Multifunctional Isosteric Pyridine Analogs-Based 2-Aminothiazole: Design, Synthesis, and Potential Phosphodiesterase-5 Inhibitory Activity
Abstract
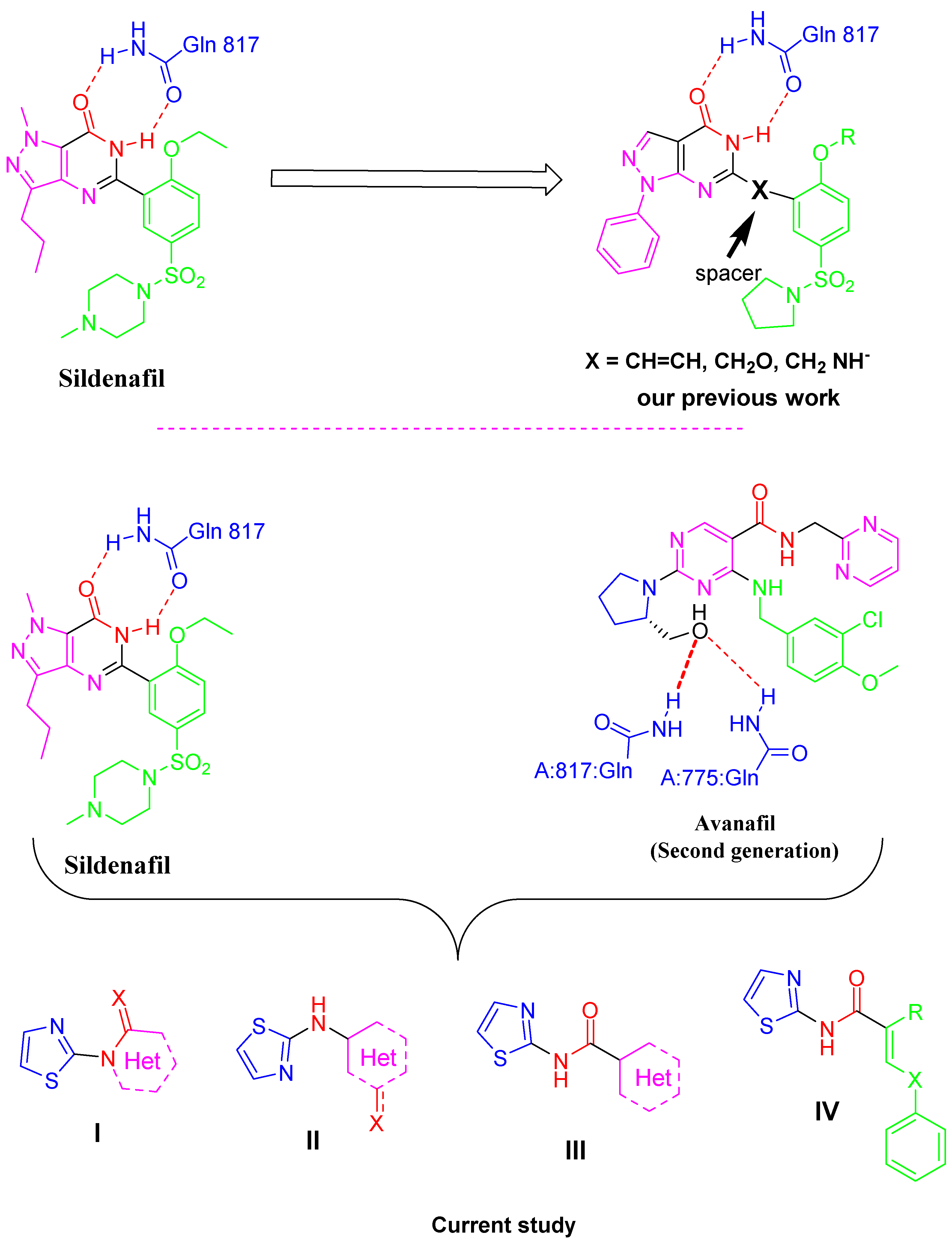
1. Introduction
2. Results
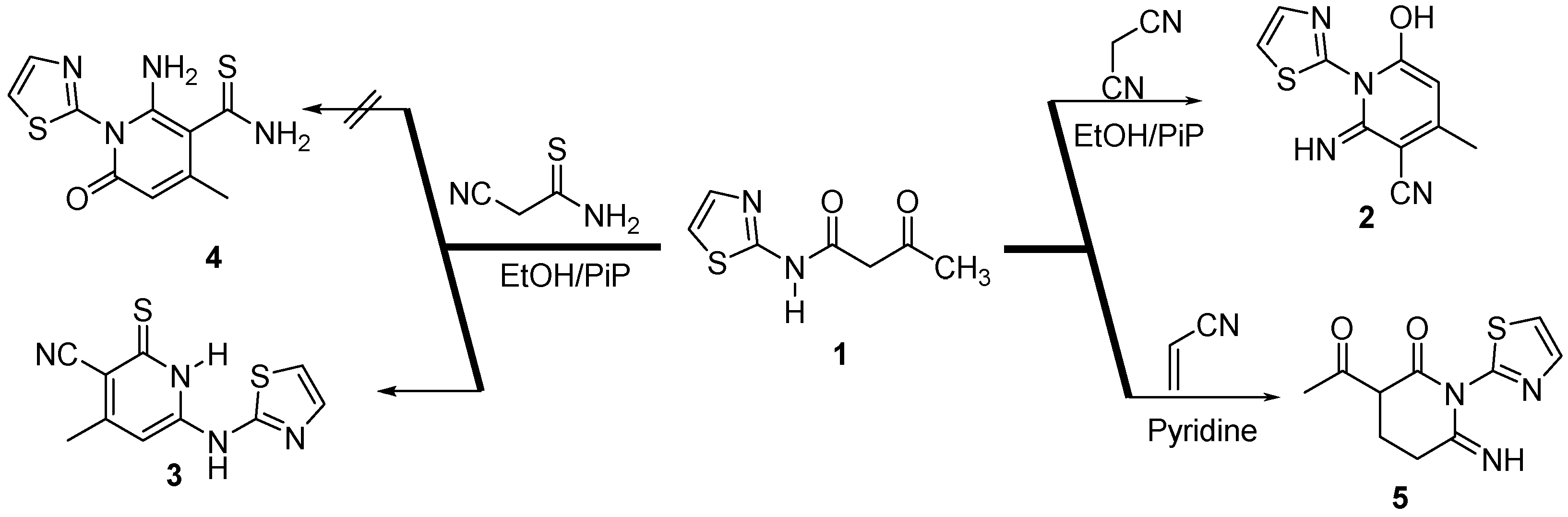
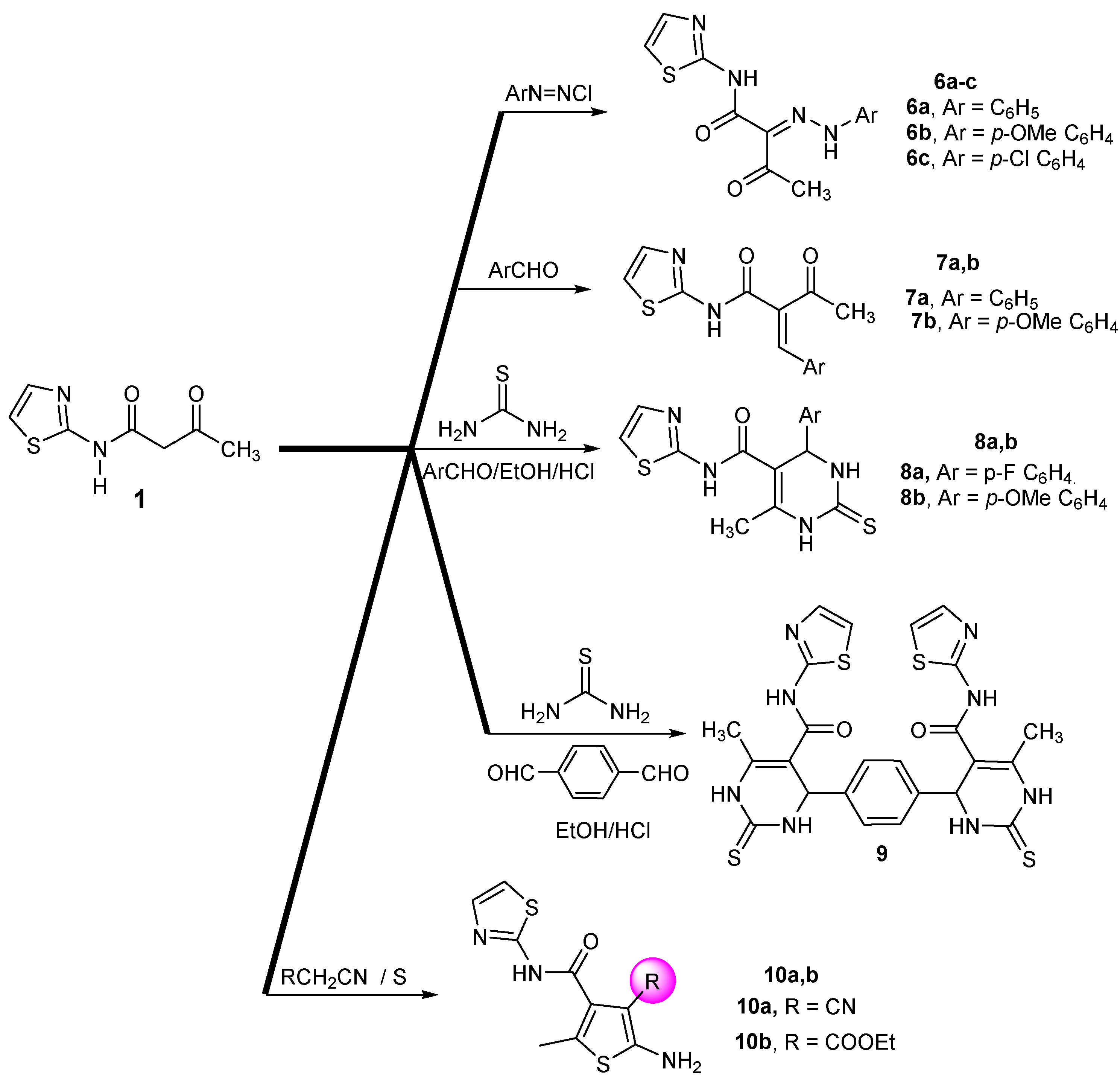
2.1. Chemistry
2.2. In Vitro PDE5 Inhibitory Activity Assay
2.3. Pharmacology
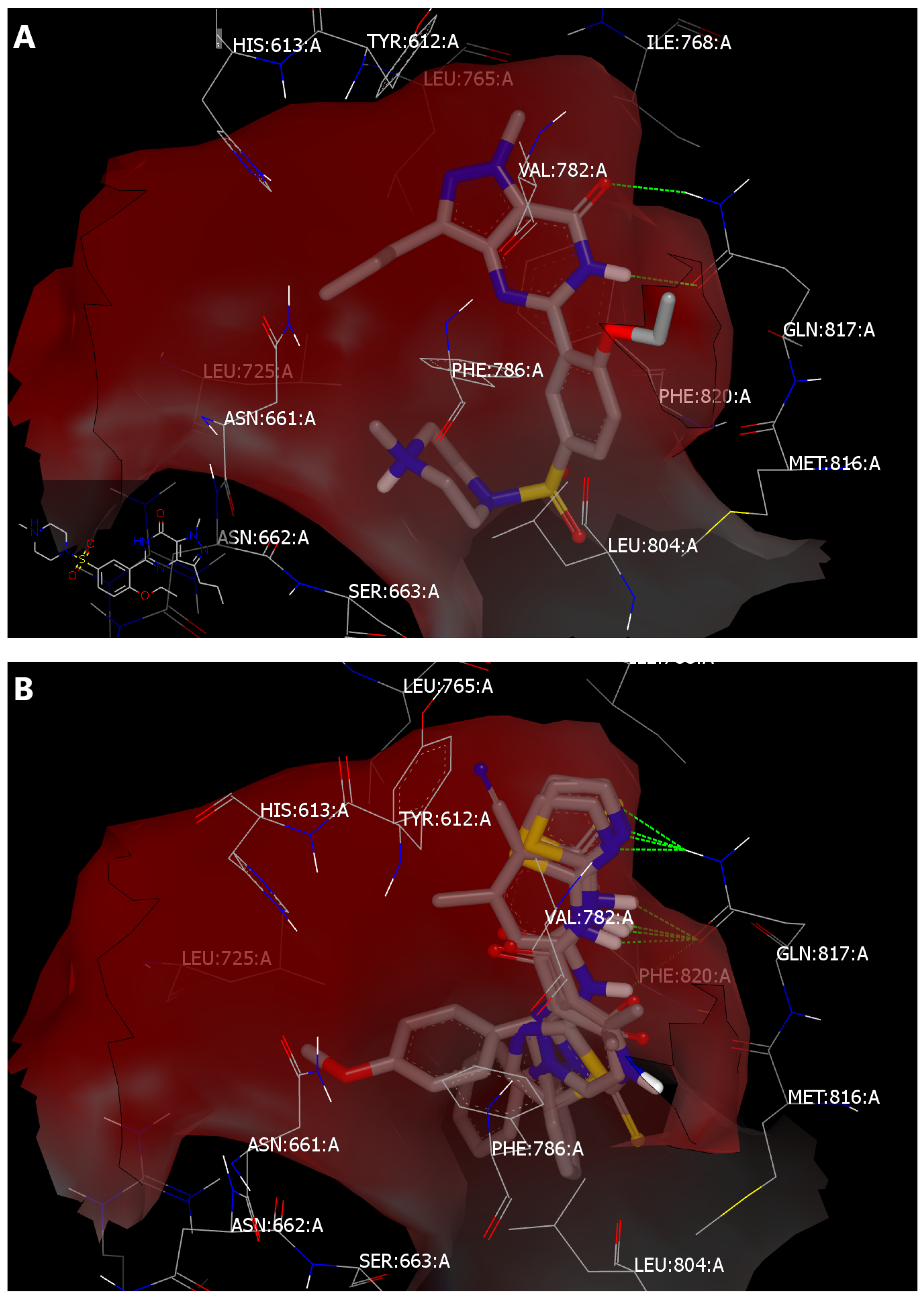
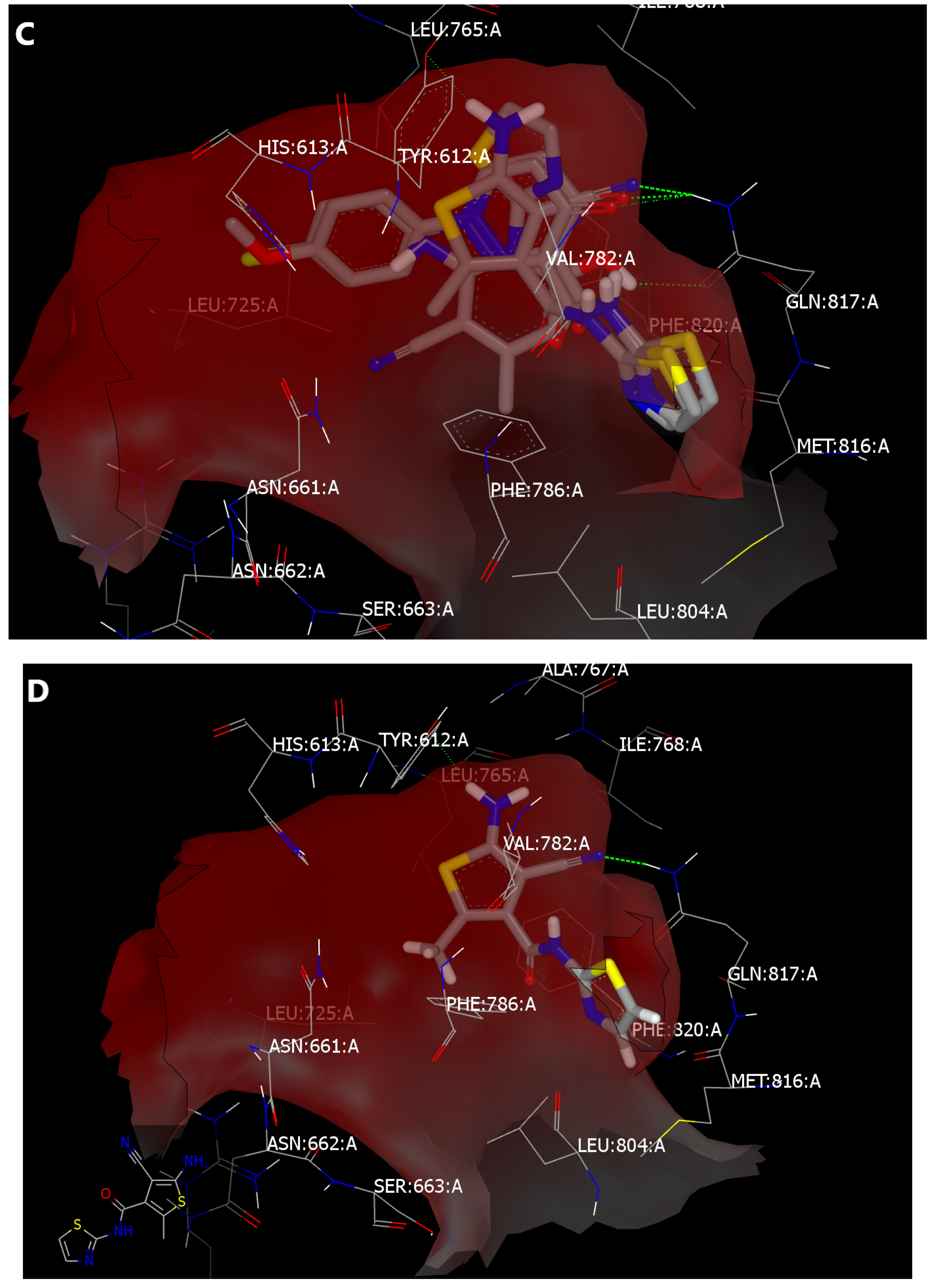
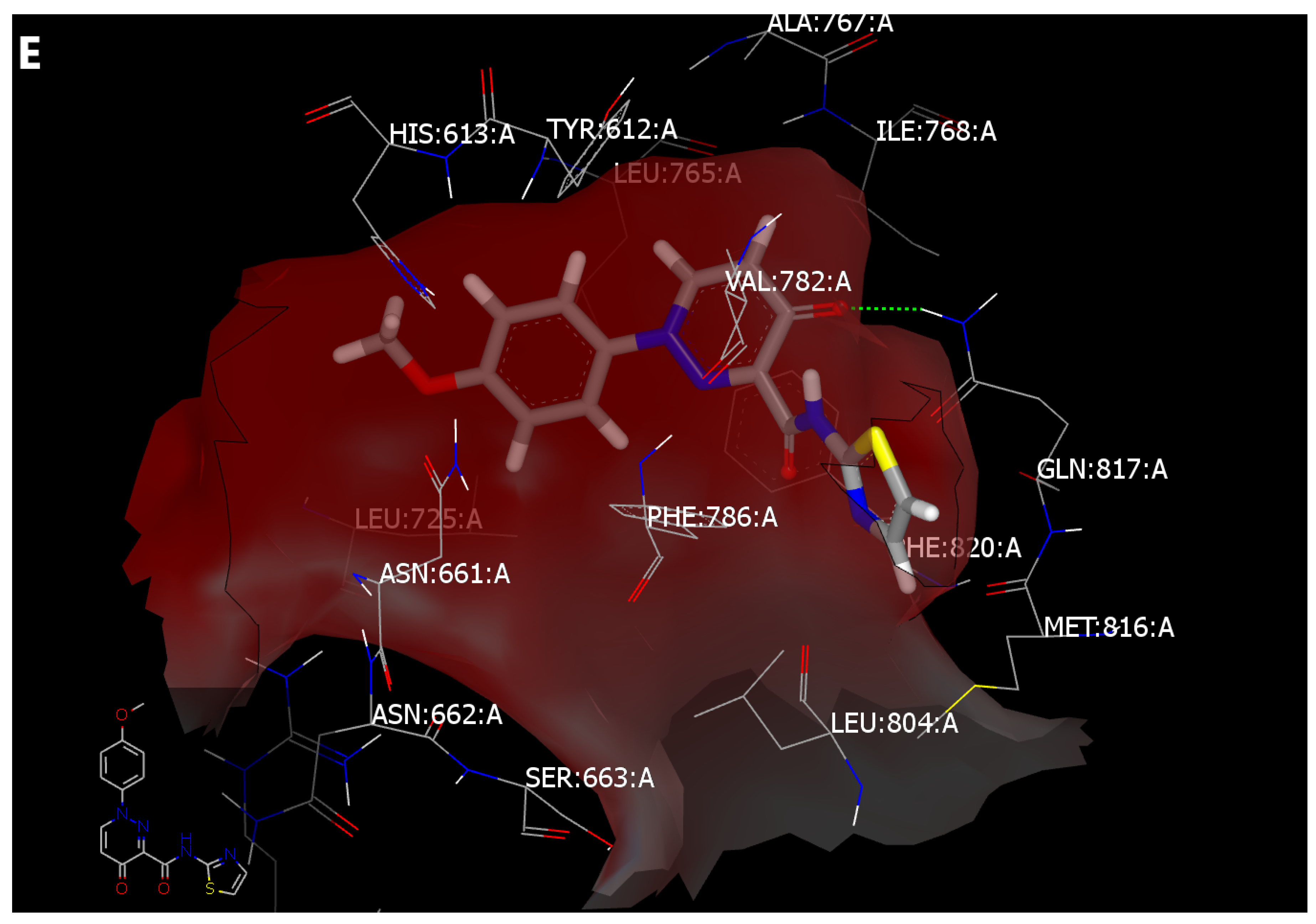
2.4. Molecular Modeling
