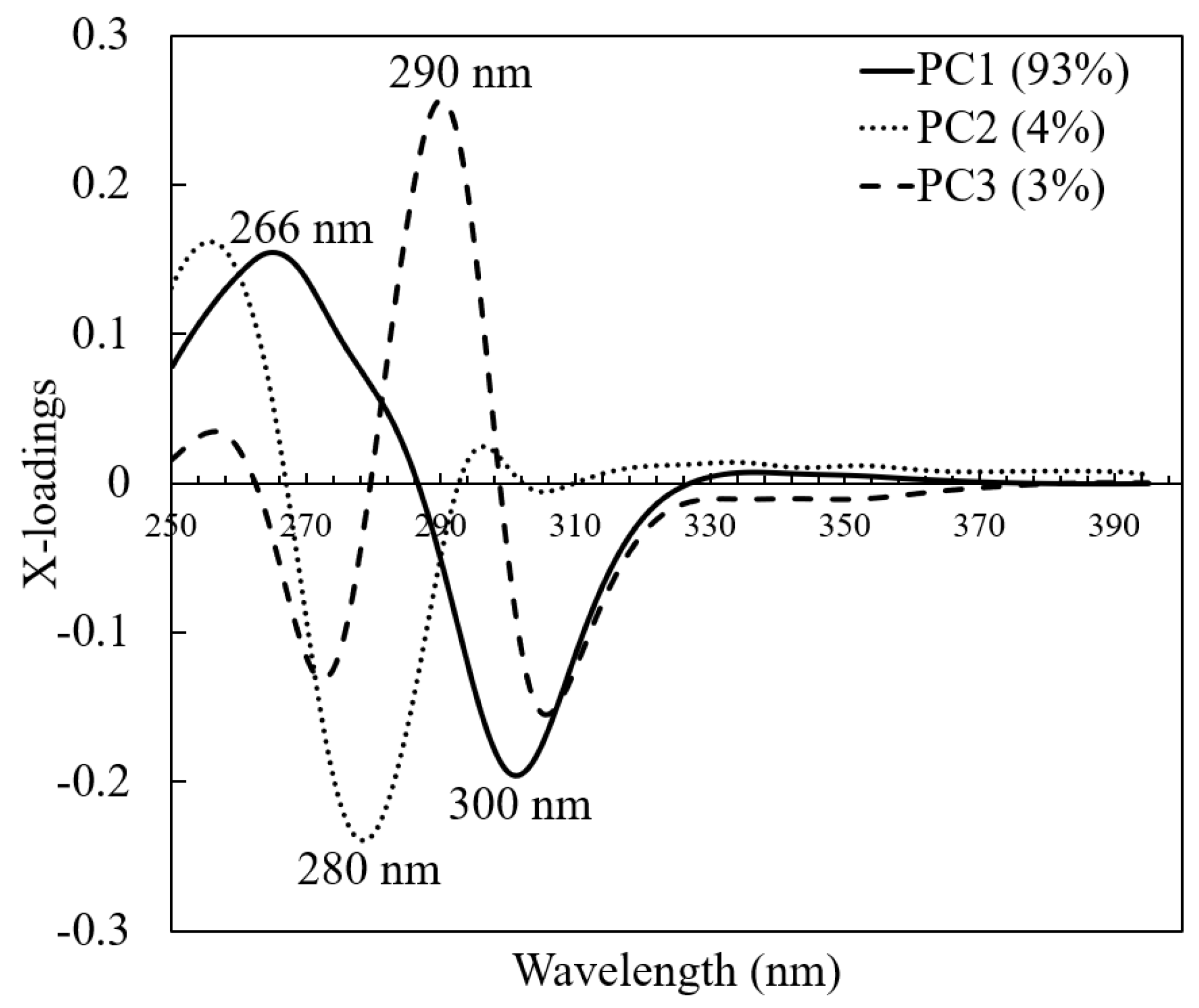
3.2. PCA Analysis
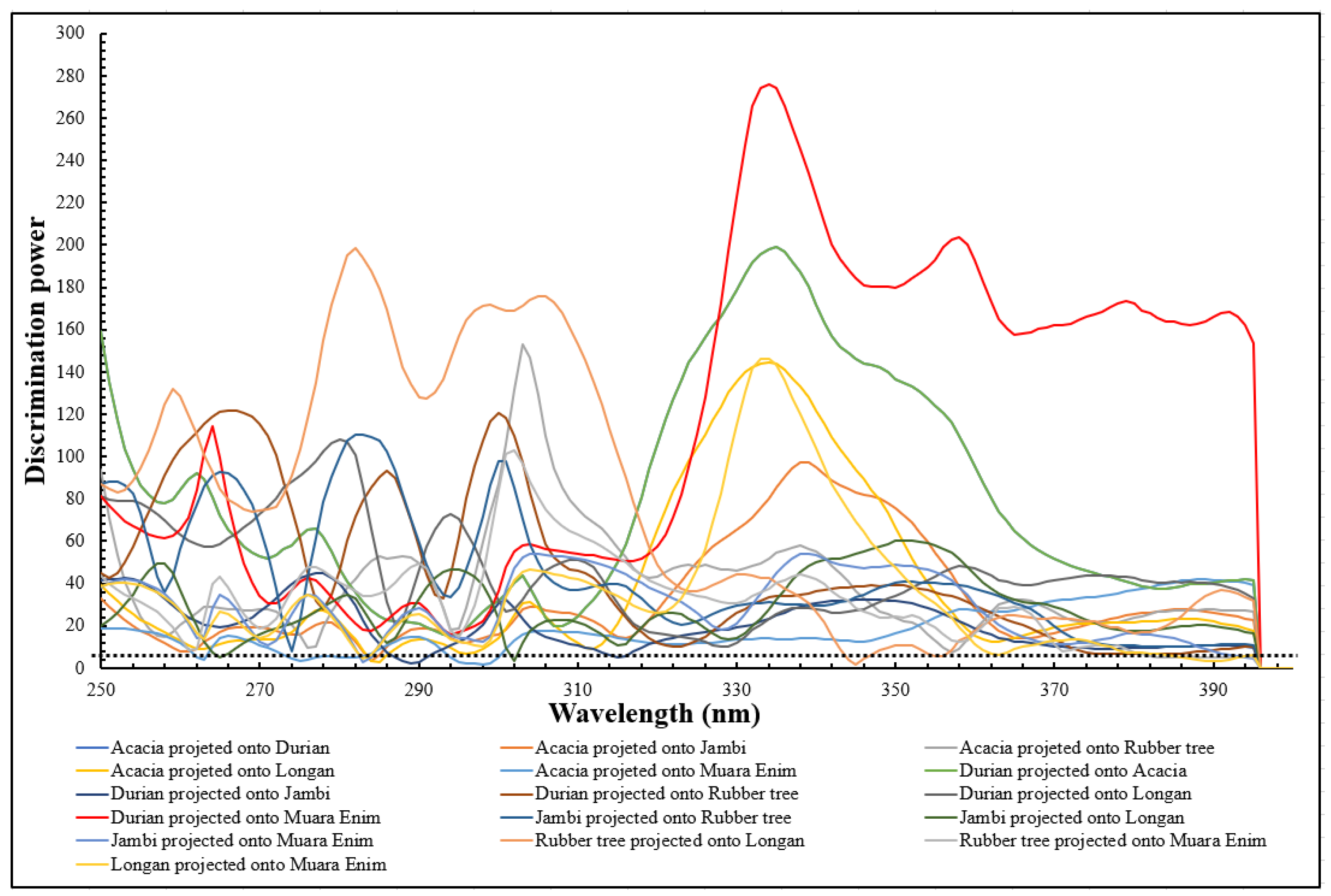
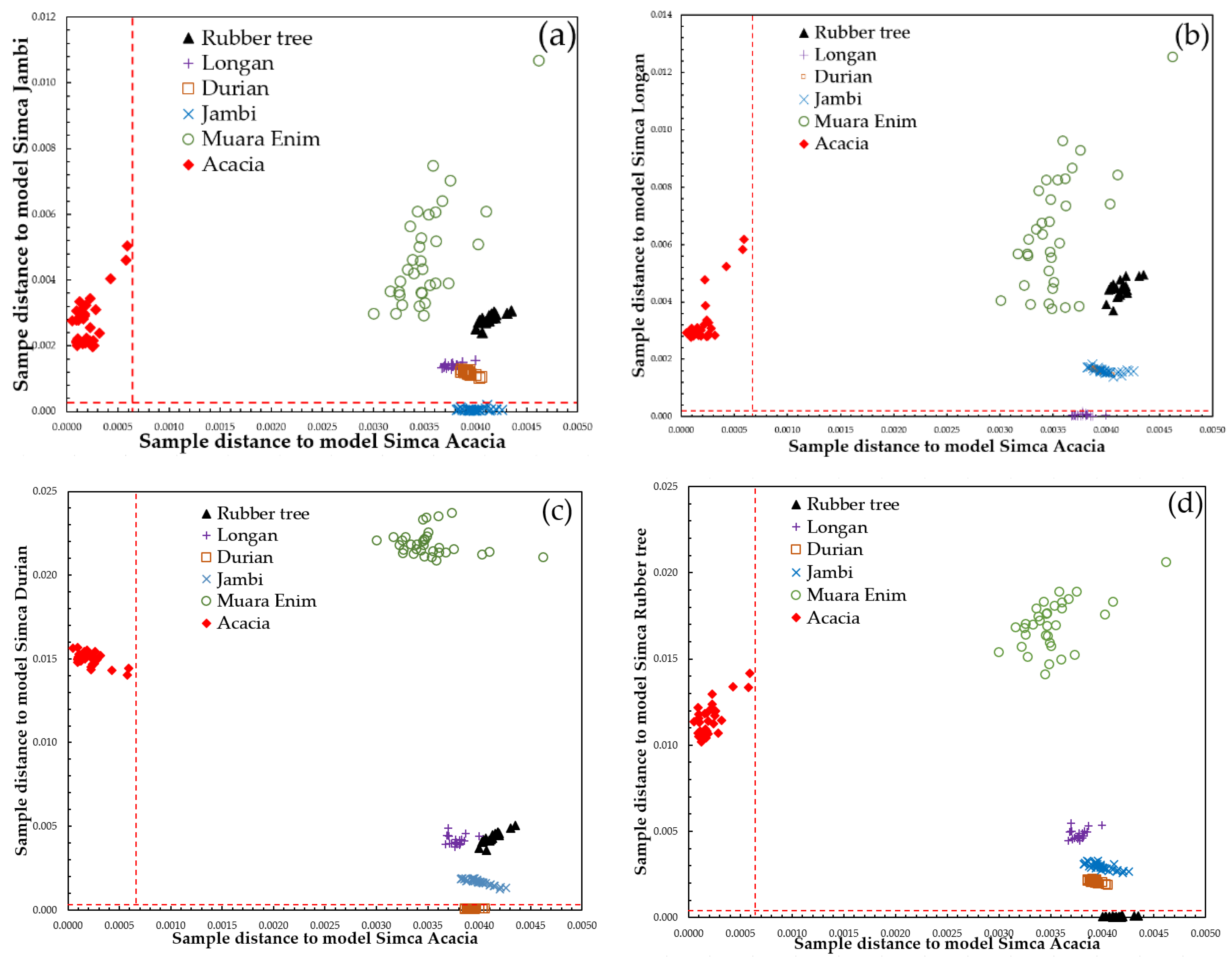
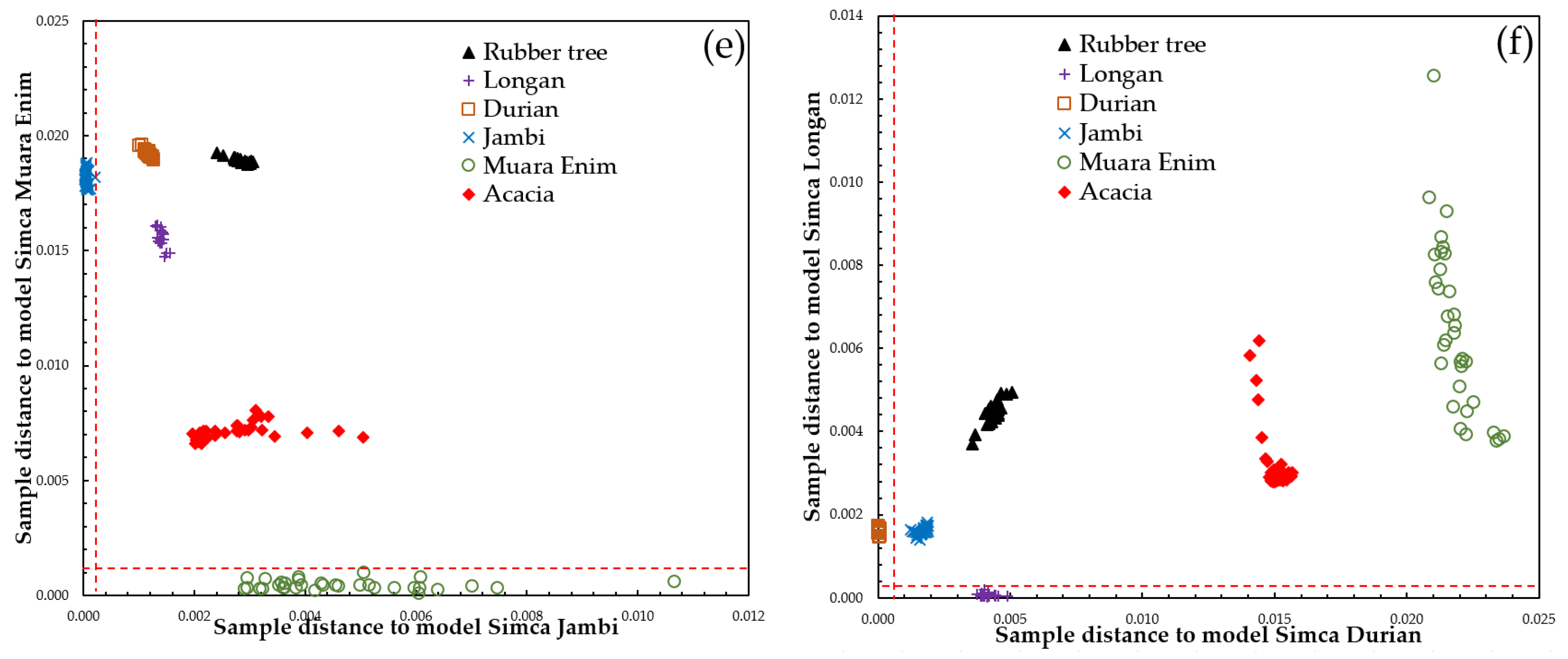
3.3. Supervised Classification of SIMCA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Codex Alimentarius Commission. Revised Codex Standard for Honey, Codex Stan 12-1981, Rev. 1 (1987), Rev. 2 (2001); Food and Agriculture Organization (FAO): Rome, Italy, 2001; pp. 1–7. [Google Scholar]
- Puścion-Jakubik, A.; Borawska, M.H.; Socha, K. Modern Methods for Assessing the Quality of Bee Honey and Botanical Origin Identification. Foods 2020, 9, 1028. [Google Scholar] [CrossRef]
- Pita-Calvo, C.; Guerra-Rodríguez, M.E.; Vázquez, M. Analytical Methods Used in the Quality Control of Honey. J. Agric. Food Chem. 2017, 65, 690–703. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant Activity, Total Phenolic Content, Individual Phenolics and Physicochemical Parameters Suitability for Romanian Honey Authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M.C. Botanical Origin, Pollen Profile, and Physicochemical Properties of Algerian Honey from Different Bioclimatic Areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Ansari, M.J.; Ghamdi, A.A.; Khan, K.A.; Adgaba, N.; El-Ahmady, S.H.; Gad, H.A.; Roshan, A.; Meo, S.A.; Kolyali, S. Validation of botanical origins and geographical sources of some Saudi honeys using ultraviolet spectroscopy and chemometric analysis. Saudi J. Biol. Sci. 2017, 25, 377–382. [Google Scholar] [CrossRef]
