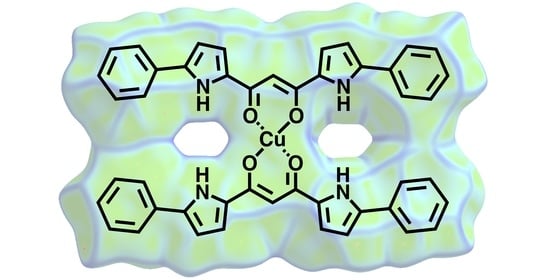
Supramolecular Assemblies of Dipyrrolyldiketone CuII Complexes
Abstract
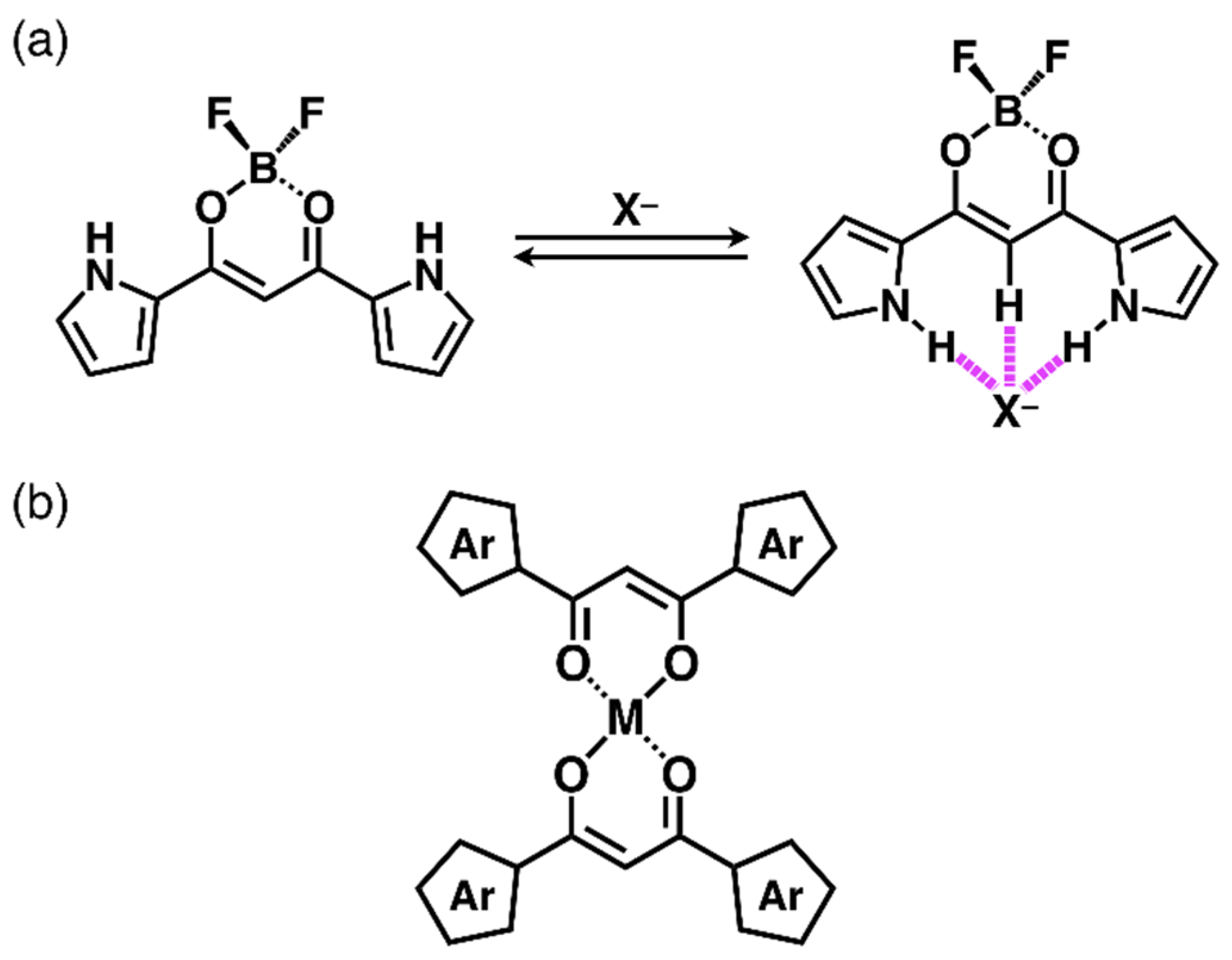
1. Introduction
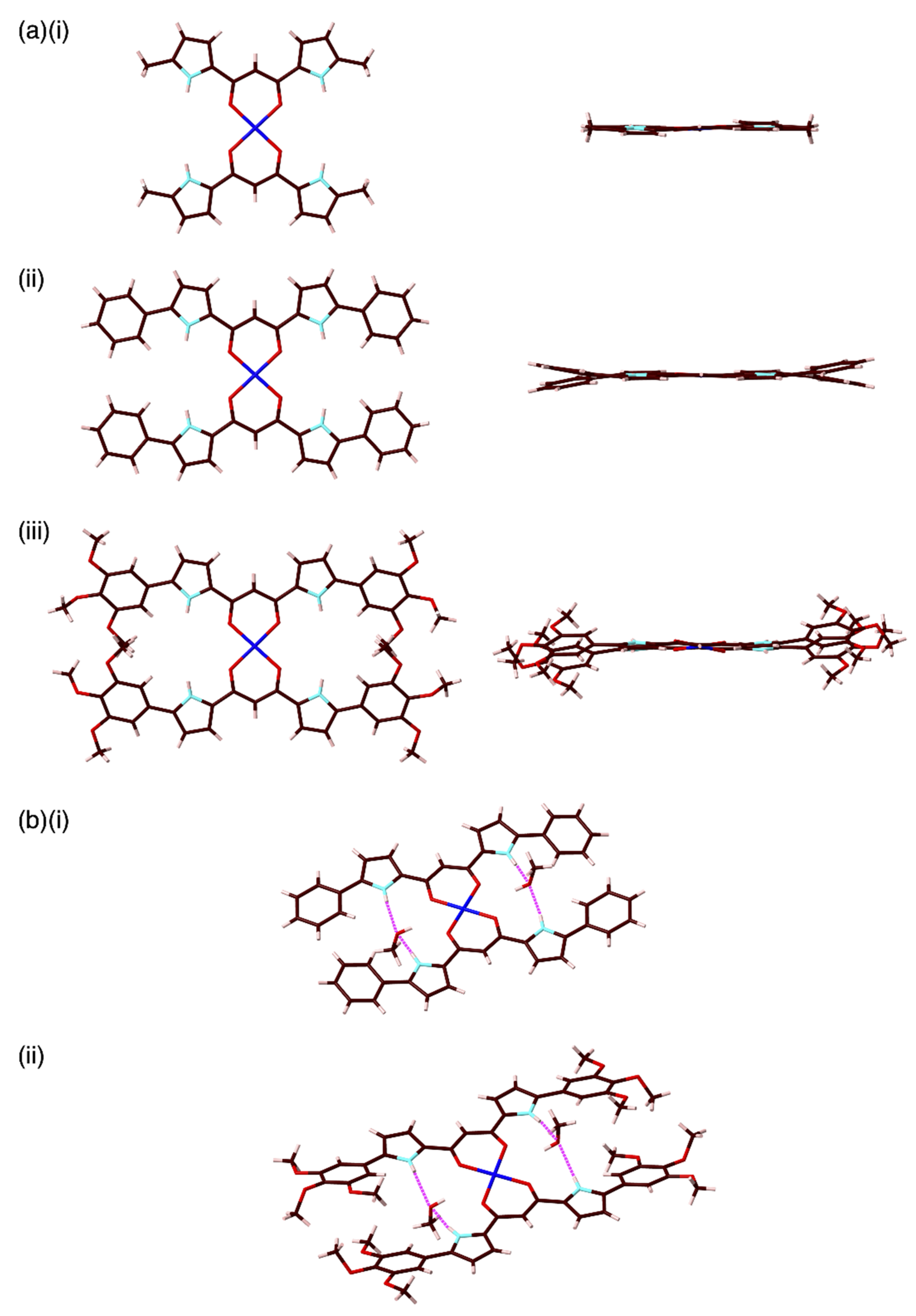
2. Results and Discussion
3. Materials and Methods
3.1. Synthetic Procedures and Spectroscopic Data
3.1.1. General Procedures
3.1.2. 1,3-Bis(5-(3,4-dimethoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2e′)
3.1.3. 1-tert-Butoxycarbonyl-2-(3,4-dihexadecyloxyphenyl)pyrrole and 2-(3,4-dihexadecyloxyphenyl)pyrrole
3.1.4. 1,3-Bis(5-(3,4-dihexadecyloxyphenyl)pyrrol-2-yl)-1,3-propanedione (3a′)
3.1.5. CuII complex of 1,3-di(5-methylpyrrol-2-yl)-1,3-propanedione (1a)
3.1.6. CuII Complex of 1,3-bis(3,4-diethylpyrrol-2-yl)-1,3-propanedione (1b)
3.1.7. CuII Complex of 1,3-di(5-phenylpyrrol-2-yl)-1,3-propanedione (2a)
3.1.8. CuII complex of 1,3-di(5-(2-methoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2b)
3.1.9. CuII complex of 1,3-di(5-(3-methoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2c)
3.1.10. CuII complex of 1,3-di(5-(4-methoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2d)
3.1.11. CuII complex of 1,3-bis(5-(3,4-dimethoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2e)
3.1.12. CuII complex of 1,3-bis(5-(3,4,5-trimethoxyphenyl)pyrrol-2-yl)-1,3-propanedione (2f)
3.1.13. CuII complex of 1,3-bis(5-(3,4-dihexadecyloxyphenyl)pyrrol-2-yl)-1,3-propanedione (3a)
3.1.14. CuII complex of 1,3-bis(5-(3,4,5-trihexadecyloxyphenyl)pyrrol-2-yl)-1,3-propanedione (3b)