2.4.1. Lead Optimization by Scaffold Hopping
2.4.2. Structure-Based Lead Discovery
3. Materials and Methods
3.1. General
3.2. Syntheses
3.2.1. 6-Hydroxy-2-imino-4-methyl-1-(thiazol-2-yl)-1,2-dihydro-pyridine-3-carbonitrile (2)
3.2.2. 4-Methyl-6-(thiazol-2-ylamino)-2-thioxo-1,2-dihydropyridine-3-carbonitrile (3)
3.2.3. 3-Acetyl-6-imino-1-(thiazol-2-yl)piperidin-2-one (5)
3.2.4. Typical Procedure for Preparing 6a–c
3.2.5. 1 3-Oxo-2-(2-phenylhydrazono)-N-(thiazol-2-yl)butanamide (6a)
3.2.6. 2 2-[2-(4-Methoxyphenyl)hydrazono]-3-oxo-N-(thiazol-2-yl)-butanamide(6b)
3.2.7. 3 2-(2-(4-Chlorophenyl)hydrazono)-3-oxo-N-(thiazol-2-yl)-butanamide (6c)
3.2.8. Typical Procedure for Preparing 7a,b
3.2.9. 1 2-Benzylidene-3-oxo-N-(thiazol-2-yl)butanamide (7a)
3.2.10. 2 2-(4-Methoxybenzylidene)-3-oxo-N-(thiazol-2-yl)butanamide (7b)
3.2.11. Typical Procedure for Preparing 8a,b
3.2.12. 4,4′-(1,4-.Phenylene)bis(6-methyl-N-(thiazol-2-yl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide) (9)
3.2.13. Typical Procedure for Preparing 10a,b
3.2.14. Typical Procedure for Preparing 11a–c