- Valand, R.; Tanna, S.; Lawson, G.; Bengtström, L. A review of Fourier Transform Infrared (FTIR) spectroscopy used in food adulteration and authenticity investigations. Food Addit. Contam. Part A 2019, 37, 19–38. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T.; Costinel, D.; Ionete, R.E. Evaluation of honey in terms of quality and authenticity based on the general physicochemical pattern, major sugar composition and δ13C signature. Food Control 2020, 109, 106919. [Google Scholar] [CrossRef]
- Anklam, E. A review of the analytical methods to determine the geographical and botanical origin of honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Karabagias, I.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced in Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2019, 245, 23–39. [Google Scholar] [CrossRef]
- Maione, C.; Barbosa, F.; Barbosa, R.M. Predicting the botanical and geographical origin of honey with multivariate data analysis and machine learning techniques: A review. Comput. Electron. Agric. 2019, 157, 436–446. [Google Scholar] [CrossRef]
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR Profiling of North Macedonian and Bulgarian Honeys for Detection of Botanical and Geographical Origin. Molecules 2020, 25, 4687. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Oertel, E.; Serra, R.; Farre, R.; Spano, N.; Caredda, M.; Malfatti, L.; Sanna, G. Classification of Unifloral Honeys from SARDINIA (Italy) by ATR-FTIR Spectroscopy and Random Forest. Molecules 2020, 26, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Rogers, K.; Li, Y.; Yang, S.; Chen, L.; Zhou, J. Untargeted and Targeted Discrimination of Honey Collected by Apis cerana and Apis mellifera Based on Volatiles Using HS-GC-IMS and HS-SPME-GC–MS. J. Agric. Food Chem. 2019, 67, 12144–12152. [Google Scholar] [CrossRef] [PubMed]