3.2. Method for Single-Crystal X-ray Analysis
3.3. DFT Caluculations
3.4. Differential Scanning Calorimetry (DSC)
3.5. Polarizing Optical Microscopy (POM)
3.6. Synchrotron X-ray Diffraction Analysis (XRD)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| NMR | Nuclear magnetic resonance |
| MALDI-TOF-MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| ESI-TOF-MS | Electrospray ionization time-of-flight mass spectrometry |
| DFT | Density functional theory |
| DSC | Differential scanning calorimetry |
| POM | Polarized optical microscopy |
| XRD | X-ray diffraction |
| TLC | Thin-layer chromatography |
References and Notes
- Koch, N. Supramolecular Materials for Opto-Electronics; The Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar] [CrossRef]
- Haketa, Y.; Maeda, H. Dimension-Controlled π-Electronic Ion-Pairing Assemblies. Bull. Chem. Soc. Jpn. 2018, 91, 420–436. [Google Scholar] [CrossRef]
- Haketa, Y.; Urakawa, K.; Maeda, H. First decade of π-electronic ion-pairing assemblies. Mol. Syst. Des. Eng. 2020, 5, 757–771. [Google Scholar] [CrossRef]
- Haketa, Y.; Sasaki, S.; Ohta, N.; Masunaga, H.; Ogawa, H.; Mizuno, N.; Araoka, F.; Takezoe, H.; Maeda, H. Oriented salts: Dimension-controlled charge-by-charge assemblies from planar receptor–anion complexes. Angew. Chem. Int. Ed. 2010, 49, 10079–10083. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Naritani, K.; Honsho, Y.; Seki, S. Anion modules: Building blocks of supramolecular assemblies by combination with π-conjugated anion receptors. J. Am. Chem. Soc. 2011, 133, 8896–8899. [Google Scholar] [CrossRef]