3.2.15. N-(5-(4-Chlorophenyl)-5H-pyrazolo[4,3-c]pyridazin-3-yl)-thiazol-2-amine (12)
3.2.16. (Z)-N-(5-(4-Chlorophenyl)-2-phenyl-2H-pyrazolo[4,3-c]-pyridazin-3(5H)-ylidene)thiazol-2-amine (13)
3.3. Pharmacological Assessment
3.3.1. PDE5 Enzyme Activity Assay Procedure
3.3.2. ABP Measurement
3.4. Molecular Modeling
3.4.1. Lead Optimization by Scaffold Hopping
3.4.2. Structure-Based Lead Discovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beavo, J.A.; Conti, M.; Heaslip, R.J. Multiple cyclic nucleotide phosphodiesterases. Mol. Pharmacol. 1994, 46, 399–405. [Google Scholar] [PubMed]
- Corbin, J.D.; Francis, S.H. Cyclic GMP Phosphodiesterase-5: Target of Sildenafil. J. Biol. Chem. 1999, 274, 13729–13732. [Google Scholar] [CrossRef]
- Card, G.L.; England, B.P.; Suzuki, Y.; Fong, D.; Powell, B.; Lee, B.; Luu, C.; Tabrizizad, M.; Gillette, S.; Ibrahim, P.N.; et al. Structural Basis for the Activity of Drugs that Inhibit Phosphodiesterases. Structure 2004, 12, 2233–2247. [Google Scholar] [CrossRef]
- Bank, D. Avanafil. Available online: https://www.drugbank.ca/drugs/DB06237 (accessed on 15 September 2020).