- Zuccato, V.; Finotello, C.; Menegazzo, I.; Peccolo, G.; Schievano, E. Entomological authentication of stingless bee honey by 1H NMR-based metabolomics approach. Food Control 2017, 82, 145–153. [Google Scholar] [CrossRef]
- Karabagias, I. Advances of Spectrometric Techniques in Food Analysis and Food Authentication Implemented with Chemometrics. Foods 2020, 9, 1550. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Se, K.W.; Wahab, R.A.; Yaacob, S.N.S.; Ghoshal, S. Detection techniques for adulterants in honey: Challenges and recent trends. J. Food Compos. Anal. 2019, 80, 16–32. [Google Scholar] [CrossRef]
- Chin, N.L.; Sowndhararajan, K. A Review on Analytical Methods for Honey Classification, Identification and Authentication. IntechOpen 2020. [Google Scholar] [CrossRef]
- Scholz, M.B.D.S.; Júnior, A.Q.; Delamuta, B.H.; Nakamura, J.M.; Baudraz, M.C.; Reis, M.O.; Kato, T.; Pedrão, M.R.; Dias, L.F.; Dos Santos, D.T.R.; et al. Indication of the geographical origin of honey using its physicochemical characteristics and multivariate analysis. J. Food Sci. Technol. 2020, 57, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- El-Sofany, A.; Al Naggar, Y.; Naiem, E.; Giesy, J.P.; Seif, A. Authentication of the botanical and geographic origin of Egyptian honey using pollen analysis methods. J. Apic. Res. 2020, 59, 946–955. [Google Scholar] [CrossRef]
