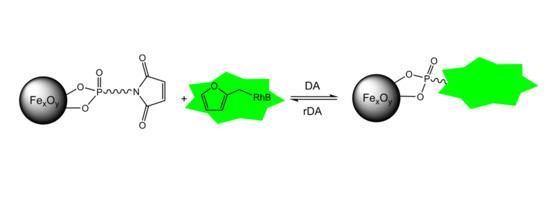
Reversible Diels–Alder Reactions with a Fluorescent Dye on the Surface of Magnetite Nanoparticles
Abstract
1. Introduction
2. Results
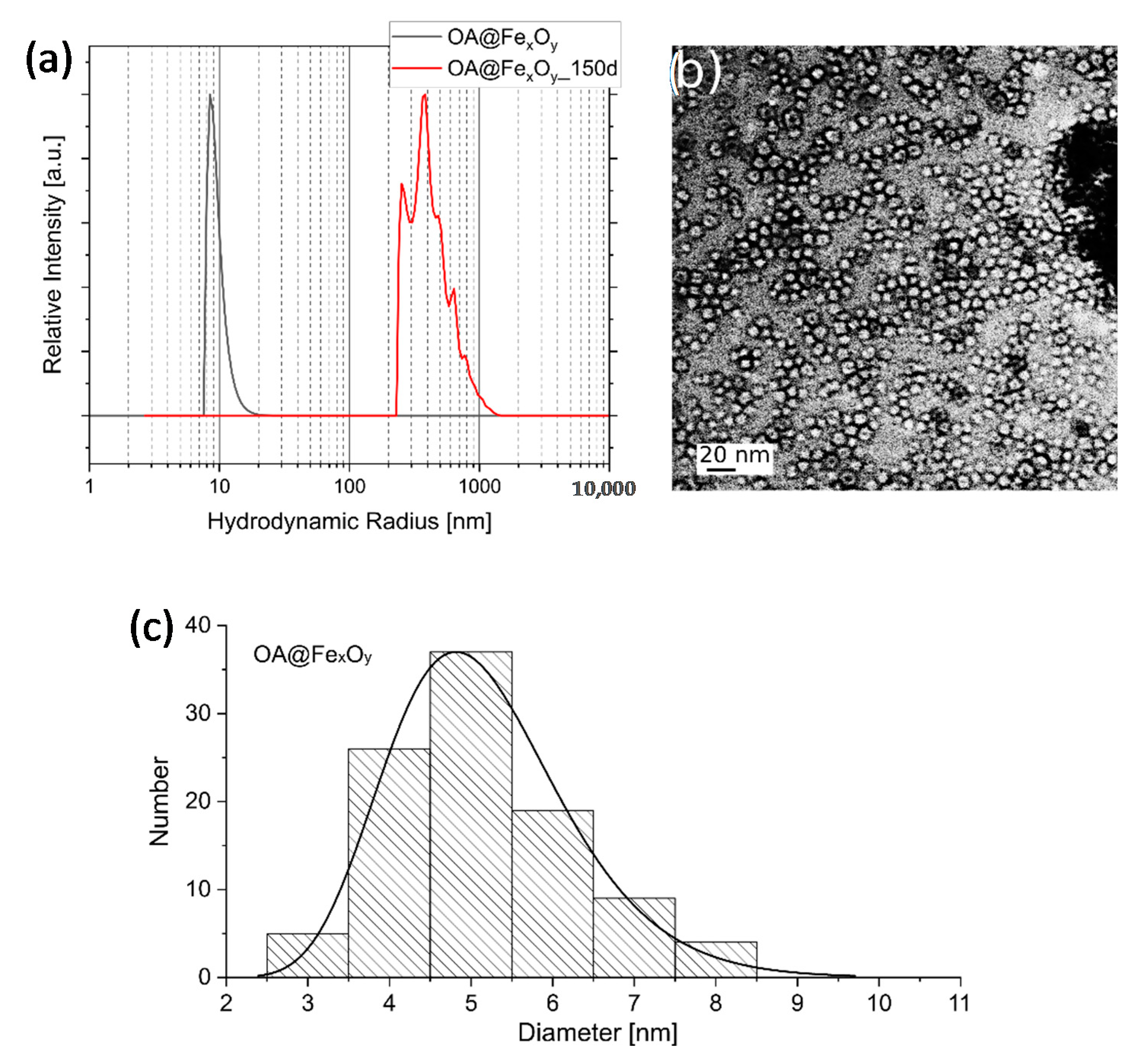
2.1. Synthesis of Iron Oxide Nanoparticles
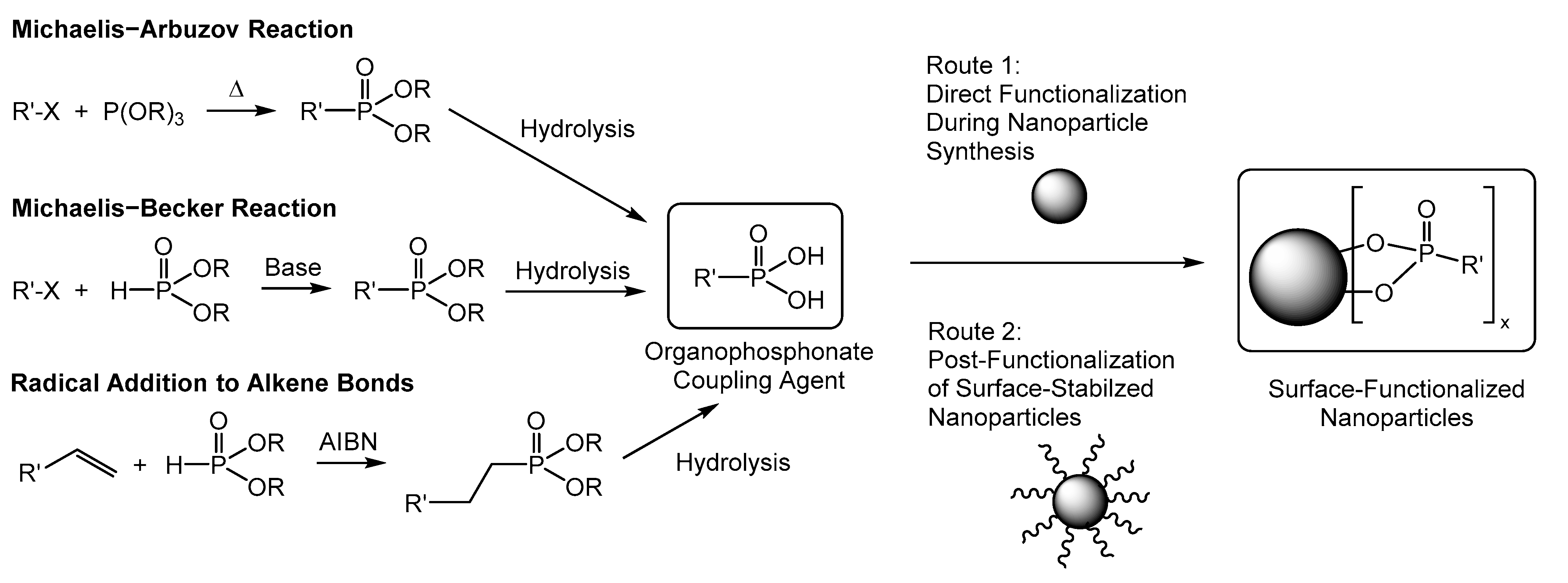
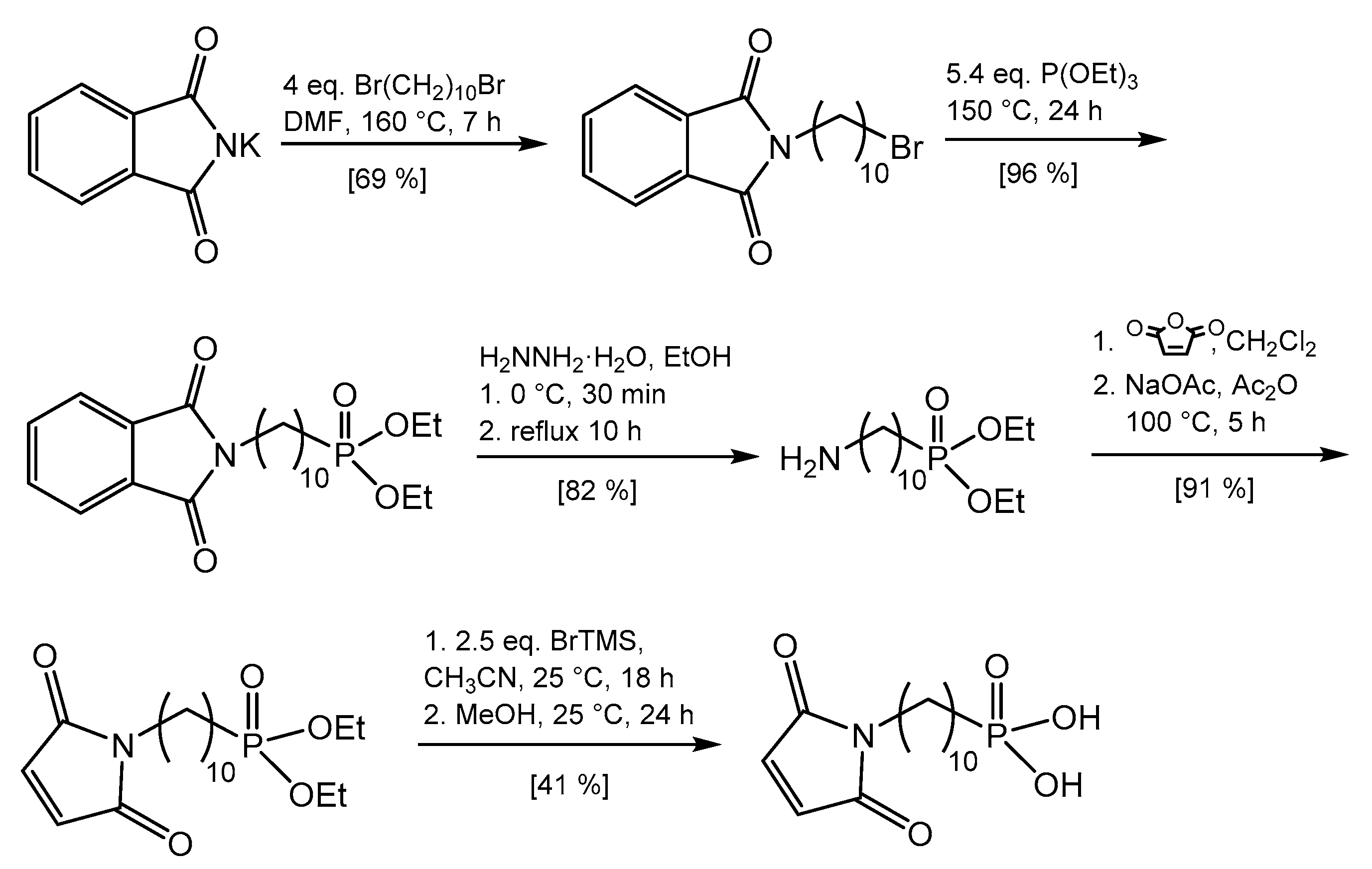
2.2. Preparation of N-(10-Maleimidodecyl)-Phosphonic Acid
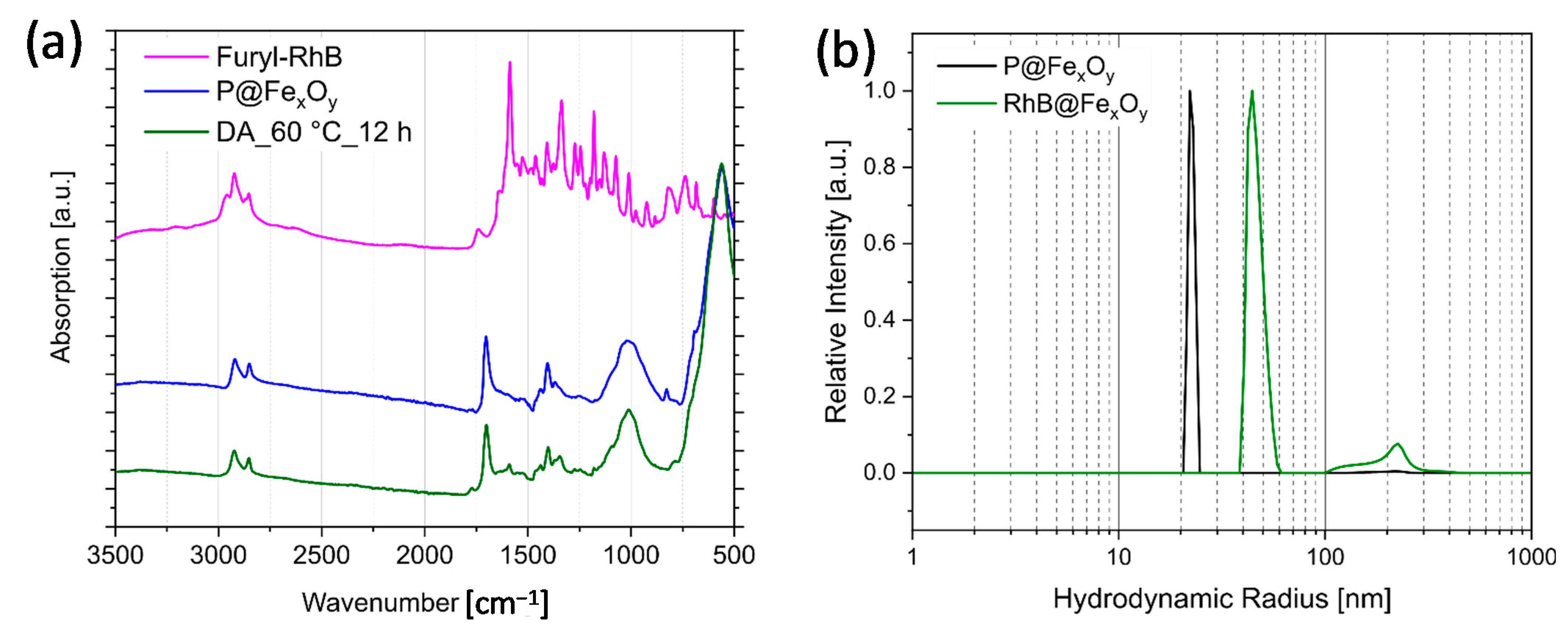
2.3. Maleimide-Functionalized Iron Oxide Nanoparticles
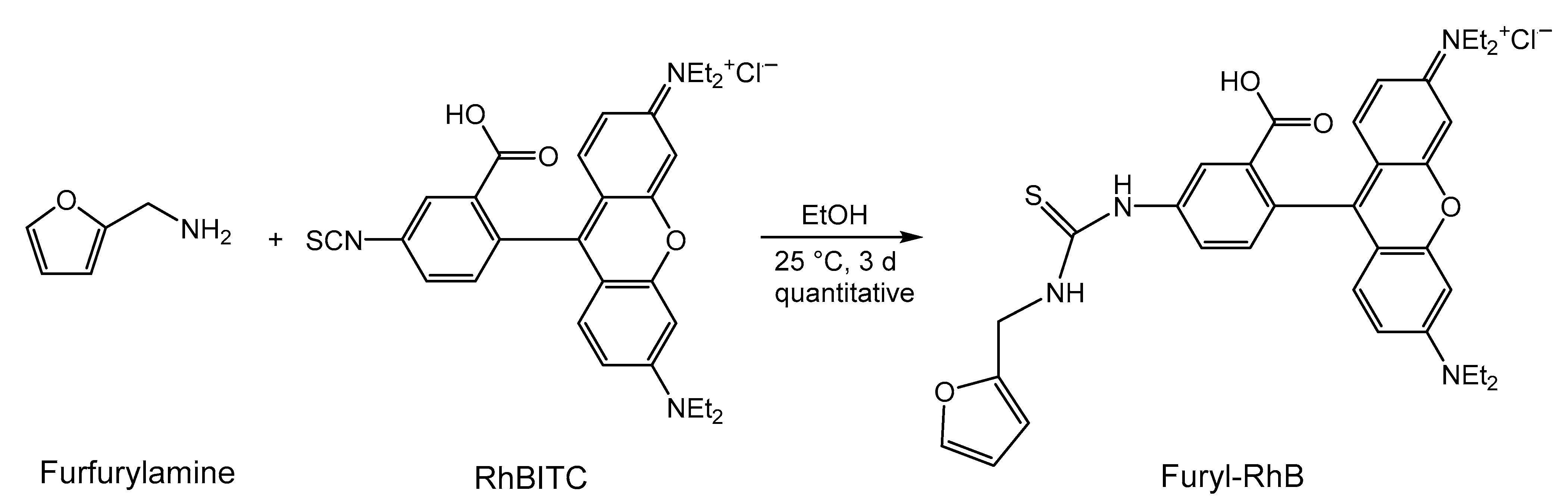
2.4. Modification of Rhodamine B Isothiocyanate with Furfurylamine
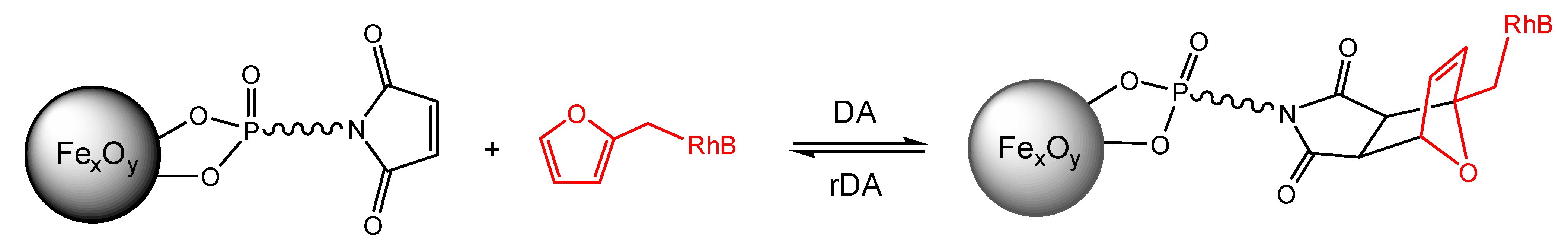
2.5. Investigation of the Diels–Alder Reactions
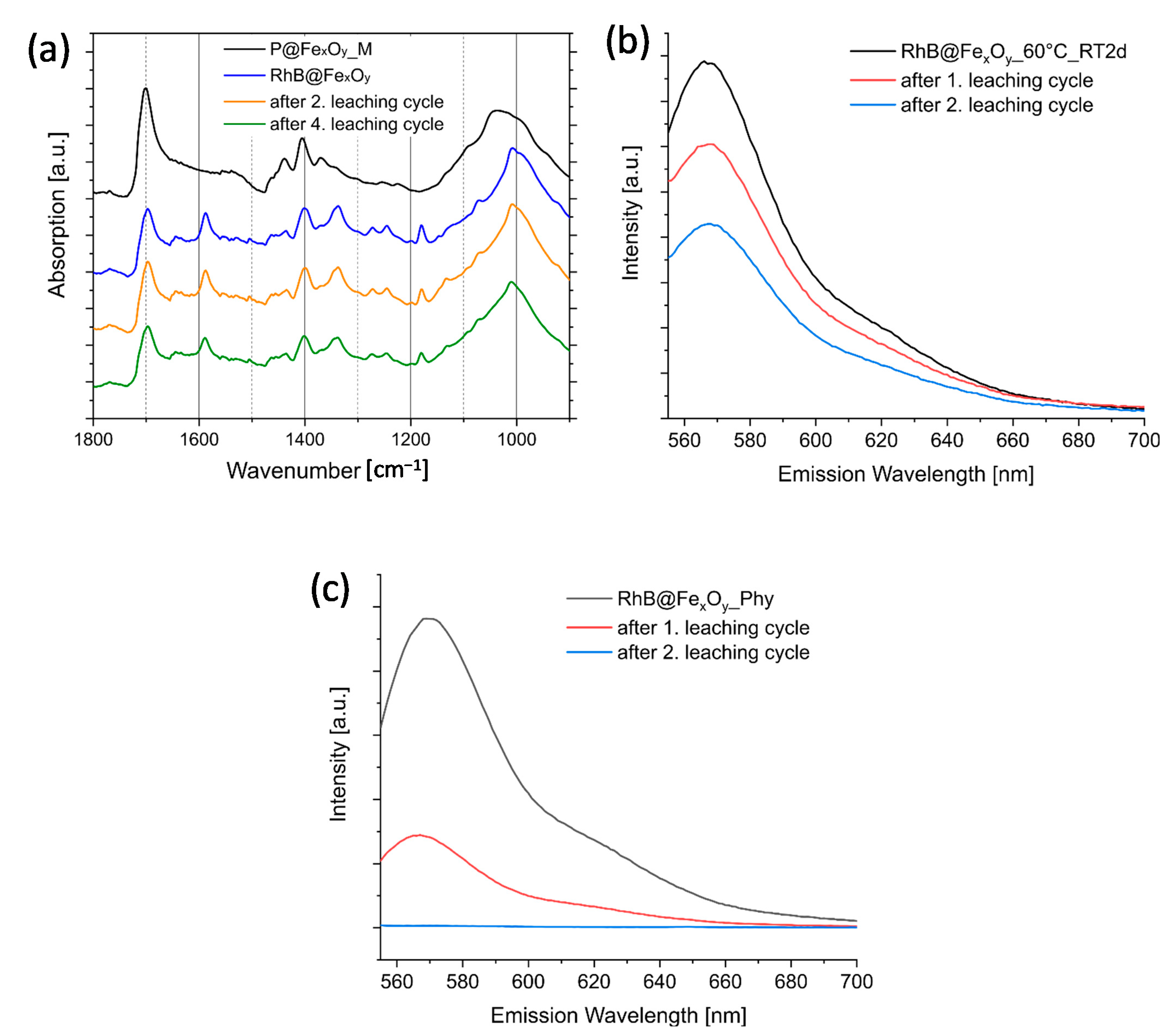
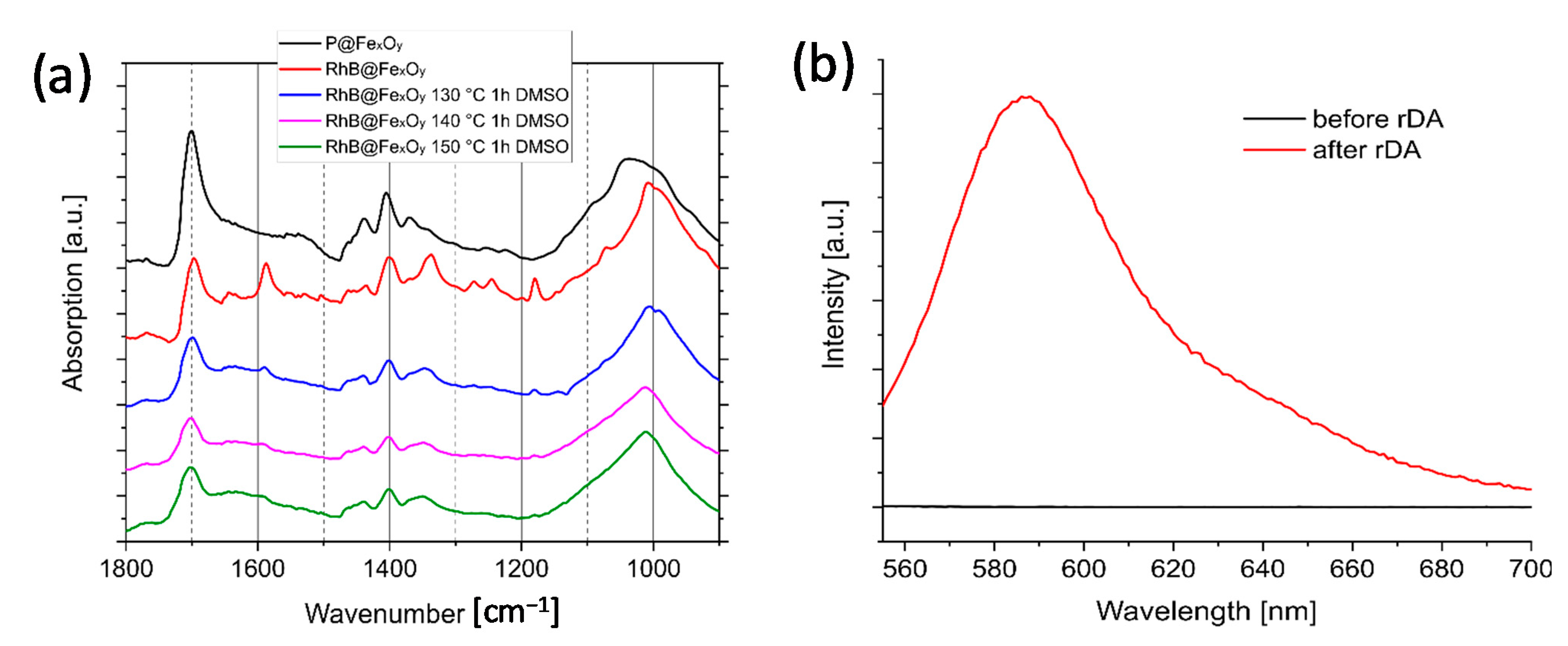
2.6. Investigation of retro-Diels–Alder Reactions at the Particle Surface
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Characterization Methods
4.3. Syntheses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement.
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Sant, S.; Wang, B.; Laurent, S.; Sen, T. Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy. Adv. Drug Deliv. Rev. 2011, 63, 24–46. [Google Scholar] [CrossRef]
- Ulbrich, K.; Hola, K.; Subr, V.; Bakandritsos, A.; Tucek, J.; Zboril, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed]
- Alsoraya, M.; Zhao, M.; Fan, D. Engineered Nanomaterials for Sustainable Oil Separation and Recovery. ChemNanoMat 2020, 6, 1539–1552. [Google Scholar] [CrossRef]