- Beavo, J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995, 75, 725–748. [Google Scholar] [CrossRef]
- Corbin, J.D. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int. J. Impot. Res. 2004, 16, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.A.; Radwan, A.A.; Mosa, Y.A.; Abd-Elwahab, B.A. Synthesis and Docking Study of Some Pyrazolo[3,4-d]Pyrimidin-4 (5H)-One Derivatives as Phosphodiesterase-5 Inhibitors. Saudi Pharm. 2009, 17, 109–129. [Google Scholar]
- Tsai, C.-Y.; Kapoor, M.; Huang, Y.-P.; Lin, H.-H.; Liang, Y.-C.; Lin, Y.-L.; Huang, S.-C.; Liao, W.-N.; Chen, J.-K.; Huang, J.-S.; et al. Synthesis and Evaluation of Aminothiazole-Paeonol Derivatives as Potential Anticancer Agents. Mololecules 2016, 21, 145. [Google Scholar] [CrossRef]
- Kesicki, E.A.; Bailey, M.A.; Ovechkina, Y.; Early, J.V.; Alling, T.; Bowman, J.; Zuniga, E.S.; Dalai, S.; Kumar, N.; Masquelin, T. Synthesis and evaluation of the 2-aminothiazoles as anti-tubercular agents. PLoS ONE 2016, 11, e0155209. [Google Scholar] [CrossRef]
- Ghodse, S.M.; Telvekar, V.N. Synthesis of 2-aminothiazole derivatives from easily available thiourea and alkyl/aryl ketones using aqueous NaICl2. Tetrahedron Lett. 2015, 56, 472–474. [Google Scholar] [CrossRef]
- Kayagil, I.; Demirayak, S. Synthesis and Anticancer Activities of Some Thiazole Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2197–2207. [Google Scholar] [CrossRef]
- Abouzeid, L.A.; El-Subbagh, H.I. DNA binding of ethyl 2-substituted aminothiazole-4-carboxylate analogues: A molecular modeling approach to predict their antitumor activity. Future J. Pharm. Sci. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Ghasemi, B.; Najimi, M. Antibacterial effect of thiazole derivatives on Rhodoccocus equi, Brucella abortus, and Pasteurella multocida. Iranian J. Vet. Med. 2016, 10, 47–52. [Google Scholar]
- Mishra, R.; Tomer, I.; Sharma, P.; Jha, K. Synthesis and antimicrobial evaluation of some novel thiazole derivatives. Der. Pharm. Sin. 2012, 3, 361–366. [Google Scholar]
- Mishra, R.; Sharma, P.K.; Verma, P.K.; Tomer, I.; Mathur, G.; Dhakad, P.K. Biological Potential of Thiazole Derivatives of Synthetic Origin. J. Heterocycl. Chem. 2017, 54, 2103–2116. [Google Scholar] [CrossRef]
- Hassan, F.A. Synthesis, characterization, anti-inflammatory, and antioxidant activities of some new thiazole derivatives. Int. J. Appl. Sci. Technol. 2012, 2, 180–187. [Google Scholar]
- Lin, P.-Y.; Hou, R.-S.; Wang, H.-M.; Kang, I.-J.; Chen, L.-C. Efficient Synthesis of 2-Aminothiazoles and Fanetizole in Liquid PEG-400 at Ambient Conditions. J. Chin. Chem. Soc. 2009, 56, 455–458. [Google Scholar] [CrossRef]
- Rodrigues, C.A.; Dos Santos, P.F.; Da Costa, M.O.L.; Pavani, T.F.A.; Xander, P.; Geraldo, M.M.; Mengarda, A.C.; De Moraes, J.; Rando, D.G. 4-Phenyl-1,3-thiazole-2-amines as scaffolds for new antileishmanial agents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2018, 24, 26. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Gong, J.; Fang, H.; Liu, A.; Du, G.; Xu, W. Design, synthesis, and biological activity of thiazole derivatives as novel influenza neuraminidase inhibitors. J. Enzym. Inhib. Med. Chem. 2010, 26, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.M.; Khames, A.A.; El-Adasy, A.-B.A.; Atalla, A.A.; Abdel-Rady, M.; Hassan, M.I.A.; Nemr, M.T.M.; Elshaier, Y.A.M.M. Design, synthesis and biological evaluation of new 2-aminothiazole scaffolds as phosphodiesterase type 5 regulators and COX-1/COX-2 inhibitors. RSC Adv. 2020, 10, 29723–29736. [Google Scholar] [CrossRef]