- Sobrino-Gregorio, L.; Vilanova, S.; Prohens, J.; Escriche, I. Detection of honey adulteration by conventional and real-time PCR. Food Control 2019, 95, 57–62. [Google Scholar] [CrossRef]
- Souto, U.T.C.P.; Pontes, M.J.C.; Silva, E.C.; Galvão, R.K.H.; De Araujo, M.C.U.; Sanches, F.A.C.; Cunha, F.; Oliveira, M.S.R. UV–Vis spectrometric classification of coffees by SPA–LDA. Food Chem. 2010, 119, 368–371. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Ahmady, S.H.; Abou-Shoer, M.I.; Al-Azizi, M.M. A Modern Approach to the Authentication and Quality Assessment of Thyme Using UV Spectroscopy and Chemometric Analysis. Phytochem. Anal. 2013, 24, 520–526. [Google Scholar] [CrossRef]
- Roshan, A.-R.A.; Gad, H.A.; El-Ahmady, S.H.; Khanbash, M.S.; Abou-Shoer, M.I.; Al-Azizi, M.M. Authentication of Monofloral Yemeni Sidr Honey Using Ultraviolet Spectroscopy and Chemometric Analysis. J. Agric. Food Chem. 2013, 61, 7722–7729. [Google Scholar] [CrossRef] [PubMed]
- Suhandy, D.; Yulia, M. Peaberry coffee discrimination using UV-visible spectroscopy combined with SIMCA and PLS-DA. Int. J. Food Prop. 2017, 20, S331–S339. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. The Use of Partial Least Square Regression and Spectral Data in UV-Visible Region for Quantification of Adulteration in Indonesian Palm Civet Coffee. Int. J. Food Sci. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Yulia, M.; Suhandy, D. Indonesian palm civet coffee discrimination using UV-visible spectroscopy and several chemometrics methods. J. Phys. Conf. Ser. 2017, 835, 012010. [Google Scholar] [CrossRef]