- Corot, C.; Robert, P.; Idee, J.-M.; Port, M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv. Drug Deliv. Rev. 2006, 58, 1471–1504. [Google Scholar] [CrossRef]
- Jordan, A.; Scholz, R.; Wust, P.; Fahling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Haefeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Amstad, E.; Textor, M.; Reimhult, E. Stabilization and functionalization of iron oxide nanoparticles for biomedical applications. Nanoscale 2011, 3, 2819–2843. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D.C. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G. Alkyl Phosphonate/Phosphate Coating on Magnetite Nanoparticles: A Comparison with Fatty Acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Ceylan, S.; Friese, C.; Lammel, C.; Mazac, K.; Kirschning, A. Inductive heating for organic synthesis by using functionalized magnetic nanoparticles inside microreactors. Angew. Chem. Int. Ed. 2008, 47, 8950–8953. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Yamamoto, K.; Aoyagi, T. New liquid chromatography method combining thermo-responsive material and inductive heating via alternating magnetic field. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2008, 876, 97–102. [Google Scholar] [CrossRef]
- Hubbard, J.W.; Orange, F.; Guinel, M.J.F.; Guenthner, A.J.; Mabry, J.M.; Sahagun, C.M.; Rinaldi, C. Curing of a Bisphenol E Based Cyanate Ester Using Magnetic Nanoparticles as an Internal Heat Source through Induction Heating. ACS Appl. Mater. Interfaces 2013, 5, 11329–11335. [Google Scholar] [CrossRef]