- Zoraghi, R.; Corbin, J.D.; Francis, S.H. Phosphodiesterase-5 Gln817 Is Critical for cGMP, Vardenafil, or Sildenafil Affinity its orientation impacts cGMP but not cAMP affinity. J. Biol. Chem. 2006, 281, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Zoraghi, R.; Francis, S.H.; Corbin, J.D. Critical Amino Acids in Phosphodiesterase-5 Catalytic Site That Provide for High-Affinity Interaction with Cyclic Guanosine Monophosphate and Inhibitors. Biochemistry 2007, 46, 13554–13563. [Google Scholar] [CrossRef]
- Hussein, A.H.M.; El-Adasy, A.-B.A.; Khames, A.A.; Atalla, A.A.; Abdel-Rady, M.; El-Adasy, A.P.A.-B.A.; Khames, A.P.A.A. 3-Oxobutanamides in Heterocyclic Synthesis, Synthesis Approaches for new Pyridines, Pyrimidines and their Fused Derivatives. ChemistrySelect 2017, 2, 1625–1629. [Google Scholar] [CrossRef]
- Price, C.P.; Grzesiak, A.L.; Kampf, J.W.; Matzger, A.J. Maize 1: A Trimorphic Azo Pigment. Cryst. Growth Des. 2003, 3, 1021–1025. [Google Scholar] [CrossRef]
- Hussein, A.H.M.; Gad-Elkareem, M.A.M.; El-Adasy, A.-B.A.A.M.; Khames, A.A.; Othman, I.M.M. β-Oxoanilides in Heterocyclic Synthesis: Synthesis and Antimicrobial Activity of Pyridines, Pyrans, Pyrimidines and Azolo, Azinopyrimidines Incorporating Antipyrine Moiety. Int. J. Org. Chem. 2012, 2, 341–351. [Google Scholar] [CrossRef]
- Folkers, K.; Harwood, H.; Johnson, T.B. Researches on pyrimidines. Cxxx. Synthesis of 2-keto-1, 2, 3, 4-tetrahydropyrimidines. J. Am. Chem. Soc. 1932, 54, 3751–3758. [Google Scholar] [CrossRef]
- Spoto, G.; Berardi, S.; Ajerba, G.; De Laurentiis, V. A reverse-phase HPLC method for cyclic nucleotide phosphodiesterases activity and classification. In Purine and Pyrimidine Metabolism in Man VIII; Springer: Boston, MA, USA, 1995; Volume 370, pp. 815–820. [Google Scholar]
- OpenEye Lead Optimization EON. Available online: https://www.eyesopen.com/eon (accessed on 12 October 2020).
- Wang, H.; Liu, Y.; Huai, Q.; Cai, J.; Zoraghi, R.; Francis, S.H.; Corbin, J.D.; Robinson, H.; Xin, Z.; Lin, G. Multiple Conformations of Phosphodiesterase-5 implications for enzyme function and drug development. J. Biol. Chem. 2006, 281, 21469–21479. [Google Scholar] [CrossRef]
- OpenEye Database Preparation. Filter. Available online: https://www.eyesopen.com/filter (accessed on 5 October 2020).
- McGann, M. FRED Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2011, 51, 578–596. [Google Scholar] [CrossRef]
- OpeEye Fast Rigid Exhaustive Docking (FRED) Receptor; Version 2.2.5; OpenEye Scientific Software: Santa Fe, NM, USA; Available online: http://www.eyesopen.com (accessed on 15 October 2020).
- OpenEye Scientific Software: OEApplications 2020.2. Available online: https://docs.eyesopen.com/applications (accessed on 25 December 2020).