- Suhandy, D.; Yulia, M. The development of classification model of peaberry and civet ground roasted coffee using UV-visible spectroscopy and two different compensation methods. J. Phys. Conf. Ser. 2019, 1341, 022007. [Google Scholar] [CrossRef]
- Yulia, M.; Suhandy, D. Authentication of organic Lampung robusta ground roasted coffee by UV-visible spectroscopy and PLS-DA method. J. Phys. Conf. Ser. 2019, 1341, 022006. [Google Scholar] [CrossRef]
- Frausto-Reyes, C.; Casillas-Peñuelas, R.; Quintanar-Stephano, J.L.; Macías-López, E.; Bujdud-Pérez, J.M.; Medina-Ramírez, I. Spectroscopic study of honey from Apis mellifera from different regions in Mexico. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 178, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Guijarro, M.; Rubio, D.; Aucatoma, B.; Guillén, T.; Jentzsch, P.V.; Ciobotă, V.; Stolker, L.; Ulic, S.E.; Vásquez, L.; et al. Exploratory Monitoring of the Quality and Authenticity of Commercial Honey in Ecuador. Foods 2019, 8, 105. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Bro, R.; Smilde, A.K. Principal component analysis. Anal. Methods 2014, 6, 2812–2831. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Xu, L.; Cai, C.; Cui, H.; Ye, Z.; Yu, X. Rapid discrimination of pork in Halal and non-Halal Chinese ham sausages by Fourier transform infrared (FTIR) spectroscopy and chemometrics. Meat Sci. 2012, 92, 506–510. [Google Scholar] [CrossRef]
- Xing, J.; Guyer, D.; Ariana, D.; Lu, R. Determining optimal wavebands using genetic algorithm for detection of internal insect infestation in tart cherry. Sens. Instrum. Food Qual. Saf. 2008, 2, 161–167. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Wang, Y.