- Park, C.-H.; Kang, S.-J.; Tijing, L.D.; Pant, H.R.; Kim, C.S. Inductive heating of electrospun Fe2O3/polyurethane composite mat under high-frequency magnetic field. Ceram. Int. 2013, 39, 9785–9790. [Google Scholar] [CrossRef]
- Uchida, S.; Yamagata, M.; Ishikawa, M. Novel rapid synthesis method of LiFePO4/C cathode material by high-frequency induction heating. J. Power Sources 2013, 243, 481–487. [Google Scholar] [CrossRef]
- Bai, S.; Zou, H.; Dietsch, H.; Simon, Y.C.; Weder, C. Functional Iron Oxide Nanoparticles as Reversible Crosslinks for Magnetically Addressable Shape-Memory Polymers. Macromol. Chem. Phys. 2014, 215, 398–404. [Google Scholar] [CrossRef]
- Bayerl, T.; Duhovic, M.; Mitschang, P.; Bhattacharyya, D. The heating of polymer composites by electromagnetic induction—A review. Compos. Part A 2014, 57, 27–40. [Google Scholar] [CrossRef]
- Sharma, P.; Holliger, N.; Pfromm, P.H.; Liu, B.; Chikan, V. Size-Controlled Synthesis of Iron and Iron Oxide Nanoparticles by the Rapid Inductive Heating Method. ACS Omega 2020, 5, 19853–19860. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Sadat, M.E.; Dunn, A.W.; Mast, D.B. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale 2015, 7, 8209–8232. [Google Scholar] [CrossRef]
- Hatanaka, S.; Matsushita, N.; Abe, M.; Nishimura, K.; Hasegawa, M.; Handa, H. Direct immobilization of fluorescent dyes onto ferrite nanoparticles during their synthesis from aqueous solution. J. Appl. Phys. 2003, 93, 7569–7570. [Google Scholar] [CrossRef]