- Ahmed, M.S.; Kopel, L.C.; Halaweish, F. Structural Optimization and Biological Screening of a Steroidal Scaffold Possessing Cucurbitacin-Like Functionalities as B-Raf Inhibitors. Chem. Med. Chem. 2014, 9, 1361–1367. [Google Scholar] [CrossRef]
- Elshaier, Y.A.; Shaaban, M.A.; El Hamid, M.K.A.; Abdelrahman, M.H.; Abou-Salim, M.A.; Elgazwi, S.M.; Halaweish, F. Design and synthesis of pyrazolo[3,4- d ]pyrimidines: Nitric oxide releasing compounds targeting hepatocellular carcinoma. Bioorganic Med. Chem. 2017, 25, 2956–2970. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H. A comparative QSAR analysis and molecular docking studies of quinazoline derivatives as tyrosine kinase (EGFR) inhibitors: A rational approach to anticancer drug design. J. Saudi Chem. Soc. 2013, 17, 361–379. [Google Scholar] [CrossRef]
- Prasad, V.R.; Reddy, G.D.; Kathmann, I.; Amareswararao, M.; Peters, G. Nitric oxide releasing acridone carboxamide derivatives as reverters of doxorubicin resistance in MCF7/Dx cancer cells. Bioorganic Chem. 2016, 64, 51–58. [Google Scholar] [CrossRef] [PubMed]


| Compound No. | PDE5 (Inhibition %) | MABP |
|---|---|---|
| 1 | 13 | 103.1 ± 1.85 # |
| 2 | 100 | 64.7 ± 2.98 ### |
| 3 | 2 | 79.3 ± 2.57 ## |
| 5 | 100 | 115.95 ± 2.91 ### |
| 6a | 20 | 110.1 ± 3.5 ## |
| 6c | 5 | 108.8 ± 3.9 # |
| 7a | 38 | 87.2 ± 2.68 * |
| 7b | 62 | 90.4 ± 2.84 ** |
| 8b | 23 | 88.1 ± 1.62 ** |
| 9 | 10 | 90.1 ± 1.62 ** |
| 10a | 100 | 110.3 ± 2.84 # |
| 10b | 82 | 105.3 ± 2.84 # |
| 11a | 8 | 120.1 ± 2.96 ### |
| 11b | 100 | 78.3 ± 2.57 ## |
| 12 | 7 | 90.3 ± 1.43 ** |
| 11c | 13 | 81.3 ± 2.57 ## |
| 13 | 8 | 81.10 ± 3.78 # |
| Control | - | 93.10 ± 1.55 |
| Nitroprusside | - | 52.10 ± 1.11 ### |
| Sildenafil | 100 | 72.50 ± 2.92 ## |
| Compound No. | EON ET Combo | EON Rank |
|---|---|---|
| Sildenafil | 0.494 | 1 |
| 9 | 0.370 | 2 |
| 5 | 0.298 | 3 |
| 6a | 0.290 | 4 |
| 12 | 0.247 | 5 |
| 8a | 0.235 | 6 |
| 13 | 0.234 | 7 |
| 6b | 0.203 | 8 |
| 11b | 0.198 | 9 |
| 11a | 0.186 | 10 |
| 8b | 0.181 | 11 |
| 6c | 0.167 | 12 |
| 7a | 0.147 | 13 |
| 7b | 0.136 | 14 |
| 2 | 0.129 | 15 |
| 11c | 0.115 | 16 |
| 10a | 0.107 | 17 |
| 10b | 0.106 | 18 |
| 3 | 0.041 | 19 |
| Comp. No | FRED Chemgauss4 Score |
|---|---|
| 12 | −12.9335 |
| 3 | −12.3931 |
| 11b | −11.3665 |
| 8b | −11.1262 |
| 11c | −11.0972 |
| 11a | −10.9461 |
| 7a | −10.9088 |
| 10b | −10.1529 |
| 8a | −9.7921 |
| 10a | −9.594 |
| 7b | −9.0632 |
| 2 | −8.4895 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, A.H.M.; Khames, A.A.; El-Adasy, A.-B.A.; Atalla, A.A.; Abdel-Rady, M.; Hassan, M.I.A.; Abou-Salim, M.A.; Elshaier, Y.A.M.M.; Barakat, A. Multifunctional Isosteric Pyridine Analogs-Based 2-Aminothiazole: Design, Synthesis, and Potential Phosphodiesterase-5 Inhibitory Activity. Molecules 2021, 26, 902. https://doi.org/10.3390/molecules26040902
Hussein AHM, Khames AA, El-Adasy A-BA, Atalla AA, Abdel-Rady M, Hassan MIA, Abou-Salim MA, Elshaier YAMM, Barakat A. Multifunctional Isosteric Pyridine Analogs-Based 2-Aminothiazole: Design, Synthesis, and Potential Phosphodiesterase-5 Inhibitory Activity. Molecules. 2021; 26(4):902. https://doi.org/10.3390/molecules26040902
Chicago/Turabian StyleHussein, Abdel Haleem M., Ahmed A. Khames, Abu-Bakr A. El-Adasy, Ahmed A. Atalla, Mohamed Abdel-Rady, Mohamed I. A. Hassan, Mahrous A. Abou-Salim, Yaseen A. M. M. Elshaier, and Assem Barakat. 2021. "Multifunctional Isosteric Pyridine Analogs-Based 2-Aminothiazole: Design, Synthesis, and Potential Phosphodiesterase-5 Inhibitory Activity" Molecules 26, no. 4: 902. https://doi.org/10.3390/molecules26040902
APA StyleHussein, A. H. M., Khames, A. A., El-Adasy, A.-B. A., Atalla, A. A., Abdel-Rady, M., Hassan, M. I. A., Abou-Salim, M. A., Elshaier, Y. A. M. M., & Barakat, A. (2021). Multifunctional Isosteric Pyridine Analogs-Based 2-Aminothiazole: Design, Synthesis, and Potential Phosphodiesterase-5 Inhibitory Activity. Molecules, 26(4), 902. https://doi.org/10.3390/molecules26040902