-J.; Yan, H.; Chang, X.-W.; Zhou, G.-S.; Zhu, L.; Liu, P.; Guo, S.; Dong, T.T.X.; Duan, J.-A. Rapid Geographical Origin Identification and Quality Assessment of Angelicae Sinensis Radix by FT-NIR Spectroscopy. J. Anal. Methods Chem. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Shawky, E.; Selim, D.A. NIR spectroscopy-multivariate analysis for discrimination and bioactive compounds prediction of different Citrus species peels. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 1–7. [Google Scholar] [CrossRef]
- Dimitrova, B.; Gevrenova, R.; Anklam, E. Analysis of phenolic acids in honeys of different floral origin by solid-pase extraction and high-performance liquid chromatography. Phytochem. Anal. 2006, 18, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Parri, E.; Santinami, G.; Domenici, V. Front-Face Fluorescence of Honey of Different Botanic Origin: A Case Study from Tuscany (Italy). Appl. Sci. 2020, 10, 1776. [Google Scholar] [CrossRef]
- Shirzadifar, A.; Bajwa, S.; Mireei, S.A.; Howatt, K.; Nowatzki, J. Weed species discrimination based on SIMCA analysis of plant canopy spectral data. Biosyst. Eng. 2018, 171, 143–154. [Google Scholar] [CrossRef]
- Mireei, S.A.; Amini-Pozveh, S.; Nazeri, M. Selecting optimal wavelengths for detection of insect infested tomatoes based on SIMCA-aided CFS algorithm. Postharvest Biol. Technol. 2017, 123, 22–32. [Google Scholar] [CrossRef]
- Moomkesh, S.; Mireei, S.A.; Sadeghi, M.; Nazeri, M. Early detection of freezing damage in sweet lemons using Vis/SWNIR spectroscopy. Biosyst. Eng. 2017, 164, 157–170. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; Bosset, J.-O.; De Baerdemaeker, J. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar] [CrossRef]