- Benbenishty-Shamir, H.; Gilert, R.; Gotman, I.; Gutmanas, E.Y.; Sukenik, C.N. Phosphonate-Anchored Monolayers for Antibody Binding to Magnetic Nanoparticles. Langmuir 2011, 27, 12082–12089. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Avti, P.K.; Pouliot, P.; Tardif, J.-C.; Rheaume, E.; Lesage, F.; Kakkar, A. Magnetic resonance imaging/fluorescence dual modality protocol using designed phosphonate ligands coupled to superparamagnetic iron oxide nanoparticles. J. Mater. Chem. B 2016, 4, 3969–3981. [Google Scholar] [CrossRef] [PubMed]
- Qiao, R.; Esser, L.; Fu, C.; Zhang, C.; Hu, J.; Ramirez-arcia, P.; Li, Y.; Quinn, J.F.; Whittaker, M.R.; Whittaker, A.K.; et al. Bioconjugation and Fluorescence Labeling of Iron Oxide Nanoparticles Grafted with Bromomaleimide-Terminal Polymers. Biomacromolecules 2018, 19, 4423–4429. [Google Scholar] [CrossRef]
- Boissezon, R.; Muller, J.; Beaugeard, V.; Monge, S.; Robin, J.-J. Organophosphonates as anchoring agents onto metal oxide-based materials: Synthesis and applications. RSC Adv. 2014, 4, 35690–35707. [Google Scholar] [CrossRef]
- Guerrero, G.; Alauzun, J.G.; Granier, M.; Laurencin, D.; Mutin, P.H. Phosphonate coupling molecules for the control of surface/interface properties and the synthesis of nanomaterials. Dalton Trans. 2013, 42, 12569–12585. [Google Scholar] [CrossRef]
- Queffelec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Bachinger, A.; Kickelbick, G. Pickering emulsions stabilized by anatase nanoparticles. Monatsh. Chem. 2010, 141, 685–690. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Lomoschitz, C.J.; Kickelbick, G. Tuning the self-assembled monolayer formation on nanoparticle surfaces with different curvatures: Investigations on spherical silica particles and plane-crystal-shaped zirconia particles. J. Colloid Interface Sci. 2011, 360, 15–25. [Google Scholar] [CrossRef]