- Haketa, Y.; Honsho, Y.; Seki, S.; Maeda, H. Ion materials comprising planar charged species. Chem. Eur. J. 2012, 18, 7016–7020. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Sakurai, T.; Honsho, Y.; Seki, S.; Maeda, H. Cation modules as building blocks forming supramolecular assemblies with planar receptor-anion complexes. J. Am. Chem. Soc. 2013, 135, 1284–1287. [Google Scholar] [CrossRef]
- Dong, B.; Sakurai, T.; Bando, Y.; Seki, S.; Takaishi, K.; Uchiyama, M.; Muranaka, A.; Maeda, H. Ion-based materials derived from positively and negatively charged chloride complexes of π-conjugated molecules. J. Am. Chem. Soc. 2013, 135, 14797–14805. [Google Scholar] [CrossRef] [PubMed]
- Vigato, P.A.; Peruzzo, V.; Tamburini, S. The evolution of β-diketophenol ligands and related complexes. Coord. Chem. Rev. 2009, 253, 1099–1201. [Google Scholar] [CrossRef]
- Brock, A.J.; Clegg, J.K.; Li, F.; Lindoy, L.F. Recent developments in the metallo-supramolecular chemistry of oligo-β-diketonato ligands. Coord. Chem. Rev. 2018, 375, 106–133. [Google Scholar] [CrossRef]
- Zheng, H.; Lai, C.K.; Swager, T.M. Controlling intermolecular interactions between metallomesogens: Side-chain effects in discotic copper, palladium, and vanadyl bis(β-diketonates. Chem. Mater. 1995, 7, 2067–2077. [Google Scholar] [CrossRef]