- Lenhardt, L.; Bro, R.; Zeković, I.; Dramićanin, T.; Dramićanin, M.D. Fluorescence spectroscopy coupled with PARAFAC and PLS DA for characterization and classification of honey. Food Chem. 2015, 175, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Mehretie, S.; Al Riza, D.F.; Yoshito, S.; Kondo, N. Classification of raw Ethiopian honeys using front face fluorescence spectra with multivariate analysis. Food Control 2018, 84, 83–88. [Google Scholar] [CrossRef]
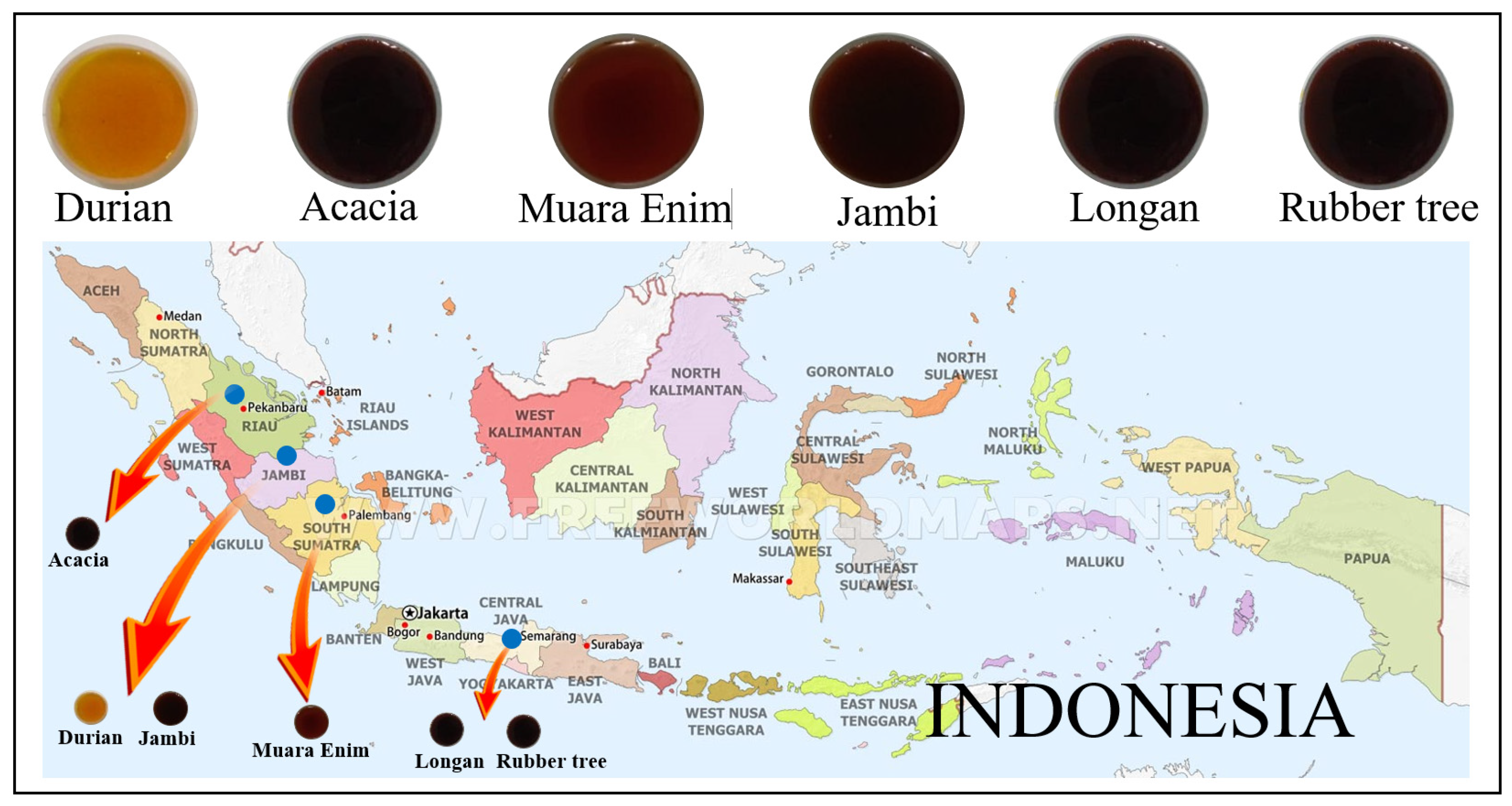


| Honey Sample | Floral Types | Floral Sources | Bee Types | Geographical Origin | Number of Samples |
|---|---|---|---|---|---|
| Rubber tree | Monofloral | Hevea brasiliensis | Apis mellifera | Central Java | 120 |
| Longan | Monofloral | Euphorbia longan | Apis mellifera | Central Java | 120 |
| Durian | Monofloral | Durio zibethinus | Apis dorsata | Jambi | 200 |
| Jambi | Multifloral | - | Apis dorsata | Jambi | 200 |
| Muara Enim | Multifloral | - | Apis dorsata | South Sumatera | 200 |
| Acacia | Monofloral | Acacia mangium | Apis dorsata | Riau | 200 |
| SIMCA Model | Number of Calibration and Validation Samples | Number of Principal Components (PCs) | The Cumulative Informative Variance (CIV) (%) | |
|---|---|---|---|---|
| Calibration | Validation | |||
| Rubber tree | 100 | 3 | 99.489 | 98.893 |
| Longan | 100 | 3 | 99.520 | 98.962 |
| Durian | 167 | 2 | 98.054 | 98.155 |
| Jambi | 167 | 3 | 99.113 | 99.279 |