- Feichtenschlager, B.; Pabisch, S.; Peterlik, H.; Kickelbick, G. Nanoparticle Assemblies as Probes for Self-Assembled Monolayer Characterization: Correlation between Surface Functionalization and Agglomeration Behavior. Langmuir 2012, 28, 741–750. [Google Scholar] [CrossRef]
- Bachinger, A.; Ivanovici, S.; Kickelbick, G. Formation of Janus TiO2 nanoparticles by a pickering emulsion approach applying phosphonate coupling agents. J. Nanosci. Nanotechnol. 2011, 11, 8599–8608. [Google Scholar] [CrossRef] [PubMed]
- Betke, A.; Kickelbick, G. Long alkyl chain organophosphorus coupling agents for in situ surface functionalization by reactive milling. Inorganics 2014, 2, 410–423. [Google Scholar] [CrossRef]
- Heinrich, C.; Niedner, L.; Oberhausen, B.; Kickelbick, G. Surface-charged zirconia nanoparticles prepared by organophosphorus surface functionalization with ammonium or sulfonate groups. Langmuir 2019, 35, 11369–11379. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Kickelbick, G. Thermoreversible Reactions on Inorganic Nanoparticle Surfaces: Diels-Alder Reactions on Sterically Crowded Surfaces. Chem. Mater. 2013, 25, 149–157. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Self-healing polymer nanocomposites based on Diels-Alder-reactions with silica nanoparticles: The role of the polymer matrix. Polymer 2015, 69, 357–368. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Double Reversible Networks: Improvement of Self-Healing in Hybrid Materials via Combination of Diels-Alder Cross-Linking and Hydrogen Bonds. Macromolecules 2018, 51, 6099–6110. [Google Scholar] [CrossRef]