- Ohta, K.; Yokoyama, M.; Kusubayashi, S.; Mikawa, H. Square-planar trans-Bis-(1-p-n-octylphenylbutane-1,3-dionato)copper(II), a new compound exhibiting three kinds of ‘double melting′ behaviour. J. Chem. Soc. Chem. Commun. 1980, 392–393. [Google Scholar] [CrossRef]
- Ohta, K.; Ishii, A.; Yamamoto, I.; Matsuzaki, K. Discotic liquid crystals of organocopper complexes: The substituent effects. J. Chem. Soc. Chem. Commun. 1984, 1099–1101. [Google Scholar] [CrossRef]
- Thompson, N.J.; Goodby, J.W.; Toyne, K.J. Liquid-crystalline polymesomorphism in copper(II) complexes of β-diketones. Mol. Cryst. Liq. Cryst. 1992, 213, 187–205. [Google Scholar] [CrossRef]
- Poelsma, S.N.; Servante, A.H.; Fanizzi, F.P.; Maitlis, P.M. Discotic metallomesogens: Synthesis and properties of square planar metal bis(β-diketonate) complexes. Liq. Cryst. 1994, 16, 675–685. [Google Scholar] [CrossRef]
- Prasad, V.; Sadashiva, B.K. Liquid crystalline behaviour in some homologous series of β-diketones and a few of their copper(II) and palladium(II) complexes. Mol. Cryst. Liq. Cryst. 1995, 268, 89–100. [Google Scholar] [CrossRef]
- Schmidt, A.; Heinrich, B.; Kirscher, G.; Chaumont, A.; Henry, M.; Kyritsakas, N.; Haketa, Y.; Maeda, H.; Mobian, P. Dipyrrolyldiketonato Titanium(IV) complexes from monomeric to multinuclear architectures: Synthesis, stability and liquid crystal properties. Inorg. Chem. 2020, 59, 12802–12816. [Google Scholar] [CrossRef]
- PtII complexes of dipyrrolyldiketones have also been prepared and will be reported elsewhere.