| Muara Enim | 167 | 2 | 98.667 | 98.910 |
| Acacia | 167 | 3 | 99.059 | 98.441 |
| Predicted Classes | |||||||
|---|---|---|---|---|---|---|---|
| Rubber Tree | Longan | Durian | Jambi | Muara Enim | Acacia | ||
| Actual Classes | Rubber tree | 18 | 0 | 0 | 0 | 0 | 0 |
| Longan | 0 | 19 | 0 | 0 | 0 | 0 | |
| Durian | 0 | 0 | 33 | 0 | 0 | 0 | |
| Jambi | 0 | 0 | 0 | 31 | 0 | 0 | |
| Muara Enim | 0 | 0 | 0 | 0 | 32 | 0 | |
| Acacia | 0 | 0 | 0 | 0 | 0 | 32 | |
| Model Distance | |||||||
|---|---|---|---|---|---|---|---|
| Rubber Tree | Longan | Durian | Jambi | Muara Enim | Acacia | ||
| Classes | Rubber tree | 1 | 9139 | 4090 | 2783 | 2034 | 2403 |
| Longan | 1 | 3774 | 802 | 915 | 427 | ||
| Durian | 1 | 653 | 2573 | 3711 | |||
| Jambi | 1 | 1121 | 386 | ||||
| Muara Enim | 1 | 167 | |||||
| Acacia | 1 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhandy, D.; Yulia, M. The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins. Molecules 2021, 26, 915. https://doi.org/10.3390/molecules26040915
Suhandy D, Yulia M. The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins. Molecules. 2021; 26(4):915. https://doi.org/10.3390/molecules26040915
Chicago/Turabian StyleSuhandy, Diding, and Meinilwita Yulia. 2021. "The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins" Molecules 26, no. 4: 915. https://doi.org/10.3390/molecules26040915
APA StyleSuhandy, D., & Yulia, M. (2021). The Use of UV Spectroscopy and SIMCA for the Authentication of Indonesian Honeys According to Botanical, Entomological and Geographical Origins. Molecules, 26(4), 915. https://doi.org/10.3390/molecules26040915