- Schäfer, S.; Kickelbick, G. Diels-Alder Reactions on Surface-Modified Magnetite/Maghemite Nanoparticles: Application in Self-Healing Nanocomposites. ACS Appl. Nano Mater. 2018, 1, 2640–2652. [Google Scholar] [CrossRef]
- Maleki, A.; Taheri-Ledari, R.; Soroushnejad, M. Surface functionalization of magnetic nanoparticles via palladium-catalyzed Diels-Alder approach. ChemistrySelect 2018, 3, 13057–13062. [Google Scholar] [CrossRef]
- Casey, J.S.; Arrizabalaga, J.H.; Abu-Laban, M.; Becca, J.C.; Rose, B.J.; Strickland, K.T.; Bursavich, J.B.; McCann, J.S.; Pacheco, C.N.; Jessen, L.; et al. Alternating magnetic field mediated release of fluorophores from magnetic nanoparticles by hysteretic heating. J. Colloid Interface Sci. 2020, 571, 348–355. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Harris, M.T. Surface modification of magnetic nanoparticles capped by oleic acids: Characterization and colloidal stability in polar solvents. J. Colloid Interface Sci. 2006, 293, 401–408. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Gao, W.; Dickinson, L.; Grozinger, C.; Morin, F.G.; Reven, L. Self-Assembled Monolayers of Alkylphosphonic Acids on Metal Oxides. Langmuir 1996, 12, 6429–6435. [Google Scholar] [CrossRef]