- Maeda, H.; Terasaki, M.; Haketa, Y.; Mihashi, Y.; Kusunose, Y. BF2 complexes of α-alkyl-substituted dipyrrolyldiketones as acyclic anion receptors. Org. Biomol. Chem. 2008, 6, 433–436. [Google Scholar] [CrossRef]
- Maeda, H.; Kusunose, Y.; Mihashi, Y.; Mizoguchi, T. BF2 Complexes of β-tetraethyl-substituted dipyrrolyldiketones as anion receptors: Potential building subunits for oligomeric systems. J. Org. Chem. 2007, 72, 2612–2616. [Google Scholar] [CrossRef]
- Maeda, H.; Haketa, Y.; Nakanishi, T. Aryl-substituted C3-bridged oligopyrroles as anion receptors for formation of supramolecular organogels. J. Am. Chem. Soc. 2007, 129, 13661–13674. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Eifuku, N. Alkoxy-substituted derivatives of π-conjugated acyclic anion receptors: Effects of substituted positions. Chem. Lett. 2009, 38, 208–209. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017. [Google Scholar]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R.T. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. Sect. E 2019, 75, 308–318. [Google Scholar] [CrossRef]
- Similar characteristic Hirshfeld surfaces were observed in the crystal-state π–π stacking structures of large aromatic hydrocarbons such as hexabenzocoronene: See also [26].
- Tiekink, E.R.T.; Zukerman-Schpector, J. The importance of Pi-Interactions in Crystal Engineering: Frontiers in Crystal Engineering; Wiley: Chichester, UK, 2012. [Google Scholar] [CrossRef]
- For the elucidation of the mesophase structures: Ohta, K. Physics and Chemistry of Molecular Assemblies; World Scientific: Singapore, 2020. [Google Scholar] [CrossRef]
- Belov, D.S.; Ivanov, V.N.; Curreli, F.; Kurkin, A.V.; Altieri, A.; Debnath, A.K. Synthesis of 5-arylpyrrole-2-carboxylic acids as key intermediates for nbd series hiv-1-entry inhibitors. Synthesis 2017, 49, 3692–3699. [Google Scholar] [CrossRef]
- Herod, J.D.; Bates, M.A.; Whitwood, A.C.; Bruce, D.W. Ionic N-phenylpyridinium tetracatenar mesogens: Competing driving forces in mesophase formation and unprecedented difference in phase stabilization within a homologous series. Soft Matter 2019, 15, 4432–4436. [Google Scholar] [CrossRef]
- Maeda, H.; Kusunose, Y.; Terasaki, M.; Ito, Y.; Fujimoto, C.; Fujii, R.; Nakanishi, T. Micro- and nanometer-scale porous, fibrous, and sheet architectures constructed by supramolecular assemblies of dipyrrolyldiketones. Chem. Asian J. 2007, 2, 350–357. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Yadokari-XG; Software for Crystal Structure Analyses; Wakita, OK, USA, 2001.
- Kabuto, C.; Akine, S.; Nemoto, T.; Kwon, E. Release of software (Yadokari-XG 2009) for crystal structure analyses. J. Cryst. Soc. Jpn. 2009, 51, 218–224. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haketa, Y.; Maeda, H. Supramolecular Assemblies of Dipyrrolyldiketone CuII Complexes. Molecules 2021, 26, 861. https://doi.org/10.3390/molecules26040861
Haketa Y, Maeda H. Supramolecular Assemblies of Dipyrrolyldiketone CuII Complexes. Molecules. 2021; 26(4):861. https://doi.org/10.3390/molecules26040861
Chicago/Turabian StyleHaketa, Yohei, and Hiromitsu Maeda. 2021. "Supramolecular Assemblies of Dipyrrolyldiketone CuII Complexes" Molecules 26, no. 4: 861. https://doi.org/10.3390/molecules26040861
APA StyleHaketa, Y., & Maeda, H. (2021). Supramolecular Assemblies of Dipyrrolyldiketone CuII Complexes. Molecules, 26(4), 861. https://doi.org/10.3390/molecules26040861