- McElwee, J.; Helmy, R.; Fadeev, A.Y. Thermal stability of organic monolayers chemically grafted to minerals. J. Colloid Interface Sci. 2005, 285, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Kalska-Szostko, B.; Wykowska, U.; Satula, D.; Nordblad, P. Thermal treatment of magnetite nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, Q.; Wang, J. Thermodynamic Analysis of Decomposition of Thiourea and Thiourea Oxides. J. Phys. Chem. B 2005, 109, 17281–17289. [Google Scholar] [CrossRef] [PubMed]
- Roca, A.G.; Marco, J.F.; Morales, M.d.P.; Serna, C.J. Effect of Nature and Particle Size on Properties of Uniform Magnetite and Maghemite Nanoparticles. J. Phys. Chem. C 2007, 111, 18577–18584. [Google Scholar] [CrossRef]
- Patsula, V.; Moskvin, M.; Dutz, S.; Horák, D. Size-dependent magnetic properties of iron oxide nanoparticles. J. Phys. Chem. Solids 2016, 88, 24–30. [Google Scholar] [CrossRef]
- Radoń, A.; Drygała, A.; Hawełek, Ł.; Łukowiec, D. Structure and optical properties of Fe3O4 nanoparticles synthesized by co-precipitation method with different organic modifiers. Mater. Charact. 2017, 131, 148–156. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Davis, K.; Qi, B.; Witmer, M.; Kitchens, C.L.; Powell, B.A.; Mefford, O.T. Quantitative Measurement of Ligand Exchange on Iron Oxides via Radiolabeled Oleic Acid. Langmuir 2014, 30, 10918–10925. [Google Scholar] [CrossRef]
- Davis, K.; Cole, B.; Ghelardini, M.; Powell, B.A.; Mefford, O.T. Quantitative Measurement of Ligand Exchange with Small-Molecule Ligands on Iron Oxide Nanoparticles via Radioanalytical Techniques. Langmuir 2016, 32, 13716–13727. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Karlsruhe, B.A. Topas 5.1. General Profile and Structure Analysis Software for Powder Diffraction Data; Bruker AXS Karlsruhe: Karlsruhe, Germany, 2014. [Google Scholar]

| Samples or Fragments | Results from CHN Analysis [Mass %] | Mass % Relation in the Samples or Fragments | ||
|---|---|---|---|---|
| C | H | N | C:H:N | |
| Oleic acid (C18H34O2) 1 | 76.54 | 12.13 | 0 | 1:0.16:0 |
| N-(10-Maleimidodecyl) fragment (C14H22NO2) 1 | 71.15 | 9.38 | 5.92 | 1:0.13:0.83 |
| Furfuryl-modified rhodamine B attached to N-(10-maleimidodecyl) fragment (C34H37ClN4O4S) 1 | 64.49 | 5.89 | 8.85 | 1:0.91:0.13 |
| OA@FexOy | 9.77 | 1.56 | 0 | 1:0.16:0 |
| P@FexOy | 14.43 | 2.32 | 0.74 | 1:0.16:0.51 |
| RhB@FexOy | 16.85 | 2.52 | 1.03 | 1:0.14:0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Kickelbick, G. Reversible Diels–Alder Reactions with a Fluorescent Dye on the Surface of Magnetite Nanoparticles. Molecules 2021, 26, 877. https://doi.org/10.3390/molecules26040877
He S, Kickelbick G. Reversible Diels–Alder Reactions with a Fluorescent Dye on the Surface of Magnetite Nanoparticles. Molecules. 2021; 26(4):877. https://doi.org/10.3390/molecules26040877
Chicago/Turabian StyleHe, Siyang, and Guido Kickelbick. 2021. "Reversible Diels–Alder Reactions with a Fluorescent Dye on the Surface of Magnetite Nanoparticles" Molecules 26, no. 4: 877. https://doi.org/10.3390/molecules26040877
APA StyleHe, S., & Kickelbick, G. (2021). Reversible Diels–Alder Reactions with a Fluorescent Dye on the Surface of Magnetite Nanoparticles. Molecules, 26(4), 877. https://doi.org/10.3390/molecules26040877